1. Introduction
Negative pressure pulmonary edema (NPPE) is a
noncardiogenic pulmonary edema caused by a rapid increase in
negative intrathoracic pressure. It can occur due to acute or
chronic upper airway obstruction, which can lead to
life-threatening hypoxemia (1);
upper airway obstruction may be caused by a number of potential
factors, including laryngeal spasm, foreign bodies and tracheal
intubation, resulting in breathing difficulties. Negative pressure
in the capillaries occurs when the patient breathes, causing
transudative fluid from the pulmonary capillaries to seep into the
alveolar and interstitial tissues and leading to alveolar and
interstitial edema (1-6).
The relationship between upper airway obstruction and pulmonary
edema was first described in 1927 by Moore and Binger (7). However, the first clinical case was
not reported until 1973(2). Since
then, NPPE has been reported multiple times with varying incidence
statistics. In a study of 176 children with severe upper airway
obstruction, the incidence rate of NPPE was 9.6% (8). In another study, the incidence rate
of NPPE in patients with acute upper airway obstruction was
reported to be as high as 12% (9).
From the statistics of the Australian Incident Monitoring Study
(AIMS), the reported incidence rate of NPPE in patients with
laryngospasm was 3% (10). The
incidence rate of NPPE has also been reported to be as low as 0.1%
in laryngospasm cases (11). Thus,
the estimated incidence rate of NPPE is between 0.1 and 12%
(9,12-14).
However, considering the frequent occurrence of upper airway
obstruction during the peri-anesthesia period, it was speculated
that the actual incidence rate may be much higher than that
reported so far, since several cases are misdiagnosed or overlooked
(1). Reports on mortality also
vary. The mortality rate of NPPE has previously been described as
11 to 40%, with a more recent literature review showing a mortality
rate of only 2% (15-25).
A recent systematic review of NPPE in adult ear, nose and throat
(ENT) surgery reported a mortality rate of 5% and identified age
and ICU admission as the main risks for increased mortality
(3). Due to the high incidence and
misdiagnosis rate of NPPE, further improvements in the level of
diagnosis, treatment and management of NPPE are expected in
clinical practice. The present review aims to summarize the latest
advances in the epidemiology, pathophysiology, clinical
manifestations and treatment of NPPE.
2. Etiology
Several acute or chronic diseases can cause upper
airway obstruction and each of them could lead to NPPE (26). Upper airway trauma and, more
commonly, laryngospasm account for ~50% of NPPE after obstruction
(4). In addition, upper airway
infections and vocal cord dysfunction are also potential causes of
NPPE (1,4).
Acute airway obstruction
In adult patients, airway obstruction leading to
NPPE occurs most commonly in the event of a post-extubation
laryngospasm (4,27). Most cases in children are
subglottic obstructions caused by the glottis, acute infectious
croup or epiglottitis (28,29).
In these pediatric cases, patients who present with ventilatory
failure due to glottic or supraglottic obstruction with prolonged
stridor (30) and pulmonary edema
are usually diagnosed after the initiation of mechanical
ventilation.
Chronic airway obstruction
Chronic upper airway obstruction is a prevalent
condition observed in patients with various underlying factors,
including obesity, obstructive sleep apnea, tonsil or gland
hypertrophy, upper airway tumor, mediastinal tumor, nasopharyngeal
mass, goiter and acromegaly (1,31-34).
In particular, adult patients with obesity and obstructive sleep
apnea, characterized by episodes of hypopnea and hypoxemia, have
demonstrated a propensity for intermittent pulmonary edema stemming
from recurrent upper airway obstruction (3).
Other risk factors
Several studies have reviewed cases that included
risk factors for perianaesthesia-induced NPPE, such as obesity with
obstructive sleep apnea and anatomical intubation difficulties, as
well as nasal, pharyngeal and laryngeal surgery or disease
(35-37).
Patients with the aforementioned risk factors should preferably
undergo awake intubation, as they may be at increased risk of
airway complications following extubation performed after general
anesthesia; therefore, optimal upper airway muscle tone is ensured
in these patients (36). These
patients should be closely observed in the post-anesthesia care
unit after surgery. The duration of the observation varies
depending on the specific individual (36).
