Introduction
In the modern industrial society, environmental
pollutants, including particulate matter (PM) such as fine dust,
have a significant impact on public health (1). PM is typically categorized into dust
with a diameter of ≤10 µm and dust with a diameter of ≤2.5 µm [fine
PM (PM2.5)]. It is primarily generated from industrial
facilities and vehicles, and consists of organic components, such
as dioxins and benzene, as well as inorganic components such as
nitrates, sulfates and metal compounds (2). PM infiltrates the respiratory and
circulatory systems of the human body, leading to various health
issues. Particularly, PM2.5 is recognized as a key
factor causing severe diseases in humans, including respiratory
diseases, cardiovascular diseases and cancer (3,4).
Health issues related to environmental pollution are exponentially
increasing, and pollution has a lethal impact on individuals with
respiratory and cardiovascular diseases, including the elderly,
resulting in an increase in mortality rates (5). Therefore, there is a marked emphasis
on research, not only on the mechanistic aspects of the impact of
PM2.5 on human health, but also on the development of
materials that can effectively control its presence (6).
Oxidative stress in the human body has been reported
to play a crucial role in causing genetic mutations in cells and
tissues, as well as in exerting lethal effects on cellular
organelles, ultimately leading to various human diseases (7). Reactive oxygen species (ROS), a key
factor contributing to oxidative stress, are generated not only
during physiological conditions such as immune responses, but also
due to physical and chemical environmental pollutants.
Specifically, PM2.5, when inhaled through the
respiratory system, has been reported to directly generate large
amounts of ROS along the bloodstream, affecting various tissues in
the human body and inducing oxidative stress (8,9).
Since ROS are considered essential factors in inducing both acute
and chronic inflammatory diseases, there is an increasing need to
effectively control them. This has led to a concentration of
interest not only in the field of biomedicine, but also in the
health food sector, prompting numerous researchers to focus on
developing natural food materials for effective ROS control
(10). Consequently, research in
the food industry is actively pursuing the development of natural
food materials that can control ROS effectively with minimal or no
side-effects (11,12). However, research on natural food
materials specifically aimed at managing and improving oxidative
stress and inflammation caused by PM2.5 is still
insufficiently advanced.
Propolis, a natural resin collected by bees to
protect their hives, contains various bioactive components, such as
flavonoids, phenolic acids, esters, terpenes, amino acids and
vitamins (13-15).
These components exhibit potent antioxidant effects by neutralizing
ROS and eliminating free radicals. Propolis is well-known for its
anti-inflammatory effects, inhibiting the generation of
inflammatory mediators and reducing inflammatory responses
(16). Red bean (Vigna
angularis), a 1-year vine plant cultivated in East Asia, has
been reported to have anticancer, antioxidant, anti-inflammatory
and anti-obesity effects (17).
Red beans are rich in polyphenols and flavonoids, which help
prevent oxidative damage and contribute to maintaining cellular
health (18). Furthermore,
polyphenols and flavonoid components derived from red beans have
been reported to regulate inflammatory responses and contribute to
the prevention and management of chronic inflammatory-related
diseases (19). Additionally,
tomatoes (Solanum lycopersicum) contain antioxidants, such
as polyphenols, flavonoids and lycopene. These components protect
cells from free radicals, reduce DNA damage and contribute to
inhibiting inflammatory responses (20,21).
Lycopene, in particular, provides protective effects against
various health issues related to oxidative stress (22). Despite the well-known benefits of
propolis, red beans and tomatoes, there is a consistent increase in
consumer demand due to a growing interest in health. However,
despite their efficacy, the utilization of extracts from these
three sources is relatively low based on consumer preferences,
resulting in a slow growth rate in demand.
Therefore, the aim of the present study was to
develop a mixture of propolis, red bean and tomato extracts (PRTE)
that could alleviate oxidative stress and inflammatory responses
caused by PM2.5. The aim was to manufacture PRTE, verify
its antioxidant abilities based on the ratios of each extract, and
create a novel natural extract. In order to achieve this, optimal
ratios were determined, and the antioxidant and anti-inflammatory
effects were investigated using keratinocyte cells (HaCaT cells)
and macrophages (RAW264.7 cells) following treatment with the
developed mixture.
Materials and methods
Cells and materials
HaCaT cells (cat. no. 300493-SF) were acquired from
the CLS Cell Lines Service GmbH. RAW264.7 cells (cat. no. TIB-71)
were purchased from ATCC. Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS), penicillin-streptomycin, RIPA
buffer, and trypsin-EDTA were purchased from Thermo Fisher
Scientific, Inc. The Quanti-MAX™ WST-8 cell viability assay kit,
and TBST buffer was obtained from BIOMAX, Inc. 2,2'-Azino-bis
(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium
persulfate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), Griess reagent,
lipopolysaccharide (LPS), PM2.5 and goat anti-rabbit IgG
HRP-conjugated antibody (cat. no. 31458) were purchased from
MilliporeSigma. The prostaglandin E2 (PGE2),
interleukin (IL)-1β), IL-6 and tumor necrosis factor-α (TNF-α)
ELISA kits were obtained from R&D Systems, Inc. The superoxide
dismutase (SOD), glutathione peroxidase (GPx) and glutathione (GSH)
assay kits were purchased from Cayman Chemical Company. Inducible
nitric oxide synthase (iNOS; sc-7271), cyclooxygenase-2 (COX-2;
sc-514489) and β-actin (cat. no. sc-8432) antibodies, along with
goat anti-mouse IgG HRP-conjugated antibody (cat. no. sc-2354),
were acquired from Santa Cruz Biotechnology, Inc. The Bradford
assay reagent and SDS-PAGE sample loading buffer were purchased
from Bio-Rad Laboratories, Inc.
