Introduction
Stanozolol is a performance-enhancing anabolic
androgenic steroid (AAS). Among all AASs, stanozolol is one of the
most frequently abused steroids by professional athletes and young
adults in order to ameliorate physical appearance and performance.
Stanozolol is a 17α-alkylated derivative of testosterone with
anabolic and high androgenic properties (1,2)
and its use is prohibited in sports by the World Anti-doping Agency
(WADA) (3).
In the past, AASs were used only by elite athletes
and bodybuilders for doping purposes. However, nowadays even young
adults are abusing AASs at supraphysiological doses in order to
improve physical appearance (4,5).
Stanozolol has been reported to be one of the most commonly abused
AAS (6) and it is responsible for
several medical and behavioral adverse effects, being a recognized
risk factor for liver diseases, both in experimental animals and in
human beings (7–13). Stanozolol is extensively
biotransformed by enzymatic pathways in the liver. The major
metabolites of stanozolol have been reported to be
3′-hydroxystanozolol, 4-β-hydroxystanozolol and
16-β-hydroxystanozolol (14,15). In general, AASs exert their
effects through several different mechanisms, such as by modulating
androgen receptor expression (16). Liver-related adverse effects are
more commonly associated with the 17α-alkyl derivatives of AASs and
have been reported not to be related with the route of
administration. However, the exact mechanisms are not yet fully
understood (17).
Telomeres are heterochromatin nucleoprotein
complexes on the chromosome ends involved in a number of basic
biological functions (Fig. 1). It
is known that telomeres play a key role in the formation and
progression of up to 90% of malignancies. Telomerase activity plays
a key role in cellular aging and tumorigenesis (18). An increased telomerase activity is
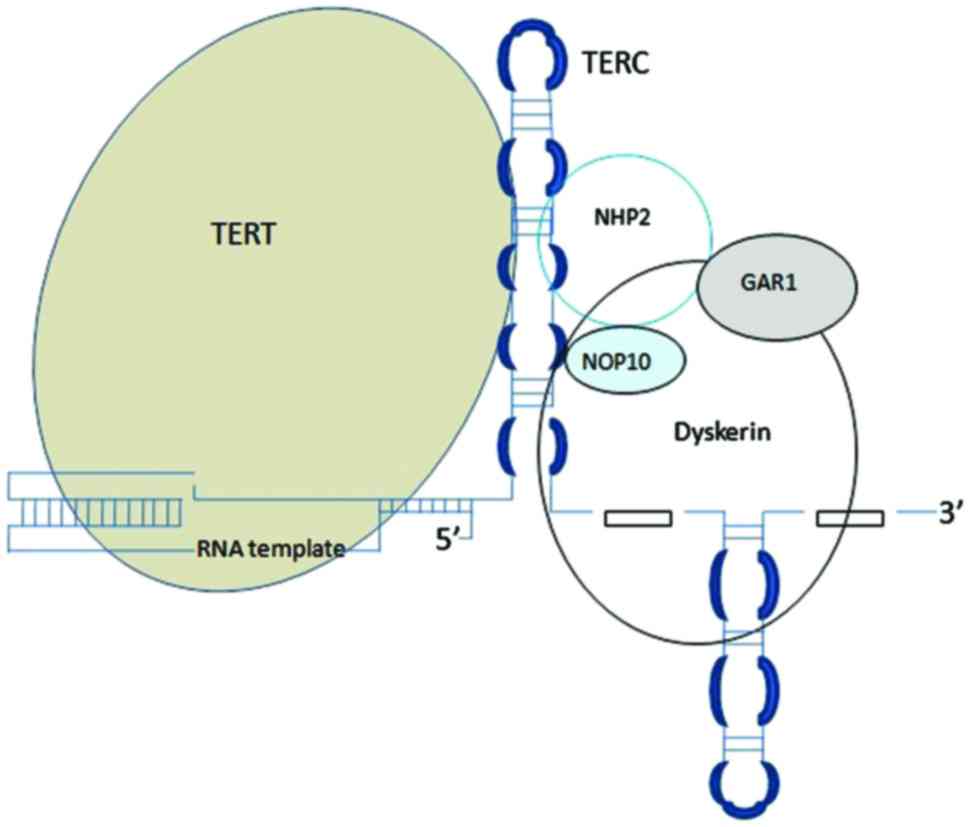
detected in the majority of human cancers (19). Telomerase is a ribonucleoprotein
responsible for maintaining telomere length. The core of telomerase
has two components: Catalytic telomerase reverse transcriptase
(TERT) and telomerase RNA component (TERC) (Fig. 2).
The TERT mRNA expression level has been studied as a
biomarker, as it has been demonstrated to be the rate-limiting
determinant of telomerase activity in various malignancies
(20). The phosphatase and tensin
homolog protein (PTEN) gene encodes a tumor suppressor protein with
phosphatase activity. It has been reported that PTEN has a loss of
heterozygosity frequency incidence in human hepatocellular
carcinoma (HCC) of up to 33% (21). PTEN is involved in the
downregulation of telomerase activity via TERT activity regulation
(22). PTEN is a negative
regulator protein of the phosphoinositide 3-kinase/AKT signaling
pathway of the cell survival regulatory mechanism and induces
cellular apoptosis (23). PTEN
prevents the activation of AKT via the de-phosphorilation of
phosphatidylinositol (3,4,5)-trisphosphate (PIP3) to
phosphatidylinositol 4,5-bisphosphate (PIP2). The suppression of
PTEN is associated with oncogenic activity in the cell (24).
The aim of this study was to investigate, for the
first time, at least to the best of our knowledge, the role of
telomerase in stanozolol-induced hepatotoxicity by investigating
the correlation between telomerase activity and PTEN-TERT gene
expression levels. The bioaccumulation of stanozolol and its two
major metabolites (3′-hydroxystanozolol and 16-β-hydroxystanozolol)
in the liver tissue was also examined, as well as its association
with telomerase activity.
Materials and methods
Animal experiments
A total of 34 male Sprague-Dawley rats, 8 weeks old,
were obtained and housed in the laboratory animal house facilities
of the Department of Laboratory Animal Sciences, Institute of
Experimental Medicine, Istanbul University (Istanbul, Turkey), in
accordance with the Ethics Committee on Animal Experimentation of
Istanbul University, HADYEK (approval no. 2013/100). The rats were
divided into 5 groups as follows: i) The control (C) group; ii) the
propylene treatment (PG) group; iii) the stanozolol treatment (ST)
group; iv) the propylene treatment and exercise (PGE) group; and v)
the stanozolol treatment and exercise (STE) group. The animals were
housed as 4 animals per one metal cage and kept in a 12-h
dark/light cycle at a temperature of 20–23°C. The number of rats
per experimental group, rat care, handling and employed
experimental procedures were in accordance with the guidelines of
HADYEK. The weight of the rats upon purchase was recorded and used
for dose adjustments (Table I).
