Introduction
Bone is a dynamic structure that is undergoing
constant change and remodeling in response to the changing
environment. Under conditions such as long bed rest, lack of
exercise or microgravity, there is a long-term reduction in the
external force acting on the bone, resulting in a significant
reduction of bone mass (1).
Conversely, an increase in external forces acting upon the bone can
increase the net accumulation of bone (2). Various in vitro loading
techniques, such as cell swelling, substrate stretch loading and
fluid shear stress (FSS), are used in the study of osteocyte
response to mechanical stimulation. However, the majority of
studies do not recapitulate osteoblast anabolism under mechanical
stimulation, including the activation of intracellular chloride
ions, chloride channel genes and channel proteins (3–7). In fact,
studies have shown that numerous ion channels [such as
Ca2+ channels (8) and
K+ channels (9)] have a
role in osteogenic differentiation. ClC-3 is a subtype of the
volume-sensitive chloride channel family that has a role in cell
volume regulation (10–12), as well as in cell proliferation
(13), apoptosis (10), cell cycle progression (14) and intracellular acidification
regulation in the osteoclast (15).
Studies have also reported the involvement of chloride channels in
the metabolism of osteoblasts, for example, ClC-3 protein
expression can promote osteogenesis via Runx2 gene expression in
mouse osteoblasts (16). However, the
physiological mechanism of CLC-3 in the dynamic structure of
osteoblast remains to be elucidated. In the present study, the role
of ClC-3 was investigated in osteoblast response to mechanical
stress and report FSS regulatory cell volume decrease (RVD) via
ClC-3.
Materials and methods
Cell lines
Mouse embryonic osteogenic precursor cells
(MC3T3-E1) were obtained from the Institute of Basic Medical
Sciences, Chinese Academy of Medical Sciences (Beijing, China).
Extracellular perfusate and glass
microelectrode internal fluid
The isosmotic perfusate had an osmotic pressure of
300 mOsmol/l and was composed mainly of the following: 70 mmol/l
NaCl, 0.5 mmol/l MgCl2, 2 mmol/l CaCl2, 10
mmol/l HEPES and 140 mmol/l D-mannitol. The extracellular perfusate
was adjusted to pH 7.4 using a Tris-base solution. The hypotonic
solution (Hypo) had an osmotic pressure of 160 mOsmol/l and had the
same composition as the isosmotic perfusate, except for the
omission of D-mannitol. The electrode internal liquid contained 70
mmol/l NMDG-Cl, 1.2 mmol/l MgCl2, 10 mmol/l HEPES, 1
mmol/l EGTA, 140 mmol/l D-mannitol and 2 mmol/l ATP. Hydrochloric
acid was used to adjust to pH 7.25. A freezing point osmometer
(Advanced™ 3250; Advanced Instruments, Inc., Norwood, MA, USA) was
used to detect osmotic pressure following liquid preparation.
Cell culture
MC3T3-E1 cells were cultured in DMEM (Gibco, Grand
Island, NY, USA) containing 10% fetal bovine serum (Gibco) in an
incubator maintained at 5% CO2, 37°C and saturated
humidity. Routine cell passaging was performed to maintain the cell
line. Cells were digested prior to the experiment, and the cell
suspension was seeded in a plastic plate with a diameter of 35 mm.
The cells attached as a monolayer and were placed in the incubator.
After 10 min of cell adherence, patch clamping was performed. The
experimental group was stimulated with FSS using the FSS device.
The cells were stimulated once every 24 h for a total of 48 h, and
each stimulus comprised a loading time of 30 min. Cells in the
control group were not administered mechanical stimulation,
however, they were also cultured in the square groove in the middle
of the flow chamber system. Aside from the lack of FSS stimulation,
the control group had the same physical environment as the
experimental group.