PRTE
The propolis used in the present study was provided
by Unique BioTech Co., Ltd. Red beans and tomatoes were purchased
from a local market, and following verification by Professor
Hong-Jun Kim at the College of Oriental Medicine, Woosuk University
(Wanju-gun, Korea) the samples (voucher specimen; #2023-06-07) were
stored at the research laboratory of SIJ at Jeonju University
(Jeonju, Korea). Red bean and tomato extracts were prepared by
mixing them in a 1:20 ratio with 70% ethanol and subjecting them to
vibration extraction at 161 x g for 3 days at 50˚C. The extracts
were filtered once through a nylon mesh and twice through a 0.45-µm
filter paper. The filtered extracts were concentrated under a
rotary vacuum (A-3S; EYELA) at 50˚C and then freeze-dried to obtain
powder samples. The obtained powder samples were stored at -80˚C
and used in the following experiments.
The derivation of the mixture ratio of
propolis, red bean and tomato
PRTE were mixed under four different conditions as
follows: Mixture 1 (M1-PRTE) was prepared by combining propolis,
red bean and tomato at a ratio of 1:1:1. Mixture 2 (M2-PRTE) was
prepared at a ratio of 1.5:1:0.5, mixture 3 (M3-PRTE) at a ratio of
1.5:0.5:1 and mixture 4 (M4-PRTE) at a ratio of 1.2:0.9:0.9. In
order to determine the optimal mixture ratio, the
radical-scavenging efficacy of each mixture was evaluated, as
described below. Based on these results, the optimal mixture was
determined.
DPPH radical scavenging activity
The DPPH radical scavenging activity experiment was
conducted with a slight modification of the method proposed in the
study by Blois (23). Each extract
and mixture were dissolved in distilled water. Subsequently, 100 µl
of each sample solution and 100 µl of 0.3 mM DPPH solution were
mixed in a 96-well plate and allowed to react at room temperature
for 20 min. The absorbance was then measured at 540 nm (Sunrise™,
Tecan Group, Ltd.), and the percentage difference in absorbance
between the sample solution and the blank solution was
calculated.
ABTS radical scavenging activity
The ABTS radical scavenging activity was measured
according to the method described in the study by Re et al
(24). A mixture of 7 mM ABTS and
2.45 mM potassium persulfate
(K2S2O8) at a 1:1 ratio was
allowed to stand for 24 h at room temperature to generate radicals.
The resulting radical solution was diluted with distilled water to
achieve an absorbance of 0.70±0.04 at 720 nm. Subsequently, 50 µl
of each extract and mixture were mixed with 950 µl of the prepared
ABTS solution and allowed to react for 30 min at 23˚C. After the
reaction, 100 µl of the mixture were transferred to a 96-well
plate, and the absorbance was measured at 720 nm (Sunrise™, Tecan
Group, Ltd.). The percentage difference in absorbance between the
sample solution and the blank solution was calculated.
Cell culture
The human-derived keratinocyte cell line (HaCaT) was
obtained from CLS Cell Lines Service GmbH, and the murine
macrophage cell line (RAW264.7) was acquired from ATCC. The cells
were cultured in DMEM containing 10% FBS and 1% antibiotics
(penicillin and streptomycin) in a humidified atmosphere at 37˚C
with 5% CO2.
Cell viability
The HaCaT cells were seeded at a concentration of
2x105 cells/ml in a 96-well plate and the cells were
cultured at 37˚C and 5% CO2 for 24 h. The cells were
then exposed to with various concentrations of PM2.5
(0-100 µg/ml) or M2-PRTE (0-50 µg/ml). Following 24 h of
incubation, the cells were cultured at 37˚C and 5% CO2,
WST-8 solution (10 µl per well) was added, and after 4 h, the
absorbance was measured at 450 nm (Sunrise™, Tecan Group, Ltd.) to
calculate the cell viability. The RAW264.7 cells were seeded at a
final concentration of 2x105 cells/ml in a 96-well
plate, then cultured for 24 h at 37˚C with 5% CO2.
Subsequently, they were treated with M2-PRTE at concentrations of
25 and 50 µg/ml, followed by exposed to LPS at a concentration of 1
µg/ml after 1 h. Following 24 h of incubation, the cells were
cultured at 37˚C and 5% CO2, WST-8 solution (10 µl per
well) was added, and after 4 h, the absorbance was measured at 450
nm (Sunrise™, Tecan Group, Ltd.) to calculate cell viability.
