Introduction
Lung complications are common after general
anesthesia surgery, significantly increasing mortality and
morbidity (1,2). Low tidal volume (VT) mechanical
ventilation in anaesthetized patients undergoing abdominal surgery
can minimize the chances of postoperative pulmonary complications
(3). Initial evidence of protective
ventilation with low VT ventilation has been obtained from acute
respiratory distress syndrome (ARDS) (4). Research has shown consistent results
concerning applying low VT ventilation to the surgical population
without ARDS (5). Patients
undergoing thoracic surgery with one-lung ventilation (OLV) face
physiologically and clinically challenging circumstances that
complicate lung-protective ventilation application with low VT.
Due to the significant physiological changes caused
by OLV, the clinical prognosis of patients is affected. These
changes include the following (6-8):
The obligate collapse of the non-dependent lung and overdistention
of the dependent lung indicate an inflammatory cascade and can be
related to high airway pressures; increase in shunt fraction when
ventilation is switched from two-lung to OLV, resulting in
hypoxemia; frequent occurrence of pulmonary atelectasis owing to
lower chest wall compliance due to lateral decubitus position
compared with two-lung ventilation. Therefore, patients undergoing
thoracic surgery with OLV are vulnerable to ventilator-induced lung
injury and an ultimate increase in the length of their hospital
stay.
Mostly, OLV studies involve small-sized samples and
primarily focus on reporting physiological outcomes. Furthermore,
owing to the paucity of evidence, anesthesiologists have tried
implementing various ventilation strategies during OLV (9). A study demonstrated the improvement in
physiological outcomes in OLV for thoracic surgery through
lung-protective ventilation with recruitment maneuvers and positive
end-expiratory pressure (PEEP) (10). However, it is still unclear if low
VT improves the clinical outcomes when used during OLV in patients
undergoing thoracic surgery. In 2017, El Tahan et al
(11) noted that low VT of OLV did
not affect the length of hospital stay, while a subsequent study
(12) showed that the duration of
hospital stay was shorter in the low VT group. The present study
assessed the effects of low VT on the physiological and clinical
outcomes of surgery in adults undergoing OLV.
Materials and methods
The methods used for writing the meta-analysis were
according to the guidelines provided by the Preferred Reporting
Items for Systematic Reviews and Meta-Analyses guidelines (13).
Search strategies and study
screening
PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com/) and Cochrane Library
databases (https://www.cochranelibrary.com) were extensively
searched from inception to February 2023. Studies were related to
the intraoperative use of OLV with low VT in patients undergoing
thoracic surgery. This was not limited to articles published in
just one language. The terms searched included ‘protective
ventilation or low tidal volume’ and ‘one-lung ventilation or
thoracic surgery’. In addition, the authors manually searched the
reference lists of relevant studies.
Then two reviewers (FX and ZL) independently
assessed all the titles and abstracts and excluded irrelevant
studies. Further, according to the inclusion and exclusion
criteria, the full texts of the remaining articles were
independently reviewed. The inclusion criteria were as follows: i)
Patients receiving OLV undergoing surgery; ii) a clear report of
VT; iii) different VT compared during intraoperative ventilation of
patients; iv) randomized controlled trials (RCT). Studies involving
children, lung transplantation, cardiopulmonary bypass, airway
device comparison, indefinite time of measurement and patients with
COVID-19 were excluded. The discrepancies were resolved through
agreement and after discussion with a third reviewer to reach a
consensus on inclusion. The included studies defined low and high
VT as 3-6 and 8-10 ml/kg of ideal body weight, respectively. These
studies were analyzed to identify outcome measures.
Data extraction and quality
assessment
Two authors (FX and JJ) performed data extraction
according to a standardized author-developed data extraction form
in Microsoft Excel. The following data were extracted from the
included trials: Year of publication, first author, type of
patients, operating side, VT category, PEEP setting, fraction of
inspired oxygen (FiO2) during OLV, recruitment maneuver,
and physiology or clinical outcomes. The primary outcome was the
risk of acute lung injury and the length of stay at the hospital.
Secondary outcomes were focused on physiology outcomes, including
the driving pressure (ΔP), peak pressure (Ppeak), arterial oxygen
pressure (PaO2)/FiO2, atelectasis and blood
IL-6. Acute lung injury was defined as the sudden onset of
respiratory distress and impaired oxygenation with a
PaO2/FiO2 ratio of <300 mm Hg. Atelectasis was
defined as new pulmonary infiltrates on a chest radiograph. The
data presented as a median range was converted to mean standard
deviation (14).
The evaluation of the present study involved RCTs
using the Cochrane Risk of Bias tool, which included the following
items: Random sequence generation (selection bias), allocation
concealment (selection bias), blinding of participants and
personnel (performance bias), blinding of outcome assessment
(detection bias), incomplete outcome data (attrition bias),
selective reporting (reporting bias) and other biases (15). Visual inspection of funnel plots was
applied for the evaluation of publication bias. Data extraction and
bias assessment of the included studies were performed by JJ and
confirmed by FX. In case of discrepancy, a consensus was reached by
discussion.
Statistical analysis
All statistical pooling of the meta-analysis was
conducted using RevMan (version 5.1; The Nordic Cochrane Centre).