The humane endpoints defined in our study were pain, distress,
abnormal posture and seizures in accordance with the OECD Guidance
Document (25). No animals
exhibited clinical signs of humane endpoints that justified their
sacrifice prior to the end of the experiment. The experimental
design of the study is presented in Table II.
 | Table IWeight of the rats upon purchase. |
Table I
Weight of the rats upon purchase.
| Number of rats | Weight of rats upon
purchase (g) |
|---|
| Control |
| 1 | 259 |
| 2 | 268 |
| 3 | 271 |
| 4 | 264 |
| 5 | 282 |
| Propylene
glycol |
| 1 | 255 |
| 2 | 276 |
| 3 | 273 |
| 4 | 261 |
| 5 | 272 |
| Propylene glycol
and exercise |
| 1 | 262 |
| 2 | 276 |
| 3 | 284 |
| 4 | 264 |
| 5 | 278 |
| 6 | 263 |
| 7 | 260 |
| 8 | 277 |
| Steroid group |
| 1 | 280 |
| 2 | 265 |
| 3 | 275 |
| 4 | 272 |
| 5 | 277 |
| 6 | 272 |
| 7 | 263 |
| 8 | 290 |
| Steroid and
exercise group |
| 1 | 275 |
| 2 | 277 |
| 3 | 290 |
| 4 | 263 |
| 5 | 280 |
| 6 | 285 |
| 7 | 279 |
| 8 | 273 |
 | Table IIExperimental design of the study. |
Table II
Experimental design of the study.
| Groups | No. of rats | Subcutaneous
injections | Exercise |
|---|
| Control | 5 | No injection | No exercise |
| Propylene glycol
treatment | 5 | 1 ml/kg propylene
glycol per day | No exercise |
| Stanozolol
treatment | 8 | 5 mg/kg stanozolol
per day | No exercise |
| Propylene glycol
treatment and exercise | 8 | 1 ml/kg propylene
glycol per day | Swimming: 20
min/day, 5 days/week |
| Stanozolol
treatment and exercise | 8 | 5 mg/kg stanozolol
per day | Swimming: 20
min/day, 5 days/week |
Swimming was selected as a model of exercise
(26,27) and began 1 week prior to the
treatment scheme in order for the animals to adapt. The rats were
subjected to swimming in a rectangular polyethylene tank
(120-cm-long × 50-cm-deep × 43-cm-wide) filled with water at
29±1°C. During the experiments, for 20 min/day, 5 days/week, the
rats were subjected to swimming following an adaptation period of 1
week. The animals were adapted to the process by swimming in water
for 5 min during the first 2 days, and swimming time was then
gradually increased to 5 min per day up to a final duration of 20
min on day 5.
Propylene glycol (PG) (Tekkim, Istanbul, Turkey) was
used as a vehicle for stanozolol (Sigma, Schnelldorf, Germany). PG
is known to be a good vehicle for in vivo experimental
studies (28,29). However, it has been reported that
high concentrations of PG can induce DNA damage in eukaryotic cells
and mouse oocytes (30,31). Subcutaneous administration was
selected and the doses were selected in accordance with previous
studies (32–34). The exposed groups received a
single dose of PG (1 ml/kg) and ST (5 ml/kg) subcutaneously for 5
days per week.
After 28 days of treatment, the animals underwent
light anesthesia using a percentage of 1.9% diethyl-ether in an
anesthesia chamber and euthanized by cervical dislocation carried
out properly trained personnel. Liver tissue samples were collected
and divided into 2 sections. One section was immediately frozen in
liquid nitrogen and stored at −80°C, and the other was fixed with
10% buffered formalin and embedded paraffin for histochemical
analysis.
Liquid chromatography-mass spectrometry
(LC-MS) analysis
Standards of stanozolol, 3′-hydroxystanozolol and
16-β-hydroxystanozolol at concentrations of 0, 0.1, 0.25, 0.5 and 1
ppm were prepared from 20 ppm standard stock solutions. Turinabol
(LGC, Leeds, UK) was used as an internal standard (IS) with target
ions m/z 317.25 and m/z 335.25. Calibration curves were obtained by
measuring the peak of target ions areas ratio to IS as follows: For
stanozolol m/z 370.4, 352.3 and 329.35, for
3′-hydroxystanozolol m/z 386.4, 345.35, for
16-β-hydroxystanozolol m/z 386.4, 366.3 and 345.35 for IS
turinabol (Table III) (the m/z
ion used for quantification is shown in bold font). The liver
samples of the untreated animals that yielded negative results
(<LOD) during screening were used as blank matrices for the
preparation of spiked standard samples at various concentrations
(0, 1, 2, 5 and 10 ng/mg). The spiked samples were used for the
preparation of spiked curves and furthermore for the determination
of stanozolol, 3′-hydroxystanozolol and 16-β-hydroxystanozolol
levels in the liver samples.
 | Table IIILC-MS analysis parameters. |
Table III
LC-MS analysis parameters.
| Agent | Rt | m/z target | m/z | m/z | Mw |
|---|
|
4α-hydroxystanozolol | 9 | 386.4 | 345.35 | | |
|
4β-hydroxystanozolol | 9.3 | 386.4 | 345.35 | | |
|
3-hydroxystanozolol | 10.15 | 386.4 | 345.35 | | 344.49 |
| 16-β
hydroxystanozolol | 10.45 | 386.4 | 366.3 | 345.35 | 344.49 |
| Stanozolol | 12.95 | 370.4 | 352.3 | 329.35 | 328.49 |
| Turinabol (IS) | 13.9 | 317.25 | 335.25 | | 334.9 |
Approximately 0.1 g of liver sample from each animal
were mechanical homogenized at high speed for 2 min with 1.0 ml of
water. The homogenates were strongly vortexed and then incubated in
an ultrasonic bath for 10 min. The addition of 1.5 ml of ethyl
acetate followed and the extraction of the analytes was performed
for 10 min. The samples were centrifuged at 1,820 × g for 2 min at
4°C. The supernatants were transferred to an empty tube and
evaporated to dryness under nitrogen at 30°C. Following
evaporation, 100 μl acetonitrile were added and strongly
vortexed. The supernatants were transferred to vials and 10
μl of these were injected to the LC-MS system for
analysis.
The LC-MS system consists of a binary LC pump
(Shimadzu Prominence, Kyoto Japan), a vacuum degasser, an
autosampler and a column oven. A gradient of 0.1% formic acid in
water (solvent A) and acetonitrile (solvent B) were selected as the
mobile phase. The separation of analytes was achieved on a
Discovery C18 HPLC column (250×4.6 mm, 5 μm) thermostated at
30°C. A mass spectrometer (LCMS-2010 EV; Shimadzu Prominence),
coupled with an atmospheric pressure chemical ionization (APCI)
interface and a single quadrupole mass filter was used in a
selected ion monitoring (SIM) positive mode. The interface, CDL and
heat block temperatures were 400, 200 and 200°C, respectively. The
detector voltage was 1.5 kV, the nebulizing gas flow was 2.5 l/min
and the drying gas was set at 0.02 MPa.