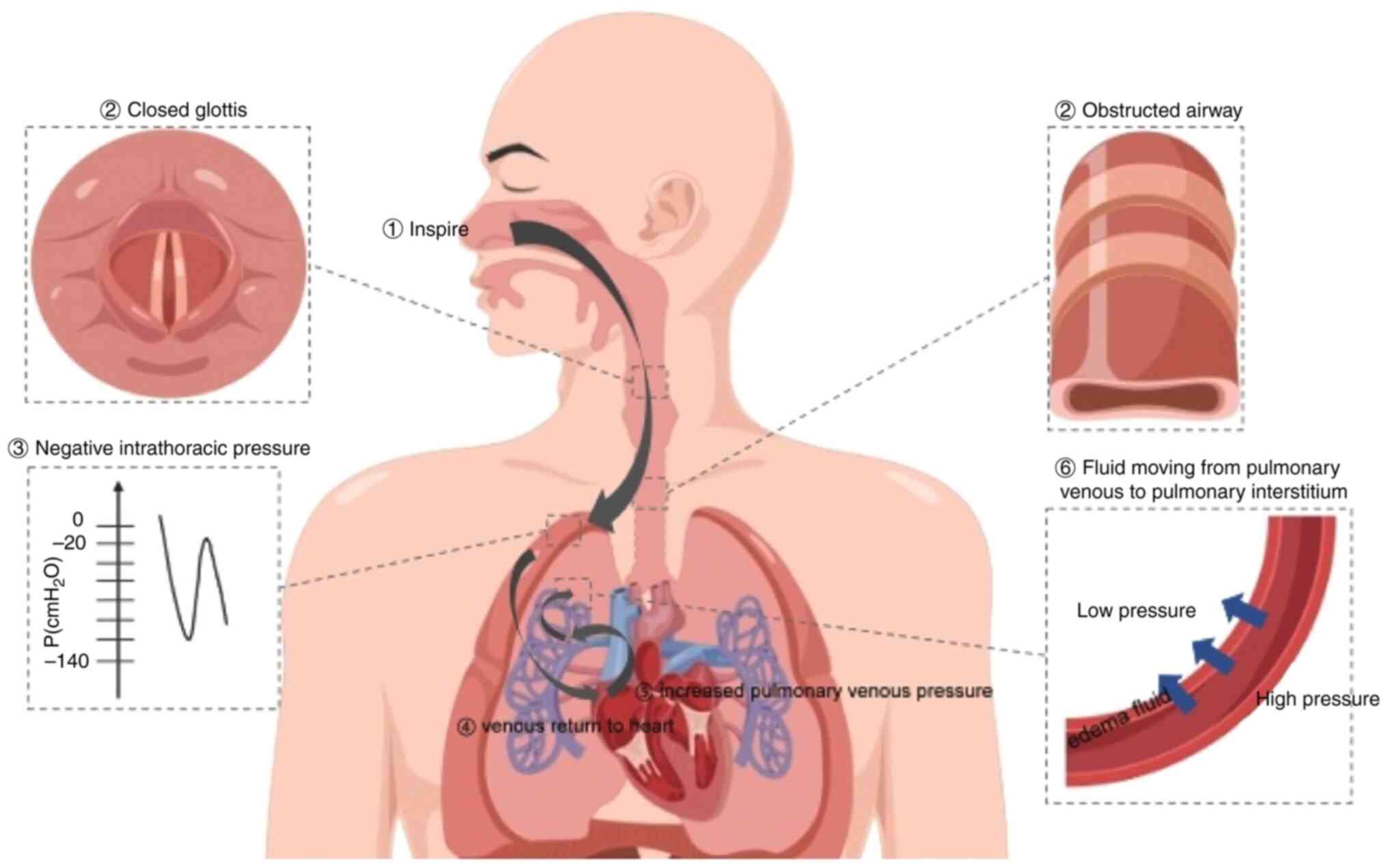
3. Pathogenesis and pathophysiological
features
The occurrence of NPPE is the result of a
combination of factors. There are two main pathogenic mechanisms in
NPPE: Hydrostatic and increased permeability pulmonary edema. The
mechanism behind hydrostatic pulmonary edema suggests that NPPE is
caused by marked fluid shifts triggered by changes in the
intrathoracic pressure (Fig. 1)
(1,4,38).
The mechanism behind increased permeability pulmonary edema
suggests that the destruction of alveolar epithelium and pulmonary
microvascular membranes caused by severe mechanical stress results
in increased pulmonary capillary permeability and protein-rich
pulmonary edema (26,38). Pulmonary edema fluid/plasma protein
ratio measurement is routinely used to distinguish hydrostatic
pulmonary edema from increased permeability pulmonary edema, since
the ability of the alveolar epithelial barrier to filter out the
alveolar edema fluid is usually impaired in patients with acute
lung injury but not in patients with hydrostatic pulmonary edema
(26). For example, Fremont et
al (26) collected edema fluid
and plasma samples from 10 patients with NPPE during the first 8 h
after intubation. The mean pulmonary edema fluid/plasma protein
ratio in these patients was 0.54±0.15, and the mean rate of
alveolar fluid clearance within 8 h was 14.0±17.4%/h (26). The pulmonary edema fluid/plasma
protein ratio and the presence of net alveolar fluid clearance
support the mechanism of hydrostatic pulmonary edema.