Measurement of SOD and GPx, and
determination of the GSH content
After seeding tbe HaCaT cells in a 60-mm dish at a
final concentration of 2x105 cells/ml, the cells were
cultured at 37˚C and 5% CO2 for 24 h. Subsequently, the
cells were treated with M2-PRTE at concentrations of 25 and 50
µg/ml. After 1 h, PM2.5 was added at a concentration of
100 µg/ml, and the cells were then cultured at 37˚C and 5%
CO2 for an additional 24 h. Subsequently, the cells were
washed twice with PBS and protein extraction was performed using
RIPA buffer. The extracted proteins were quantified by measuring
the absorbance at 595 nm using Bradford protein assay reagent, and
the activities of SOD and GPx, as well as the GSH content, were
measured according to the manufacturer's instructions.
Measurement of nitric oxide (NO)
production
After seeding the RAW264.7 cells in a 48-well plate
at a final concentration of 2x105 cells/ml, the cells
were cultured in an incubator at 37˚C and 5% CO2 for 24
h. Following this, the cells were treated with M2-PRTE at
concentrations of 25 and 50 µg/ml, and 1 h later, LPS was added at
a concentration of 1 µg/ml. After 24 h, a mixture of 100 µl Griess
reagent and 100 µl cell culture supernatant was prepared in a
96-well plate, and the absorbance was measured at 540 nm using a
microplate reader (Tecan Group Ltd.) at room temperature. A
standard curve was constructed using sodium nitrate, and the amount
of NO production was calculated.
Western blot analysis
After seeding the RAW264.7 cells in a 60-mm dish at
a final concentration of 2x105 cells/ml, the cells were
cultured in an incubator at 37˚C and 5% CO2 for 24 h.
Following this, the cells were treated with M2-PRTE at
concentrations of 25 and 50 µg/ml. Subsequently, 1 h later, LPS was
added at a concentration of 1 µg/ml, and the cells were cultured at
37˚C and 5% CO2 for 24 h. Subsequently, the cells were
washed twice with PBS and protein extraction was performed using
RIPA buffer. The extracted proteins were quantified by measuring
the absorbance at 595 nm using Bradford protein assay reagent. The
quantified proteins were separated by SDS-PAGE (7.5%) at 100 V for
1 h and transferred onto a PVDF (polyvinylidene difluoride)
membrane (Bio-Rad Laboratories, Inc.). The membrane was blocked
with 5% skim milk at room temperature for 1 h, followed by three
washes with TBST buffer for 10 min each. Primary antibodies for
iNOS (1:200), COX-2 (1:100) and β-actin (1:2,000) were then
applied, and the membrane was incubated at 4˚C for 24 h. The
membrane was then washed three times with TBST for 10 min each. The
secondary antibody (mouse IgG HRP; 1:5,000) was applied at room
temperature for 2 h, followed by three washes with TBST for 10 min
each. Subsequently, images were obtained using a UV imaging system
(ALLIANCE LD4; UVITEC). Protein band intensity was analyzed using
ImageJ (1.53a) gel analysis software (National Institutes of
Health).
Measurement of TNF-α, IL-1β and IL-6
cytokines, and PGE2 levels
After seeding the RAW264.7 cells in a 12-well plate
at a final concentration of 2x105 cells/ml, the cells
were cultured in an incubator at 37˚C and 5% CO2 for 24
h. Following this, the cells were treated with M2-PRTE at
concentrations of 25 and 50 µg/ml. Subsequently, 1 h later, LPS was
added at a concentration of 1 µg/ml. After 24 h, the supernatant
was collected, and the levels of TNF-α, IL-1β, IL-6 and
PGE2 were measured according to the protocol of the
ELISA assay kits provided by the manufacturer.
High-performance liquid chromatography
(HPLC) analysis
Solvent extracts of propolis, red bean and tomato
were filtered using a 0.45-µm syringe filter and then used for HPLC
analysis. HPLC analysis was performed using a Waters e2695 Alliance
HPLC System (Waters Corporation) equipped with a binary pump
delivery system, degasser (G1379A), autosampler (G1313A) and PDA
detector (G1315B) operating at 330 nm. Separation was performed
with a gradient elution (0 min-10% B, 13 min-10% B, 20 min-25% B,
24 min-30% B, 28 min-35% B, 32 min-45% B, 35 min-45% B, 40 min-50%
B, 43 min-55% B, 47 min-60% B, 50 min-60% B, 55 min-10% B) and flow
rate and sample consisting of 0.1% formic acid in acetonitrile and
0.1% acetic acid in distilled H2O over an Xbridge C18
column (Waters Corporation, 4.6x250 mm, 5 µm). The injection volume
was fixed at 0.5 ml/min and 15 µl, respectively. The column
temperature was 35˚C. Standards were identified based on retention
time, and the concentrations of caffeic acid, ferulic acid,
chlorogenic acid, caffeic acid phenethyl ester, isoquercetin, rutin
and lycopene were calculated by comparing the peak area with that
of the standard.
