Introduction
When performing personal identification by short
tandem repeat (STR) analysis, the source of the DNA extracted from
the cadaver varies depending on the time passed since the point of
expiration (1–5). If fewer than 24 h have passed, DNA is
obtained from white blood cells. For bodies found within 2–5 days
of expiration, cartilage is typically used for extracting DNA; if
more than 5 days have passed, bone and other hard tissues are the
final option (1,3).
The use of poor quality DNA is a common challenge
for STR analysis (2,3,6). Tissue
necrosis, for example, results in DNA degradation and shortening
(7). Necrosis generally occurs more
readily in soft tissues than in hard tissues (1,3,8). For STR analysis, the most commonly used
hard tissue is bone, which preserves DNA more effectively than soft
tissue (1,9). However, extracting DNA from bone can be
a difficult and time-consuming process (1,3,9).
There are instances when bodies cannot be personally
identified using DNA extracted from bone. The environment at the
crime scene and the time elapsed since passing are the largest
contributing factors to identification failure (2,6,9). This failure in personal identification
can contribute toward the grief of relatives by disrupting aspects,
including inheritance management and life insurance claims. These
failures may also affect lawsuits in the case of murder.
Since enamel is the hardest tissue in the human body
(10), we hypothesized that teeth may
preserve DNA better than bone. In the present study, experiments
used porcine teeth and bone as mimics for human teeth and bone. The
environments of known crime scenes were imitated using 11 different
conditions and various timeframes, the longest being 1 year.
Polymerase chain reaction (PCR) was performed as the first step of
STR analysis and DNA quality was evaluated using primers for
porcine β-actin (ACTB) and mitochondrial DNA (mtDNA).
Additionally, inhibitors of PCR were tested in certain select
conditions.
Materials and methods
Experimental design
In the current study, the two hard tissues of bones
and teeth were compared. Porcine teeth and bones were selected
since they are easily obtained and have macroscopic features
similar to human teeth and bones. The environmental conditions for
teeth and bone exposure were designed by imitating the conditions
of various crime scenes based on biological evidence. DNA quality