Physiology outcomes for the meta-analysis of VT were blood IL-6,
ΔP, Ppeak, PaO2/FiO2, and atelectasis.
Clinical outcomes for meta-analysis of VT were the length of stay
at the hospital and the incidence of acute lung injury. Subgroup
analyses were conducted with stratification by TV of predicted body
weight (6 ml/kg vs. <6 ml/kg). Relative risk (RRs) with 95%
corresponding confidence intervals (CIs) were calculated for
dichotomous outcomes. The random-effects model was considered for
clinical heterogeneity (16). The
author quantified the existence of heterogeneity between the
studies using the I2 statistic (17). One study was excluded from
sensitivity testing and the process was repeated to analyze the
robustness of the aggregated results. P<0.05 was considered to
indicate a statistically significant difference.
Results
Search results
A total of 2,842 relevant articles were initially
retrieved and 25 additional records were identified through other
sources. The titles and abstracts were screened to eliminate
duplicates, which left 1,023 records. Among these, 943 publications
were discarded for being irrelevant. The full text of the remaining
80 publications was assessed. Based on the exclusion criteria, 68
publications were excluded. Finally, 12 studies were included in
the meta-analysis (Fig. 1).
Study characteristics
The included 12 studies compared low and high VT in
patients undergoing thoracic surgery with OLV. The characteristics
of each study are listed in Table
I. Basic characteristics of patients are provided in Table SI. The included studies were
published between 2005 and 2023. The analysis involved individual
studies on 876 participants using different sample sizes that
ranged between 26 and 343. Low and high VT varied between 3-6 and
8-10 ml/kg of ideal body weight, respectively. In 10 studies PEEP
was applied varying from 3-8 cm H2O in the low VT
groups, whereas PEEP was set to zero in the high VT groups. The
FiO2 applied in eight studies during the surgery was
adjusted based on oxygen saturation or protocol. Three and two
studies administered recruitment manoeuvres (RM) in the low and
high VT groups, respectively. A total of 12 (12,18-28)
of the studies reported data on physiology outcomes (IL-6, ΔP,
Ppeak, PaO2/FiO2 and atelectasis) and seven
studies (12,18,22-24,26,27)
included data on clinical outcomes (length of stay at the hospital
and the incidence of acute lung injury). The details of the bias
assessment risk are outlined in Fig.
2.
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| | Low VT | High VT | |
|---|
| First author,
year | Type of
surgery | Number of
patients | Time of ventilation
(min) | Vt (ml/kg) IBW | PEEP (cm
H2O) | RM |
FiO2 | Number of
patients | Time of ventilation
(min) | Vt (ml/kg) IBW | PEEP (cm
H2O) | RM |
FiO2 | Time of
measurement | Main outcomes | (Refs.) |
|---|
| Ahn, 2012 | Video-assisted
thoracic surgery | 25 | 108.6±36.5 | 6 | 5 | NA | 0.5 | 25 | 115.9±44.0 | 10 | 0 | NA | 1 | 60 min after
