Introduction
In recent years, mental illness has become one of
the major diseases seriously threatening human health. It is
estimated that 45-50 million individuals (1%) are suffering from
schizophrenia worldwide, 33 million of whom are from developing
countries (1,2). As a common mental illness,
schizophrenia can be found in various social cultures and
geographic regions, and its incidence and prevalence are roughly
the same all over the world. Its life-time prevalence rate is ~1%
(3-5).
Clinical symptoms of schizophrenia are complex and diverse.
Clinical features vary among different individuals, disease types
and stages (6). The etiology of
schizophrenia has not yet been fully elucidated. However, in terms
of behavior, the majority of patients exhibit perceptive, thinking,
emotional, volitional and behavioral disorders (7-10).
At present, drug therapy is a major option for schizophrenia. Since
the advent of chlorpromazine in 1952, antipsychotics have been
developed for more than 60 years. Great progress has been made in
their research, and a large number of novel anti-psychotics have
entered into different phases of clinical trials. Atypical
antipsychotics have become the first-line medication for
schizophrenia due to their outstanding efficacy and safety
(11). Lurasidone (trade name,
Latuda™), a novel atypical antipsychotic, was approved
by the FDA on October 28, 2010. It mainly functions through the
complete antagonistic action on dopamine D2 and
5-hydroxytryptamine2A (5-HT)2A receptors
(12), and is another novel
antipsychotic after iloperidone and asenapine (13). One study showed lurasidone is safe
in treating schizophrenia, making it a compelling option for
patients with schizophrenia (14).
Lurasidone greatly enhances patient compliance and its clinical
application rate is increasing due to its oral, once-daily
administration mode. However, there are some inconsistent
conclusions of efficacy and tolerability of lurasidone in treating
schizophrenia. A network meta-analysis suggested that lurasidone 80
mg/day decreases body weight. Conversely, lurasidone 40 mg/day was
associated with weight increase (15). A meta-analysis found that lurasidone
reduced most psychopathology symptoms, but it did not analyze the
PANSS score, CGI-S score and MADRS which can be useful for the
clinicians (16). Therefore,
studies of re-evaluation of the post-marketing efficacy and safety
of lurasidone are receiving growing attention from clinicians and
pharmacists. By searching a large amount of bibliographic
databases, a number of RCTs regarding the clinical efficacy and
safety of lurasidone in schizophrenia were identified; however, the
efficacy and safety caused by dosage discrepancies were also
prominent. The aim of the present systematic review was to
ascertain the efficacy and safety of different doses of lurasidone
in schizophrenia using evidence-based medicine methods and strict
quality standards for the included references (17), providing an evidence-based basis for
the clinical application of this drug.
Materials and methods
Methods
The present study was conducted and reported
according to The Cochrane Collaboration's recommendations and
guidelines for conducting systematic reviews and meta-analyses for
observational studies (18,19), as well as the Preferred Reporting
Items for Systematic Reviews and Meta-Analyses (20,21)
statement.
Study eligibility criteria
The a priori inclusion criteria for the
present meta-analysis included: i) Double-blind,
parallel-controlled, randomized clinical trials; ii) included
patients of any age or disease severity [meeting criteria for the
diagnosis of acute or chronic schizophrenia, psychosis,
schizoaffective disorder, schizophreniform disorder and other
psychotic disorders as defined by the Diagnostic and Statistical
Manual of Mental Disorders, Fourth Edition (DSM-IV) (22), DSM-IV Text Revision (23) and International Classification of
Diseases, Tenth Revision Classification of Mental and Behavioral
Disorders (24-26)];
iii) relevant interventions assigned to lurasidone 40, 80, 120 and
160 mg/day were selected for primary comparison compared with
placebo independently; iv) the primary efficacy outcome measure was
a change from baseline in the Positive and Negative Syndrome Scale
(PANSS) (27), Clinical Global
Impression of Severity (CGI-S) (28) and Montgomery-Asberg Depression
Rating Scale (MADRS) (29); and v)
the safety outcome was the rate of discontinuation due to adverse
effects (>5%). Exclusion criteria included the following: i)
Systematic reviews; ii) review article; iii) case-control study
designs; iv) animal studies; v) comment; vi) incomplete data; vii)
case reports; viii) improper statistical method; and, ix) duplicate
publications.
Data sources and search strategy
PubMed\Medline (https://pubmed.ncbi.nlm.nih.gov), EBSCO (https://search.ebscohost.com), Embase (https://www.em-base.com), Cochrane library (https://www.cochranelibrary.com/?content-Language=eng),
OVID (https://ovidsp.ovid.com) and Web of
Science (https://www.webofscience.com/wos) were searched up to
May 2023. No limits were placed on this search and there were no
language restrictions. Potentially relevant unpublished data were
searched using ClinicalTrials.gov (Drugs@FDA; https://www.accessdata.fda.gov/), the Chinese Clinical
Trial Registry (http://www.chictr.org.cn/), European Union Drug
Regulating Authorities Clinical Trials (https://eudract.ema.europa.eu/index.html), and the
World Health Organization International Clinical Trials Registry
Platform (http://www.who.int/ictrp/en/). Reference lists of
included and excluded articles were searched for randomized
clinical trials matching the inclusion criteria. The search was
performed using the terms: ‘lurasidone’, ‘Latuda’, ‘Latuda’,
‘lurasidone hydrochloride’, ‘placebo’, ‘schizophrenia’,
‘schizoaffective disorder’, ‘schizophreniform disorder’,
‘efficacy’, ‘safety’, ‘tolerability’, ‘Clinical trial’, ‘randomized
controlled trial’, ‘RCT’, ‘double-blind’ and ‘parallel-controlled’.
The PubMed search string used was as follows: ‘(lurasidone OR
Latuda OR lurasidone hydrochloride) AND (placebo) AND
(schizophrenia OR schizoaffective disorder OR schizophreniform
disorder OR) AND (efficacy) AND (safety OR tolerability) AND
(Clinical trial OR randomized controlled trial OR RCT) AND
(double-blind) AND (parallel-controlled)’ AND (human OR
humans).
Study selection
Each search was conducted separately and then
downloaded as a separate file. In order to minimize selection bias,
two researchers (SG and LF) independently screened the titles,
abstracts, full-texts and extracted data based on the pre-defined
eligibility criteria, and evaluated the quality of the literatures.
If they had a disagreement, a third researcher was used to solve
the disagreement when necessary.
Data extraction
For each study, two researchers extracted
information on study characteristics, baseline characteristics of
patients such as age, duration of illness, duration of treatment,
PANSS total score and CGI-severity score, MADRS total score,
ethnicity, study location, interventions of the trial, end points
such as primary efficacy outcomes (PANSS total score change, CGI-S
score change and MADRS total score change) and safety outcomes
(adverse effects).