was measured by the presence or absence of PCR products. PCR was
performed using primers for porcine ACTB to mimic human STR
analysis and porcine mtDNA to mimic human mitochondrial analysis.
The porcine ACTB PCR product size was designed to be longer
than the longest PCR product size derived from human STR analysis
(2). Similarly, the mitochondrial PCR
product size was designed by considering the length of the
mitochondria variable region (11).
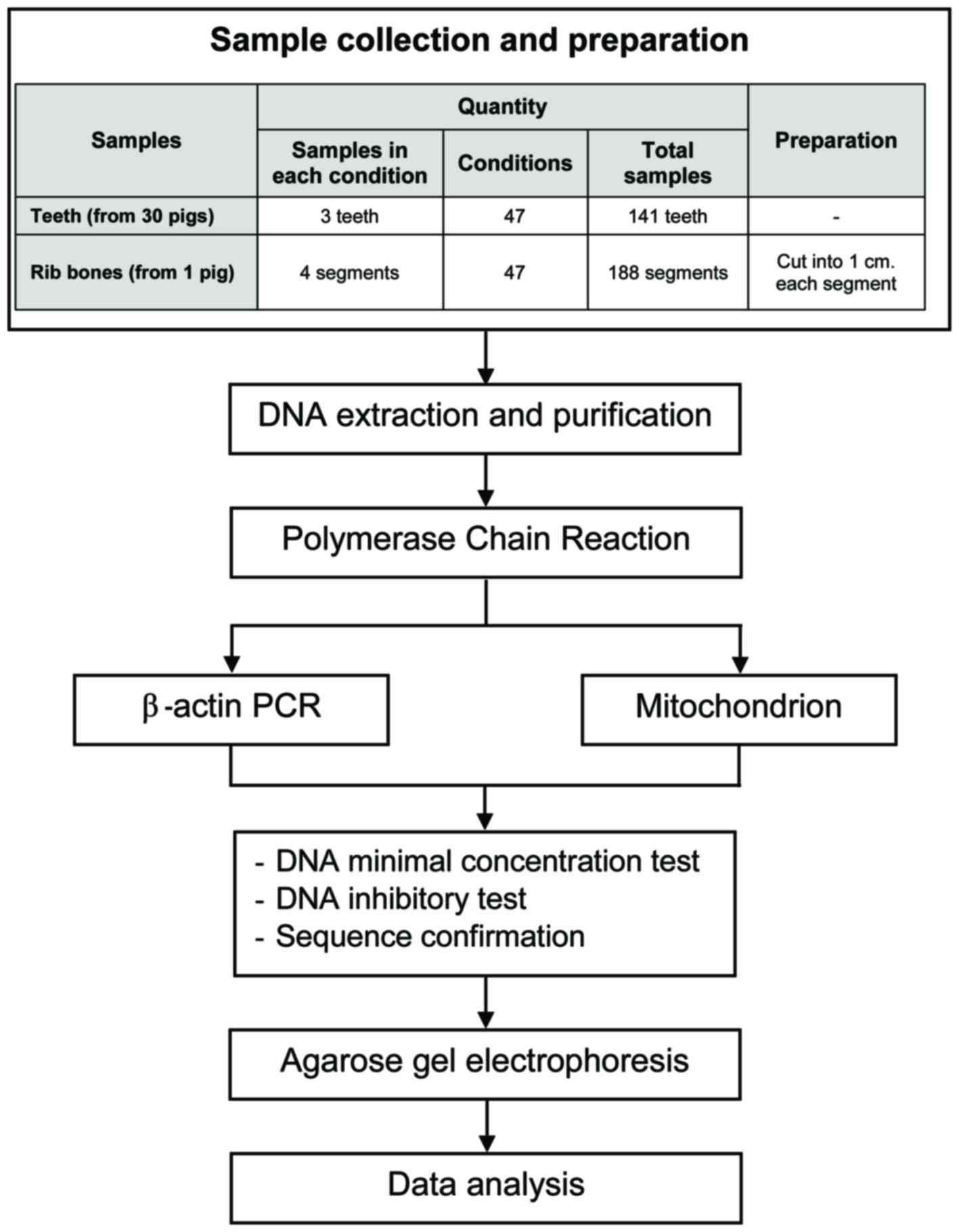
Additional details of the experimental design are depicted in
Fig. 1.
Sample selection and preparation
Porcine teeth and bones were obtained from remains
obtained from a local farm in Phanat Nikom, Chonburi; a period of
approximately 8–10 h transpired from slaughter to the extraction of
samples from the carcasses. A total of 141 teeth were extracted
from 30 animals; three teeth were selected randomly for testing in
each environmental condition. For the bone samples, four rib bones
were extracted from one animal and cut into 188 pieces of 1 cm
thickness; four bone samples were randomly selected for testing in
each environmental condition. As a positive control for the PCR
reactions, a soft tissue sample of porcine muscle was segmented and
stored at −20°C until DNA extraction. All animals were 3 to 4
months of age.
Environmental conditions and timelines
for the samples
The samples were exposed to 11 different
environmental conditions under the normal tropical climate
conditions of Bangkok, Thailand. They were left in open-air, buried
in soil, buried in sand (in situ in Phayathai, Bangkok,
Thailand), submerged in water (in situ in Bang Sue Canal,
Phayathai, Bangkok, Thailand), submerged in seawater (in
situ at Samaesan Pier, Sattahib, Chonburi, Thailand), soaked in
caustic soda (Merck KGaA, Darmstadt, Germany), soaked in sulfuric
acid (Merck KGaA), burnt with rubber (30 pieces of rubber sized 0.2
× 5.0 × 5.0 cm each, duration 3 h), and stored at 4, −20 and −80°C,
respectively (Table I). For the
majority of the conditions, DNA was extracted after 1 week, 1, 3
and 6 months, and 1 year. For the samples soaked in caustic soda or
sulfuric acid, DNA was extracted after 1 day, 1 week and 2 weeks.
For the samples burnt with rubber, DNA was extracted immediately
following the experiment.
 | Table I.Results of ACTB and mtDNA PCR
from bone and teeth DNA under various conditions. |
Table I.