OLV | IL-6; atelectasis;
ALI; hospital length of stay | (18) |
| Jung, 2014 | Video-assisted
thoracostomy | 30 | NA | 6 | 8 | Yes | 1 | 30 | NA | 10 | 0 | No | 1 | 45 min after
OLV |
PaO2/FiO2;
Ppeak | (19) |
| Kim, 2019 | Thoracoscopic
lobectomy | 20 | 121±34 | 6 | 5 | No | Adjusted according
to oxygen saturation | 20 | 131±40 | 10 | 0 | No | Adjusted according
to oxygen saturation | OLV end | IL-6;
PaO2/FiO2; ΔP; Ppeak; atelectasis | (20) |
| Lin, 2008 | Esophagectomy | 20 | 142±21 | 5-6 | 3-5 | NA | NA | 20 | 154±32 | 10 | 0 | NA | NA | 120 min after
OLV | IL-6;
PaO2/FiO2; Ppeak | (21) |
| Marret, 2018 | Lung cancer
surgery | 172 | NA | 5 | 5-8 | Yes | According to local
protocol | 171 | NA | 10 | 0 | Yes | According to local
protocol | 20 min after
OLV | ΔP; Ppeak;
atelectasis; ALI; Hospital length of stay | (12) |
| Maslow, 2013 | Elective pulmonary
resection | 16 | 42±8.3 | 5 | 5 | NA | Adjusted according
to oxygen saturation | 16 | 46±9.5 | 10 | 0 | NA | Adjusted according
to oxygen saturation | 20 min after
OLV | ΔP; Ppeak;
atelectasis; hospital length of stay | (22) |
| Michelet 2006 | Esophagectomy for
cancer | 26 | 85±29 | 5 | 5 | NA | Adjusted according
to oxygen saturation | 26 | 85±29 | 9 | 0 | NA | Adjusted according
to oxygen saturation | OLV end | IL-6; ALI | (23) |
| Qutub, 2014 | Video-assisted
thoracic surgery | 13 | NA | 4 | 5 | Yes | Adjusted according
to oxygen saturation | 13 | NA | 8 | 5 | Yes | Adjusted according
to oxygen saturation | 15 min after
OLV |
PaO2/FiO2;
atelectasis; ALI; hospital length of stay | (24) |
| Schilling,
2005 | Thoracic
surgery | 16 | 68±71.9 | 5 | 0 | NA | Adjusted to achieve
a PaO2 >80 mm Hg | 16 | 71±60.7 | 10 | 0 | NA | Adjusted to achieve
a PaO2 > 80 mm Hg | 30 min after
OLV | ΔP; Ppeak | (25) |
| Shen, 2013 | Esophagectomy | 53 | 72.2±23.6 | 5 | 5 | NA | Adjusted according
to oxygen saturation | 48 | 75.0±18.8 | 8 | 0 | NA | Adjusted according
to oxygen saturation | 18-h
postoperative |
PaO2/FiO2; hospital
length of stay | (26) |
| Yang, 2011 | Lung cancer
surgery | 50 | 120±41 | 6 | 5 | NA | Adjusted according
to oxygen saturation | 50 | 126±53 | 10 | 0 | NA | 1 | 60 min after
OLV | ΔP; Ppeak;
atelectasis; ALI; hospital length of stay | (27) |
| Ye, 2011 | Lung cancer
surgery | 10 | NA | 6 | 5 | NA | 1 | 10 | NA | 8 | 0 | NA | 1 | 70 min after
OLV | Ppeak | (28) |
Physiology outcomes
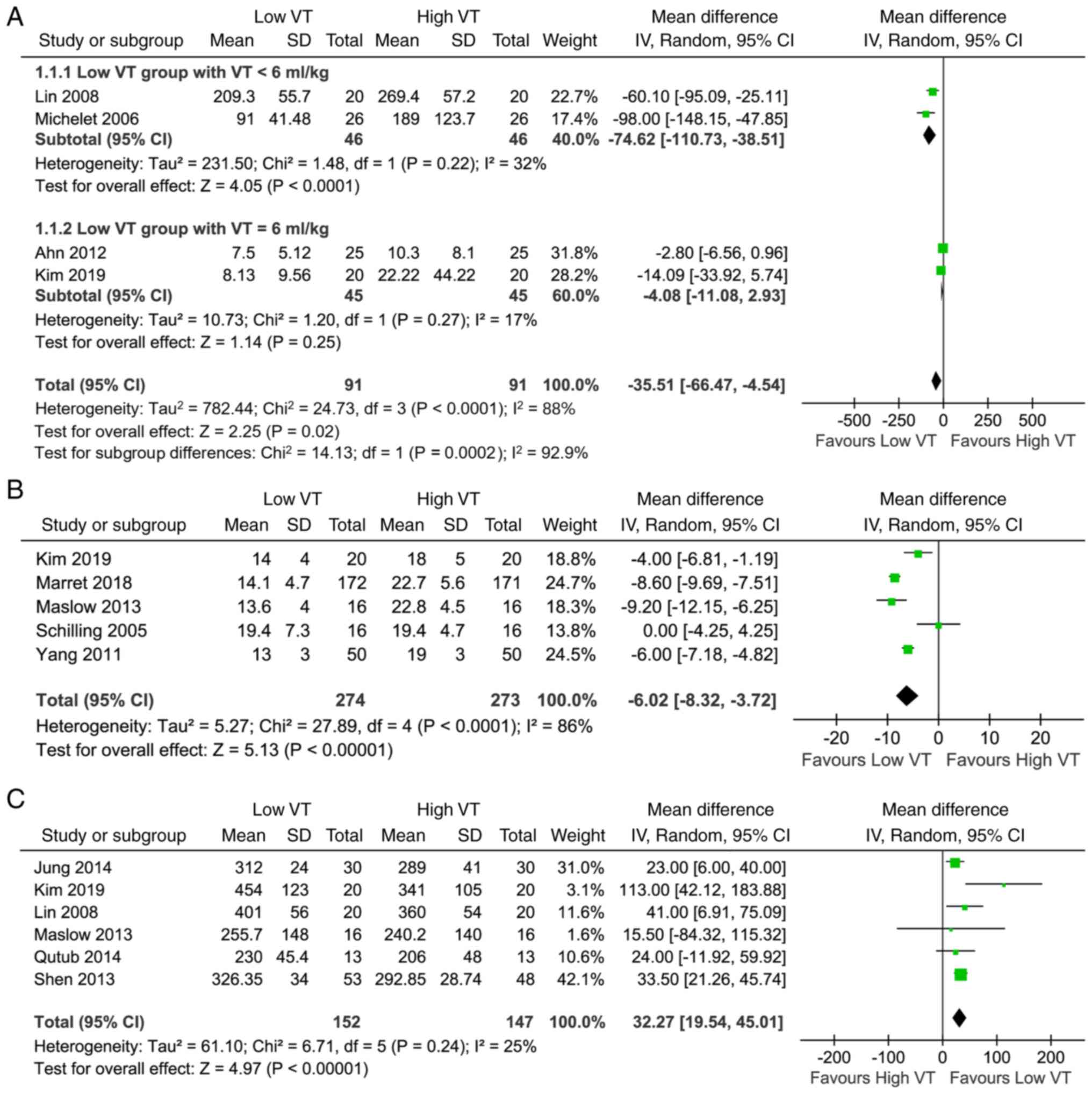
A total of 12 studies with 876 participants reported
the effect of low VT on physiology outcomes, including IL-6, ΔP,
Ppeak, PaO2/FiO2 and atelectasis. The results
suggested that OLV with low VT was associated with decreased IL-6
[mean difference (MD), -35.51 pg/ml; 95% CI (-66.47, -4.54 pg/ml);
P=0.02; Fig. 3A), ΔP [MD, -6.02
cmH2O; 95% CI (-8.32, -3.72 cmH2O);
P<0.0001; Fig. 3B], Ppeak [MD,
-2.88 cmH2O; 95% CI (-4.60, -1.16 cmH2O);
P=0.001; Fig. S1) and increased
PaO2/FiO2 [MD, 32.27 mmHg; 95% CI (19.54,
45.01 mmHg); P<0.00001; Fig.