FSS loading
MC3T3-E1 cells were seeded on rectangular cover
glass slips (50×20×0.20 mm) at a density of 2×104
cells/cm2, and the coverslips were placed in culture
plates with 5 ml of complete medium. Cells were cultured overnight
to allow for cell adherence and fusion. In the experimental group,
the cover glass was subsequently placed in a square groove in the
middle of the flow chamber system (which was previously sterilized
by ultraviolet irradiation for 1 h), thus forming a closed loop
with the other components of the perfusion system. Circulation of
the perfusate was promoted by the power resulting from the negative
pressure of the peristaltic pump. The association between FSS and
the flow rate of the osteoblast and the flow chamber was τ =
6Qµ/bh2, where τ was the FSS at the bottom of the
chamber (namely, the forces acting upon the cells)
(dyne/cm2), Q was the flow rate of circulation fluid
(cm3/sec), µ was the viscosity of the circulation fluid
(0.01 dynes/cm2), b was the width of the chamber (2 cm)
and h was the height of the chamber (0.025 cm). A horizontal FSS
magnitude that was in accordance with the most effective way to
stimulate osteoblast differentiation was employed (8). The rotational speed of the peristaltic
pump was adjusted to 28 rpm, and the flow rate in the flow chamber
(Q) was 49.7 ml/min (0.833 cm3/sec). The magnitude of
the FSS (τ) at the bottom of the chamber was 1.2 Pa (1.2 Pa = 12
dyne/cm2).
Effect of FSS simulation on mRNA
expression of ClC-3 was quantified by quantitative polymerase chain
reaction (qPCR)
Cells from the experimental and control groups were
collected prior and subsequent to mechanical stimulation and washed
two times with phosphate-buffered saline. TRIzol (Invitrogen Life
Technologies, Carlsbad, CA, USA) was added to the samples and the
total RNA was extracted. Reverse transcription kits (DRR037S;
Takara Bio, Dalian, China) were used for cDNA synthesis, and SYBR®
Premix Ex Taq™ (Takara Bio) was used to detect the mRNA expression
levels of ClC-3. The total volume of the cDNA (10–30 ng) reaction
system was 20 µl. The thermal cycling reaction conditions were as
follows: 95°C for 30 sec; followed by 95°C for 5 sec and 60°C for
34 sec, for a total of 45 cycles. Fluorescence quantitative
analysis was used for relative quantitation. The
2−ΔΔCt formula was used for data
analysis.
Cell volume measurement
MC3T3-E1 cells in the logarithmic growth phase were
digested by conventional methods and prepared as a cell suspension.
Cells from the experimental and control groups were separately
seeded in the perfusion groove of a custom-made culture plate, and
after 20 min of cell adhesion, the experiment was conducted. An
inverted phase contrast microscope (DMI6000 B; Leica, Mannheim,
Germany) was used in fixed view mode to continuously acquire cell
images. Image-Pro Plus (Media Cybernetics, Rockville, MD, USA) was
used to control image acquisition. The perfusion speed was set at 4
ml/min. Cells were soaked in isotonic solution for 5 min, and the
baseline cell volume was observed. The cells were subsequently
perfused with Hypo for 20 min and subsequently re-perfused with
isotonic solution for 5 min. Subsequent to the experiment,
Image-Pro Plus was used to analyze and measure cell volume.
Standardized cell volume (Vst) was calculated according to the
formula Vst = (Vt/Vo) × 100, where Vt was the cell volume measured
in real time and Vo was the average volume of the same cell under
isotonic conditions. All the experiments were performed at room
temperature (20–24°C).
Determination of intracellular
chloride ion concentration
A chloride ion fluorescence probe was used to detect
and analyze the chloride channel activity of MC3T3-E1 cells prior
and subsequent to mechanical stimulation. The chloride ion
fluorescent probe
N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide (MQAE)
(Beyotime Institute of Biotechnology, Shanghai, China) was excited
at a wavelength of 355 nm, and emitted light was collected at a
wavelength of 460 nm. The fluorescence intensity of the probe was
inversely proportional to the chloride ion concentration. MC3T3-E1
cells were digested with trypsin prior and subsequent to mechanical
stimulation and were subsequently seeded in a culture plate
specifically designed for confocal microscopy. After 12 h of cell
adhesion, the medium was replaced with culture medium containing 5
mmol/l MQAE, and the cells were cultured in a 37°C incubator for 1
h. The cells were washed, and the intracellular MQAE fluorescence
was observed with a fluorescence microscope.