Starling principle
Starling's principle is an important theory that
describes the behavior of the fluid passing through the pulmonary
capillary endothelial-alveolar wall barrier (1). The Starling equation includes factors
that determine the formation of NPPE:
Qf=[Kx(Pmv-Pi)]-[σx(πmv-πi)],
where Qf is the net fluid flux between capillary
membranes, K is the coefficient of capillary permeability,
Pmv is the hydrostatic pressure of the capillary
membrane, Pi is the hydrostatic pressure of the alveolar
interstitium, σ is the reflection coefficient (that is, the ability
of a membrane to block protein passage), πmv is the
osmotic pressure of microvascular proteins, and πi is
the interstitial protein osmotic pressure. In physiologic
conditions, when the hydrostatic pressure is at equilibrium, the
primary plasma fluid flow from the capillaries to the lung
interstitium is minimal and the fluid is then reabsorbed by the
lymphatic vessels. However, when the rate of interstitial fluid
formation is higher than the reabsorption capacity, edema forms
(1,4,39).
Müller's maneuver
Müller's maneuver is a forced attempt to inhale
after the glottis has been closed. This action can markedly
increase the negative pressure in the pleural cavity (1). The pressure around the pulmonary
blood vessels and in the pulmonary interstitium can also be
markedly reduced through pressure transmission to the surrounding
structures (1). In healthy
individuals, the range of intrathoracic pressure is between -8 and
4 cmH2O at the end of calm inhalation and between -4 and
2 cmH2O at the end of exhalation. However, when the
airway is obstructed, a strong inspiration can generate negative
intrathoracic pressure as low as -50 to -100 cmH2O
(1,23). Strong inspiratory efforts may
generate even more negative pressures (-140 cmH2O)
(23). Although the exact
threshold of negative intrathoracic pressure to induce pulmonary
edema remains unclear, it is hypothesized that there may be
individual differences, since the parameters of each individual in
the Starling equation are different (1).
Ventricular preload and afterload
The decrease in intrathoracic pressure is
transmitted to the pulmonary interstitium, eventually causing an
increase in venous blood flow back to the right atrium (40). The increased workload in the right
side of the heart results in an increase in pulmonary blood volume,
pulmonary capillary hydrostatic pressure and hydrostatic pressure
gradient across the capillaries (17). This promotes the formation of
pulmonary edema. By contrast, a decrease in intrathoracic pressure
can increase left ventricular (LV) afterload, increasing both LV
end-diastolic volume and pressure (17). This reduces the LV ejection volume
and ejection fraction, ultimately leading to increased pulmonary
microvascular pressure (17).
Interventricular association
Increased venous return leads to increased filling
of the right ventricle, compressing the interventricular septum
toward the left ventricle. This results in a decrease in LV volume
and compliance (an ‘interventricular correlation’), which
ultimately leads to an increase in LV end-diastolic pressure.
Negative intrathoracic pressure increases the pressure gradient
between the left ventricle and the aorta, thereby increasing LV
afterload (4).
Pulmonary capillary permeability
It is commonly considered that the permeability of
pulmonary capillaries increases under the condition of negative
thoracic pressure (41). In
general, when pulmonary vascular compliance is low, an increase in
pulmonary blood volume can alter membrane permeability and even
disrupt membrane integrity, which ultimately leads to leakage of
body fluids and blood cells into the alveoli (20,41).
This damaging effect has been demonstrated both experimentally and
clinically (1,42,43).
Hypoxemia and hyperadrenergic
states
Hypoxemic and hyperadrenergic states that accompany
upper airway obstruction can also contribute to the development of
pulmonary edema (44,45). The hyperadrenergic state is induced
by upper airway obstruction alone and is enhanced by hypoxemia.
Hypoxia and metabolic acidosis (occurring during hypoxia due to
anaerobic metabolism, impaired circulation or impaired oxygen
utilization) lead to vasoconstriction in the pulmonary
microcirculation, which increases pulmonary circulatory resistance,
thus altering pulmonary capillary membrane permeability and
inhibiting myocardial function (46). Increased excitability of the
sympathetic nervous system results in the centralization of the
circulation and the movement of systemic blood toward the pulmonary
circulation, thereby increasing pulmonary microvascular pressure
(46).
The hyperadrenergic state can also alter the
integrity of the pulmonary capillary membranes (1). In addition, autonomic
hyperexcitability promotes the development of neurogenic pulmonary
edema, a condition characterized by the accumulation of fluid in
the lungs due to dysfunction or injury of the central nervous
system, occurring as a result of various neurological conditions or
insults, such as traumatic brain injury, intracranial hemorrhage,
seizures, brain tumors, or infections affecting the central nervous
system (1). Increased pulmonary
capillary membrane permeability and pulmonary microvascular
pressure, which result from the strong release of catecholamines
from the adrenal medulla, are currently considered to be the main
pathogenic mechanisms of pulmonary edema (19).
4. Clinical manifestations
Symptoms
Notably, patients may present with predisposing
factors for developing upper airway obstruction. Symptoms of upper
airway obstruction include stridor, respiratory distress,
paradoxical chest movements and the involvement of accessory
muscles in breathing (36,41). Clinical manifestations of acute
pulmonary edema include dyspnea, tachypnea, cyanosis, asthma and
the production of a profuse pink foamy sputum (1). Consequently, medical complications,
including hypoxemia, hypercapnia and metabolic acidosis, may occur
as a consequence of the underlying respiratory dysfunction and
disturbed gas exchange in acute pulmonary edema (1).