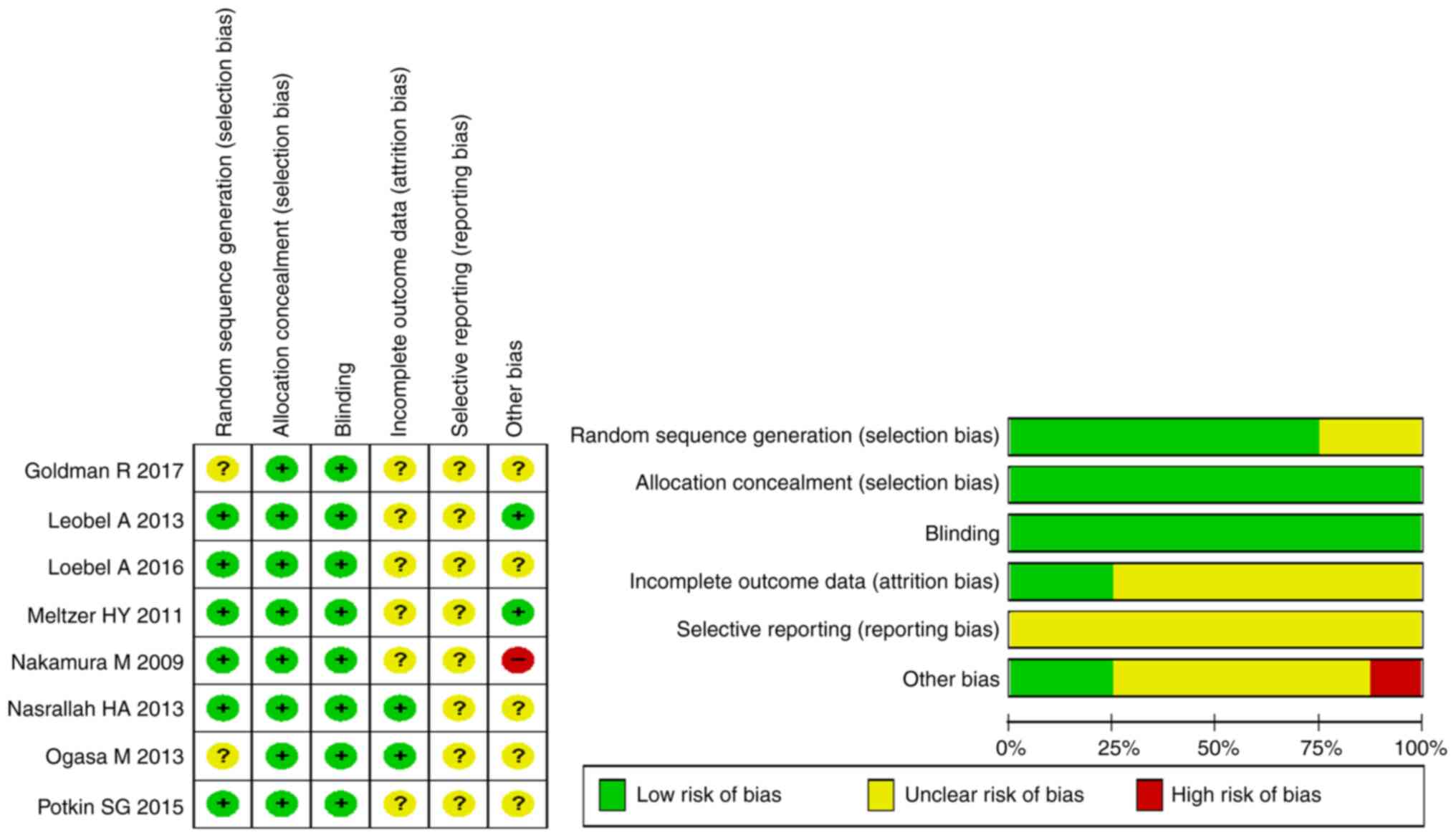
Quality assessment
The quality of literature was assessed using the
Cochrane system evaluation (version 5.1.0) RCTs bias risk
assessment tool (30). The
predefined key domains included random sequence generation,
allocation concealment, blinding, incomplete outcome data
addressed, intention-to-treat analysis, free of selective reporting
and free of other bias. Each item was classified as ‘yes’ (low risk
of bias), ‘no’ (high risk of bias) or ‘unclear’.
Statistical analysis
All outcomes were pooled using RevMan 5.3 software
(download from http://www.cochrane.org/). Risk ratios (RRs) with 95%
confidence intervals (CIs) were calculated for dichotomous outcomes
(such as response rates) and the standardized mean difference (SMD)
was used to report continuous outcomes (such as scale scores). The
I2 statistic was calculated to estimate
heterogeneity. If I2 was ≤50%, the fixed-effects
model with the Mantel-Haenszel (M-H) method was selected;
otherwise, the random-effect model (REM) was adopted. The risk of
publication bias was shown as a funnel plot. P<0.05 was
considered to indicate a statistically significant difference.
Results
Literature search and study
characteristics
The search yielded 744 citations, of which eight
articles (2,456 patients) met the inclusion criteria (31-38).
A total of five articles defined response using the PANNS total
score change and CGI-S score change (32-35,37)
and three articles defined response using the MADRS total score
change (34,35,37) in
the lurasidone (40 mg/day) group compared with placebo group. A
total of five articles defined response using the PANNS total score
change and CGI-S score change (31,33,35-37)
and four articles defined response using the MADRS total score
change (31,35-37)
in the lurasidone (80 mg/day) group compared with placebo group. A
total of three articles defined response using the PANNS total
score change and CGI-S score change (32,34,35),
and two articles defined response using the MADRS total score
change (34,35) in the lurasidone (120 mg/day) group
compared with placebo group. One article defined response using the
PANNS total score change, CGI-S score change and MADRS total score
change in the lurasidone (160 mg/day) group compared with placebo
group (31). A total of eight
articles defined response using the safety of different doses of
lurasidone compared with placebo (31-38).
A total of eight articles were published in public databases. The
eight articles were conducted in 14 different countries (United
States, Ukraine, Russia, Bulgaria, Romania, Colombia, Mexico,
Poland, Philippines, South Korea, Malaysia, Spain, France and
Hungary). A total of five articles were multicenter clinical
studies in the United States (32,35-38).
A total of three articles were multi-country, multi-center clinical
studies (31,33,34).