Telomerase activity assay
The determination of telomerase activity in rat
liver tissue samples was performed quantitatively using the
teloTAGGG telomerase PCR ELISA PLUS kit (Roche Diagnostic GmbH,
Mannheim, Germany). The kit protocol was followed for telomerase
activity assessment as previously described (35,36).
Gene expression assessment
RNA isolation was performed from paraffin-embedded
rat liver tissue sections using the High Pure FFPET RNA isolation
(Roche Diagnostic GmbH), according to the manufacturer's
instructions. A fixed amount of RNA from each sample was used for
cDNA synthesis. cDNA was prepared using the Transcriptor First
Strand cDNA Synthesis kit (Roche Diagnostic GmbH) according to the
manufacturer's instructions. The gene expression levels of TERT and
PTEN were analyzed by quantitative (real-time) polymerase chain
reaction (qPCR) using Light Cycler 480 machine (Roche Diagnostic
GmbH) with Real Time Ready Catalog Assay (Roche Diagnostic GmbH)
according to the manufacturer's instructions. The primer sequences
were as follows: PTEN forward, 5′-AGAACAAGATGCTCAAAAAGGACAA-3′ and
reverse, 5′-TGTCAGGGTGAGCACAAGAT-3′; TERT forward,
5′-GACATGGAGAACAAGCTGTTTGC-3′; and reverse,
5′-ACAGGGAAGTTCACCACTGTC-3′; and GAPDH forward,
5′-TTCAACGGCACAGTCAAGG-3′ and reverse, 5′-CTCAGCACCAGCATCACC-3′.
PCR amplifications were performed according to manufacturer's
instructions in triplicate. A reaction mixture without cDNA
template was used as a negative control. The expression levels
(2−ΔΔCt) was calculated as described previously
(37,38).
Immunohistochemistry (IHC) analysis
IHC analyses were performed using the Ultra
Streptavidin HRP Detection kits [BioLegend Sig-32248, Ultra
Streptavidin HRP Detection kit (Multi-species, DAB)] and BioLegend
Sig-32250, Ultra Streptavidin HRP Detection kit (Multi-species,
AEC) (BioLegend, San Diego, CA, USA) for PTEN and TERT expression
levels, respectively. The paraffin-embedded sections were mounted
on Superfrost microscope slides (Menzel-Gläser, Braunschweig,
Germany). After drying overnight, IHC analysis of PTEN and TERT was
performed using the labeled streptavidin-biotin-peroxidase method.
The slides were treated with xylene and rehydrated in increasing
grades of ethanol solutions. Antigen retrieval was performed by
boiling the slides for 5 min/3 times in citrate buffer (0.01 M). In
order to quench endogenous peroxidase activity, the tissue sections
were treated with Blocking Reagent 1 for 15 min and washed with
PBS. All the sections were incubated with Blocking Reagent 2 for 5
min at room temperature to avoid any non-specific binding. PTEN
(251264) and TERT (250509) (both from Abbiotec, Aachen, Germany)
polyclonal antibody incubations were performed overnight at 4°C
with 1/100 dilutions. The slides were incubated with Linking
Reagent 4 and then Labeling Reagent 5 for 20 min at room
temperature. The slides were visualized with DAB and AEC
chromogens, counterstained with Mayer's hematoxylin and finally
mounted. The expression levels of PTEN and TERT were evaluated
under a light microscope (Olympus BX40F4; Olympus, Tokyo, Japan).
TERT and PTEN IHC analyses were classified by the naked eye into 4
categories on the basis of the staining intensity as follows: 0, no
staining; +, weak staining; ++, moderate staining; and +++, strong
staining). Analysis was performed using a one slide reader for
minimizing variability due to subjective scoring.
Statistical analysis
The means ± SD and the median were used for the
expression of levels of stanozolol and its metabolites and for
PTEN, TERT and percentage relative telomerase activity. Changes
between two values were expressed as percentage relative changes or
otherwise based on the following formula: (actual change/reference
value) *100%.
The Kolmogorov-Smirnov with Liliefors correction
test was applied for examining the normality of continuous
variables. Spearman's R was applied to measure bivariate
correlations between two continuous variables (e.g., percentage
relative telomerase activity vs. the levels of 3′-hydroxystanozolol
TERT gene expression). The non-parametric Kruskal-Wallis test and
parametric one-way ANOVA were applied for comparing differences in
levels of stanozolol and its metabolites between the study groups
(control, stanozolol, PG and exercise groups). Non-parametric post
hoc comparisons were assessed by using Dunn's (non-paremetric) and
Tukey's HSD tests for parametric tests. IBM SPSS Statistics 21.0
software (IBM Corp., Armonk, NY, USA) was used for statistical
analysis. A level of 0.05 was set for accepting or rejecting the
null hypothesis (statistical significance). The sample sizes for
the individual analyses differed slightly due to some missing
values arose from experimental conditions.
Results
Normality tests
Tests for normality revealed that only percentage
relative telomerase activity retained the null hypothesis,
suggesting a normal distribution of data (P=0.137). All other
continuous variables tested, such as TERT, PTEN and
3′-hydroxystanozolol did not follow a normal distribution
(P<0.01) (data not shown).
Bioaccumulation of stanozolol and its
metabolites in liver tissues
The results are summarized in Table IV. The levels of stanozolol and
its metabolites were non-significantly higher in the STE group
compared to the ST group (P>0.05).
 | Table IVConcentration levels (ng/mg) of
stanozolol and its metabolites in the stanozolol (ST) and
stanozolol plus exercise (STE) groups. |
Table IV
Concentration levels (ng/mg) of
stanozolol and its metabolites in the stanozolol (ST) and
stanozolol plus exercise (STE) groups.
| Agent | ST group (means ±
SD) | STE group (means ±
SD) | Mann-Whitney
(P-value) | % relative
change |
|---|
| Stanozolol
(ng/mg) | 2.98±1.01 | 3.89±1.09 | 0.240 | 30.5 |
|
3′-hydroxystanozolol (ng/mg) | 0.34±0.06 | 0.44±0.18 | 0.485 | 29.4 |
|
16-β-hydroxystanozolol (ng/mg) | 0.25± 0.11 | 0.32±0.15 | 0.485 | 28.0 |
Telomerase activity and gene expression
assessment
The PTEN and TERT gene expression levels and
percentage relative telomerase activity in the study groups are
presented in Table V. A
significant difference was observed for TERT gene expression in the
various groups (χ2=17.585, df=4, P<0.001). Based on
the Dunn's test, exercise reduced TERT expression by (71.0%;
P=0.001) and ST administration increased TERT expression by (160%;
P<0.001) compared to the PG group. Of note, the
stanozolol-induced increase in TERT expression vs. the stanozolol
group was restricted by (68.0%; P=0.042) in the animals subjected
to exercise. A similar pattern was observed for percentage
telomerase activity, as well. PTEN gene expression was practically
unaffected either by exercise or stanozolol administration. It
should be noted that not all values presented above are shown in
Table V due to the large number
of pairwise comparisons.