Disease progression
Cases of delayed onset are rare, but pulmonary edema
has been reported to occur 1-6 h after upper airway obstruction
(44,47,48).
Since the severity of clinical manifestations is variable, some
mild cases may not be recognized. The severity of the condition is
related to the duration of the obstruction and the degree of
pulmonary capillary damage (3,46).
It has been suggested that the observation period
following extubation should be extended in patients with
pre-existing or predisposing factors for upper airway obstruction,
even if clinical manifestations of pulmonary edema have not been
observed (44,46). For several patients, pulse oximetry
monitoring in the operating room and post-anesthesia care can
discern any risk factors (1). The
symptoms and signs of NPPE are atypical. Occasionally, the clinical
manifestation may only cause a slight decrease in pulse oximetry.
This may be more evident when the patient is breathing room air
and, in some cases, only chest imaging can identify the risk for
NPPE after the examination (1).
Symptoms subside
Once the airway is protected, the negative airway
pressure is relieved by positive-pressure mechanical ventilation
(1,26). Imaging analysis of alveolar or
interstitial edema has indicated that NPPE usually resolves within
12-24 h (1). Occasionally, some
cases take longer for the NPPE to resolve, which is related to the
degree of pulmonary microvascular damage. In most cases, NPPE
resolves within 12-24 h (26).
Consequences of NPPE
Some patients with NPPE may suffer from associated
long-term complications, such as myocardial infarction, transient
ischemic attack, non-ST-elevation myocardial infarction, hypoxic
brain injury and pulmonary hemorrhage. A small number of patients
may die from causes such as septic shock and cardiac arrest
(3).
5. Differential diagnosis
Clinically, it is difficult to diagnose NPPE in
patients with mild symptoms such as mild respiratory distress, a
mild decrease in oxygen saturation, a mild cough or cough with
frothy sputum or mild chest discomfort. The diagnosis can often be
made when the upper airway obstruction is relieved, but the
condition is not yet completely resolved (1,46).
NPPE should be differentiated from the diseases described below, as
their treatment differs from that of NPPE.
Aspiration of gastric contents
If NPPE is excluded, aspiration of gastric contents
and subsequent pneumonia should be considered first. Aspiration
pneumonia is occasionally self-limiting with only the use of
supportive care, such as oxygen therapy, fluid and nutritional
support, symptom management, respiratory support, and monitoring
and observation, depending on the quantity and quality of the
inhaled material, such as pH and type of particulate matter
inhaled. Direct laryngoscopy or fiberoptic bronchoscopy can help
confirm the diagnosis of particulate aspiration (5,49).
Chest X-ray imaging does not help to distinguish between pulmonary
edema and aspiration pneumonia (49).
Acute respiratory distress syndrome
(ARDS)
Based on the risk factors of the patient, ARDS must
be excluded. ARDS is a severe lung condition characterized by
sudden and widespread inflammation in the lungs, leading to
impaired oxygenation and difficulty in breathing. While both ARDS
and NPPE involve impaired oxygenation and respiratory distress,
their underlying mechanisms differ. ARDS is primarily caused by
inflammation and injury to the alveoli (air sacs) and lung tissue,
resulting in increased permeability of the lung's blood vessels and
leakage of fluid into the airspaces. By contrast, NPPE is caused by
negative pressure in the lungs due to upper airway obstruction,
leading to fluid accumulation and pulmonary edema. Rapid clinical
improvement also helps to confirm the diagnosis of post-obstructive
NPPE (1).
Hypervolemia
A measurement of the central venous pressure and
pulmonary capillary wedge pressure can be conducted to account for
the possibility of circulating hypervolemia. Treatments include
limiting fluid volume (ensuring that fluid intake is balanced or
reduced compared to fluid output), dilating blood vessels and using
diuretics.
Abnormal cardiac function
A diagnosis of cardiac insufficiency must be
excluded, especially in patients with a prior history of cardiac
disease or significant cardiac risk factors, such as hypertension,
diabetes, obesity and age. These cardiac insufficiencies include
myocardial ischemia, arrhythmia and cardiac decompensation caused
by valvular heart disease. Imaging aids can be used, with NPPE
often showing prominent bilateral perihilar alveolar infiltrates
(5). Cardiogenic pulmonary edema
infiltrates follow a more interstitial pattern and typically show
marked shunting of blood flow to the lungs (5,11).
Coronavirus disease 2019
(COVID-19)
Sudden respiratory failure and bilateral pulmonary
patchy infiltrates are the most common manifestations of NPPE. The
clinical diagnosis of NPPE may be confused with that of COVID-19.
Both NPPE and COVID-19 cause ground-glass opacities on chest CT
scans (50). The characteristic
changes of NPPE mainly appear in the central area, whereas COVID-19
mainly manifests in the peripheral area of the lungs (50). In addition, NPPE results in
decreased vascular clarity, whereas COVID-19 causes vasodilation in
the lesion area (1,51). These differences, along with the
medical history of the patient, are critical to distinguish between
these two similar imaging findings. Clinicians must understand the
differences between COVID-19 and NPPE to ensure proper diagnosis
and treatment (50).