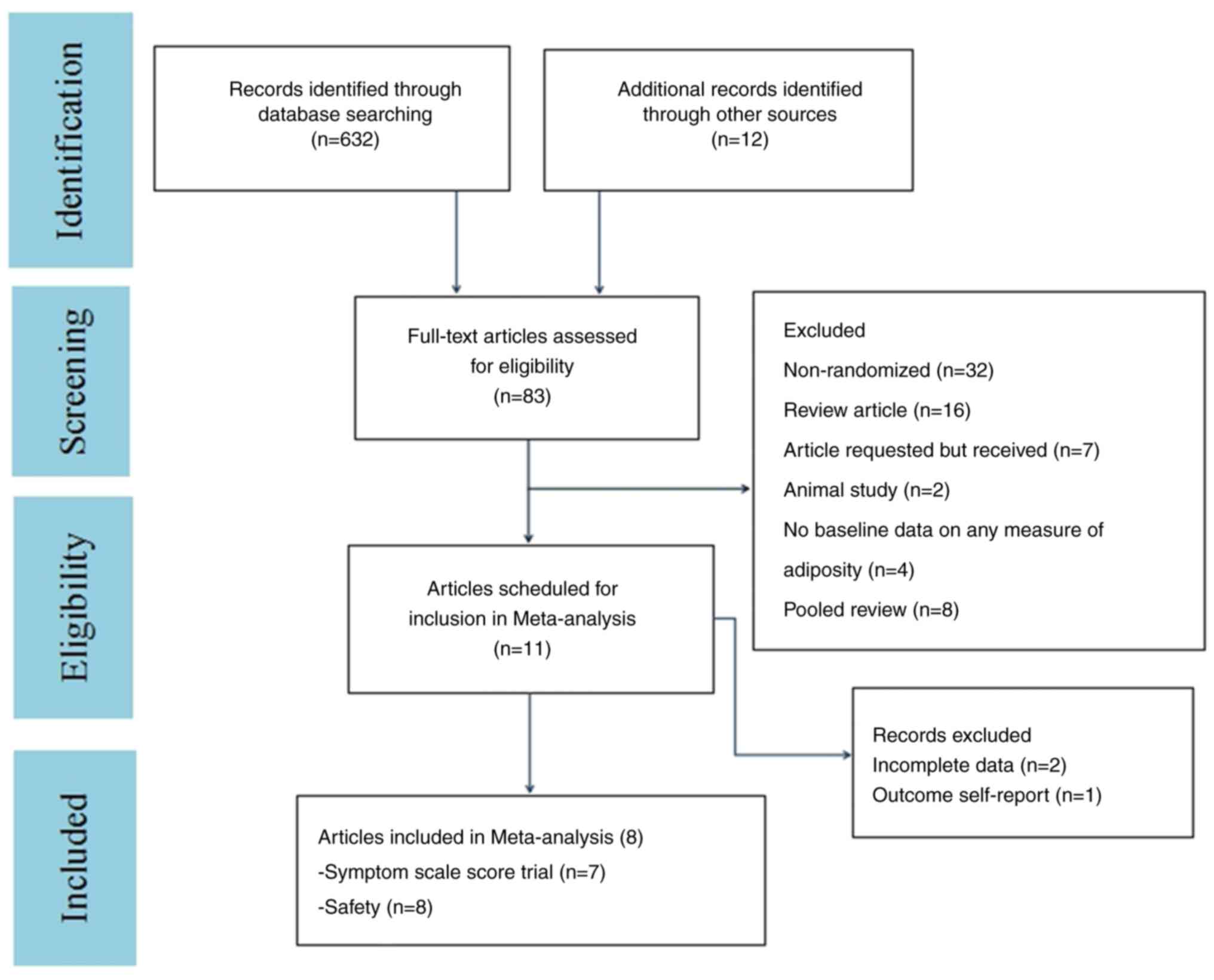
The flow diagram in Fig. 1 shows
additional details regarding trial selection and Table I shows the characteristics of the
included studies. A total of eight articles were of a double-blind
parallel-controlled design (Yes) (31-38).
For all eight articles, it was not clear if these were selective
reports (31-38).
One article was relatively vague on the other bias (No) (36). Details of the risk of bias
assessment are shown in Fig. 2.
 | Table IBasic characteristics of literatures
(mean ± standard deviation). |
Table I
Basic characteristics of literatures
(mean ± standard deviation).
| First author/s,
year | Interventions | Patients (n) | Age (years) | Duration of illness
(years) | Treatment duration
(weeks) | PANSS total
score | CGI-severity
score | MADRS total
score | Ethnicity | Outcome | (Refs.) |
|---|
| Goldman, et
al, 2017 | Lu 40 mg Lu | 108 106 | 15.5±1.3 | Not reported | 6 | 94.5±11.0 | 4.9±0.6 | Not reported | White, Black,
Asian, Other | ①②③④ | (33) |
| | 80 mg Placebo | 112 | 15.3±1.4 | | | 94.0±11.1 | 4.8±0.7 | | | | |
| | | | 15.3±1.4 | | | 92.8±11.1 | 4.6±0.7 | | | | |
| Loebel, et
al, 2013 | Lu 80 mg | 125 121 | 36.2±10.9 | 11.1±9.2 | 6 | 97.7±9.7 | 5.0±0.5 | 11.6±7.6 | White, Black,
Asian, Other | ①②③④ | (31) |
| | Lu 160 mg | 121 | 37.9±11.3 | 11.8±8.8 | | 97.5±11.8 | 5.0±0.6 | 11.2±7.8 | | | |
| | Placebo | | 37.4±10.8 | 11.3±9.3 | | 96.6±10.2 | 4.9±0.5 | 11.3±6.7 | | | |
| Loebel, et
al, 2016 | Lu 80 mg | 52 43 | 42.0±10.9 | Not reported | 6 | 93.3±9.4 | 4.9±0.5 | Not reported | White, Black,
Asian, Other | ④ | (38) |
| | Lu 160 mg | 112 | 41.3±9.0 | | | 96.0±9.6 | 5.0±0.6 | | | | |
| | Placebo | | 40.7±11.6 | | | 97.8±10.3 | 4.9±0.6 | | | | |
| Meltzer, et
al, 2011 | Lu 40 mg | 119 118 | 37.7±11.0 | 13.3±9.9 | 6 | 96.6±10.7 | 5.0±0.7 | 10.8±7.0 | White, Black,
Asian, Other | ①②③④ | (34) |
| | Lu 120 mg | 114 | 37.9±11.2 | 14.7±11.0 | | 97.9±11.3 | 5.0±0.6 | 14.4±7.2 | | | |
| | Placebo | | 37.0±11.3 | 12.6±9.6 | | 95.8±10.8 | 4.9±0.7 | 10.6±6.1 | | | |
| Nakamura, et
al, 2009 | Lu 80 mg | 90 90 | 39.7±9.9 | Not reported | 6 | 94.4±10.9 | 4.8±0.7 | 14.2±8.0 | White, Black,
Other | ①②③④ | (36) |
| | Placebo | | 41.9±9.8 | | | 96.0±11.6 | 4.8±0.7 | 14.5±8.3 | | | |
| Nasrallah, et
al, 2013 | Lu 40 mg | 122 119 | 40.3±11.3 | Not reported | 6 | 96.5±11.5 | 5.0±0.7 | 11.2±6.4 | White, Black,
Asian, Other | ①②③④ | (35) |
| | Lu 80 mg | 124 124 | 38.6±9.6 | | | 96.0±10.8 | 4.9±0.6 | 11.1±7.1 | | | |
| | Lu 120 mg | | 37.6±11.1 | | | 96.0±9.7 | 4.9±0.6 | 11.3±7.3 | | | |
| | Placebo | | 38.2 9.9 | | | 96.8±11.1 | 4.9±0.6 | 11.9±6.8 | | | |
| Ogasa, et
al, 2013 | Lu 40 mg | 108 106 | 39.8±9.5 | Not reported | 6 | 92.8±16.1 | 4.8 0.7 | Not reported | White, Black,
Other | ①②③④ | (32) |
| | Lu 120 mg | 112 | 41.0±9.0 | | | 89.6±13.4 | 4.7±0.6 | | | | |
| | Placebo | | 38.1±9.7 | | | 93.3±16.4 | 4.6±0.7 | | | | |
| Potkin, et
al, 2015 | Lu 40 mg | 67 71 72 | 42.0±10.9 | Not Reported | 6 | 93.4±15.0 | 4.8±0.8 | 13.1±7.5 | White, Black,
Asian, Other | ①②③④ | (37) |
| | Lu 80 mg | | 42.2±8.3 | | | 93.1±13.6 | 4.7±0.8 | 13.6±8.0 | | | |
| | Placebo | | 41.0±9.7 | | | 96.5±15.2 | 4.8±0.7 | 14.7±8.6 | | | |
Primary efficacy outcomes
Articles used the PANSS, CGI-S and MADRS scale to
measure the efficacy of lurasidone. A total of seven articles
reported the response using the PANSS, CGI-S or MADRS scale at six
weeks. Patients were considered to be PANSS, CGI-S and MADRS scale
responders if they achieved a decrease in their total score from
baseline at the end of the study.
Lurasidone (40 mg/day). There were no
statistically significantly changes associated with PANSS total
score change (P>0.05; SMD, -2.57; 95% CI, -5.19 to 0.06;
I2, 99%), CGI-S score change (P>0.05; SMD,
1.64; 95% CI, -1.88 to 5.15; I2, 100%) or MADRS
total score change (P>0.05; SMD, -1.53; 95% CI, -4.02 to 0.97;
I2, 99%) in the lurasidone (40 mg/day) group