3C]. Furthermore, the risk of atelectasis [RR, 0.79; 95% CI
(0.53, 1.17); P=0.24; Fig. S2)
with low VT did not show any increase.
During the analysis of IL-6, for those who received
a low VT of below 6 ml/kg, there was a significant decrease in the
low VT group compared with the high VT group [MD, -74.62 pg/ml, 95%
CI (-110.73, -38.51 pg/ml), P<0.0001; Fig. 3A], with possible moderate
heterogeneity (I2=32%). However, for those who received
a low VT of 6 ml/kg, there was no significant difference in IL-6
between both the groups [MD, -4.08 pg/ml, 95% CI (-11.08, 2.93
pg/ml), P=0.25; Fig. 3A]. There was
also a possibility of substantial heterogeneity
(I2=84.7%) between the subgroups (Fig. 3A).
Clinical outcomes
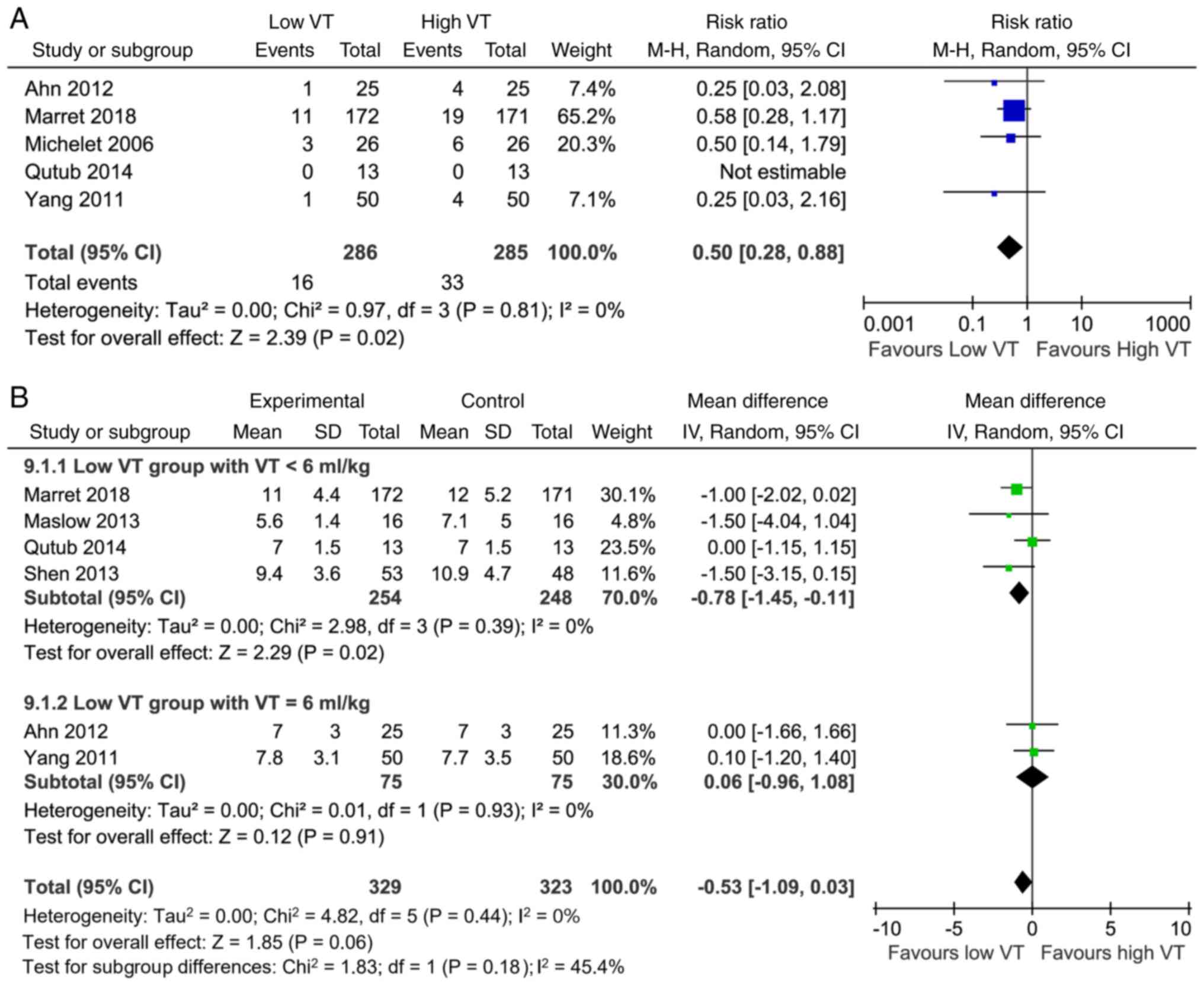
A total of 13 studies reported acute lung injury and
length of stay at the hospital as clinical outcomes. Overall, there
was a significant decrease in the risk of acute lung injury with
low VT [RR, 0.50; 95% CI (0.28, 0.88); P=0.02; Fig. 4A]. A possibility of low
heterogeneity (I2=0%) existed between the low and high
VT groups (Fig. 4A). Meanwhile, the
results suggested that low VT was not associated with the length of
stay at the hospital [MD, -0.53 days; 95% CI (-1.09, -0.03) days;
P=0.06; Fig. 4B].