Whole-cell patch clamp recording
Borosilicate glass capillary tubes (outside
diameter, 1.5 mm; inside diameter, 0.86 mm) were used for
multiple-step drawing in an electrode puller (P-2000; Sutter
Instruments, Novato, CA, USA). The electrode was filled with
electrode fluid, and the resistance at the electrode tip was 5–10
MΩ. The whole-cell membrane current of a single cell was amplified
through an EPC-9 amplifier (HEKA Elektronik Dr. Schulze GmbH,
Lambrecht, Germany), and via filtering by 2.9 kHz, the currents
were recorded to the Pulse and Pulsefit software system (HEKA
Elektronik Dr. Schulze GmbH). Whole-cell patch clamp recording
parameters were as follows (11,17): Under
the voltage clamp mode, the cells were clamped at 0, ±40 and ±80
mV. Each clamp pulse was held for 200 msec with an interval gap of
4 sec. The experiment was performed at room temperature (20–24°C).
Following recording the whole-cell current, a stable background
current was recorded. Hypo solution was perfused over the cells to
induce a volume-sensitive chloride current. When the current
reached a stable peak value, a chloride-channel blocker was added,
and the recording was ended when the current reached a stable
minimum value.
Statistical analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used
for variance analysis on all the data with a significance level of
α=0.05, and P<0.05 was considered to indicate a statistically
significant difference. The data are presented as mean ± standard
deviation.
Results
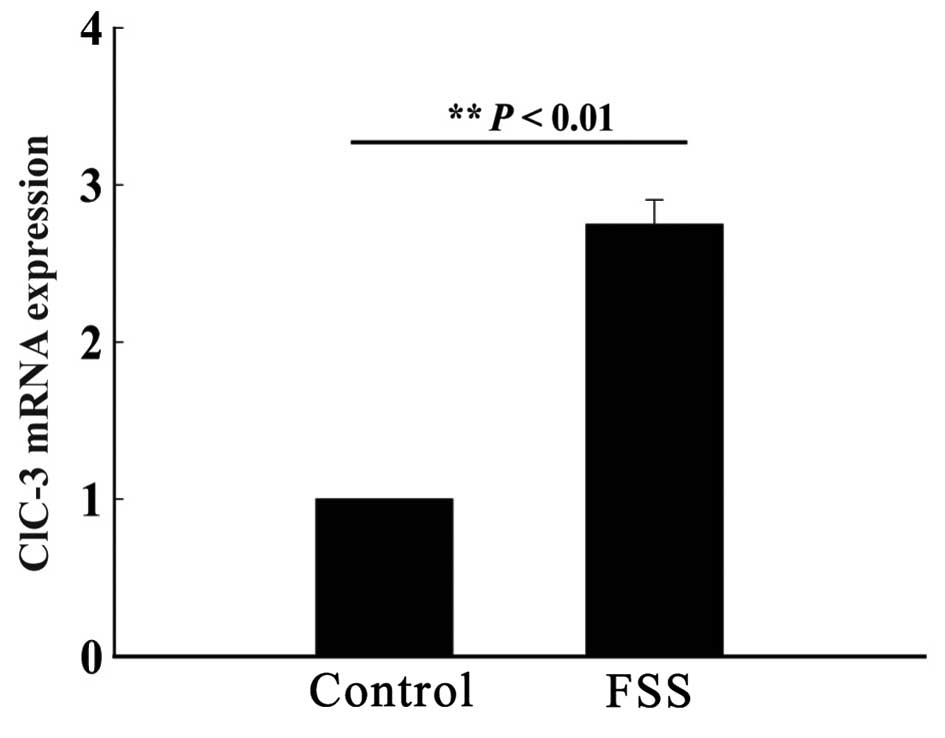
FSS simulation increases mRNA
expression of ClC-3
An FSS device was used to stimulate MC3T3-E1
osteoblasts by FSS once every 24 h for a total of 48 h, with each
stimulation having a duration of 30 min. A control group of
osteoblasts was cultured in the same physical environment, without
FSS stimulation. After 48 h, the mRNA expression of ClC-3 was
quantified. The results showed a significantly higher mRNA
expression in the experimental group compared to the control group,
suggesting that osteoblasts are sensitive to FSS and can increase
the gene expression of ClC-3 (n=3; P<0.01; Fig. 1).