compared with the placebo group (Table
II).
 | Table IIComparison of therapeutic effect
analysis in each trial group. |
Table II
Comparison of therapeutic effect
analysis in each trial group.
| Therapeutic effect
analysis | Lurasidone 40
mg | Lurasidone 80
mg | Lurasidone 120
mg | lurasidone 160
mg |
|---|
| PANNS total score
change | -2.57 (-5.19 to
0.06) Z=1.92 (P=0.06) 5 trials | -3.90 (-5.94 to
-1.86)a Z=3.75
(P=0.0002) 5 trials | -3.15 (-4.49 to
-1.81)a Z=4.61
(P<0.00001) 5 trials | -9.69 (-10.61 to
-8.77)a Z=20.69
(P<0.00001) 1 trial |
| CGI-S score
change | 1.64 (-1.88 to
5.15) Z=0.91 (P=0.36) 5 trials | -3.78 (-5.46 to
-2.10)a Z=4.41
(P<0.0001) 5 trials | -3.86 (-5.55 to
-2.17)a Z=4.47
(P<0.00001) 5 trials | -7.97 (-8.74 to
-7.21)a Z=4.47
(P<0.00001) 5 trials |
| MADRS total score
change | -1.53 (-4.02 to
0.97) Z=1.20 (P=0.23) 3 trials | -2.90 (-4.79 to
-1.02)a Z=3.02
(P=0.003) 4 trial | 0.17 (-1.46 to
1.79) Z=0.20 (P=0.84) 2 trials | -6.78 (-7.44 to
-6.12) Z=0.20 (P=0.84) 1 trial |
Lurasidone (80 mg/day). There were
statistically significant changes on PANSS total score change
(P<0.05; SMD, -3.90; 95% CI, -5.94 to -1.86;
I2, 99%), CGI-S score change (P<0.05; SMD,
-3.78; 95% CI, -5.46 to -2.10; I2, 99%) or MADRS
total score change (P<0.05; SMD, -2.90; 95% CI, -4.79 to -1.02;
I2, 99%) in the lurasidone (80 mg/day) group
compared with the placebo group (Table
II).
Lurasidone (120 mg/day). With the
exception that there was no statistical difference in MADRS total
score change (P>0.05), lurasidone (120 mg) had a statistically
significant superior effect on PANSS total score change (P<0.05;
SMD, -3.15; 95% CI, -4.49 to -1.81; I2, 96%) or
CGI-S score change (P<0.05; SMD, -3.86; 95% CI, -5.55 to -2.17;
I2, 98%) in the lurasidone (120 mg/day) group
compared with the placebo group (Table
II).
Lurasidone (160 mg/day). There were
statistically significant changes on PANSS total score change
(P<0.05; SMD, -9.69; 95% CI, -10.61 to -8.77), CGI-S score
change (P<0.05; SMD, -7.79; 95% CI, -8.74 to -7.21) or MADRS
total score change (P<0.05; SMD, -6.78; 95% CI, -7.44 to -6.12)
in the lurasidone (1,600 mg/day) group compared with the placebo
group (Table II).
Safety outcomes
In terms of safety, a parallel, independent
meta-analysis of 11 adverse reactions with a rate of >5%
in eight articles, including headache, insomnia, akathisia, nausea,
vomiting, anxiety, somnolence, agitation, dyspepsia, constipation
and extrapyramidal disorder was conducted. The results of the
meta-analysis of adverse reactions showed that, compared with the
placebo group, i) The incidence of akathisia (response rate; M-H
RR, 3.66; 95% CI, 2.07 to 6.47), nausea (response rate; M-H RR,
2.00; 95% CI, 1.28 to 3.13), somnolence (response rate; M-H RR,
1.82; 95% CI, 1.12 to 2.95), agitation (response rate; M-H RR,
2.09; 95% CI, 1.17 to 3.73) and extrapyramidal disorder (response
rate; M-H RR, 2.8; 95% CI, 1.55 to 5.06) was higher in the
lurasidone (40 mg/day) group (Table
III); ii) the incidence of akathisia (response rate; M-H RR,
3.56; 95% CI, 2.15 to 5.88), nausea (response rate; M-H RR, 2.38;
95% CI, 1.58 to 3.59), vomiting (response rate; M-H RR, 2.06; 95%
CI, 1.28 to 3.30), somnolence (response rate; M-H RR, 2.05; 95% CI,
1.29 to 3.28) and extrapyramidal disorder (response rate; M-H RR,
2.36; 95% CI, 1.11 to 5.04) was higher in the lurasidone (80
mg/day) group (Table III); iii)
the incidence of akathisia (response rate; M-H RR, 11.86; 95% CI,
5.03 to 27.94), nausea (response rate; M-H RR, 2.21; 95% CI, 1.25
to 3.90), somnolence (response rate; M-H RR, 2.95; 95% CI, 1.64 to
5.29) and extrapyramidal disorder (response rate; M-H RR, 5.18; 95%
CI, 2.62 to 10.52) was higher in the lurasidone (120 mg/day) group
(Table III); and iv) the
incidence of akathisia (response rate; M-H RR, 6.32; 95% CI, 1.61
to 24.83) was higher in the lurasidone (160 mg/day) group (Table III).