In the low VT group, four studies with a VT of 4-5
ml/kg showed a shorter length of stay at the hospital [MD, -0.78
days; 95% CI (-1.45, -0.11) days; P=0.02; Fig. 4B], whereas two studies with a VT of
6 ml/kg showed no difference compared with the high VT group [MD
-0.06 days; 95% CI (-0.96, 1.08) days; P=0.91; Fig. 4B]. A possibility of low
heterogeneity existed between the two subgroups
(I2=45.4%; Fig. 4B).
Discussion
Meta-analysis was conducted to elaborate on the
physiology and clinical impacts of VT in patients undergoing
thoracic surgery with OLV. The results demonstrated that low VT
ventilation during OLV significantly improves
PaO2/FiO2 and decreases blood IL-6, ΔP, Ppeak
and the risk of acute lung injury. Furthermore, the length of stay
at the hospital decreases in low VT ventilation with VT set to 4-5
ml/kg. Meanwhile, low VT ventilation does not impact the risk of
atelectasis.
Low VT ventilation strategy aims at limiting lung
overdistension, leading to a reduction in the incidence of ALI
along with a shorter hospital stay. Two-lung ventilation is a
conventional VT technique that may lead to overdistension of the
aerated lung and increase the shear forces generated owing to the
repetitive opening and collapse of the atelectatic areas. In
comparison, a low VT ventilation strategy is beneficial for both
the injured lungs (4) and
anaesthetized patients (3).
However, large-sample randomized controlled studies do not exist to
evaluate the effect of low VT on ALI. According to Hu and Du
(29), the incidence of ALI was low
when patients had one-lung ventilation during the surgery. However,
this study did not explore the length of stay at the hospital. This
limitation was overcome in the present study, which noted that the
length of stay at the hospital decreased when VT was set to 4-5
ml/kg. However, this factor did not differ between the ventilatory
strategies in Lee et al (30) and this could be attributed to using
the actual body weight to set VT in their research. Ahn et
al (18) showed that low VT did
not have any positive effect on the length of stay at the hospital.
In the present study, the negative results could be attributed to
the fact that although ventilated with low VT, the platform
pressure is <20 cmH2O. This result implies that
pressure during ventilation needs to be taken into account while
determining the factors affecting the outcomes of OLV patients.
In a retrospective study, Amato et al
(31) identified the risk of high
ΔP as an outcome in ARDS patients. Among the surgical population,
either two-lung (32) or one-lung
(33,34) ventilation, ∆P is identified as a
risk factor for the development of postoperative pulmonary
complications. ΔP equals elastance times the VT. Thus, it may serve
as a surrogate for dynamic alveolar injury. The results of the
present study show that low VT significantly reduces both the ΔP
and Ppeak. Therefore, it can be hypothesized that low VT
ventilation is associated with postoperative pulmonary
complications in patients. Further studies are needed to clearly
understand the relationship between VT, ΔP and patient outcomes. In
addition, the results of the present study give strong indications
that lung injury is attenuated by the application of low VT. These
12 studies excluded patients with chronic obstructive disease and
obstructive pulmonary dysfunction, possibly mainly considering that
the tolerance and efficacy of low TV may vary depending on the
background conditions of the lungs. Unfortunately, only five
studies mentioned the proportion of smokers among the included
patients, while the remaining studies did not (Table SII). From a pathophysiological
perspective, a smoking history may be related to the patient's
tolerance to low VT.
High VT is associated with deformation of the
alveolar epithelium and cyclic opening of collapsed areas during
OLV, which leads to local production and release of inflammatory
mediators resulting in ALI. Inflammatory biomarkers directly assess
lung damage. The inflammatory response was observed to decrease in
healthy lungs after low VT ventilation compared with conventional
VT (35). A previous meta-analysis
(11) evaluated the impact of low
VT without the use of inflammatory biomarkers. In the present
study, in the patients who received low VT ventilation, serum IL-6
was found to decrease, which was indicated as a useful marker of
induced injury (36). The present
study was consistent with previous findings (23) of patients undergoing esophagectomy.
This referred to VT of 5 ml/kg being combined with PEEP 5 cm
H2O during one-lung ventilation, which resulted in the
release of low levels of IL-6 into the serum after the surgery.