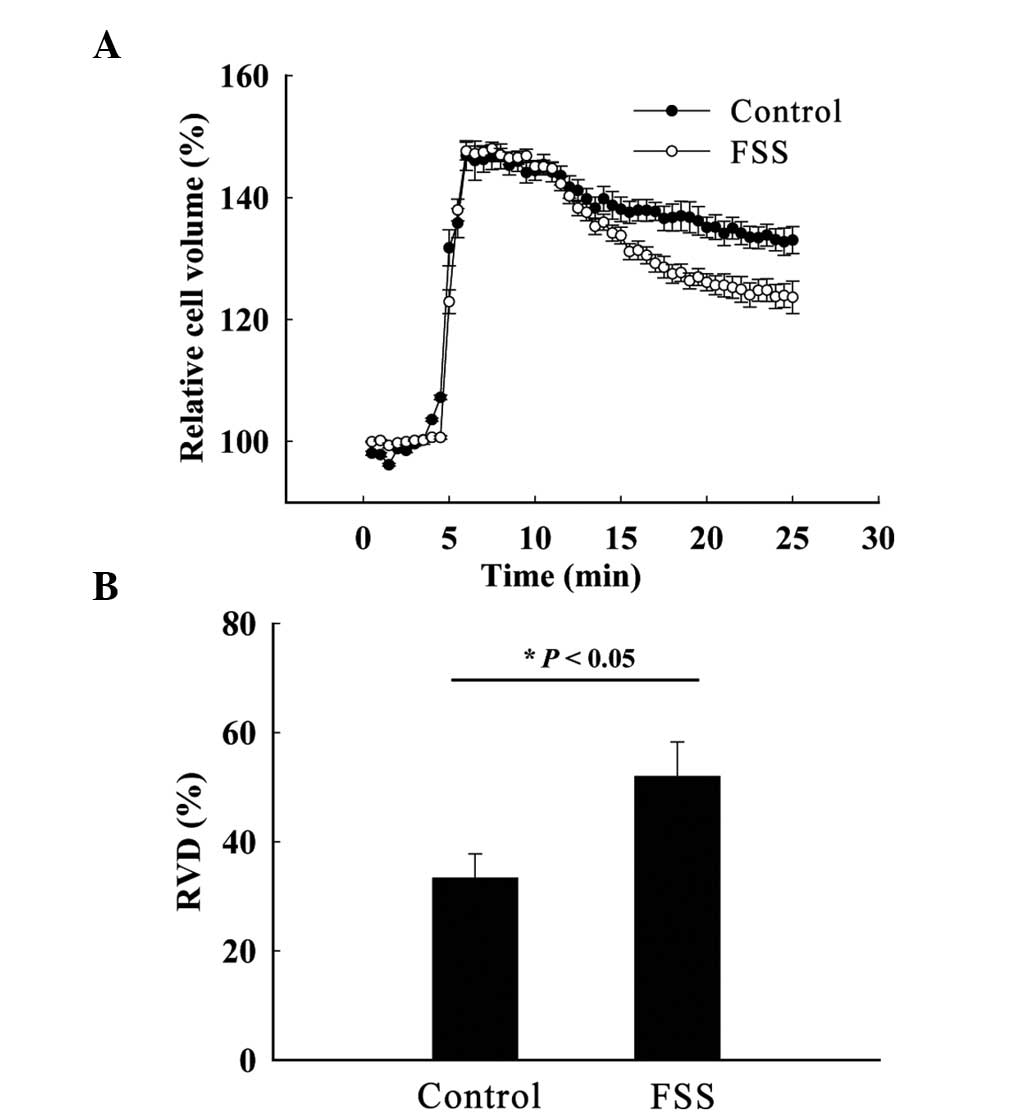
FSS stimulation enhances RVD in
MC3T3-E1 osteoblasts
Cell volumes of the control and experimental groups
were maximally swollen ~5 min after hypotonic stimulation and the
standardized cell volumes were 148.00±1.05 and 146.63±2.45%,
respectively; chloride channels are subsequently activated by cell
volume swelling, allowing chloride ions to flow out of the cell and
to drive the efflux of water, and the RVD is activated (Fig. 2A). Following stimulation, the RVD of
the control and experimental groups were 33.33±4.45 and
51.94±6.35%, respectively (n=3; P<0.05) (Fig. 2B). Combined with the qPCR data, these
results suggest that the expression of osteoblast chloride channels
is increased by mechanical stimulation. The increase in chloride
channel expression results in increased chloride ion efflux and
enhanced osteoblast RVD.
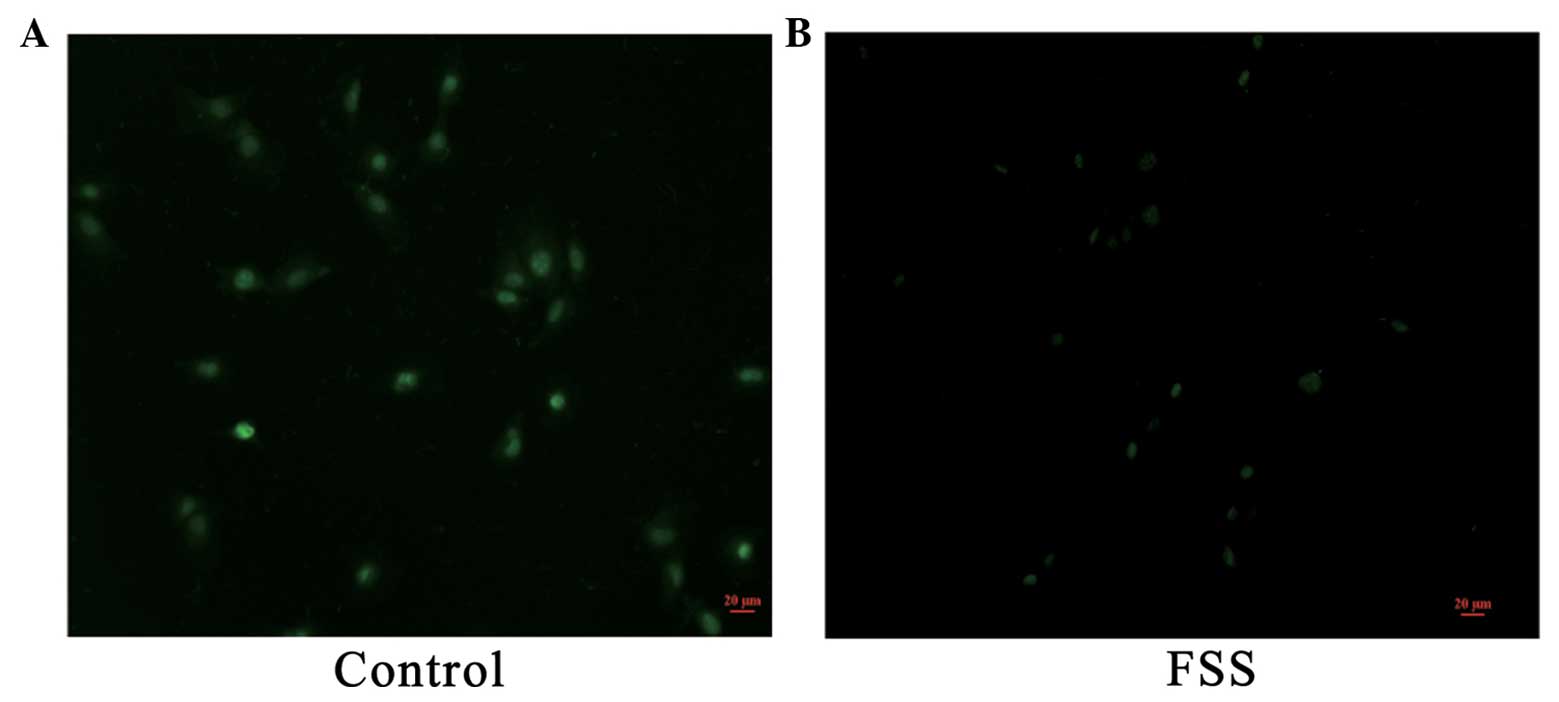
FSS stimulation induces chloride ion
efflux in MC3T3-E1 osteoblasts
MQAE is a widely used chloride ion fluorescent
probe. MQAE fluorescence intensity decreases proportionally with
the increase of chloride ion concentration. MQAE was used to detect
and analyze the chloride channel activity of MC3T3-E1 cells prior
and subsequent to mechanical stimulation. The excitation wavelength
of MQAE was 355 nm, and the emission wavelength was 460 nm (green).
After 48 h of FSS stimulation, the MQAE fluorescence intensity of