Results of ACTB and mtDNA PCR
from bone and teeth DNA under various conditions.
|
| ACTB PCR | mthNA PCR |
|---|
|
|
|
|
|---|
| Condition | Bone | Teeth | Bone | Teeth |
|---|
| Positive control | + | + | + | + |
| Left in
open-air |
|
1 w | + | + | + | + |
|
1 m | + | + | + | + |
|
3 m | + | + | + | + |
|
6 m | + | + | + | + |
|
1 y | − | − | + | + |
| Buried in
soil |
|
1 w | + | − | + | − |
|
1 m | + | − | + | − |
|
3 m | − | − | + | − |
|
6 m | − | − | − | − |
|
1 y | − | + | + | + |
| Buried
in sand |
|
1 w | + | + | + | + |
|
1 m | + | + | + | + |
|
3 m | + | + | + | + |
|
6 m | − | − | − | + |
|
1 y | − | + | − | + |
|
Submerged in water |
|
1 w | + | + | + | + |
|
1 m | − | − | + | − |
|
3 m | − | − | − | − |
|
6 m | − | − | − | − |
|
1 y | − | − | − | − |
|
Submerged in seawater |
|
1 w | + | + | + | + |
|
1 m | − | − | − | − |
|
3 m | − | − | − | − |
|
6 m | − | − | − | − |
|
1 y | − | − | − | − |
| Soaked
in caustic soda |
|
1 d | − | + | − | + |
|
1 w | Samples could not
be collected |
|
2 w | Samples could not
be collected |
| Soaked
in sulfuric acid |
|
1 d | + | + | + | + |
|
1 w | − | − | − | − |
|
2 w | Samples could not
be collected |
| Burnt with
rubber | − | + | − | + |
| Stored
at 4°C |
|
1 w | + | + | + | + |
|
1 m | + | + | + | + |
|
3 m | + | + | + | + |
|
6 m | + | + | + | + |
|
1 y | + | + | + | + |
| Stored
at −20°C |
|
1 w | + | + | + | + |
|
1 m | + | + | + | + |
|
3 m | + | + | + | + |
|
6 m | + | + | + | + |
|
1 y | + | + | + | + |
| Stored
at −80°C |
|
1 w | + | + | + | + |
|
1 m | + | + | + | + |
|
3 m | + | + | + | + |
|
6 m | + | + | + | + |
|
1 y | + | + | + | + |
DNA extraction
Following removal of each sample from its respective
environmental condition at the designated time, it was crushed with
a hammer and stored overnight at 50°C with a lysis buffer (0.75
mol/l NaCl, 0.024 mol/l EDTA, pH 8.0) containing 10% sodium dodecyl
sulfate and 20 mg/ml proteinase K (Sigma-Aldrich; Merck KGaA). DNA
was extracted by a standard phenol-chloroform extraction protocol
(12,13). Following alcohol precipitation, the
DNA samples were air-dried at room temperature and resuspended in
50 µl distilled water. The DNA concentration was measured using a
NanoDrop 2000 spectrophotometer (ND-1000 Spectrophotometer;
NanoDrop Technologies; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Next, the isolated genomic DNA was eluted in distilled water.
The optical density 260/280 ratio was greater than 1.8, which is
acceptable for DNA purity and PCR (14). The detailed procedure is presented in
Fig. 2.
Primer design and PCR conditions
Primers to amplify ACTB were designed from the Sus
scrofa ACTB gene, partial coding sequence (NCBI sequence ID:
DQ452569.1; http://www.ncbi.nlm.nih.gov), which imitates the
product of the STR marker PCR reference from FBI CODIS Core STR
Loci, retrieved from https://strbase.nist.gov/coreSTRs.htm (April 1, 2017).
The length of the ACTB PCR product in the presesnt study was 475
bp, longer than the maximum PCR length of 464 bp, retrieved from
https://strbase.nist.gov/fbicore.htm
(April 1, 2017). The primer sequences were porcine ACTB
forward, 5′-AGATCGTGCGGGACATCAAG-3′ and porcine ACTB
reverse, 5′-GAGAGAAGCCCGACTGAGC-3′.