 | Table VPTEN and TERT gene expression levels
(2−ΔΔCt) and % relative telomerase activity per
group. |
Table V
PTEN and TERT gene expression levels
(2−ΔΔCt) and % relative telomerase activity per
group.
| Parameters | Groups | N | Mean | SD | Group
comparison |
|---|
| PTEN gene
expression (2−ΔΔCt) | Control | 5 | 1.13 | 1.77 | Kruskal-Wallis |
| Propylene glycol
treatment | 5 | 0.37 | 0.25 |
χ2=3.643, |
| Stanozolol
treatment | 6 | 0.40 | 0.42 | df=4, P=0.456 |
| Propylene glycol
treatment and exercise | 5 | 0.98 | 1.62 | |
| Stanozolol
treatment and exercise | 6 | 1.13 | 0.86 | |
| TERT gene
expression (2−ΔΔCt) | Control | 5 | 0.40 | 0.41 | Kruskal-Wallis |
| Propylene glycol
treatment | 5 | 2.78 | 2.66 |
χ2=17.585, |
| Stanozolol
treatment | 6 | 7.25 | 1.40 | df=4, P=0.001 |
| Propylene glycol
treatment and exercise | 5 | 0.81 | 0.96 | |
| Stanozolol
treatment and exercise | 6 | 2.29 | 0.97 | |
| % relative
telomerase activity | Control | 5 | 1.30 | 0.58 | ANOVA: |
| Propylene glycol
treatment | 5 | 1.92 | 0.96 | F=3.015, df=4, |
| Stanozolol
treatment | 6 | 2.59 | 1.30 | P=0.040 |
| Propylene glycol
treatment and exercise | 5 | 0.76 | 0.61 | |
| Stanozolol
treatment and exercise | 6 | 1.33 | 0.96 | |
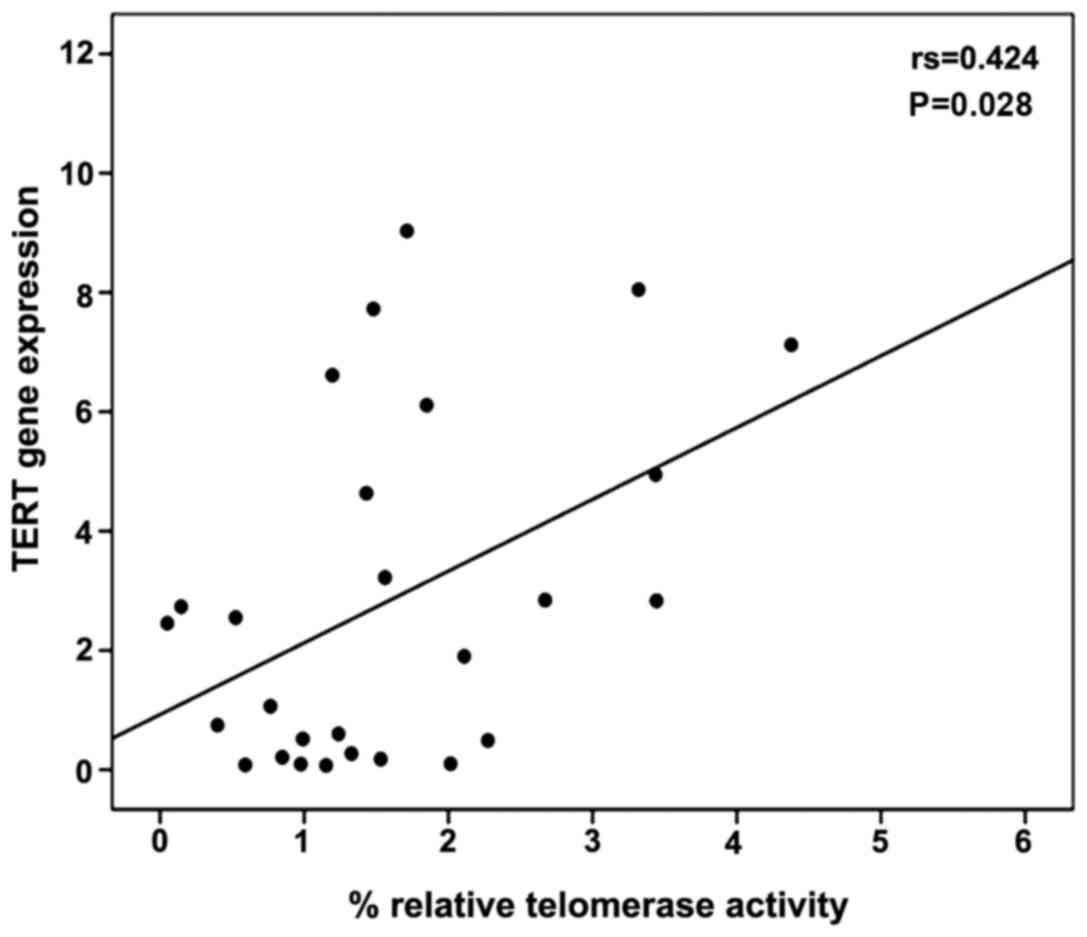
A moderate correlation between percentage relative
telomerase activity and TERT gene expression levels was observed
using Spearman's correlation coefficient (r=0.424, P=0.028)
(Fig. 3). The levels of
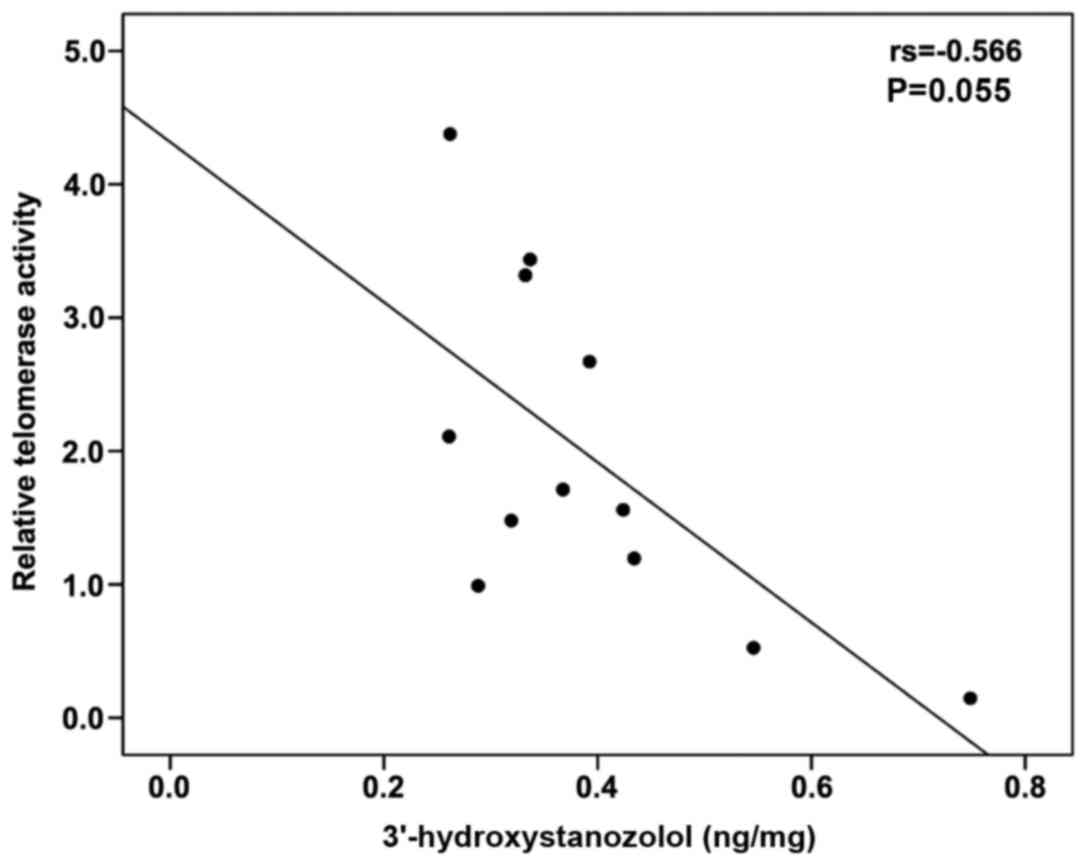
3′-hydroxystanozolol measured in the ST and STE groups tended to
negatively correlate with percentage relative telomerase activity
(Spearman's r=−0.566, P=0.055) (Fig.
4). No correlation was observed between any of the parameters
monitored with stanozolol and 16-β-hydroxystanozolol (data not
shown).
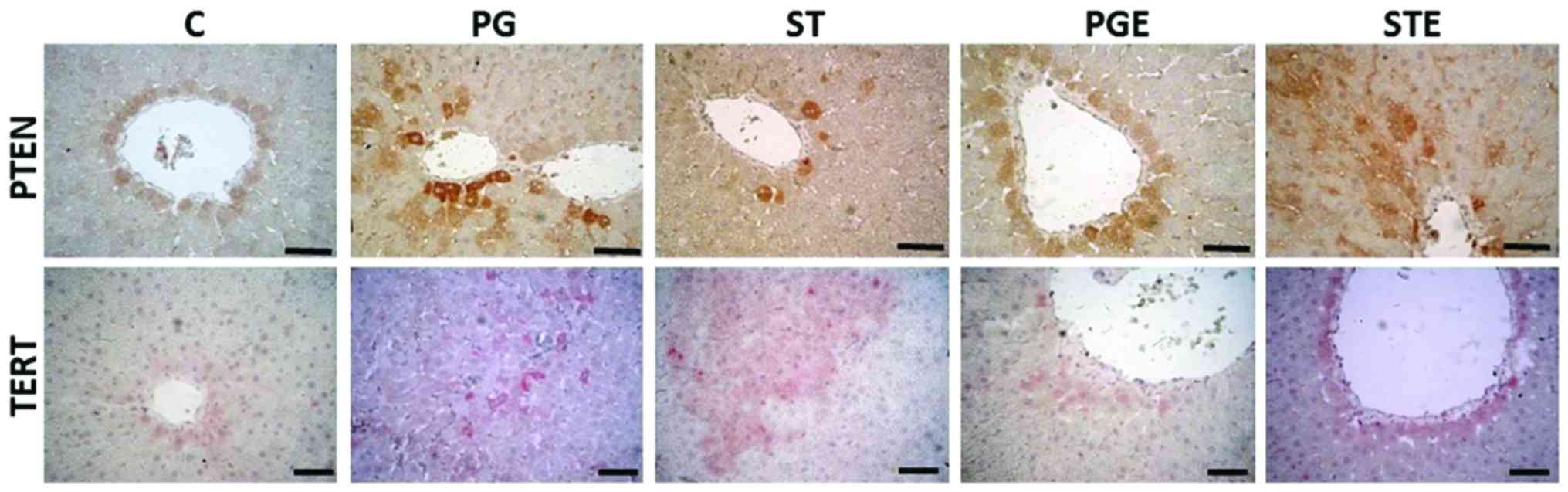
IHC analyses
The IHC staining images are shown in Fig. 5 and the results are summarized in