6. Treatments
Treatment of NPPE depends on its severity and cause.
In mild cases, oxygen therapy alone may be sufficient. With proper
diagnosis and treatment, patients can be expected to recover from
NPPE within 24 h.
Ventilatory support
The primary method for treating NPPE is to maintain
airway patency and provide oxygen support. Invasive tests are
generally not required when considering the diagnosis of NPPE, but
close observation in the intensive care unit is required until the
condition is stable. Endotracheal intubation is usually required to
open the airway and support mechanical ventilation. Among the cases
reported by Lang et al (44), 85% of adult patients and children
required intubation, 50% required mechanical ventilation and 50%
required continuous positive end-expiratory pressure (PEEP)
ventilation. Initial attempts were made to maintain airway patency
and oxygen without intubation. Some patients required only oxygen
support, only continuous PEEP with a mask or both (1). Patients with severe pulmonary edema
often required reintubation in the operating room or
post-anesthesia care unit. Most patients treated with mechanical
ventilation were able to resolve pulmonary edema and extubation
within 24 h. For those patients treated with mechanical
ventilation, PEEP could improve oxygenation and reduce the required
oxygen concentration (1).
It can be challenging to perform PEEP if the upper
airway obstruction makes endotracheal intubation difficult
(52,53). Difficulties with endotracheal
intubation can also be encountered in patients with acute
epiglottitis. In these cases, clinicians need to be prepared to
obtain adequate rapid airway access via cricothyroidotomy or
tracheostomy (52,53).
Fluid management
Diuretics and steroids are often used as adjunctive
agents in the treatment of cardiogenic pulmonary edema to promote
fluid clearance (54,55). However, the use of some diuretics,
such as furosemide, is controversial (56). Some studies suggested that
diuretics are only useful in patients with ARDS and their effect in
other patients is limited (1,4,45).
In the past, fluid intake restriction and the use of diuretics were
not recommended for patients who were diagnosed with NPPE (4,45,57).
Since these patients are deficient in effective circulating blood
volume owing to the transfer of large amounts of fluid to the
lungs, this may aggravate their present state (57).
Other treatment measures
Invasive hemodynamic monitoring is usually not
required but it can be helpful in differential diagnosis (58). Adrenocorticoid therapy was used in
the past but this approach is not currently supported or
recommended, as it does not directly address the underlying
mechanism of NPPE (14,42). The use of bronchodilators has also
been studied and, although the obstruction may not be caused by
bronchospasm, these studies suggested that bronchodilators, such as
β-agonists, could increase alveolar fluid clearance to reduce
symptoms of pulmonary edema (1,59).
As aforementioned, patients who have developed upper airway
obstruction or have predisposing factors, but have not yet
exhibited pulmonary edema, require close observation in the
post-anesthesia care unit, although the duration of observation
remains controversial (1).
Previously, Grant et al (60) reported the case of a 26-year-old
female patient with laryngeal papillomatosis who developed
laryngospasm after direct laryngoscopy. The patient developed
severe NPPE, for which mechanical ventilator support was
ineffective. The patient was successfully treated with venovenous
extracorporeal membrane oxygenation.
Prevention
To reduce or eliminate NPPE, several prevention
strategies have been investigated. Oropharyngeal secretions of
patients should be thoroughly aspirated before extubation, as
bloody secretions may induce laryngospasm (61,62).
An increased number of laryngoscopy attempts is associated with an
increased incidence of laryngospasm (63). A dose of 5 mg of dexamethasone may
be used before extubation to reduce laryngeal edema caused by
multiple intubation attempts (64,65).
This effect is associated with a reduction in the frequency of
laryngospasms by deepening anesthesia and enhancing muscle
relaxation (66). A dose of 1-2
mg/kg of lidocaine 5 min before tracheal extubation has been
reported to reduce laryngospasm in children (67,68).
Propofol at a dose of 0.5 mg/kg administered 60 sec before
extubation was also effective in reducing the incidence of
laryngospasm (66,69). In addition, the cuff leak test
could help prevent the risk of post-extubation edema (62). The cuff leak test is based on the
principle that air leaks around the tracheal tube where the cuff is
deflated will be inversely proportional to the degree of laryngeal
obstruction resulting from laryngeal edema (70). Extubation may be successful if air
leaks can be heard when the patient coughs during PEEP (70).
7. Conclusion
NPPE is a rare but potentially fatal complication of
upper airway obstruction; patients with atypical symptoms are often
misdiagnosed. NPPE should be considered in patients presenting with
symptoms of pulmonary edema following upper airway obstruction,
after excluding other contributing factors. The treatment of NPPE
includes close monitoring, prompt relief of airway obstruction,
administration of supplemental oxygen and, if necessary, assisted
ventilation. Due to interindividual variations, the epidemiology,
etiology and pathophysiological processes of NPPE have been
subjects of controversy and pose ongoing challenges.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Kunshan Science Project
(grant no. KSZ2169).