Statistical analysis
All experimental values are expressed as the mean ±
standard deviation (mean ± SD). Statistical comparisons were
performed using IBM SPSS Statistics 22 (IBM Corp.). Comparisons
between different experimental groups were conducted using one-way
analysis of variance (ANOVA), and post hoc multiple comparisons
were carried out using Tukey's test to identify significant
differences among the experimental groups. P-value <0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
Determination of the ratio of
propolis, red bean and tomato mixture, and the measurement of the
antioxidant activity
Prior to assessing the intracellular antioxidant and
anti-inflammatory efficacy of PRTE, the PRTE were mixed under four
conditions as follows: M1-PRTE was mixed at a 1:1:1 ratio, M2-PRTE
at a ratio of 1.5:1:0.5, M3-PRTE at a ratio of 1.5:0.5:1 and
M4-PRTE at a ratio of 1.2:0.9:0.9. Free radicals in an unstable
state can cause damage to cells within the body, and the
antioxidant efficacy of using antioxidant substances can be
measured by evaluating radical scavenging ability (25). In the present study, in order to
determine the optimal mixture ratio, the DPPH and ABTS radical
scavenging abilities of each mixture were evaluated. As presented
in Table I, among the four
combinations, M2-PRTE at a ratio of 1.5:1:0.5 exhibited the most
superior DPPH and ABTS radical scavenging abilities compared to the
other ratios. The radical scavenging abilities (IC50) of
DPPH and ABTS radicals for M2-PRTE were confirmed as 192.86±3.34
µg/ml and 554.28±4.78 µg/ml, respectively (Table I). Furthermore, when comparing the
radical scavenging abilities of DPPH and ABTS with the individual
extracts of propolis, red bean and tomato, M2-PRTE at a ratio of
1.5:1:0.5 exhibited enhanced radical scavenging abilities compared
to the individual extracts. Based on these results, M2-PRTE was
selected for confirming the antioxidant efficacy in HaCaT
keratinocytes.
 | Table IRadical scavenging ability of
different ratios of PRTE and each extract. |
Table I
Radical scavenging ability of
different ratios of PRTE and each extract.
| Samples | DPPH
(IC50) | ABTS
(IC50) |
|---|
| M1-PRTE |
385.13±7.22e |
704.06±5.56d |
| M2-PRTE |
192.86±3.34a |
554.28±4.78a |
| M3-PRTE |
197.54±2.86a |
674.75±6.92c |
| M4-PRTE |
296.18±4.62b |
683.65±5.66c |
| Propolis
extract |
197.21±4.11a |
566.43±9.26a |
| Red bean
extract |
316.12±3.83c |
598.93±8.61b |
| Tomato extract |
336.28±11.26d |
974.24±8.26e |
Antioxidant effects of M2-PRTE on
PM2.5-induced oxidative stress
Before measuring the antioxidant effects, the
cytotoxicity of PM2.5 and M2-PRTE on the human-derived
keratinocyte cell line, HaCaT, was evaluated using the WST-8 assay
to assess cell viability. The results revealed no cytotoxicity at
all concentrations tested for both PM2.5 and M2-PRTE
(Fig. 1). Based on these results,
subsequent experiments were performed using the HaCaT cells with a
non-cytotoxic concentration of PM2.5 at 100 µg/ml and
PRTE at concentrations <50 µg/ml. To investigate the effects of
M2-PRTE on the activity of antioxidant enzymes, the HaCaT cells
were pre-treated with M2-PRTE (25 and 50 µg/ml) for 1 h, followed
by the induction of oxidative stress with PM2.5.
Subsequently, the activities of SOD and GPx, as well as the GSH
content, were measured. The results revealed that exposure to
PM2.5 significantly depleted the enzymatic activities of
SOD and GPx, and reduced the GSH content compared to the control
group (Fig. 2). However, following
treatment with M2-PRTE at a concentration of 25 µg/ml, the GPx
activity exhibited no significant change; however, a substantial
increase was observed following treatment at a concentration of 50
µg/ml (Fig. 2A). SOD activity,
which decreased in a concentration-dependent manner following
exposure to PM2.5, was restored and significantly
increased at a concentration of 50 µg/ml M2-PRTE (Fig. 2B). Finally, the GSH content
exhibited a modest restorative effect at a concentration of 25
µg/ml M2-PRTE; notably, at a concentration of 50 µg/ml M2-PRTE,
there was a marked restorative effect in the GSH content (Fig. 2C).
SOD, GPx and GSH are essential antioxidant enzymes
that protect cells from oxidative stress and ROS. They
significantly contribute to maintaining the health and stability of
cells in unique ways (26). SOD,
as an endogenous antioxidant enzyme, effectively removes reactive
oxygen species such as O2-, thus playing a crucial role
in protecting cells from oxidative stress (27). Furthermore, GPx collaborates with
GSH to prevent cellular damage by eliminating ROS and organic
peroxides (28). GSH, in turn,
functions as an antioxidant responding to oxidative stress within
cells. It is essential for neutralizing and detoxifying toxic
substances, and is known to be involved in the proper response and
protection mechanisms of cells (29). Therefore, M2-PRTE appears to be a
bioactive material contributing to the restoration of antioxidant
enzyme activities, such as SOD and GPx, which are depleted by
oxidative stress such as PM2.5, as well as the
replenishment of the antioxidant substance GSH. Hence, the superior
antioxidant effects of M2-PRTE suggest its potential use as a
natural antioxidant agent.