Table VI. PTEN gene expression
levels were observed around the vena centralis and the parenchyma.
In the STE group, the staining was moderate in the hepatocytes
surrounding these areas. TERT IHC analysis revealed strong staining
in the ST group around the portal field, vena centralis and
parenchyma, while exercise attenuated the increase in TERT gene
expression (moderate staining in the STE group). Our results thus
indicated that exercise exerted positive effects on PTEN gene
expression, as shown in Table
V.
 | Table VIScoring results for PTEN and TERT
immunohistochemical analyses. |
Table VI
Scoring results for PTEN and TERT
immunohistochemical analyses.
| Groups | PTEN IHC
scoringa | TERT IHC
scoringa |
|---|
| Control | + | + |
| Propylene glycol
treatment | ++ | ++ |
| Stanozolol
treatment | + | +++ |
| Propylene glycol
treatment and exercise | + | + |
| Stanozolol
treatment and exercise | ++ | ++ |
Discussion
Stanozolol is a widely abused and most potent AAS
responsible for a number of side-effects, including cardiovascular,
reproductive, behavioral effects and hepatotoxicity (17). To the best of our knowledge, this
is the first study to investigate stanozolol-induced molecular
pathways of telomerase activity in rat liver and any relevant
effect of exercise. Stanozolol induces intrahepatic structural
changes with cholestasis and increases the risk of HCC (37). In addition, AAS abuse in general
has been found to be responsible for hepatocellular adenomas
(12,39). Even though the mechanisms
responsible for stanozolol-induced hepatotoxicity have not yet been
clearly identified, proliferative effects on liver cells may play a
central role in the observed hepatotoxicity (12,40,41). In our previous study, we
demonstrated that stanozolol exerted DNA-damaging effects in
peripheral blood lymphocytes, probably related to telomerase
activity alterations (35).