Availability of data and materials
Not applicable.
Authors' contributions
JM, TL, QW and HY conceived, designed and
coordinated the study. TL, QW, JM, HY, ZG, QF, YZ and XX drafted
this manuscript. All authors substantially contributed to the
conception, writing and revision of the work. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bhattacharya M, Kallet RH, Ware LB and
Matthay MA: Negative-pressure pulmonary edema. Chest. 150:927–933.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Capitanio MA and Kirkpatrick JA:
Obstructions of the upper airway in children as reflected on the
chest radiograph. Radiology. 107:159–161. 1973.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Din-Lovinescu C, Trivedi U, Zhang K,
Barinsky GL, Grube JG, Eloy JA and Hsueh WD: Systematic review of
negative pressure pulmonary edema in otolaryngology procedures. Ann
Otol Rhinol Laryngol. 130:245–253. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lemyze M and Mallat J: Understanding
negative pressure pulmonary edema. Intensive Care Med.
40:1140–1143. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Holzgreve A, Fabritius MP and Conter P: CT
findings in negative pressure pulmonary edema. Diagnostics (Basel).
10(749)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu R, Wang J, Zhao G and Su Z: Negative
pressure pulmonary edema after general anesthesia: A case report
and literature review. Medicine (Baltimore).
98(e15389)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moore RL and Binger CA: The response to
respiratory resistance: A comparison of the effects produced by
partial obstruction in the inspiratory and expiratory phases of
respiration. J Exp Med. 45:1065–1080. 1927.PubMed/NCBI View Article : Google Scholar
|
|
8
|
McGowan FX, Kenna MA, Fleming JA and
O'Connor T: Adenotonsillectomy for upper airway obstruction carries
increased risk in children with a history of prematurity. Pediatr
Pulmonol. 13:222–226. 1992.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tami TA, Chu F, Wildes TO and Kaplan M:
Pulmonary edema and acute upper airway obstruction. Laryngoscope.
96:506–509. 1986.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kluger MT, Visvanathan T, Myburgh JA and
Westhorpe RN: Crisis management during anaesthesia: regurgitation,
vomiting, and aspiration. Qual Saf Health Care.
14(e4)2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Deepika K, Kenaan CA, Barrocas AM, Fonseca
JJ and Bikazi GB: Negative pressure pulmonary edema after acute
upper airway obstruction. J Clin Anesth. 9:403–408. 1997.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Park H, Nam S, Jang YJ, Ku S and Choi SS:
Negative pressure pulmonary edema in a patient undergoing open
rhinoplasty: A case report. Medicine. 100(e24240)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tebay A, Bouti K and Tebay N: Negative
pressure pulmonary edema following a cholecystectomy-A case report.
Rev Pneumol Clin. 73:267–271. 2017.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
14
|
Xiong J and Sun Y: Negative pressure
pulmonary edema: A case report. BMC Anesthesiol.
19(63)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen Y and Zhang X: Acute postobstructive
pulmonary edema following laryngospasm in elderly patients: a case
report. J Perianesth Nurs. 34:250–258. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ikeda-Miyagawa Y, Kihara T and Matsuda R:
Case of negative pressure pulmonary edema after administration of
sugammadex under general anesthesia with laryngeal mask airway.
Masui. 63:1362–1365. 2014.PubMed/NCBI(In Japanese).
|
|
17
|
Contou D, Voiriot G, Djibré M, Labbé V,
Fartoukh M and Parrot A: Clinical features of patients with diffuse
alveolar hemorrhage due to negative-pressure pulmonary edema. Lung.
195:477–487. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sakamoto T, Sato R, Endo A, Iwashita Y and
Tanabe K: Negative-Pressure pulmonary edema and takotsubo
cardiomyopathy in the older adults. Cureus.
14(e22661)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Udeshi A, Cantie SM and Pierre E:
Postobstructive pulmonary edema. J Crit Care. 25:508.e1–e5.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kuramoto K, Matsuyama M, Nonaka M,
Takeishi T, Oshima H, Matsumura S, Nakajima M, Sakai C, Shiozawa T,
Kiwamoto T, et al: Negative-pressure pulmonary hemorrhaging due to
severe obstructive sleep apnea. Intern Med. 60:2291–2296.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Patton WC and Baker CL Jr: Prevalence of
negative-pressure pulmonary edema at an orthopaedic hospital. J
South Orthop Assoc. 9:248–253. 2000.PubMed/NCBI
|
|
22
|
Chen G, Wang XD, Nie HF, Yang ZQ, Chen K,
Li ZH, Song YM, Pei FX and Zeng JC: Negative pressure pulmonary
edema after percutaneous endoscopic interlaminar lumbar
discectomy-a case report. BMC Musculoskelet Disord.
19(401)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Goldenberg JD, Portugal LG, Wenig BL and
Weingarten RT: Negative-pressure pulmonary edema in the
otolaryngology patient. Otolaryngol Head Neck Surg. 117:62–66.