However, Kim et al (20) did
not observe a difference in plasma IL-6. This could be attributed
to the calculation of the sample size of the study not being based
on postoperative outcomes, resulting in its small size. Therefore,
the clinical impact on the severity of the surgical trauma needs to
be further investigated. In addition to IL-6, commonly used
inflammatory markers also include C-reactive protein (CRP) and
white blood cell (WBC). Unfortunately, none of the four studies
mentioned (18,20,21,23)
detected CRP and WBC. One possible reason is that in these studies,
the total surgical time was between 120 and 300 min; IL-6 was
tested before surgery, during single lung ventilation and 15 min-2
h postoperatively, with only one study retesting IL-6 at 18 h
postoperatively. It is known that IL-6 is one of the earliest
inflammatory factors to appear in the acute phase of inflammation,
reaching its peak within 2 h with a half-life of only 1 h, which
can reflect the rapid changes in inflammation in a timely manner
(37). However, CRP is induced by
IL-6 and reaches its peak ~48 h, while white blood cells begin to
rise as early as 6-24 h after inflammation. Therefore, researchers
may consider that IL-6 gives an improved reflection of the early
inflammatory status of surgical patients. If CRP and WBC data can
be reported in these studies, it will help a more comprehensive and
detailed evaluation of the patient's inflammatory status.
Hypoxemia during OLV can be prevented by applying a
ventilation strategy that can avoid alveolar collapse while
minimally impairing perfusion of the dependent lung. The use of low
VT and PEEP to the ventilated lung and titrating inspired
FiO2 to maintain a pulse oximetric oxygen saturation can
serve as strategies to improve the ventilation/perfusion ratio and
maintain arterial oxygen tension during OLV (8) in thoracic surgery. In the present
study, PaO2/FiO2 was improved under low VT
ventilation. These results are consistent with Lee et al
(30); that low VT ventilation is
associated with improved oxygenation compared with conventional
ventilation requiring OLV. In Liu et al (38), owing to the comparison of different
modes rather than different VTs of ventilation, there is no
difference in PaO2/FiO2 between
pressure-controlled ventilation and volume-controlled ventilation.
Therefore, in this case, compared with low VT, high VT, which is
potentially injurious to the lung, did not translate into improved
oxygenation. Low VT ventilation, which keeps the lung open without
impeding perfusion, improves oxygenation during OLV.
It has also been found that postoperative
atelectasis is not evident in the low VT group compared with the
conventional VT group. During intraoperative ventilation,
atelectasis may occur due to ventilator-associated lung injuries
(39,40), leading to a reduction in the
functional residual capacity consequent to OLV and muscle
paralysis. A previous study (30)
has shown that lung atelectasis can be reduced by low VT
ventilation when assessed using lung ultrasound.
The main complication of low VT is atelectasis,
which also leads to an increase in arterial oxygen pressure
(PaO2) level and an increase in dead space fraction due
to atelectasis. In nine studies, PaCO2 levels were
compared between two groups, with three studies showing higher
PaCO2 levels in the low VT group and the remaining
studies showing no significant difference between the two groups.
Only one study compared the VD/VT between two groups, and the
results showed no significant difference (Table SIII).
Apart from VT, the application of 3-8 cm
H2O PEEP (41) in the
low VT group contributed to the prevention of atelectasis. This
physiological level of PEEP is mainly aimed at reversing the
sustained opening of the glottis caused by tracheal intubation, in
order to maintain physiological positive airway pressure and
functional residual air volume. In only one study (25), the low VT group was not given PEEP
and the results showed no increase in the incidence of atelectasis.
However, the number of cases in this study is small (16/16) and
more research results are needed to confirm whether low TV without
PEEP increases the risk of atelectasis.
The present meta-analysis also has some limitations.
First, the magnitude of hypoxemia generally peaks ~20 min after OLV
begins. However, in the present study, data were collected for
times between 15 min and 2 h from the different studies, which
would overestimate the effect of low VT on oxygenation. Second,
heterogeneity was identified in the use of PEEP and RM between the
two groups for the included studies. In most studies, low VT
ventilation during OLV is often accompanied by PEEP, and the actual
effect of PEEP cannot be separated from low VT to a certain extent.
The current study cannot clearly demonstrate the specific
advantages of low VT ventilation in the absence of other
ventilation strategies (PEEP and recruitment maneuvers). Therefore,
analyses need to be cautiously interpreted due to heterogeneity.
Third, the risk of bias and publication bias was assessed, but may
be affected by the study design and outcome reported of the
original article.
The present study assessed the physiology and
clinical impact of low VT ventilation during OLV. In OLV patients,
low VT improves PaO2/FiO2 and decreases blood
IL-6, ΔP, Ppeak, and risk of acute lung injury. Furthermore, low VT
can reduce the length of hospital stay when set to 4-5 ml/kg,
implying that low VT should be applied in patients with OLV.
However, further research on this might be required for
confirmation.
Supplementary Material
Association of low tidal volume
ventilation and peak pressure in patients with one-lung ventilation
undergoing surgery. SD, standard deviation; CI, confidence
interval; df, degrees of freedom; IV, inverse variance.