The mitochondrial primers were designed from the Sus
scrofa mtDNA sequence from mitochondrial isolate Y1, complete
genome (sequence ID: KT372134.1), which imitates the human
mitochondrial marker used to confirm the maternal lineage when
personal identification has failed (15). The length of the PCR product was 357
bp, which covers the hypervariable (HV) regions (HV1:16024-16365
and HV2:73-340). The primer sequences were porcine mtDNA forward,
5′-GGAGCAGTGTTCGCCATTAT-3′ and porcine mtDNA reverse,
5′-TTCTCGTTTTGATGCGAATG-3′.
The ACTB and mtDNA PCR reactions contained 1X
PCR buffer, 200 mM dNTPs, 0.2 mM of each primer, 0.5 U Taq DNA
polymerase (Qiagen, Inc., Valencia, CA, USA), and 50 ng template
DNA. The polymerase was activated by incubation at 95°C for 15 min,
followed by 40 cycles at 95°C for 1 min, 68°C for 1 min and 72°C
for 1 min, and a final extension at 72°C for 7 min. DNA extracted
from porcine muscle and distilled water were used as the positive
and negative PCR controls, respectively. Following amplification,
the PCR products were separated by gel electrophoresis using a 2%
agarose gel in Tris-borate-EDTA buffer, and then stained with
SYBR-Green nucleic acid gel stain (GelStar™; Lonza Group, Ltd.,
Basel, Switzerland) for 40 min at room temperature.
Designation of positive and negative DNA quality
depended upon visualization of the PCR products. The confirmation
of a PCR product from a minimum of one sample of a specific
environmental condition resulted in that sample type being counted
as positive for the condition. The results from a selection of the
DNA samples were confirmed by direct sequencing of the PCR products
(16) to verify the sequence
accuracy.
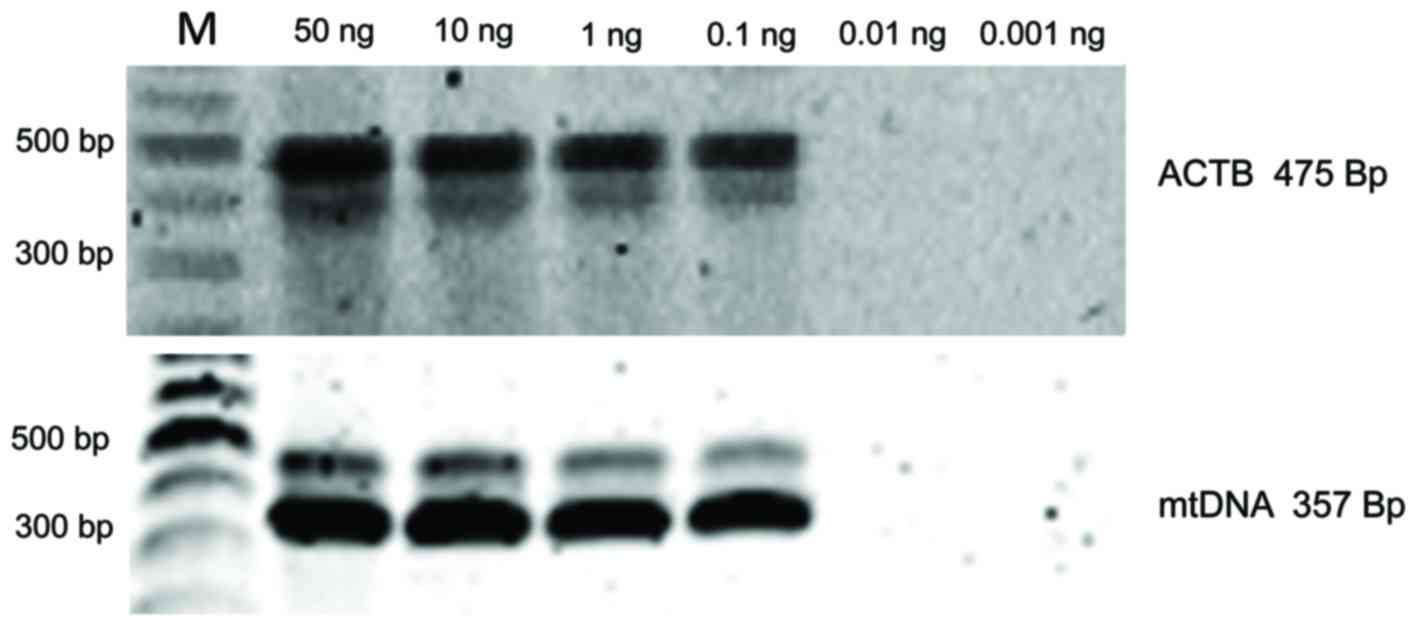
Tests to determine the minimal DNA
concentration and PCR inhibitory factors
The sensitivity of the ACTB and mtDNA PCR reactions
was tested by 10-fold serial dilution of the DNA template from 10
to 0.001 ng/µl. Certain unamplified DNA samples were randomly
tested for the presence of inhibitor components by mixing positive
control DNA and individual unamplified DNA templates equally in PCR
reactions.