 | Table IIIAbsolute risk ratio of adverse
reactions for lurasidone compared with placebo. |
Table III
Absolute risk ratio of adverse
reactions for lurasidone compared with placebo.
| Adverse
reaction | Headache | Insomnia | Akathisia | Nausea | Vomiting | Anxiety | Somnolence | Agitation | Dyspepsia | Constipation | Extrapyramidal
disorder |
|---|
| Lurasidone 40
mg | 1.00% (0.73-1.37%)
I2=13% 5 trials | 1.01% (0.65-1.56%)
I2=0% 5 trials | 3.66%
(2.07-6.47%)a
I2=38% 5 trials | 2.00%
(1.28-3.13%)a
I2=19% 5 trials | 1.24% (0.72-2.12%)
I2=14% 5 trials | 1.35% (0.74-2.46%)
I2=0% 2 trials | 1.82%
(1.12-2.95%)a
I2=0% 5 trials | 2.09%
(1.17-3.73%)a
I2=0% 4 trial | 0.98% (0.59-1.63%)
I2=0% 4 trial | 0.66% (0.19-2.30%)
I2=53% 3 trials | 2.8%
(1.55-5.06%)a
I2=0% 5 trials |
| Lurasidone 80
mg | 0.85% (0.64-1.14%)
I2=0% 6 trials | 0.94% (0.64-1.36%)
I2=27% 6 trials | 3.56%
(2.15-5.88%)a
I2=0% 6 trials | 2.38%
(1.58-3.59%)a
I2=38% 6 trials | 2.06%
(1.28-3.30%)a
I2=0% 6 trials | 0.76% (0.24-2.39%)
I2=54% 4 trial | 2.05%
(1.29-3.28%)a
I2=0% 6 trials | 0.76% (0.43-1.33%)
I2=0% 5 trials | 1.38% (0.81-2.34%)
I2=0% 4 trial | 1.12% (0.60-2.10%)
I2=12% 3 trials | 2.36%
(1.11-5.04%)a
I2=0% 4 trial |
| Lurasidone 120
mg | 0.90% (0.63-1.30%)
I2=0% 3 trials | 1.04% (0.61-1.79%)
I2=39% 3 trials | 11.86%
(5.03-27.94%)a
I2=0% 3 trials | 2.21%
(1.25-3.90%)a
I2=0% 3 trials | 1.63% (0.90-2.98%)
I2=0% 3 trials | 1.47% (0.63-3.47%)
1 trial | 2.95%
(1.64-5.29%)a
I2=0% 3 trials | 1.75% (0.75-4.08%)
I2=30% 2 trials | 1.22% (0.68-2.20%)
I2=47% 3 trials | 0.70% (0.08-6.13%)
I2=55% 2 trials | 5.18%
(2.62-10.52%)a
I2=0% 3 trials |
| Lurasidone 160
mg | 0.94% (0.49-1.78%)
I2=0% 2 trials | 0.63% (0.34-1.17%)
I2=0% 2 trials | 6.32%
(1.61-24.83%)a
I2=0% 2 trials | 1.75% (0.67-4.55%)
I2=0% 2 trials | 1.81% (0.74-4.46%)
I2=0% 2 trials | 0.76% (0.21-2.79%)
I2=60% 2 trials | 2.38% (0.26-22.12%)
I2=67% 2 trials | 0.55% (0.24-1.25%)
I2=30% 2 trials | 1.75% (0.53-5.82%)
1 trial | 0.33% (0.04-3.16%)
1 trial | 1.04% (0.21-5.17%)
1 trial |
Discussion
Lurasidone is a benzothiazole atypical
antipsychotic. The US FDA has recommended an initial dose of 40
mg/day, which can be increased to 80 mg/day (39,40).
Lurasidone has been approved to treat the patients with
schizophrenia in the Canada, USA, Europe and Australia (41,42).
Lurasidone has a potent binding affinity for dopamine D2,
5-hydroxytryptamine (5-HT)2A, 5-HT7, 5-HT1A and noradrenaline α2C
receptors (43), which make
lurasidone be reduction in negative symptoms, improvement in
cognition and circadian rhythm regulation, improvement in
depressive symptoms, reduced drowsiness and somnolence (44,45).
Compared with other second generation antipsychotics, lurasidone
has affinity for to muscarinic M1 receptors and histamine H1
receptors, both of which can make lurasidone exert weight gain,
impaired glucose metabolism and increased plasma lipid levels
caused by other antipsychotics (43). A meta-analysis also concluded that
lurasidone had the lowest risk of weight gain and glucose changes
compared with other antipsychotics (46). Lurasidone offers an obvious
advantage to patients as it has promising effects on glucose and
lipid parameters and body weight (40,47),
which make lurasidone a good choice for clinicians and patients. A
review concluded that clinical trials showed the effectiveness of
lurasidone is very promising in treating these disorders such as
bipolar depression disorder (48).
Some clinical trials show that lurasidone is an effective treatment
option for patients with schizophrenia (49,50).
Miura et al (51) noted that
lurasidone improves functional and cognitive behaviors of patients
with schizophrenia, especially in long-term treatment. Patients
with schizophrenia can use the antipsychotics to prevent
schizophrenia in the long-term treatment. Most adverse reactions of
lurasidone are only mild to moderate (49,50),
and lurasidone is found to cause less weight gain but higher rates
of extrapyramidal side-effects, anxiety and akathisia compared with
olanzapine (35). Compared with
quetiapine, lurasidone also shows a higher incidence of
extrapyramidal side-effects (37).