1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Westreich R, Sampson I, Shaari CM and
Lawson W: Negative-pressure pulmonary edema after routine
septorhinoplasty: Discussion of pathophysiology, treatment, and
prevention. Arch Facial Plast Surg. 8:8–15. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Karakaya MA and Karakaya AD: As a rare
reason of alveolar consolidation, negative pressure pulmonary
edema: Case report. Medeni Med J. 35:75–78. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fremont RD, Kallet RH, Matthay MA and Ware
LB: Postobstructive pulmonary edema: A case for hydrostatic
mechanisms. Chest. 131:1742–1746. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li X, Tan L, He M and Zhu T: Subcutaneous
emphysema and negative pressure pulmonary edema after
robot-assisted laparoscopic bladder enlargement: A case report.
Asian J Surg. 45:1753–1754. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Toukan Y, Gur M, Keshet D and Bentur L:
Negative pressure pulmonary edema in a child following laryngospasm
triggered by a laryngeal mask. Isr Med Assoc J. 21:56–57.
2019.PubMed/NCBI
|
|
29
|
Thiagarajan RR and Laussen PC: Negative
pressure pulmonary edema in children-pathogenesis and clinical
management. Paediatr Anaesth. 17:307–310. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chaudhry H, Nimmala S, Papudesi BN, Sajjad
F, Paul S, Gohar Z, Azad R, Naveen H and Demidovich J: Negative
pressure pulmonary oedema due to rigors and chills associated with
liver abscess. Respirol Case Rep. 9(e0826)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Watanabe Y, Nagata H, Ichige H and Kojima
M: Negative pressure pulmonary edema related with severe sleep
apnea syndrome: A case report. Respir Med Case Rep.
31(101153)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Medford A: Negative pressure pulmonary
edema: Consider undiagnosed obstructive sleep apnea too. Chest.
141(1365)2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chaudhary BA, Nadimi M, Chaudhary TK and
Speir WA: Pulmonary edema due to obstructive sleep apnea. South Med
J. 77:499–501. 1984.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chaudhary BA, Ferguson DS and Speir WA Jr:
Pulmonary edema as a presenting feature of sleep apnea syndrome.
Chest. 82:122–124. 1982.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Masui D, Fukahori S, Nakahara H, Tsuruhisa
S, Sakamoto S, Higashidate N, Hashizume N, Koga Y, Saikusa N, Ishii
S and Tanaka Y: Negative-Pressure pulmonary edema after difficult
endotracheal intubation in a patient with juvenile rheumatoid
arthritis undergoing spigelian hernia surgery: A case report. Am J
Case Rep. 23(e934678)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lorch DG and Sahn SA: Post-extubation
pulmonary edema following anesthesia induced by upper airway
obstruction. Are certain patients at increased risk? Chest.
90:802–805. 1986.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yamanashi H, Koyamatsu J, Nobuyoshi M,
Murase K and Maeda T: Exercise-Induced pulmonary edema in a
triathlon. Case Rep Med. 2015(968152)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang Q, Vayalumkal J, Ricely J, Elrod S
and Raza A: The awareness of negative pressure pulmonary edema in
the medical intensive care unit. Cureus. 12(e10251)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Matthay MA, Folkesson HG and Clerici C:
Lung epithelial fluid transport and the resolution of pulmonary
edema. Physiol Rev. 82:569–600. 2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kuo JS, Ruan SY, Huang CT and Chen WT:
Postobstructive pulmonary edema after nonlethal suicidal hanging.
Am J Respir Crit Care Med. 204:e113–e114. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Milet P and Louis O: Negative pressure
pulmonary oedema and pulmonary haemorrhage following upper airway
obstruction. Rev Med Liege. 70:371–373. 2015.PubMed/NCBI(In French).
|
|
42
|
Toukan Y, Gur M and Bentur L: Negative
pressure pulmonary edema following choking on a cookie. Pediatr
Pulmonol. 51:E25–E27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bhaskar B and Fraser JF: Negative pressure
pulmonary edema revisited: Pathophysiology and review of
management. Saudi J Anaesth. 5:308–313. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lang SA, Duncan PG, Shephard DA and Ha HC:
Pulmonary oedema associated with airway obstruction. Can J Anaesth.
37:210–218. 1990.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Guru PK, Agarwal A, Pimentel M, McLaughlin
DC and Bansal V: Postoperative pulmonary edema conundrum: A case of
negative pressure pulmonary edema. Case Rep Crit Care.
2018(1584134)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bisinotto FM, Cardoso R and Abud TM: Acute
pulmonary edema associated with obstruction of the airways. Rev
Bras Anestesiol. 58:165–171. 2008.PubMed/NCBI View Article : Google Scholar : (In English).
|
|
47
|
Rosero-Britton B, Uribe A, Stoicea N,
Periel L and Bergese SD: Negative pressure pulmonary edema
postextubation following medial nerve repair with sural graft
surgery in a young patient: A case report. Medicine.