Results
PCR amplification comparing DNA
samples purified from porcine bones and teeth
Certain PCR samples were submitted for direct
sequencing. The sequencing results revealed 100% similarity to the
original template DNA. All results are summarized in Fig. 3 and Table
I.
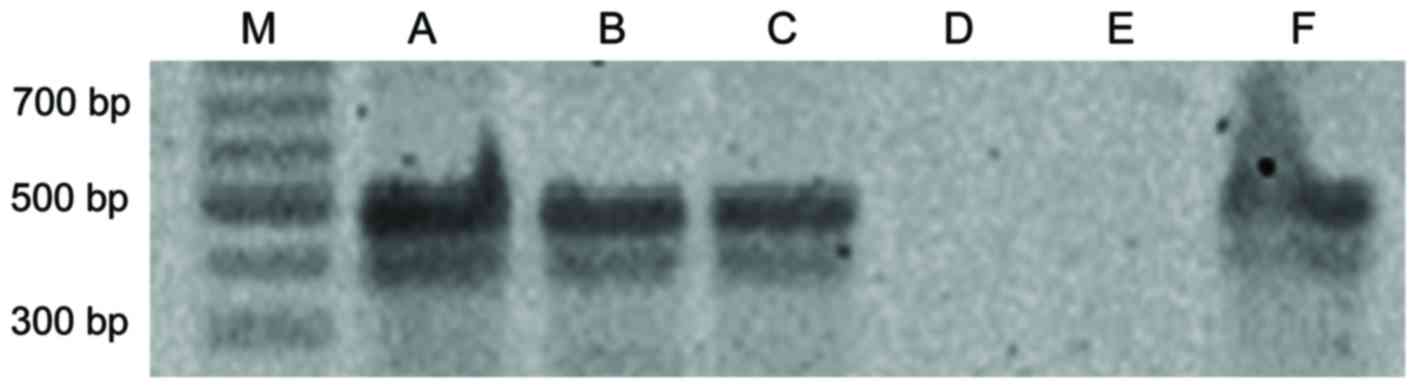
 | Figure 3.Comparison of DNA samples purified
from porcine bones and teeth following ACTB and mtDNA
polymerase chain reaction. M, 100 bp ladder; Neg, distilled water;
A, positive control; B, left in open-air for 1 year; C, buried in
soil for 1 year; D, buried in sand for 1 year; E, submerged in
water for 1 year; F, submerged in seawater 1 year; G, soaked in
caustic soda for 1 day; H, soaked in sulfuric acid for 1 week; I,
burnt with rubber; J, stored at −80°C. ACTB, β-actin; mtDNA,
mitochondrial DNA. |
For the samples left in open-air, submerged in
seawater or soaked in sulfuric acid, DNA extracted from bones and
teeth had similar quality, and was capable of PCR amplification for
both ACTB and mtDNA. For the samples buried in soil for 3
months or less, bones displayed positive PCR results for ACTB
(samples buried ≤1 month) and mtDNA (samples buried ≤3 months).