the control group was stronger compared to the experimental groups
(Fig. 3), which implied that the
chloride channel activity of experimental groups was increased.
FSS stimulation increases the current
density of the volume-activated chloride current in MC3T3-E1
osteoblasts
Whole-cell patch clamping was used to monitor
changes in the volume-sensitive chloride current activated by a
hypotonic environment prior and subsequent to FSS stimulation. The
background current was low and stable in cells perfused with
isotonic solution, and the current was activated by hypotonic
solution (Fig. 4A). Under isotonic
solution, clamped at a voltage of +80 and −80 mV, the currents of
the control group were 5.56±1.39 and −3.67±2.25 pA/pF,
respectively, and the experimental group were 6.33±1.65 and
−3.85±2.73 pA/pF, respectively (n=6; P>0.05) (Fig. 4B). When the perfusion fluid was changed
to hypotonic solution, the cells swelled and the current increased,
potentially activating the volume-sensitive chloride current
increased significantly. Under clamping at +80 mV, the current
density of the control group was 58.16±3.20 pA/pF, and for the
experimental group was 81.34±5.87 pA/pF, (n=6; P<0.01). Under
clamping at −80 mV, the current density was −39.41±4.86 pA/pF in
the control group and −42.29±4.51 pA/pF in the experimental group
(n=6; P<0.05) (Fig. 4C).
Discussion
Bone metabolism has a strong regulatory system for
mechanical homeostasis, which adapts to the changed biomechanical
circumstances by constant self-regulation (18). Bone remodeling partly contributes to
the mechanical environment, such as body weight, loading, muscle
tension and physical activity. In bone tissue, sensors such as
osteoblasts, bone cells and bone lining cells can convert a
mechanical stimulation signal into biochemical signals. Bone can be
generated and reconstructed under mechanical stimulation. Cells
under static stretching do not engage in active bone remodeling;
only dynamic mechanical loading can cause significant anabolic
effects in vivo. The effects of tension on bone tissue are
affected by numerous factors, such as the magnitude, duration and
rate of the applied force. Generally, smaller and longer loading
forces result in similar protein synthesis as larger and shorter
forces (2). The majority of
investigators have hypothesized that the regulation of cell volume
relies on the participation of the chloride channel superfamily,
including the ClC-3. Mutations in these channels produce several
genetic diseases, including cardiovascular disease (19), hypertension (20), stroke (21) and osteosarcoma (22). ClC-7 has been reported as a late
endosomal to lysosomal chloride channel that has a key role in
acidifying the extracellular lysosome between osteoclasts and the
bone (23). The physiological role of
CLC-3 in the dynamic structure of bone remains to be
elucidated.