Inhibitory effects of M2-PRTE on NO
production
Prior to confirming whether the superior antioxidant
efficacy of M2-PRTE translates to anti-inflammatory effects, the
present study first evaluated the NO scavenging ability of PRTE
mixtures under four conditions in RAW264.7 cells. This was
performed to verify whether the anti-inflammatory efficacy of
M2-PRTE aligns with its antioxidant efficacy. The cellular
environment has a marked impact on the conditions the cell
experiences. In the present study, the RAW 264.7 cells exhibited
variable NO production rates that were associated with the number
of passages. To ensure accuracy, experiments were repeated with the
same number of passages (10-11)
to ensure the consistency of NO production levels. The results
revealed that the NO scavenging ability followed the order of
M2-PRTE > M1-PRTE > M3-PRTE > M4-PRTE, and consistent with
the antioxidant efficacy experiments, M2-PRTE exhibited the most
superior performance (Fig. 3A).
Additionally, when comparing the NO scavenging ability with the
individual extracts of propolis, red bean and tomato, the ratio of
M2-PRTE at 1.5:1:0.5 exhibited superior NO scavenging ability
compared to the individual extracts (Fig. 3B). Subsequently, to investigate
whether the observed NO scavenging ability resulted from toxicity
induced by LPS and the extracts, cell toxicity was examined using
WST-8. The results revealed no cytotoxicity under all conditions,
confirming the absence of toxic effects (Fig. 3C and D). Based on these results, M2-PRTE was
selected for further confirmation of its anti-inflammatory efficacy
in RAW264.7 mouse macrophages.
 | Figure 3Inhibitory effects of different
ratios of PRTE on (A and B) NO levels and (C and D) on the
viability of LPS-stimulated RAW264.7 cells. The cells
(2x105 cells/ml) were cultured and pre-treated with 25
or 50 µg/ml M2-PRTE for 1 h, and then stimulated with LPS (1 µg/ml)
for 24 h. NO levels were examined in the culture supernatants, and
relative cell viability was assessed using WST-8 assay. The results
are presented as the mean ± SD of three different experiments. Bars
with different lowercase letters (a-d) indicate statistically
significant differences between groups (P<0.05). Bars with the
same lowercase letter (a) indicate that there were no statistically
significant differences between groups (P>0.05). PRTE, propolis,
red bean and tomato extracts; M1-PRTE, 1:1:1 ratio; M2-PRTE,
1.5:1:0.5 ratio; M3-PRTE, 1.5:0.5:1 ratio; M4-PRTE, 1.2:0.9:0.9
ratio; NO, nitric oxide; LPS, lipopolysaccharide; P, propolis; R,
red bean; T, tomato. |
The inhibitory effects of M2-PRTE on inflammatory
mediators, NO and PGE2, in LPS-stimulated RAW264.7 cells
were then investigated. Initially, in the LPS-stimulated RAW264.7
cells, the production of NO and PGE2 significantly
increased compared to the untreated control group. However, in the
cells pre-treated with M2-PRTE, a concentration-dependent and
significant inhibitory effect on both NO and PGE2
production were observed (Fig. 4A
and B). In acute inflammation, NO
promotes vasodilation and increases blood flow to the inflammatory
site, aiding in the defense against invading microorganisms
(30). However, the chronic
overproduction of NO can lead to tissue damage and inflammation in
diseases, such as chronic lung conditions (31). At the same time, the
pro-inflammatory mediator, PGE2, is involved in
vasodilation, increased vascular permeability and the infiltration
of immune cells into the inflammatory site. Environmental
pollutants, such as fine dust can induce the excessive production
of PGE2, potentially serving as a cause for chronic
inflammatory diseases such as chronic bronchitis and atopic
dermatitis (32). Therefore, the
regulation of the excessive production of NO and PGE2 is
considered a crucial therapeutic target in the management of
chronic inflammatory conditions. The present study then
investigated the mechanisms of action of M2-PRTE in the inhibition
of NO and PGE2 production; the effects on the expression
of iNOS and COX-2 proteins were examined using western blot
analysis. The results revealed an increase in the protein
expression of iNOS due to LPS exposure (Fig. 4C). However, following treatment
with two concentrations of M2-PRTE, 25 and 50 µg/ml, the protein
expression of both iNOS and COX-2 significantly decreased (Fig. 4C-E). The activation of iNOS can
have negative effects on health by increasing inflammation and
oxidative stress. COX-2 plays a crucial role in converting
arachidonic acid to PGE2, directly participating in the
inflammatory process (33).
Therefore, M2-PRTE was found to inhibit the expression of iNOS and
COX-2, leading to the suppression of NO and PGE2
production. Consequently, M2-PRTE may be considered as a bioactive
food material that can effectively inhibit mediators causing
inflammatory diseases in the human body.
 | Figure 4Inhibitory effects of M2-PRTE on (A)
NO levels, (B) PGE2 levels, and (C-E) on iNOS and COX-2
expression in LPS-stimulated RAW264.7 cells. The cells
(2x105 cells/ml) were cultured and pre-treated with 25
or 50 µg/ml M2-PRTE for 1 h, and then stimulated with LPS (1 µg/ml)
for 24 h. The NO and PGE2 levels were examined in the
culture supernatants. iNOS and COX-2 expression levels were
examined in the total cell extracts, and the relative density of
iNOS and COX-2 was then calculated using ImageJ software. The
results are presented as the mean ± SD of three different
experiments. Bars with different lowercase letters (a-ds) indicate
statistically significant differences between groups (P<0.05).