When buried in soil for 6 months, bones and teeth were negative for
ACTB and mtDNA PCR products. Notably, at 1 year, DNA
extracted from teeth amplified ACTB and mtDNA PCR products,
and DNA from bones amplified mtDNA PCR products (Table I).
For samples buried in sand, DNA extracted from bones
and teeth amplified ACTB and mtDNA PCR products during the
first 3 months; however, for the samples buried in sand for longer
periods, only DNA extracted from teeth demonstrated PCR
amplification. For samples submerged in water and seawater, DNA
extracted from bones and teeth demonstrated amplification of
ACTB and mtDNA PCR products after 1 week. Over longer
periods of time, PCR amplification was only observed for mtDNA from
bones submerged in water for 1 month (Table I).
For samples soaked in caustic soda and burnt with
rubber, ACTB and mtDNA PCR products were amplified only for
teeth. DNA from samples soaked in caustic soda for 1 or 2 weeks
could not be collected due to curd formation. Meanwhile, samples
soaked in sulfuric acid were destroyed after 1 week (Table I).
Samples stored at cold temperatures (4, −20 and
−80°C) demonstrated the best DNA preservation, with positive
results for ACTB and mtDNA PCR throughout the experimental
observation period (Table I).
Comparison of ACTB and mtDNA PCR
PCR amplification of mtDNA yielded a greater rate of
positive results compared with that of ACTB in multiple
situations, including from bone and teeth samples left in open-air
for 1 year, from bones buried in soil for 3 months, from bones
buried in soil for 1 year and from bones submerged in water for 1
month (Table I).
Testing minimal DNA concentrations and PCR
inhibitors. The minimum amount of DNA necessary for PCR
amplification of ACTB and mtDNA templates was 0.1 ng/µl
(Fig. 4). Additionally, certain DNA
samples that failed to amplify PCR products were selected to test
for the presence of reaction inhibitors. To perform this test, DNA
from samples that failed to amplify in the initial PCR reaction was
mixed with positive control DNA for an additional PCR
amplification. The samples that subsequently demonstrated PCR
amplification were: Teeth buried in soil for 1 week, teeth
submerged in water for 1 month, and bones soaked in sulfuric acid
for 1 week. By contrast, the DNA extracted from teeth and bones
submerged in seawater for 1 month still exhibited no amplification,
indicating the presence of a PCR inhibitor with the DNA template
(Fig. 5).
Discussion
The specific circumstances of crime scene
environments in which bodies are discovered can contribute toward
difficulties in personal identification due to the deterioration of
DNA quality (17,18). In the present study, crime scene
scenarios were simulated by artificially creating environments that
imitated known crime scene conditions. DNA extracted from porcine
teeth and bones was substituted, and PCR amplification of porcine
ACTB and mtDNA sequences was performed to imitate human
personal identification by PCR.
The efficiency of these PCR reactions was tested,
and the minimal concentration of DNA required for PCR amplification
was determined as 0.1 ng/µl. In the samples where amplification
from either bones or teeth was not possible, it is likely that the
DNA had deteriorated. This DNA degradation, in particular, samples
buried in soil or submerged in water were observed in our study.
Another potential explanation is the presence of reaction
inhibitors. The results of the present study demonstrated that DNA
extracted from samples submerged in seawater contained components
that interfered with PCR amplification. Seawater commonly contains
ions, including calcium, magnesium, sodium, potassium, chloride,
sulfate and nitrate, as well as other inorganic trace elements
including lead, copper, arsenic and manganese (19). The inhibitory effect of divalent ions
(Ca2+ and Mg2+), in particular, are
considerable due to sensitivity to Taq polymerase activity
(19). Further study of this
phenomenon should focus on inhibitor reduction through the
improvement of DNA preparation techniques.
Mitochondrial DNA has a higher copy number than
nuclear ACTB DNA (11), and is
therefore easier to amplify. The present results confirmed this
characteristic for DNA extracted from samples left in several
conditions, including open-air, in which mtDNA was amplified but
not ACTB after up to 1 year.