Association of low tidal volume
ventilation and the risk of atelectasis in patients with one-lung
ventilation undergoing surgery. M-H, Mantel-Haenszel; CI,
confidence interval; df, degrees of freedom.
Basic characteristics of
patients.
Smoking-related characteristics of
included studies.
Complication characteristics of
included studies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81871602).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
All authors made substantial contributions to the
present study. FX and FG designed the study. JJ was the main
contributor to the work. ZL and YT participated in the collection
and analysis of clinical data. HQ was involved in writing the
original draft. JJ has completed the manuscript revision work. YY
was involved in reviewing and substantial editing of the paper. All
authors had full access to all the data in the study and had final
responsibility for the decision to submit for publication. All
authors read and approved the final manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Lohser J and Ishikawa S: Clinical
Management of One-Lung Ventilation. In: Principles and Practice of
Anesthesia for Thoracic Surgery. Springer, New York, pp83-101,
2011.
|
|
2
|
Canet J, Gallart L, Gomar C, Paluzie G,
Vallès J, Castillo J, Sabaté S, Mazo V, Briones Z and Sanchis J:
ARISCAT Group. Prediction of postoperative pulmonary complications
in a population-based surgical cohort. Anesthesiology.
113:1338–1350. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Futier E, Constantin JM, Paugam-Burtz C,
Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C,
Lefrant JY, et al: A trial of intraoperative low-tidal-volume
ventilation in abdominal surgery. N Engl J Med. 369:428–437.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Acute Respiratory Distress Syndrome
Network. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT
and Wheeler A: Ventilation with lower tidal volumes as compared
with traditional tidal volumes for acute lung injury and the acute
respiratory distress syndrome. N Engl J Med. 342:1301–1308.
2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guay J, Ochroch EA and Kopp S:
Intraoperative use of low volume ventilation to decrease
postoperative mortality, mechanical ventilation, lengths of stay
and lung injury in adults without acute lung injury. Cochrane
Database Syst Rev. 7(Cd011151)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Grichnik KP and Shaw A: Update on one-lung
ventilation: The use of continuous positive airway pressure
ventilation and positive end-expiratory pressure
ventilation-clinical application. Curr Opin Anaesthesiol. 22:23–30.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Della Rocca G and Coccia C: Acute lung
injury in thoracic surgery. Curr Opin Anaesthesiol. 26:40–46.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Karzai W and Schwarzkopf K: Hypoxemia
during one-lung ventilation: Prediction, prevention, and treatment.
Anesthesiology. 110:1402–1411. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kidane B, Choi S, Fortin D, O'Hare T,
Nicolaou G, Badner NH, Inculet RI, Slinger P and Malthaner RA: Use
of lung-protective strategies during one-lung ventilation surgery:
A multi-institutional survey. Ann Transl Med. 6(269)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peel JK, Funk DJ, Slinger P, Srinathan S
and Kidane B: Positive end-expiratory pressure and recruitment
maneuvers during one-lung ventilation: A systematic review and
meta-analysis. J Thorac Cardiovasc Surg. 160:1112–1122.e3.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
El Tahan MR, Pasin L, Marczin N and
Landoni G: Impact of low tidal volumes during one-lung ventilation.
A meta-analysis of randomized controlled trials. J Cardiothorac
Vasc Anesth. 31:1767–1773. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Marret E, Cinotti R, Berard L, Piriou V,
Jobard J, Barrucand B, Radu D, Jaber S and Bonnet F: and the PPV
study group. Protective ventilation during anaesthesia reduces
major postoperative complications after lung cancer surgery: A
double-blind randomised controlled trial. Eur J Anaesthesiol.
35:727–735. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: Explanation and elaboration. PLoS Med.
6(e1000100)2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hozo SP, Djulbegovic B and Hozo I:
Estimating the mean and variance from the median, range, and the
size of a sample. BMC Med Res Methodol. 5(13)2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Borenstein M, Hedges LV, Higgins JP and
Rothstein HR: A basic introduction to fixed-effect and
random-effects models for meta-analysis. Res Synth Methods.
1:97–111. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ahn HJ, Kim JA, Yang M, Shim WS, Park KJ
and Lee JJ: Comparison between conventional and protective one-lung
ventilation for ventilator-assisted thoracic surgery. Anaesth
Intensive Care. 40:780–788. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jung JD, Kim SH, Yu BS and Kim HJ: Effects
of a preemptive alveolar recruitment strategy on arterial
oxygenation during one-lung ventilation with different tidal
volumes in patients with normal pulmonary function test. Korean J
Anesthesiol. 67:96–102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim HJ, Seo JH, Park KU, Kim YT, Park IK
and Bahk JH: Effect of combining a recruitment maneuver with
protective ventilation on inflammatory responses in video-assisted
thoracoscopic lobectomy: A randomized controlled trial. Surg
Endosc. 33:1403–1411. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lin WQ, Lu XY, Cao LH, Wen LL, Bai XH and
Zhong ZJ: Effects of the lung protective ventilatory strategy on
proinflammatory cytokine release during one-lung ventilation. Ai
Zheng. 27:870–873. 2008.PubMed/NCBI(In Chinese).
|
|
22
|
Maslow AD, Stafford TS, Davignon KR and Ng
T: A randomized comparison of different ventilator strategies
during thoracotomy for pulmonary resection. J Thorac Cardiovasc
Surg. 146:38–44. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Michelet P, D'Journo XB, Roch A, Doddoli
C, Marin V, Papazian L, Decamps I, Bregeon F, Thomas P and Auffray
JP: Protective ventilation influences systemic inflammation after
esophagectomy: A randomized controlled study. Anesthesiology.