M2-PRTE, propolis, red bean and tomato extracts at a ratio of
1.5:1:0.5; LPS, lipopolysaccharide; NO, nitric oxide;
PGE2, prostaglandin E2; iNOS, inducible
nitric oxide synthase; COX-2, cyclooxygenase-2. |
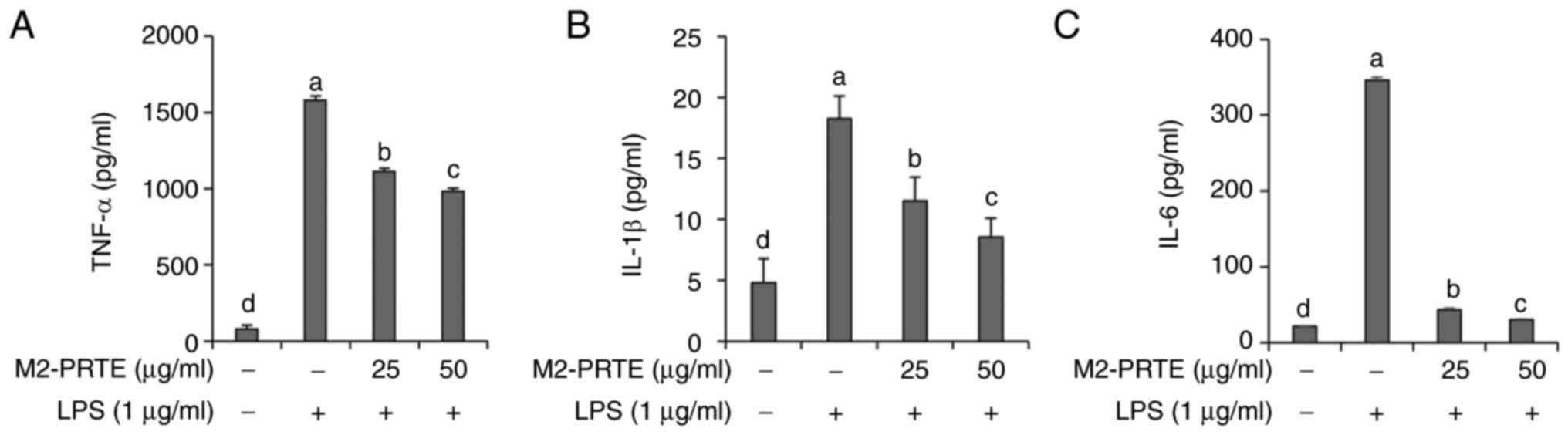
Inhibitory effects of M2-PRTE on
IL-1β, TNF-α and IL-6 production
IL-1β, TNF-α and IL-6 are well-known representative
pro-inflammatory cytokines that induce inflammatory responses
(34). Therefore, in
LPS-stimulated RAW264.7 cells, the present study investigated the
inhibitory effects of M2-PRTE on the production of the
pro-inflammatory cytokines, IL-1β, TNF-α and IL-6. The results
revealed a significant increase in the production of IL-1β, TNF-α
and IL-6 in the LPS-exposed RAW264.7 cells; however, pre-treatment
with M2-PRTE exerted a concentration-dependent and significant
inhibitory effect (Fig. 5). IL-1β
promotes immune cell migration to the inflammatory site and
triggers important responses, such as fever (35), while TNF-α increases vascular
permeability at the inflammatory site, activating the movement of
inflammatory cells (36).
Furthermore, IL-6 functions as a key factor that activates the
immune system when infection or tissue damage occurs (37). Therefore, to improve and treat
inflammatory diseases, the effective regulation of pro-inflammatory
cytokines, such as IL-1β, TNF-α and IL-6 is crucial, and there is a
need to discover substances that can modulate these inflammatory
mediators. From this perspective, M2-PRTE is considered to have the
potential to effectively alleviate inflammation by controlling the
production of IL-1β, TNF-α and IL-6. The present study demonstrated
that the mixture of propolis, tomato and red bean provided
prominent antioxidant and anti-inflammatory effects, exerting
protective effects against oxidative stress and inflammation
induced by PM2.5 pollution. With its ability to regulate
immune function and alleviate oxidative stress, propolis functions
synergistically with tomatoes (38), which are rich in lycopene, a free
radical neutralizer, to maintain cellular health (39). The addition of red beans, known for
their high antioxidant content, improves the protective efficacy of
the mixture against oxidative damage (40). This combination not only highlights
the usefulness of natural compounds in reducing health risks
associated with PM2.5, but also enhances their role in
alleviating oxidative stress and inflammation.
HPLC analysis
HPLC was conducted to determine the content of
chemical compounds contained in the propolis, red bean and tomato
extracts. The chemical compounds of the propolis extract were
chlorogenic acid (42.68±0.63 µg/g), caffeic acid (17.81±0.57 µg/g),
ferulic acid (0.28±1.48 µg/g), and caffeic acid phenethyl ester
(18.66±1.24 µg/g), respectively (Fig.