There were certain unexpected results. PCR products
were unable to be amplified using DNA extracted from samples buried
in sand or soil for 6 months, but reactions were successful using
DNA from samples buried for 1 year. This observation has been
previously reported in the literature, and reasoned as being due to
dried tissue resulting in improved DNA quality and thus easier PCR
amplification (20).
For several of the simulated crime scene
environments, DNA extracted from teeth resulted in improved PCR
amplification, compared with DNA extracted from bones. One reason
may be that tooth enamel contains more inorganic material than bone
(21,22), resulting in increased protection of
intracellular DNA. We hypothesized that teeth could be used as an
alternative DNA source in certain situations. For example, in the
case of DNA extracted from samples buried in sand for 1 year, teeth
displayed results superior to bone. The results also confirmed that
DNA extracted from all samples stored at cold temperatures (4, −20
and −80°C) was well preserved after 1 year. More stable storage
temperatures may aid in maintaining DNA stability at low
temperatures (23).
The present study has certain limitations. Firstly,
a basic phenol-chloroform DNA extraction technique was employed.
There are now more advanced technologies for DNA extraction
available, including spin-column DNA purification and magnetic bead
DNA isolation (24,25). Use of these methods may improve the
quality and quantity of DNA extracted (17,25).
However, these kits are often cost prohibitive in certain
locations; therefore, the results of the present study may aid with
the decision to amplify bone or teeth DNA in developing countries.
Secondly, levels of DNA degradation due to the exposure conditions
used in agarose gel electrophoresis were not checked. Only the
quantity of DNA by nanodrop spectrophotometry and the quality of
DNA by PCR amplification were assessed. Degradation of the DNA may
have impacted on the success of PCR amplification and should be
considered in future applications.
In conclusion, the present study imitated various
environments in tropical areas where unidentified bodies are
commonly found, with similarities in microbial composition,
temperature, humidity and pH. Therefore, the results of the present
study may prove useful for countries in tropical areas as a
preliminary reference for sample selection in various situations.
Additionally, the use of primers for porcine ACTB DNA
imitated the use of STR markers in personal identification, though
in cases where ACTB DNA amplification was not possible,
primers to amplify mtDNA appeared beneficial in the identification
of a maternal lineage relationship, due to increased mtDNA copy
number. Personal identification using DNA is a process important to
law enforcement in matters including inheritance management and
life insurance claims, as well as being used as evidence for
lawsuits in the case of murder.
Acknowledgements
The authors would like to thank the Department of
Anatomy at Chulalongkorn University, Bangkok, Thailand, for
providing equipment and technical laboratory assistance.
Funding
The present study was funded by the Thailand
Research Fund (grant no. DPG5980005). Additional support was
provided by the Anantara Siam Bangkok Hotel and Four Seasons Hotel
Care for Cancer 2017 Fun Run in coordination with the Thai Red
Cross Society and Chulalongkorn University (Bangkok, Thailand).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article. The authors permit reuse of
all illustrations in this study.
Authors' contributions
The experiments were conducted and designed by JS,
AM and NK. Sample collection and laboratory experiments were
performed by JS, TSo, TA, TSr, PP, AS and NK. The results were
analyzed and interpreted by JS and NK. JS and NK wrote the
manuscript. NK and AM reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACTB
|
β-actin
|
|
HV
|
hypervariable
|
|
mtDNA
|
mitochondrial DNA
|
|
PCR
|
polymerase chain reaction
|
|
STR
|
short tandem repeat
|
References
|
1
|
Cattaneo C, Gelsthorpe K and Sokol R: DNA
extraction methods in forensic analysisEncyclopedia of Analytical
Chemistry. John Wiley & Sons, Ltd.; Hoboken, NJ: pp. 1–18.
2017
|
|
2
|
Barbisin M and Shewale JG: Assessment of
DNA Extracted from Forensic Samples Prior to Genotyping. Forensic
Sci Rev. 22:199–214. 2010.PubMed/NCBI
|
|
3
|
Stray JE, Liu JY, Brevnov MG and Shewale
JG: Extraction of DNA from Forensic Biological Samples for
Genotyping. Forensic Sci Rev. 22:159–175. 2010.PubMed/NCBI
|
|
4
|
Johnson RN, Wilson-Wilde L and Linacre A:
Current and future directions of DNA in wildlife forensic science.