In the present study, FSS stimulation was used to
imitate the physiological stimulation of the skeleton and to
evaluate the changes in the volume-sensitive chloride channel.
After 48 h of intermittent FSS stimulation, the ClC-3 mRNA
expression of FSS group was significantly higher compared to the
control group, suggesting that osteoblasts are sensitive to
external FSS and can increase the gene expression of ClC-3. Cell
volume regulation is one of the basic physiological functions of
cells. In the hypotonic solution, cells initially swell,
subsequently decrease their volume through RVD, and finally return
to their baseline volume. Chloride ion efflux is a key component of
the RVD process, with ClC-3 considered a candidate volume-sensitive
chloride channel. The RVD capacity of FSS stimulated osteoblasts
was higher compared to that of the control group. Furthermore, the
chloride ion fluorescence probe MQAE was used to detect the
intracellular chloride ion concentration in osteoblasts following
FSS stimulation. Green fluorescence was significantly decreased
following stimulation, indicating that the chloride channel
activity was enhanced. These data, combined with the aforementioned
results, lead us to hypothesize that mechanical stimulation
increases the expression of osteoblast chloride channels. When
chloride channel expression is increased, chloride ion efflux is
increased due to hypotonic stimulation. The chloride ion
fluorescent probe in the present experiments showed that
intracellular chloride ion concentration was higher in the control
group compared to the FSS group. These experiments show that FSS
stimulation increases chloride channel expression, strengthens the
activity of chloride channels, increases chloride ion efflux,
decreases intracellular chloride ion concentration and increases
osteoblast RVD. Furthermore, whole-cell patch clamping was used to
detect changes in the volume-sensitive chloride current activated
by a hypotonic environment prior and subsequent to FSS. The results
show that following FSS treatment, the current density of the
volume-sensitive chloride current activated by a hypotonic
environment was significantly higher in the stimulated group
compared to the control group. These results suggest that FSS
treatment increases the expression of the osteoblast ClC-3 chloride
channel and increases the chloride current.
In the present study, the mechanism of mechanical
signal transduction was investigated in osteoblasts, focusing on
ion channels as signal transducers. FSS stimulation enhanced the
RVD of osteoblast cell by increasing the expression of the ClC-3
and activating the chloride channel. The study also provides
further insights into the roles of chloride channels in the
procession of osteoclasts metabolism and homeostasis.
References
|
1
|
Bikle DD, Sakata T and Halloran BP: The
impact of skeletal unloading on bone formation. Gravit Space Biol
Bull. 16:45–54. 2003.PubMed/NCBI
|
|
2
|
Burr DB, Robling AG and Turner CH: Effects
of biomechanical stress on bones in animals. Bone. 30:781–786.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dutta AK, Woo K, Khimji AK, Kresge C and
Feranchak AP: Mechanosensitive Cl-secretion in biliary epithelium
mediated through TMEM16A. Am J Physiol Gastrointest Liver Physiol.
304:G87–G98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heo J, Sachs F, Wang J and Hua SZ:
Shear-induced volume decrease in MDCK cells. Cell Physiol Biochem.
30:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qin KR, Xiang C and Cao LL: Dynamic
modeling for flow-activated chloride-selective membrane current in
vascular endothelial cells. Biomech Model Mechanobiol. 10:743–754.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dyrda A, Cytlak U, Ciuraszkiewicz A,
Lipinska A, Cueff A, Bouyer G, Egée S, Bennekou P, Lew VL and
Thomas SL: Local membrane deformations activate
Ca2+-dependent K+ and anionic currents in
intact human red blood cells. PLoS One. 5:e94472010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kefaloyianni E and Coetzee WA:
Transcriptional remodeling of ion channel subunits by flow
adaptation in human coronary artery endothelial cells. J Vasc Res.