A study reported that lurasidone had no difference in adverse
effects in patients with schizophrenia, besides lower rates of
somnolence compared with ziprasidone (52). Lurasidone has aroused the attention
of clinicians and pharmacists as an effective and safe treatment
for patients (48). A meta-analysis
concluded that lurasidone was superior to placebo in total
psychopathology, positive symptoms or negative symptoms (49). However, the present study did not
include the efficacy outcome measures, such as total PANSS score,
which can more useful the clinicians (53). Therefore, the present study analyzed
the outcome measures of total PANSS score, CGI-S score and total
MADRS score and included more safety outcome measures, which can
provide more suggestions to clinicians on the choice of drug.
Moreover, a randomized trial reported that lurasidone 80 mg is not
effective in the treatment in schizophrenia (37). However, another trial reported that
lurasidone 80 mg was superior than a placebo (35). Other trials showed significant
symptom improvement in the PANSS total score with schizophrenia in
the lurasidone 40 and 80 mg (49).
This inconsistency confused the psychiatrist and patients about
whether lurasidone 80 mg can be used to treat patients with
schizophrenia. The results of the present study analyzed the
efficacy and safety of all the doses of lurasidone in the treatment
of patients with schizophrenia and, given the suggestion of the
dose choice, will help clinicians make a clinical decision on the
dose of lurasidone.
The present systematic review and eight articles
were selected according to the inclusion and exclusion criteria
involving short-term (6-week) clinical RCTs of the efficacy and
safety of lurasidone in treatment of schizophrenia (31-38).
All eight articles were double-blind, parallel control trials with
a low risk of publication bias. A subgroup analysis of changes in
total PANSS score, CGI-S score and total MADRS score was conducted
to evaluate differences in clinical symptom improvement and adverse
reactions between each experimental group (40, 80, 120 and 160 mg
lurasidone) and the control group. In terms of efficacy evaluation,
the results demonstrated that there were no significant change in
total PANSS score, CGI-S score and MADRS between the 40 mg
lurasidone group and the placebo group (P>0.05). The 80, 120 and
160 mg lurasidone groups had significant changes in total PANSS
score, CGI-S score and MADRS compared with a placebo (P<0.05),
although changes in MADRS in the 120 mg lurasidone group were not
statistically significant (P>0.05). The results demonstrated
that the clinical symptoms of patients with schizophrenia did not
significantly improve when an initial dose (40 mg) of lurasidone
was administered. However, as the dose increased to 80-160 mg,
clinical symptom scores in enrolled patients significantly changed
compared with the placebo group. This conclusion suggests that
clinicians should choose lurasidone 80 mg/day in the treatment of
schizophrenia as the initial dose. The recommend initial dose of
lurasidone by FDA is 40 mg/day (12). However, the present authors
recommend lurasidone 80 mg/day as the initial dose because the
lurasidone 40 mg/day did not significantly improve the clinical
symptoms of schizophrenia, which is different from the previous
conclusions. In terms of safety assessment, combined meta-analyses
were independently conducted on 11 adverse reactions (headache,
insomnia, akathisia, nausea, vomiting, anxiety, somnolence,
agitation, dyspepsia, constipation and extrapyramidal disorder)
with an incidence of >5% in the eight included references.
Akathisia, nausea, somnolence and extrapyramidal disorder occurred
significantly more frequently in each experimental dose group (40,
80, 120 and 160 mg lurasidone) compared with the placebo group. As
for the relationship between adverse reactions and the dose
increase of lurasidone, there was no linear relationship observed
in the present systematic review. The results showed that the
changes in the incidence of agitation in the 40 mg lurasidone group
(P<0.05), vomiting in the 80 mg group (P<0.05) and akathisia
in the 160 mg group (P<0.05) were statistically significant and
there were also statistically significant changes in the incidence
of akathisia, nausea, somnolence and extrapyramidal disorder among
the 40, 80, 120 mg lurasidone groups (P<0.05); The incidence of
other adverse reactions was statistically insignificant (P>0.05)
as the dose of lurasidone increased to 160 mg, except for the
incidence of akathisia, which was statistically significant
(P<0.05). A dose of 160 mg lurasidone can be safer than other
doses as the incidence of adverse reactions in 160 mg lurasidone
was not statistically significant except for the incidence of
akathisia. Therefore, 80, 120 and 160 mg lurasidone can be chosen
as the treatment dose because of their outstanding efficacy and
safety. The present study showed that 160 mg lurasidone is the most
effective with the least adverse effects compared with other doses
and when clinicians choose the 80 or 120 mg lurasidone as the
treatment dose, the risk of occurrence of akathisia, nausea,
somnolence and extrapyramidal disorder should be noticed and 80 mg
lurasidone should be the initial dose rather than 40 mg. These
conclusions are different from other studies which conclude that 40
mg lurasidone is effective in the treatment of schizophrenia
(12,16,52).
The different dose choice suggestions should be helpful to guide
the dose adjustment of lurasidone for schizophrenia. However, these
results were influenced by the bibliographic bias and the integrity
of the results and data. More high-quality clinical studies are
necessary for verification.
Although the eight references included in the
present review were strictly screened on the basis of the inclusion
and exclusion criteria, with rigorous and standardized quality
evaluation, shortcomings remained in the systematic review and the
meta-analysis of measure outcomes. First, there might be a risk of
bibliographic selection bias in the bibliographic screening because
two investigators independently read and reviewed references in
parallel on the basis of the inclusion and exclusion criteria. The
risk of literature selection bias during the screening of reference
material could not be excluded. Second, some grey-literature and
non-traditional sources of evidence depending on whether there was
a confidentiality agreement were unable to properly supply the
inclusion data such as CGI-S score. Therefore, the data included in
the analysis might show some degree of publication bias. Third, in
the eight references included in the analysis (31-38),
fundamental features such as age, underlying diseases, previous
treatment conditions, BMIs and body weights of patients enrolled
were not strictly screened. There were only two studies (31,34)
that provided the duration of disease (course of disease) and there
were three studies (32,33,38)
that did not provide baseline levels of the MADRS score. Fourth,
due to the fact that the eight references (31-38)
included in the analysis were in English, rather than other
languages, there might be a risk of language bias. It was
considered that scientific and complete results could be assured by
strict screening and careful quality assessment of the references,
as well as use of proper methods, which means the results are of
high value for clinical reference.
In conclusion, existing evidence suggests that the
initial dose of lurasidone for schizophrenia can be adjusted to 80
mg and even incrementally to 160 mg as the disease aggravates. The
dose of 160 mg lurasidone is recommended as the most efficacious
and safe dose for acute schizophrenia. The risk of akathisia,
nausea, somnolence and extrapyramidal disorder is still high when
lurasidone is administered at a dose of 80-120 mg. The dose should
be promptly adjusted or the drug should be withdrawn if the
aforementioned adverse reactions worsen. Multi-center, high-quality
and long-term clinical RCTs influenced by the included references
are still necessary to support the aforementioned conclusions.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a research grant
from the technology bureau of Sichuan province (grant no.