Forensic Sci Int Genet. 10:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dumache R, Ciocan V, Muresan C and Enache
A: Molecular DNA Analysis in Forensic Identification. Clin Lab.
62:245–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Butler JM and Degraded DNA: Advanced
Topics in Forensic DNA Typing: methodology. Academic Press; San
Diego, CA: pp. 293–309. 2012, View Article : Google Scholar
|
|
7
|
Alaeddini R, Walsh SJ and Abbas A:
Forensic implications of genetic analyses from degraded DNA - a
review. Forensic Sci Int Genet. 4:148–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mader JT and Calhoun J: Bone, Joint, and
Necrotizing Soft Tissue InfectionsMedical Microbiology. Baron S:
4th edition. University of Texas Medical Branch; Galveston, TX:
Chapter 100. 1996
|
|
9
|
Anchordoquy TJ and Molina MC: Preservation
of DNA. Cell Preserv Technol. 5:180–188. 2007. View Article : Google Scholar
|
|
10
|
Bartlett JD: Dental enamel development:
Proteinases and their enamel matrix substrates. ISRN Dent.
2013:6846072013.PubMed/NCBI
|
|
11
|
Butler JM: Mitochondrial DNA
AnalysisAdvanced Topics in Forensic DNA Typing: methodology.
Academic Press; San Diego, CA: pp. 405–456. 2012, View Article : Google Scholar
|
|
12
|
Sambrook J and Russell DW: Purification of
nucleic acids by extraction with phenol: chloroform. CSH Protoc.
2006:pii: pdb.prot44552006.
|
|
13
|
McKiernan HE and Danielson PB: Molecular
Diagnostic Applications in Forensic ScienceMolecular Diagnostics.
3rd edition. Academic Press; San Diego, CA: pp. 371–394. 2017,
View Article : Google Scholar
|
|
14
|
Khare P, Raj V, Chandra S and Agarwal S:
Quantitative and qualitative assessment of DNA extracted from
saliva for its use in forensic identification. J Forensic Dent Sci.
6:81–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parsons TJ and Coble MD: Increasing the
forensic discrimination of mitochondrial DNA testing through
analysis of the entire mitochondrial DNA genome. Croat Med J.
42:304–309. 2001.PubMed/NCBI
|
|
16
|
Sanger F, Nicklen S and Coulson AR: DNA
sequencing with chain-terminating inhibitors. Proc Natl Acad Sci
USA. 74:5463–5467. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jakubowska J, Maciejewska A and Pawłowski
R: Comparison of three methods of DNA extraction from human bones
with different degrees of degradation. Int J Legal Med.
126:173–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higgins D and Austin JJ: Teeth as a source
of DNA for forensic identification of human remains: A review. Sci
Justice. 53:433–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alaeddini R: Forensic implications of PCR
inhibition - A review. Forensic Sci Int Genet. 6:297–305. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kansagara AG, McMahon HE and Hogan ME:
Dry-state, room-temperature storage of DNA and RNA. Nat Methods.
5:8502008. View Article : Google Scholar
|
|
21
|
Palmer LC, Newcomb CJ, Kaltz SR, Spoerke
ED and Stupp SI: Biomimetic systems for hydroxyapatite
mineralization inspired by bone and enamel. Chem Rev.
108:4754–4783. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brundin M, Figdor D, Sundqvist G and
Sjögren U: DNA binding to hydroxyapatite: A potential mechanism for
preservation of microbial DNA. J Endod. 39:211–216. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SB, Crouse CA and Kline MC: Optimizing
Storage and Handling of DNA Extracts. Forensic Sci Rev. 22:131–144.
2010.PubMed/NCBI
|
|
24
|
Tan SC and Yiap BC: DNA, RNA, and protein
extraction: The past and the present. J Biomed Biotechnol.
2009:5743982009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuś M, Ossowski A and Zielińska G:
Comparison of three different DNA extraction methods from a highly
degraded biological material. J Forensic Leg Med. 40:47–53. 2016.
View Article : Google Scholar : PubMed/NCBI
|