48:357–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Genetos DC, Geist DJ, Liu D, Donahue HJ
and Duncan RL: Fluid shear-induced ATP secretion mediates
prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res.
20:41–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cha SK, Kim JH and Huang CL: Flow-induced
activation of TRPV5 and TRPV6 channels stimulates Ca(2+)-activated
K(+) channel causing membrane hyperpolarization. Biochim Biophys
Acta. 1833:3046–3053. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Li H, Yang L, Deng Z, Luo H, Ye
D, Bai Z, Zhu L, Ye W, Wang L, et al: The ClC-3 chloride channel
associated with microtubules is a target of paclitaxel in its
induced-apoptosis. Sci Rep. 3:26152013.PubMed/NCBI
|
|
11
|
Wang L, Chen L and Jacob TJC: The role of
ClC-3 in volume-activated chloride currents and volume regulation
in bovine epithelial cells demonstrated by antisense inhibition. J
Physiol. 524:63–75. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duan D, Cowley S, Horowitz B and Hume JR:
A serine residue in ClC-3 links phosphorylation-dephosphorylation
to chloride channel regulation by cell volume. J Gen Physiol.
113:57–70. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu L, Yang H, Zuo W, Yang L, Zhang H, Ye
W, Mao J, Chen L and Wang L: Differential expression and roles of
volume-activated chloride channels in control of growth of normal
and cancerous nasopharyngeal epithelial cells. Biochem Pharmacol.
83:324–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao J, Li X, Chen W, Xu B, Zhang H, Li H,
Wang L, Jin X, Zhu J, Lin G, et al: Cell cycle-dependent
subcellular distribution of ClC-3 in HeLa cells. Histochem Cell
Biol. 137:763–776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Ma W, Zhu L, Ye D, Li Y, Liu S, Li
H, Zuo W, Li B, Ye W, et al: ClC-3 is a candidate of the channel
proteins mediating acid-activated chloride currents in
nasopharyngeal carcinoma cells. Am J Physiol Cell Physiol.
303:C14–C23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Mao Y, Zhang B, Wang T, Li F, Fu
S, Xue Y, Yang T, Wen X, Ding Y, et al: Chloride channel ClC-3
promotion of osteogenic differentiation through Runx2. J Cell
Biochem. 111:49–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang L, Ye D, Ye W, Jiao C, Zhu L, Mao J,
Jacob TJ, Wang L and Chen L: ClC-3 is a main component of
background chloride channels activated under isotonic conditions by
autocrine ATP in nasopharyngeal carcinoma cells. J Cell Physiol.
226:2516–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robling AG and Turner CH: Mechanical
signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene
Expr. 19:319–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duan DD: The ClC-3 chloride channels in
cardiovascular disease. Acta Pharmacol Sin. 32:675–684. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi XL, Wang GL, Zhang Z, et al:
Alteration of volume-regulated chloride movement in rat
cerebrovascular smooth muscle cells during hypertension.
Hypertension. 49:1371–1377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang YP, Zhang H and Duan DD: Chloride
channels in stroke. Acta Pharmacol Sin. 34:17–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai S, Zhang T, Zhang D, Qiu G and Liu Y:
Volume-sensitive chloride channels are involved in cisplatin
treatment of osteosarcoma. Mol Med Rep. 11:2465–2470.
2015.PubMed/NCBI
|
|
23
|
Kornak U, Kasper D, Bösl MR, Kaiser E,
Schweizer M, Schulz A, Friedrich W, Delling G and Jentsch TJ: Loss
of the ClC-7 chloride channel leads to osteopetrosis in mice and
man. Cell. 104:205–215. 2001. View Article : Google Scholar : PubMed/NCBI
|