Although various environmental factors are known to up- and
downregulate telomerase activity, the effects of exercise on
telomerase activity have not yet been clearly identified (42). Telomere length and telomerase
activity have been shown to be affected by several factors,
including oxidative stress, psychological stress and socioeconomic
status. One possible mechanism for telomere shortening is oxidative
stress by oxidized DNA base products (8-OHdG) in the guanine or
protein adducts (43,44). According to recent studies, an
increased telomerase activity is detected in almost 90% of human
cancers and in 80% of HCCs. In addition, it is well documented that
the majority of healthy cells exhibit a lack of telomerase activity
(19,20,45). The results of this study
demonstrated increased levels of percentage relative telomerase
activity in the liver tissue in the ST group, in line with
nandrolone, another well-known ASS, which has shown similar effects
by increasing telomerase activity in a dose-dependent manner both
at the heart tissue and at peripheral blood monocytes (2,46).
This may represent a compensating repair mechanism at the tissue
level, while increased circulating levels of telomerase activity
can depict systemic inflammation. The association of increased
telomerase activity and expression with proliferative effects was
not likely to occur in this study due to the short time of exposure
(28 days). In general, the mechanisms underlying the effects of
AASs on telomerase activity have not been elucidated and remain
practically unknown.
TERT is a catalytic subunit of a telomerase, which
plays a role in its regulation at transcriptional level. It has
been reported that TERT mutations are associated with
adenoma-carcinoma transitions in the liver (47). Therefore, alterations in TERT
regulation and expression play an important role in HCC (48). It has been shown that the tumor
suppressor gene, PTEN, negatively correlates with human TERT
protein in HCC tissues (21).
Therefore, PTEN and TERT play opposing roles in carcinogenesis. It
has been reported that PTEN indirectly regulates TERT activity via
the PI3K-PKB/Akt pathway in human HCC (21). According to the results of the
present study, no significant alterations were observed in PTEN
expression levels between the groups. However, TERT gene expression
was significantly increased by ST treatment. Exercise reversed the
increase in TERT expression induced by stanozolol, particularly in
the parenchyma, where metabolic zonation is reported: Glucose
release from glycogen and via gluconeogenesis, amino acid
utilization and ammonia detoxification, protective metabolism, bile
formation and the synthesis of certain plasma proteins, such as
albumin and fibrinogen occur mainly in the periportal area, whereas
glucose utilization, xenobiotic metabolism and the formation of
other plasma proteins, such as alpha 1-antitrypsin or
alpha-fetoprotein occur predominantly in the perivenous zone
(49,50).
In this study, the levels of 3′-hydroxystanozolol
and 16-β-hydroxystanozolol, the main metabolites of stanozolol,
were determined in liver tissue samples of stanozolol-treated
animals and a dose-response association between telomerase activity
and TERT/PTEN gene expressions was determined. The measured levels
of 3′-hydroxystanozolol in the ST and STE groups were associated
with the percentage relative telomerase activity, whereas no
association was observed for the stanozolol or
16-β-hydroxystanozolol levels. This may be due to the fact that
3′-hydroxystanozolol is the most potent stanozolol metabolite
(2,51).
Several studies have indicated that physical
exercise increases telomerase activity in different cell types
(52,53). However, to the best of our
knowledge, there is no study available to date investigating the
effects of stanozolol on telomerase activity in the
presence/absence of exercise, apart from our previous study which
focused on circlulating telomerase activity in peripheral blood
mononuclear cells (PBMCs) (35).
Our results indicated the elevation of telomerase activity and TERT
expression in the liver tissue, which could be associated either
with an increased proliferation risk due to stanozolol treatment
(10), rather unlikely for such a
short exposure period, or may represent a counteracting mechanism
(54). Exercise reverses the
stanozolol-induced increase in telomerase activity. A number of
studies have supported that exercise exerts hepatoprotective
effects. Huang et al demonstrated that a 12-week swimming
exercise program suppressed senescence markers and downregulated
inflammatory mediators in the liver tissues of D-galactose-induced
senescence in rats (55). Yi
et al demonstrated that both acute and chronic exercise
exerted preventive effects on the livers of rats with type 2
diabetes (56). On the other
hand, exercise has been reported to increase liver enzymes in
humans (57) and concerns exist
regarding the effects of exercise on portal hypertension in
patients with cirrhosis (58).
In conclusion, stanozolol induces telomerase
activity at a molecular level and exercise reverses this induction,
at least regarding TERT expression. This may reflect premature
tissue aging due to decreased telomerase activity Future studies
are warranted in order to investigate the mechanisms through which
exercise can be used to prevent the adverse health effects of
stanazolol and to elucidate the molecular hepatocellular mechanisms
of the stanozolol-induced adverse effects.
Abbreviations:
|
AAS
|
anabolic androgenic steroid
|
|
TERT
|
telomerase reverse transcriptase
|
|
TERC
|
telomerase RNA component
|
|
WADA
|
World Anti-doping Agency
|
|
HCC
|
hepatocellular carcinoma
|
|
PG
|
propylene glycol
|
|
IS
|
internal standard
|
|
APCI
|
atmospheric pressure chemical
ionization
|
|
PBMCs
|
peripheral blood mononuclear cells
|
Acknowledgments
The authors would like to thank Dr Alegakis
Athanasios for his valuable help on the statistical advice and
comments.
References
|
1
|
Balcells G, Matabosch X and Ventura R:
Detection of stanozolol O- and N-sulfate metabolites and their
evaluation as additional markers in doping control. Drug Test Anal.
9:1001–1010. 2017. View
Article : Google Scholar
|
|
2
|
Tsitsimpikou C, Vasilaki F, Tsarouhas K,
Fragkiadaki P, Tzardi M, Goutzourelas N, Nepka C, Kalogeraki A,
Heretis I, Epitropaki Z, et al: Nephrotoxicity in rabbits after
long-term nandrolone decanoate administration. Toxicol Lett.
259:21–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Anti-doping Agency: The 2017 list of
prohibited substances and methods. https://www.wada-ama.org/.
Accessed Feb 14, 2018.
|
|
4
|
Kioukia-Fougia N, Georgiadis N, Tsarouhas
K, Vasilaki F, Fragiadaki P, Meimeti E and Tsitsimpikou C:
Synthetic and natural nutritional supplements: Health 'allies' or
risks to public health. Recent Pat Inflamm Allergy Drug Discov.
10:72–85. 2017. View Article : Google Scholar
|
|
5
|
Tsitsimpikou C, Chrisostomou N, Papalexis
P, Tsarouhas K, Tsatsakis A and Jamurtas A: The use of nutritional
supplements among recreational athletes in Athens, Greece. Int J
Sport Nutr Exerc Metab. 21:377–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sagoe D, Molde H, Andreassen CS, Torsheim
T and Pallesen S: The global epidemiology of anabolic-androgenic
steroid use: A meta-analysis and meta-regression analysis. Ann
Epidemiol. 24:383–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ampuero J, García ES, Lorenzo MM, Calle R,
Ferrero P and Gómez MR: Stanozolol-induced bland cholestasis.