97(e13743)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Koide M, Kitada T, Kogure M, Fukui K,
Sogabe K, Kato Y, Kitajima H and Akabame S: Extraordinary
Delayed-Onset negative pressure pulmonary hemorrhage resulting in
cardiac arrest after general anesthesia for vocal cord polypectomy.
Case Rep Crit Care. 2020(8830935)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li Q and Zhou L: A rare case of type II
negative pressure pulmonary edema following extraction of inhaled
peanuts in a 21-month-old boy. J Int Med Res.
49(3000605211047779)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Karaman I and Ozkaya S: Differential
diagnosis of negative pressure pulmonary edema during COVID-19
pandemic. J Craniofac Surg. 32:e421–e423. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Luks AM and Swenson ER: COVID-19 lung
injury and high altitude pulmonary edema: A false equation with
dangerous implications. Ann Am Thorac Soc. 17:918–921.
2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Black AE, Flynn PE, Smith HL, Thomas ML
and Wilkinson KA: Association of Pediatric Anaesthetists of Great
Britain and Ireland. Development of a guideline for the management
of the unanticipated difficult airway in pediatric practice.
Paediatr Anaesth. 25:346–362. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Niven AS and Doerschug KC: Techniques for
the difficult airway. Curr Opin Crit Care. 19:9–15. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Dobbe L: Cardiogenic Pulmonary Edema. Am J
Med Sci. 358:389–397. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ingbar DH: Cardiogenic pulmonary edema:
Mechanisms and treatment-an intensivist's view. Curr Opin Crit
Care. 25:371–378. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Trabelsi B, Yedes A, Kharrat G, Abdouli H,
Mahouachi I, Saied MR and Ben Ali M: Negative-pressure pulmonary
edema following maxillofacial surgery: Recognize to prevent further
complications. Oral Maxillofac Surg: Oct 14, 2022. (Epub ahead of
print). doi: 10.1007/s10006-022-01122-6.
|
|
57
|
Liu PY, Shih ML and Chen CW:
Postobstructive pulmonary edema associated with a substernal
goitre. CMAJ. 184:2011–2014. 2012.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bateman RM, Sharpe MD, Jagger JE, Ellis
CG, Solé-Violán J, López-Rodríguez M, Herrera-Ramos E,
Ruíz-Hernández J, Borderías L, Horcajada J, et al: 36th
International Symposium on Intensive Care and Emergency Medicine:
Brussels, Belgium. 15-18 March 2016. Crit Care. 20 (Suppl
2)(S94)2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Perina DG: Noncardiogenic pulmonary edema.
Emerg Med Clin North Am. 21:385–393. 2003.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Grant BM, Ferguson DH, Aziz JE and Aziz
SM: Successful use of VV ECMO in managing negative pressure
pulmonary edema. J Card Surg. 35:930–933. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hampson-Evans D, Morgan P and Farrar M:
Pediatric laryngospasm. Paediatr Anaesth. 18:303–307.
2008.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Huang YC, Lin HY, Huang TT and Lan MC:
Negative pressure pulmonary edema after vocal augmentation. J
Formos Med Assoc. 118:1166–1167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lee JH, Turner DA, Kamat P, Nett S, Shults
J, Nadkarni VM and Nishisaki A: Pediatric Acute Lung Injury and
Sepsis Investigators (PALISI); National Emergency Airway Registry
for Children (NEAR4KIDS). The number of tracheal intubation
attempts matters! A prospective multi-institutional pediatric
observational study. BMC Pediatr. 16(58)2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Lee CH, Peng MJ and Wu CL: Dexamethasone
to prevent postextubation airway obstruction in adults: A
prospective, randomized, double-blind, placebo-controlled study.
Crit Care. 11(R72)2007.PubMed/NCBI View
Article : Google Scholar
|
|
65
|
Kuriyama A, Umakoshi N and Sun R:
Prophylactic corticosteroids for prevention of postextubation
stridor and reintubation in adults: A systematic review and
meta-analysis. Chest. 151:1002–1010. 2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Cheng JZ and Wang J: Negative pressure
pulmonary edema related to laryngospasm and upper airway
obstruction in a patient with treacher collins syndrome. Cureus.
13(e14426)2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Mihara T, Uchimoto K, Morita S and Goto T:
The efficacy of lidocaine to prevent laryngospasm in children: A
systematic review and meta-analysis. Anaesthesia. 69:1388–1396.
2014.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Qi X, Lai Z, Li S, Liu X, Wang Z and Tan
W: The Efficacy of lidocaine in laryngospasm prevention in
pediatric surgery: A network meta-analysis. Sci Rep.
6(32308)2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Batra YK, Ivanova M, Ali SS, Shamsah M, Al
Qattan AR and Belani KG: The efficacy of a subhypnotic dose of
propofol in preventing laryngospasm following tonsillectomy and
adenoidectomy in children. Paediatr Anaesth. 15:1094–1097.
2005.PubMed/NCBI View Article : Google Scholar
|
|
70
|
De Backer D: The cuff-leak test: What are
we measuring? Crit Care. 9:31–33. 2004.PubMed/NCBI View
Article : Google Scholar
|