2021YJ0445) and the Science and Technology Plan Fund from Yaan
Science and Technology Bureau (grant no. 22KJJH0039).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
SG, LF, XX and ZY were involved in the design of the
study, collected the data, undertook the statistical analyses and
drafted the manuscript. ZY and XX participated in the design of the
study, performed the statistical analyses, helped to interpret data
and drafted the manuscript. SG and LF participated in the design of
the study and helped to draft the manuscript. SG and LF collected
the data and helped to interpret data. Data authentication is not
applicable. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Henson P, D'Mello R, Vaidyam A, Keshavan M
and Torous J: Anomaly detection to predict relapse risk in
schizophrenia. Transl Psychiatry. 11(28)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hálfdánarson Ó, Zoëga H, Aagaard L,
Bernardo M, Brandt L, Fusté AC, Furu K, Garuoliené K, Hoffmann F,
Huybrechts KF, et al: International trends in antipsychotic use: A
study in 16 countries, 2005-2014. Eur Neuropsychopharmacol.
27:1064–1076. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sun ZW, Shi TT and Fu PX: Characteristics
of schizophrenia patients' homicide behaviors and their
correlations with criminal capacity. Fa Yi Xue Za Zhi. 33:32–35.
2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
4
|
Maggioni E, Crespo-Facorro B, Nenadic I,
Benedetti F, Gaser C, Sauer H, Roiz-Santiañez R, Poletti S,
Marinelli V, Bellani M, et al: Common and distinct structural
features of schizophrenia and bipolar disorder: The European
network on psychosis, affective disorders and cognitive trajectory
(ENPACT) study. PLoS One. 12(e0188000)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wimberley T, MacCabe JH, Laursen TM,
Sørensen HJ, Astrup A, Horsdal HT, Gasse C and Støvring H:
Mortality and self-harm in association with clozapine in
treatment-resistant schizophrenia. Am J Psychiatry. 174:990–998.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cai C and Yu L: Quality of life in
patients with schizophrenia in China: Relationships among
demographic characteristics, psychosocial variables, and symptom
severity. J Psychosoc Nurs Ment Health Serv. 55:48–54.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li W, Zhou FC, Zhang L, Ng CH, Ungvari GS,
Li J and Xiang YT: Comparison of cognitive dysfunction between
schizophrenia and bipolar disorder patients: A meta-analysis of
comparative studies. J Affect Disord. 274:652–661. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang LJ, Lin SK, Chen YC, Huang MC, Chen
TT, Ree SC and Chen CK: Differences in clinical features of
methamphetamine users with persistent psychosis and patients with
schizophrenia. Psychopathology. 49:108–115. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tan EJ, Neill E and Rossell SL: Assessing
the relationship between semantic processing and thought disorder
symptoms in schizophrenia. J Int Neuropsychol Soc. 21:629–638.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kurtz M, Mohring P, Förster K, Bauer M and
Kanske P: Deficits in explicit emotion regulation in bipolar
disorder: A systematic review. Int J Bipolar Disord.
9(15)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Reutfors J, Cesta CE, Cohen JM, Bateman
BT, Brauer R, Einarsdóttir K, Engeland A, Furu K, Gissler M, Havard
A, et al: Antipsychotic drug use in pregnancy: A multinational
study from ten countries. Schizophr Res. 220:106–115.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bruijnzeel D, Yazdanpanah M, Suryadevara U
and Tandon R: Lurasidone in the treatment of schizophrenia: A
critical evaluation. Expert Opin Pharmacother. 16:1559–1565.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Citrome L: Schizophrenia relapse, patient
considerations, and potential role of lurasidone. Patient Prefer
Adherence. 10:1529–1537. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Horisawa T, Ishibashi T, Nishikawa H,
Enomoto T, Toma S, Ishiyama T and Taiji M: The effects of selective
antagonists of serotonin 5-HT7 and 5-HT1A receptors on
MK-801-induced impairment of learning and memory in the passive
avoidance and morris water maze tests in rats: Mechanistic
implications for the benefcial effects of the novel atypical
antipsychotic lurasidone. Behav Brain Res. 220:83–90.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Srisurapanont M, Suttajit S, Likhitsathian
S, Maneeton B and Maneeton N: A network meta-analysis of the
dose-response effects of lurasidone on acute schizophrenia. Sci
Rep. 11(5571)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kishi T, Nosaka T, Sakuma K, Okuya M and
Iwata N: Efficacy, tolerability, and safety of lurasidone for acute
schizophrenia: A systematic review and network meta-analysis of
phase 3 trials in Japan. Neuropsychopharmacol Rep. 40:314–322.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Braga VL, Rocha LPDS, Bernardo DD, Cruz CO
and Riera R: What do Cochrane systematic reviews say about
probiotics as preventive interventions? Sao Paulo Med J.
135:578–586. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Page MJ and Moher D: Evaluations of the
uptake and impact of the preferred reporting items for systematic
reviews and meta-analyses (PRISMA) statement and extensions: A
scoping review. Syst Rev. 6(263)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Knobloch K, Yoon U and Vogt PM: Preferred
reporting items for systematic reviews and meta-analyses (PRISMA)
statement and publication bias. J Craniomaxillofac Surg. 39:91–92.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Panic N, Leoncini E, de Belvis G,
Ricciardi W and Boccia S: Evaluation of the endorsement of the
preferred reporting items for systematic reviews and meta-analysis
(PRISMA) statement on the quality of published systematic review
and meta-analyses. PLoS One. 8(e83138)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Durak S, Ercan ES, Ardic UA, Yuce D, Ercan
E and Ipci M: Effect of methylphenidate on neurocognitive test
battery: An evaluation according to the diagnostic and statistical
manual of mental disorders, fourth edition, subtypes. J Clin
Psychopharmacol. 34:467–474. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shabsigh R and Rowland D: The diagnostic
and statistical manual of mental disorders, fourth edition, text
revision as an appropriate diagnostic for premature ejaculation. J
Sex Med. 4:1468–1478. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Faiad Y, Khoury B, Daouk S, Maj M, Keeley
J, Gureje O and Reed G: Frequency of use of the international
classification of diseases ICD-10 diagnostic categories for mental
and behavioural disorders across world regions. Epidemiol Psychiatr
Sci. 9:568–576. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Janca A, Ustün TB, Early TS and Sartorius
N: The ICD-10 symptom checklist: A companion to the ICD-10
classification of mental and behavioural disorders. Soc Psychiatry
Psychiatr Epidemiol. 28:239–242. 1993.PubMed/NCBI View Article : Google Scholar
|
|
26
|
International Advisory Group for the
Revision of ICD-10 Mental and Behavioural Disorders. A conceptual
framework for the revision of the ICD-10 classification of mental
and behavioural disorders. World Psychiatry. 10:86–92.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Khan A, Lewis C and Lindenmayer JP: Use of
non-parametric item response theory to develop a shortened version
of the positive and negative syndrome scale (PANSS). BMC
Psychiatry. 11(178)2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pinna F, Deriu L, Diana E, Perra V,
Randaccio RP, Sanna L, Tusconi M and Carpiniello B: Cagliari
Recovery Study Group. Clinical global impression-severity score as
a reliable measure for routine evaluation of remission in
schizophrenia and schizoaffective disorders. Ann Gen Psychiatry.