Gastroenterol Hepatol. 37:71–72. 2014. View Article : Google Scholar
|
|
8
|
Bausserman LL, Saritelli AL and Herbert
PN: Effects of short-term stanozolol administration on serum
lipoproteins in hepatic lipase deficiency. Metabolism. 46:992–996.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El-Serag HB, Kramer J, Duan Z and Kanwal
F: Racial differences in the progression to cirrhosis and
hepatocellular carcinoma in HCV-infected veterans. Am J
Gastroenterol. 109:1427–1435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hansma P, Diaz FJ and Njiwaji C: Fatal
liver cyst rupture due to anabolic steroid use: A case
presentation. Am J Forensic Med Pathol. 37:21–22. 2016. View Article : Google Scholar
|
|
11
|
Harkin KR, Cowan LA, Andrews GA, Basaraba
RJ, Fischer JR, DeBowes LJ, Roush JK, Guglielmino ML and Kirk CA:
Hepatotoxicity of stanozolol in cats. J Am Vet Med Assoc.
217:681–684. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Socas L, Zumbado M, Pérez-Luzardo O, Ramos
A, Pérez C, Hernández JR and Boada LD: Hepatocellular adenomas
associated with anabolic androgenic steroid abuse in bodybuilders:
A report of two cases and a review of the literature. Br J Sports
Med. 39:e272005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stimac D, Milić S, Dintinjana RD, Kovac D
and Ristić S: Androgenic/anabolic steroid-induced toxic hepatitis.
J Clin Gastroenterol. 35:350–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deshmukh NI, Zachar G, Petróczi A, Székely
AD, Barker J and Naughton DP: Determination of stanozolol and
3′-hydroxystanozolol in rat hair, urine and serum using liquid
chromatography tandem mass spectrometry. Chem Cent J. 6:1622012.
View Article : Google Scholar
|
|
15
|
Mateus-Avois L, Mangin P and Saugy M: Use
of ion trap gas chromatography-multiple mass spectrometry for the
detection and confirmation of 3′hydroxystanozolol at trace levels
in urine for doping control. J Chromatogr B Analyt Technol Biomed
Life Sci. 816:193–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kicman AT: Pharmacology of anabolic
steroids. Br J Pharmacol. 154:502–521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Büttner A and Thieme D: Side effects of
anabolic androgenic steroids: Pathological findings and
structure-activity relationships. Handb Exp Pharmacol. 195:459–484.
2010. View Article : Google Scholar
|
|
18
|
Rentoukas E, Tsarouhas K, Kaplanis I,
Korou E, Nikolaou M, Marathonitis G, Kokkinou S, Haliassos A,
Mamalaki A, Kouretas D, et al: Connection between telomerase
activity in PBMC and markers of inflammation and endothelial
dysfunction in patients with metabolic syndrome. PLoS One.
7:e357392012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar M, Lechel A and Güneş Ç: Telomerase:
The devil inside. Genes (Basel). 7:E432016. View Article : Google Scholar
|
|
20
|
Xu Y and Goldkorn A: Telomere and
telomerase therapeutics in cancer. Genes (Basel). 7:E222016.
View Article : Google Scholar
|
|
21
|
Zhou X, Zhu H and Lu J: PTEN and hTERT
gene expression and the correlation with human hepatocellular
carcinoma. Pathol Res Pract. 211:316–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wojtyla A, Gladych M and Rubis B: Human
telomerase activity regulation. Mol Biol Rep. 38:3339–3349. 2011.
View Article : Google Scholar :
|
|
23
|
Yang C, Li S, Wang M, Chang AK, Liu Y,
Zhao F, Xiao L, Han L, Wang D, Li S and Wu H: PTEN suppresses the
oncogenic function of AIB1 through decreasing its protein stability
via mechanism involving Fbw7 alpha. Mol Cancer. 12:212013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung S, Li C, Jeong D, Lee S, Ohk J, Park
M, Han S, Duan J, Kim C, Yang Y, et al: Oncogenic function of
p34SEI-1 via NEDD4 1 mediated PTEN ubiquitination/degradation and
activation of the PI3K/AKT pathway. Int J Oncol. 43:1587–1595.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
OECD: Guidance document on the
recognition, assessment, and use of clinical signs as humane
endpoints for experimental animals used in safety evaluation. OECD;
Paris: 2000
|
|
26
|
Cherici Camargo IC, Barreiros de Souza R,
de Fátima Paccola Mesquita S, Chuffa LG and Frei F: Ovarian
histology and follicular score in female rats treated with
nandrolone decanoate and submitted to physical effort. Acta Biol
Hung. 60:253–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Almeida Chuffa LG, de Souza RB, Frei F,
de Fátima Paccola Mesquita S and Camargo IC: Nandrolone decanoate
and physical effort: Histological and morphometrical assessment in
adult rat uterus. Anat Rec (Hoboken). 294:335–341. 2011. View Article : Google Scholar
|
|
28
|
Gopinathan S, O'Neill E, Rodriguez LA,
Champ R, Phillips M, Nouraldeen A, Wendt M, Wilson AGE and Kramer
JA: In vivo toxicology of excipients commonly employed in drug
discovery in rats. J Pharmacol Toxicol Methods. 68:284–295. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Healing G, Sulemann T, Cotton P, Harris J,
Hargreaves A, Finney R, Kirk S, Schramm C, Garner C, Pivette P and
Burdett L: Safety data on 19 vehicles for use in 1 month oral
rodent pre-clinical studies: Administration of
hydroxypropyl-β-cyclodextrin causes renal toxicity. J Appl Toxicol.
36:140–150. 2016. View Article : Google Scholar
|
|
30
|
Aye M, Di Giorgio C, De Mo M, Botta A,
Perrin J and Courbiere B: Assessment of the genotoxicity of three
cryoprotectants used for human oocyte vitrification: Dimethyl
sulfoxide, ethylene glycol and propylene glycol. Food Chem Toxicol.
48:1905–1912. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berthelot-Ricou A, Perrin J, di Giorgio C,
de Meo M, Botta A and Courbiere B: Assessment of 1,2-propanediol
(PrOH) genotoxicity on mouse oocytes by comet assay. Fertil Steril.
96:1002–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cunningham RL and McGinnis MY: Physical
provocation of pubertal anabolic androgenic steroid exposed male
rats elicits aggression towards females. Horm Behav. 50:410–416.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matrisciano F, Modafferi AM, Togna GI,
Barone Y, Pinna G, Nicoletti F and Scaccianoce S: Repeated anabolic
androgenic steroid treatment causes antidepressant-reversible
alterations of the hypothalamic-pituitary-adrenal axis, BDNF levels
and behavior. Neuropharmacology. 58:1078–1084. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tucci P, Morgese MG, Colaianna M, Zotti M,
Schiavone S, Cuomo V and Trabace L: Neurochemical consequence of
steroid abuse: Stanozolol-induced monoaminergic changes. Steroids.