6A). In the red bean extract, isoquercetin (23.41±0.92 µg/g)
and rutin (1.38±0.58 µg/g) were detected (Fig. 6B). Moreover, lycopene (10.69±1.17
µg/g), known as a representative substance in tomato extract, was
quantified (Fig. 6C). As
aforementioned, as regards the anti-inflammatory and antioxidant
effects of PRTE, additional verification for the chemical
composition of their extracts was deemed necessary.
In conclusion, the mixture of propolis, red bean,
and tomato, known as M2-PRTE, exhibited not only DPPH and ABTS
radical scavenging abilities, but also demonstrated antioxidant
activity by inducing the activation of antioxidant enzymes, such as
SOD and GPx, as well as by increasing the intracellular GSH levels
in HaCaT cells under conditions of oxidative stress induced by
PM2.5. Additionally, M2-PRTE exerted inhibitory effects
on the expression of iNOS and COX-2 molecules in LPS-stimulated
RAW264.7 cells, leading to the suppression of NO and
PGE2 production. Moreover, M2-PRTE effectively inhibited
the production of pro-inflammatory cytokines, such as IL-1β, TNF-α
and IL-6. Therefore, M2-PRTE is anticipated to have high potential
as a functional food ingredient for alleviating oxidative stress
and inhibiting inflammatory responses. However, further research is
required in order to explore the efficacy and molecular mechanisms
of M2-PRTE at the physiological level, and additional studies on
functional components are warranted for its utilization as a health
functional food ingredient.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Collabo R&D
between Industry, Academy, and Research Institute
(RS-2023-00224909) funded by the Ministry of SMEs and Startups
(MSS, Korea).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ESK and BOC conceived and designed the experiments.
ESK and SYJ participated in the design of the study and in the
drafting of the manuscript. ESK and SYJ carried out the
experiments. ESK, SYJ, MHJ, MYK, YKH, BOC and SIJ participated in
acquisition, analysis and interpretation of the data. SIJ provided
resources, reviewed and edited the manuscript, and supervised the
study. BOC and SIJ confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Manisalidis I, Stavropoulou E,
Stavropoulos A and Bezirtzoglou E: Environmental and health impacts
of air pollution: A review. Front Public Health.
8(14)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Adams K, Greenbaum DS, Shaikh R, van Erp
AM and Russell AG: Particulate matter components, sources, and
health: Systematic approaches to testing effects. J Air Waste Manag
Assoc. 65:544–558. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bălă GP, Râjnoveanu RM, Tudorache E,
Motișan R and Oancea C: Air pollution exposure-the (in)visible risk
factor for respiratory diseases. Environ Sci Pollut Res Int.
28:19615–19628. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim H, Kim WH, Kim YY and Park HY: Air
pollution and central nervous system disease: A review of the
impact of fine particulate matter on neurological disorders. Front
Public Health. 8(575330)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Münzel T, Hahad O, Sørensen M, Lelieveld
J, Duerr GD, Nieuwenhuijsen M and Daiber A: Environmental risk
factors and cardiovascular diseases: A comprehensive expert review.
Cardiovasc Res. 118:2880–2902. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li Z, Wen Q and Zhang R: Sources, health
effects and control strategies of indoor fine particulate matter
(PM2.5): A review. Sci Total Environ. 586:610–622.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Giustarini D, Dalle-Donne I, Tsikas D and
Rossi R: Oxidative stress and human diseases: Origin, link,
measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci.
46:241–281. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu K, Hua S and Song L: PM2.5 exposure
and asthma development: The key role of oxidative stress. Oxid Med
Cell Longev. 2022(3618806)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xu Z, Ding W and Deng X: PM2.5,
fine particulate matter: A novel player in the
epithelial-mesenchymal transition? Front Physiol.
10(1404)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Muchtaridi M, Az-Zahra F, Wongso H,
Setyawati LU, Novitasari D and Ikram EHK: Molecular mechanism of
natural food antioxidants to regulate ROS in treating cancer: A
review. Antioxidants (Basel). 13(207)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shin JY, Kang ES, Park JH, Cho BO and Jang
SI: Anti-inflammatory effect of red ginseng marc, Artemisia
scoparia, Paeonia japonica and Angelica gigas extract mixture in
LPS-stimulated RAW 264.7 cells. Biomed Rep. 17(63)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sasidharan S, Nishanth KS and Nair HJ:
Ethanolic extract of Caesalpinia bonduc seeds triggers yeast
metacaspase-dependent apoptotic pathway mediated by mitochondrial
dysfunction through enhanced production of calcium and reactive
oxygen species (ROS) in Candida albicans. Front Cell Infect
Microbiol. 12(970688)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yu M, Gouvinhas I, Rocha J and Barros
AIRNA: Phytochemical and antioxidant analysis of medicinal and food
plants towards bioactive food and pharmaceutical resources. Sci
Rep. 11(10041)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Šuran J, Cepanec I, Mašek T, Radić B,
Radić S, Tlak Gajger I and Vlainić J: Propolis extract and its
bioactive compounds-from traditional to modern extraction
technologies. Molecules. 26(2930)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zullkiflee N, Taha H and Usman A:
Propolis: Its role and efficacy in human health and diseases.