14(6)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Health Quality Ontario. Psychotherapy for
Major depressive disorder and generalized anxiety disorder: A
health technology assessment. Ont Health Technol Assess Ser.
17:1–167. 2017.PubMed/NCBI
|
|
30
|
Higgins JPT and Green S (eds): Cochrane
handbook for systematic reviews of interventions version 5.1.0
[updated March 2011]. The Cochrane Collaboration, 2011. Available
from. http://handbook.cochrane.org.
|
|
31
|
Loebel A, Cucchiaro J, Sarma K, Xu L, Hsu
C, Kalali AH, Pikalov A and Potkin SG: Efficacy and safety of
lurasidone 80 and 160 mg/day in the treatment of schizophrenia: A
randomized, double-blind, placebo- and active-controlled trial.
Schizophr Res. 145:101–109. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ogasa M, Kimura T, Nakamura M and Guarino
J: Lurasidone in the treatment of schizophrenia: A 6-week,
placebo-controlled study. Psychopharmacology (Berl). 225:519–530.
2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Goldman R, Loebel A, Cucchiaro J, Deng L
and Findling RL: Efficacy and safety of lurasidone in adolescents
with schizophrenia: A 6-week, randomized placebo-controlled study.
J Child Adolesc Psychopharmacol. 27:516–525. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Meltzer HY, Cucchiaro J, Silva R, Ogasa M,
Phillips D, Xu J, Kalali AH, Schweizer E, Pikalov A and Loebel A:
Lurasidone in the treatment of schizophrenia: A randomized,
double-blind, placebo- and olanzapine-controlled study. Am J
Psychiatry. 168:957–967. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nasrallah HA, Silva R, Phillips D,
Cucchiaro J, Hsu J, Xu J and Loebel A: Lurasidone for the treatment
of acutely psychotic patients with schizophrenia: A 6-week,
randomized, placebo-controlled study. J Psychiatr Res. 47:670–677.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nakamura M, Ogasa M, Guarino J, Phillips
D, Severs J, Cucchiaro J and Loebel A: Lurasidone in the treatment
of acute schizophrenia: A double-blind, placebo-controlled trial. J
Clin Psychiatry. 70:829–836. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Potkin SG, Kimura T and Guarino J: A
6-week, double-blind, placebo- and haloperidol-controlled, phase II
study of lurasidone in patients with acute schizophrenia. Ther Adv
Psychopharmacol. 5:322–331. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Loebel A, Silva R, Goldman R, Watabe K,
Cucchiaro J, Citrome L and Kane JM: Lurasidone dose escalation in
early nonresponding patients with schizophrenia: A randomized,
placebo-controlled study. J Clin Psychiatry. 77:1672–1680.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sanford M: Lurasidone: In the treatment of
schizophrenia. CNS Drugs. 27:67–80. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Owen RT: Lurasidone: A new treatment
option for schizophrenia. Drugs Today (Barc). 47:807–816.
2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Latuda. (lurasidone HCl) Tablets US
Prescribing Information, 2012. http://www.latuda.com/LatudaPrescribingInformation.pdf.
|
|
42
|
Kane JM: Lurasidone: A clinical overview.
J Clin Psychiatry. 72 (Suppl 1):S24–S28. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ishibashi T, Horisawa T, Tokuda K,
Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y,
Toma S, et al: Pharmacological profile of lurasidone, a novel
antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and
5-HT1A receptor activity. J Pharmacol Exp Ther. 334:171–181.
2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Stahl SM, Morrissette DA, Citrome L,
Saklad SR, Cummings MA, Meyer JM, O'Day JA, Dardashti LJ and
Warburton KD: ‘Meta-guidelines’ for the management of patients with
schizophrenia. CNS Spectr. 18:150–162. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Stahl SM: Stahl's essential
psychopharmacology: Neuroscientific basis and practical
applications. 5th edition. Cambridge, UK: Cambridge University
Press, 2021.
|
|
46
|
De Hert M, Yu W, Detraux J, Sweers K, van
Winkel R and Correll CU: Body weight and metabolic adverse effects
of asenapine, iloperidone, lurasidone and paliperidone in the
treatment of schizophrenia and bipolar disorder: A systematic
review and exploratory meta-analysis. CNS Drugs. 26:733–759.
2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fukuyama K, Motomura E, Shiroyama T and
Okada M: Impact of 5-HT7 receptor inverse agonism of lurasidone on
monoaminergic tripartite synaptic transmission and pathophysiology
of lower risk of weight gain. Biomed Pharmacother.
148(112750)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Tarzian M, Soudan M, Alhajji M, Ndrio M
and Fakoya AO: Lurasidone for treating schizophrenia and bipolar
depression: A review of its efficacy. Cureus.
15(e38071)2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Higuchi T, Ishigooka J, Iyo M, Yeh CB,
Ebenezer EG, Liang KY, Lee JS, Lee SY, Lin SK, Yoon BH, et al:
Lurasidone in the treatment of schizophrenia: Results of a
double-blind, placebo-controlled trial in Asian patients. Asia Pac
Psychiatry. 11(e12352)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Stahl SM, Cucchiaro J, Simonelli D, Hsu J,
Pikalov A and Loebel A: Effectiveness of lurasidone for patients
with schizophrenia following 6 weeks of acute treatment with
lurasidone, olanzapine, or placebo: A 6-month, open-label,
extension study. J Clin Psychiatry. 74:507–515. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Miura I, Horikoshi S, Ichinose M, Suzuki Y
and Watanabe K: Lurasidone for the treatment of schizophrenia:
Design, development, and place in therapy. Drug Des Devel Ther.
17:3023–3031. 2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Harvey PD, Ogasa M, Cucchiaro J, Loebel A
and Keefe RS: Performance and interview-based assessments of
cognitive change in a randomized, double-blind comparison of
lurasidone vs ziprasidone. Schizophr Res. 127:188–194.
2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zheng W, Cai DB, Yang XH, Li L, Zhang QE,
Ng CH, Ungvari GS, Li XB, Ning YP and Xiang YT: Short-term efficacy
and tolerability of lurasidone in the treatment of acute
schizophrenia: A meta-analysis of randomized controlled trials. J
Psychiatr Res. 103:244–251. 2018.PubMed/NCBI View Article : Google Scholar
|