77:269–275. 2012. View Article : Google Scholar
|
|
35
|
Kara M, Ozcagli E, Fragkiadaki P, Kotil T,
Stivaktakis PD, Spandidos DA, Tsatsakis AM and Alpertunga B:
Determination of DNA damage and telomerase activity in
stanozolol-treated rats. Exp Ther Med. 13:614–618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsitsimpikou C, Tzatzarakis M, Fragkiadaki
P, Kovatsi L, Stivaktakis P, Kalogeraki A, Kouretas D and Tsatsakis
AM: Histopathological lesions, oxidative stress and genotoxic
effects in liver and kidneys following long term exposure of
rabbits to diazinon and propoxur. Toxicology. 307:109–114. 2013.
View Article : Google Scholar
|
|
37
|
Solbach P, Potthoff A, Raatschen HJ,
Soudah B, Lehmann U, Schneider A, Gebel MJ, Manns MP and Vogel A:
Testosterone-receptor positive hepatocellular carcinoma in a
29-year old bodybuilder with a history of anabolic androgenic
steroid abuse: A case report. BMC Gastroenterol. 15:602015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−4Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
39
|
Kesler T, Sandhu RS and Krishnamoorthy S:
Hepatology: Hepatocellular carcinoma in a young man secondary to
androgenic anabolic steroid abuse. J Gastroenterol Hepatol.
29:18522014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boada LD, Zumbado M, Torres S, López A,
Díaz-Chico BN, Cabrera JJ and Luzardo OP: Evaluation of acute and
chronic hepatotoxic effects exerted by anabolic-androgenic steroid
stanozolol in adult male rats. Arch Toxicol. 73:465–472. 1999.
View Article : Google Scholar
|
|
41
|
Kanayama G, Hudson JI and Pope HG Jr:
Long-term psychiatric and medical consequences of
anabolic-androgenic steroid abuse: A looming public health concern?
Drug Alcohol Depend. 98:1–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ornish D, Lin J, Chan JM, Epel E, Kemp C,
Weidner G, Marlin R, Frenda SJ, Magbanua MJM, Daubenmier J, et al:
Effect of comprehensive lifestyle changes on telomerase activity
and telomere length in men with biopsy-proven low-risk prostate
cancer: 5-year follow-up of a descriptive pilot study. Lancet
Oncol. 14:1112–1120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mishra S, Kumar R, Malhotra N, Singh N and
Dada R: Mild oxidative stress is beneficial for sperm telomere
length maintenance. World J Methodol. 6:163–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zar T, Graeber C and Perazella MA:
Recognition, treatment, and prevention of propylene glycol
toxicity. Semin Dial. 20:217–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Djojosubroto MW, Chin AC, Go N,
Schaetzlein S, Manns MP, Gryaznov S, Harley CB and Rudolph KL:
Telomerase antagonists GRN163 and GRN163L inhibit tumor growth and
increase chemosensitivity of human hepatoma. Hepatology.
42:1127–1136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vasilaki F, Tsitsimpikou C, Tsarouhas K,
Germanakis I, Tzardi M, Kavvalakis M, Ozcagli E, Kouretas D and
Tsatsakis AM: Cardiotoxicity in rabbits after long-term nandrolone
decanoate administration. Toxicol Lett. 241:143–151. 2016.
View Article : Google Scholar
|
|
47
|
Pilati C, Letouzé E, Nault JC, Imbeaud S,
Boulai A, Calderaro J, Poussin K, Franconi A, Couchy G, Morcrette
G, et al: Genomic profiling of hepatocellular adenomas reveals
recurrent FRK-activating mutations and the mechanisms of malignant
transformation. Cancer Cell. 25:428–441. 2014. View Article : Google Scholar
|
|
48
|
Akincilar SC, Unal B and Tergaonkar V:
Reactivation of telomerase in cancer. Cell Mol Life Sci.
73:1659–1670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jungermann K and Kietzmann T: Zonation of
parenchymal and nonparenchymal metabolism in liver. Annu Rev Nutr.
16:179–203. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jungermann K: Metabolic zonation of liver
parenchyma. Semin Liver Dis. 8:329–341. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Salvador JP, Sánchez-Baeza F and Marco MP:
Simultaneous immunochemical detection of stanozolol and the main
human metabolite, 3′-hydroxy-stanozolol, in urine and serum
samples. Anal Biochem. 376:221–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chilton WL, Marques FZ, West J,
Kannourakis G, Berzins SP, O'Brien BJ and Charchar FJ: Acute
exercise leads to regulation of telomere-associated genes and
microRNA expression in immune cells. PLoS One. 9:e920882014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ludlow AT, Gratidão L, Ludlow LW,
Spangenburg EE and Roth SM: Acute exercise activates p38 MAPK and
increases the expression of telomere-protective genes in cardiac
muscle. Exp Physiol. 102:397–410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vardavas AI, Stivaktakis PD, Tzatzarakis
MN, Fragkiadaki P, Vasilaki F, Tzardi M, Datseri G, Tsiaoussis J,
Alegakis AK, Tsitsimpikou C, et al: Long-term exposure to
cypermethrin and piperonyl butoxide cause liver and kidney
inflammation and induce genotoxicity in New Zealand white male
rabbits. Food Chem Toxicol. 94:250–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Huang CC, Chiang WD, Huang WC, Huang CY,
Hsu MC and Lin WT: Hepatoprotective effects of swimming exercise
against D-galactose-induced senescence rat model. Evid Based
Complement Alternat Med. 2013:2754312013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yi X, Cao S, Chang B, Zhao D, Gao H, Wan
Y, Shi J, Wei W and Guan Y: Effects of acute exercise and chronic
exercise on the liver leptin-AMPK-ACC signaling pathway in rats
with type 2 diabetes. J Diabetes Res. 2013:9464322013. View Article : Google Scholar
|
|
57
|
Pettersson J, Hindorf U, Persson P,
Bengtsson T, Malmqvist U, Werkström V and Ekelund M: Muscular
exercise can cause highly pathological liver function tests in
healthy men. Br J Clin Pharmacol. 65:253–259. 2008. View Article : Google Scholar
|
|
58
|
Brustia R, Savier E and Scatton O:
Physical exercise in cirrhotic patients: Towards prehabilitation on
waiting list for liver transplantation. A systematic review and
meta-analysis. Clin Res Hepatol Gastroenterol. Nov 18–2017.Epub
ahead of print. PubMed/NCBI
|
|
59
|
Tapis F: Telomeres are protective caps on
the end of chromosomes. Cell, chromosome and DNA vector
illustration. Digital image ID: 710795275, Shutterstock. https://www.shutterstock.com/image-illustration/telomeres-protective-caps-on-end-chromosomes-735264379.
|