105:911–919. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Qutub H, El-Tahan MR, Mowafi HA, El
Ghoneimy YF, Regal MA and Al Saflan AA: Effect of tidal volume on
extravascular lung water content during one-lung ventilation for
video-assisted thoracoscopic surgery: A randomised, controlled
trial. Eur J Anaesthesiol. 31:466–473. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schilling T, Kozian A, Huth C, Buhling F,
Kretzschmar M, Welte T and Hachenberg T: The pulmonary immune
effects of mechanical ventilation in patients undergoing thoracic
surgery. Anesth Analg. 101:957–965. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shen Y, Zhong M, Wu W, Wang H, Feng M, Tan
L and Wang Q: The impact of tidal volume on pulmonary complications
following minimally invasive esophagectomy: A randomized and
controlled study. J Thorac Cardiovasc Surg. 146:1267–1273;
discussion 1273-1264. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang M, Ahn HJ, Kim K, Kim JA, Yi CA, Kim
MJ and Kim HJ: Does a protective ventilation strategy reduce the
risk of pulmonary complications after lung cancer surgery?: A
randomized controlled trial. Chest. 139:530–537. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ye FF and Li LW: Effects of different
ventilation modes for one-lung ventilation anesthesia on
respiratory function and F(A)/F(I) changes during sevoflurane
inhalation. Nan Fang Yi Ke Da Xue Xue Bao. 31:714–717.
2011.PubMed/NCBI(In Chinese).
|
|
29
|
Hu XY and Du B: Lung-protective
ventilation during one-lung ventilation: Known knowns, and known
unknowns. J Thorac Dis. 11 (Suppl 3):S237–S240. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee JH, Bae JI, Jang YE, Kim EH, Kim HS
and Kim JT: Lung protective ventilation during pulmonary resection
in children: A prospective, single-centre, randomised controlled
trial. Br J Anaesth. 122:692–701. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Amato MB, Meade MO, Slutsky AS, Brochard
L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat
A, et al: Driving pressure and survival in the acute respiratory
distress syndrome. N Engl J Med. 372:747–755. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Neto AS, Hemmes SN, Barbas CS,
Beiderlinden M, Fernandez-Bustamante A, Futier E, Gajic O, El-Tahan
MR, Ghamdi AA, Günay E, et al: Association between driving pressure
and development of postoperative pulmonary complications in
patients undergoing mechanical ventilation for general anaesthesia:
A meta-analysis of individual patient data. Lancet Respir Med.
4:272–280. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Blank RS, Colquhoun DA, Durieux ME,
Kozower BD, McMurry TL, Bender SP and Naik BI: Management of
one-lung ventilation: Impact of tidal volume on complications after
thoracic surgery. Anesthesiology. 124:1286–1295. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Park M, Ahn HJ, Kim JA, Yang M, Heo BY,
Choi JW, Kim YR, Lee SH, Jeong H, Choi SJ and Song IS: Driving
pressure during thoracic surgery: A randomized clinical trial.
Anesthesiology. 130:385–393. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Terragni PP, Del Sorbo L, Mascia L, Urbino
R, Martin EL, Birocco A, Faggiano C, Quintel M, Gattinoni L and
Ranieri VM: Tidal volume lower than 6 ml/kg enhances lung
protection: role of extracorporeal carbon dioxide removal.
Anesthesiology. 111:826–835. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dreyfuss D and Rouby JJ: Mechanical
ventilation-induced lung release of cytokines: A key for the future
or pandora's box? Anesthesiology. 101:1–3. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
von Bethmann AN, Brasch F, Nüsing R, Vogt
K, Volk HD, Müller KM, Wendel A and Uhlig S: Hyperventilation
induces release of cytokines from perfused mouse lung. Am J Respir
Crit Care Med. 157:263–272. 1998.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu Z, Liu X, Huang Y and Zhao J:
Intraoperative mechanical ventilation strategies in patients
undergoing one-lung ventilation: A meta-analysis. SpringerPlus.
5(1251)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hedenstierna G and Edmark L: The effects
of anesthesia and muscle paralysis on the respiratory system.
Intensive Care Med. 31:1327–1335. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kozian A, Schilling T, Fredén F, Maripuu
E, Röcken C, Strang C, Hachenberg T and Hedenstierna G: One-lung
ventilation induces hyperperfusion and alveolar damage in the
ventilated lung: An experimental study. Br J Anaesth. 100:549–559.
2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Östberg E, Thorisson A, Enlund M,
Zetterström H, Hedenstierna G and Edmark L: Positive End-expiratory
pressure alone minimizes atelectasis formation in nonabdominal
surgery: A randomized controlled trial. Anesthesiology.
128:1117–1124. 2018.PubMed/NCBI View Article : Google Scholar
|