Molecules. 27(6120)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pahlavani N, Malekahmadi M, Firouzi S,
Rostami D, Sedaghat A, Moghaddam AB, Ferns GA, Navashenaq JG,
Reazvani R, Safarian M and Ghayour-Mobarhan M: Molecular and
cellular mechanisms of the effects of Propolis in inflammation,
oxidative stress and glycemic control in chronic diseases. Nutr
Metab (Lond). 17(65)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jiang Y, Zeng KW, David B and Massiot G:
Constituents of Vigna angularis and their in vitro
anti-inflammatory activity. Phytochemistry. 107:111–118.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yao Y, Cheng X, Wang S, Wang L and Ren G:
Influence of altitudinal variation on the antioxidant and
antidiabetic potential of azuki bean (Vigna angularis). Int J Food
Sci Nutr. 63:117–124. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chu L, Zhao P, Wang K, Zhao B, Li Y, Yang
K and Wan P: VaSDC1 is involved in modulation of flavonoid
metabolic pathways in black and red seed coats in Adzuki Bean
(Vigna angularis L.). Front Plant Sci. 12(679892)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Włodarczyk K, Smolińska B and Majak I: The
antioxidant potential of tomato plants (Solanum lycopersicum L.)
under nano-ZnO treatment. Int J Mol Sci. 24(11833)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kamiloglu S, Demirci M, Selen S, Toydemir
G, Boyacioglu D and Capanoglu E: Home processing of tomatoes
(Solanum lycopersicum): Effects on in vitro bioaccessibility of
total lycopene, phenolics, flavonoids, and antioxidant capacity. J
Sci Food Agric. 94:2225–2233. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Del Giudice R, Petruk G, Raiola A, Barone
A, Monti DM and Rigano MM: Carotenoids in fresh and processed
tomato (Solanum lycopersicum) fruits protect cells from oxidative
stress injury. J Sci Food Agric. 97:1616–1623. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Blois MS: Antioxidant determinations by
the use of a stable free radical. Nature. 181:1199–1200. 1958.
|
|
24
|
Re R, Pellegrini N, Proteggente A, Pannala
A, Yang M and Rice-Evans C: Antioxidant activity applying an
improved ABTS radical cation decolorization assay. Free Radic Biol
Med. 26:1231–1237. 1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rahman MM, Islam MB, Biswas M and Khurshid
Alam AH: In vitro antioxidant and free radical scavenging activity
of different parts of Tabebuia pallida growing in Bangladesh. BMC
Res Notes. 8(621)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ma X, Deng D and Chen W: Inhibitors and
Activators of SOD, GSH-Px, and CAT. In: Enzyme Inhibitors and
Activators. Senturk M (ed). IntechOpen, Rijeka, 2017.
|
|
27
|
Zhao H, Zhang R, Yan X and Fan K:
Superoxide dismutase nanozymes: An emerging star for
anti-oxidation. J Mater Chem B. 9:6939–6957. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pei J, Pan X, Wei G and Hua Y: Research
progress of glutathione peroxidase family (GPX) in redoxidation.
Front Pharmacol. 14(1147414)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Al-Temimi AA, Al-Mossawi AE, Al-Hilifi SA,
Korma SA, Esatbeyoglu T, Rocha JM and Agarwal V: Glutathione for
food and health applications with emphasis on extraction,
identification, and quantification methods: A review. Metabolites.
13(465)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gewalting MT and Kojda G: Vasoprotection
by nitric oxide: Mechanisms and therapeutic potential. Cardiovasc
Res. 55:250–260. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
van der Vliet A, Eiserich JP and Cross CE:
Nitric oxide: A pro-inflammatory mediator in lung disease? Respir
Res. 1:67–72. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Liu NM, Miyashita L, Sanak M, Barratt B
and Grigg J: Prostaglandin E2 and phagocytosis of
inhaled particulate matter by airway macrophages in cystic
fibrosis. J Cyst Fibros. 20:673–677. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim JB, Han AR, Park EY, Kim JY, Cho W,
Lee J, Seo EK and Lee KT: Inhibition of LPS-induced iNOS, COX-2 and
cytokines expression by poncirin through the NF-kappaB inactivation
in RAW 264.7 macrophage cells. Biol Pharm Bull. 30:2345–2351.
2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ishijima T and Nakajima K: Inflammatory
cytokines TNFα, IL-1β, and IL-6 are induced in endotoxin-stimulated
microglia through different signaling cascades. Sci Prog.
104(368504211054985)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lopez-Castejon G and Brough D:
Understanding the mechanism of IL-1β secretion. Cytokine Growth
Factor Rev. 22:189–195. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Parameswaran N and Patial S: Tumor
necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene
Expr. 20:87–103. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6(a016295)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xu W, Lu H, Yuan Y, Deng Z, Zheng L and Li
H: The antioxidant and anti-inflammatory effects of flavonoids from
propolis via Nrf2 and NF-κB pathways. Foods.
11(2439)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kulawik A, Cielecka-Piontek J and Zalewski
P: The importance of antioxidant activity for the health-promoting
effect of lycopene. Nutrients. 15(3821)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chao WW, Chung YC, Shih IP, Wang HY, Chou
ST and Hsu CK: Red bean extract inhibits lipopolysaccharide-induced
inflammation and H2O2-Induced oxidative
stress in RAW 264.7 macrophages. J Med Food. 18:724–730.
2015.PubMed/NCBI View Article : Google Scholar
|