Introduction
As one of the most common types of cancer worldwide,
gastric cancer remains the second leading cause of cancer-related
mortality (1). Although the survival
rate has improved gradually over the past 30 years, the overall
5-year survival rate of resectable gastric cancer remains high
(10–30%), which is mainly due to the fact that the majority of
gastric cancers are diagnosed at an advanced rather than an early
stage (2,3). A significant proportion of patients with
advanced cancer succumb to complications caused by metastases,
rather than the primary tumor. Numerous factors are associated with
gastric cancer prognosis, including growth factors and their
receptors, cell cycle regulators, cell adhesion molecules and
matrix-degrading enzymes, all of which play important roles in cell
proliferation, invasion and metastasis (4). Several putative tumor markers, including
p53, carbohydrate antigen (CA) 19-9, CA72-4, human epidermal growth
factor receptor 2 and pituitary tumor-transforming gene 1, have
been described as potential prognostic indicators for gastric
cancer behavior (3,5–8). However,
there is increasing interest in identifying novel prognostic
factors to improve the current treatment regimens and develop novel
therapeutic targets.
Cytokeratin (CK) is an intermediate filament
observed mainly in epithelial cells, which is involved in cell
morphology through providing mechanical and structural support. The
tissue distribution of the 20 subtypes of CK depends on the type of
epithelium and the stage of differentiation. Therefore, this CK
fingerprint may enable distinguishing primary tumors and their
metastases from other type of carcinomas, as the former two share
the same pattern (9–11). It has been demonstrated that CYFRA
21-1, the fragment of CK19, is a serum biomarker providing useful
prognostic information in lung, breast, pancreatic and colorectal
cancer (12–15). However, the role of CK19 in gastric
cancer remains unknown. As another important diagnostic marker,
CK20 is specifically found in gastrointestinal epithelium;
therefore, it normally functions as a diagnostic marker in
combination with CK7 to differentiate primary and metastatic
cancers in non-GI tissues, such as lung, prostate and ovary
(11). Urokinase plasminogen
activator (uPA) catalyzes the conversion of the proenzyme
plasminogen to the active protease plasmin, which regulates a
number of physiological processes requiring basement membrane
and/or extracellular matrix (ECM) remodeling, such as tissue
regeneration and angiogenesis, as well as cancer progression and
metastasis (16). uPA has been found
to be a poor prognostic marker for breast and other types of cancer
(17,18). Similar to uPA, members of the matrix
metalloproteinase (MMP) family are also important for tumor cell
invasion and metastasis. As a type IV collagenase, MMP-9 is able to
degrade type IV collagen, which is the major component of basement
membranes, thereby facilitating tumor spreading (19). Increased MMP-9 expression is
associated with disease progression and poor prognosis in breast,
ovarian and hepatic cancer (20–23).
Recently, a meta-analysis of 11 retrospective studies was conducted
to investigate the prognostic effect of MMP-9 on gastric cancer
(24). The authors reported that
MMP-9 protein expression may be a poor prognostic factor in gastric
cancer, although the association was weak. C-reactive protein (CRP)
is an acute-phase reactant involved in inflammatory reactions. On
the basis of the association between systemic inflammation and
poorer outcome, CRP has been shown to be an important predictor of
survival in patients with various cancers, such as colon, lung,
breast, ovarian and renal cancer (25,26). The
purpose of this study was to further investigate the role of the
abovementioned markers in the prediction of prognosis for gastric
cancer patients.
Patients and methods
Patients and peripheral blood sample
collection
Peripheral blood samples were collected from 165
gastric cancer patients who underwent gastrectomy at Zhejiang
Cancer Hospital (Hangzhou, China) between January 1, 2010 and June
30, 2011. The gastric cancers were all confirmed as
adenocarcinomas. None of the patients received any chemotherapy,
radiotherapy or other anticancer therapy prior to surgery. The
study protocol was approved by the Clinical Research Ethics
Committee of the Zhejiang Cancer Hospital and all the patients
provided written informed consent prior to specimen collection. The
histological diagnosis was based on the classification criteria of
the World Health Organization for gastric tumors (27). The tumor-node-metastasis (TNM) stage
was defined according to the 2009 guideline of the Union for
International Cancer Control (UICC) (28).
Peripheral blood samples (~3–5 ml) were collected in
EDTA tubes for each individual patient prior to chemotherapy and
following completion of three cycles of chemotherapy. Peripheral
blood mononuclear cells (PBMCs) were isolated from whole blood
using the standard Ficoll density gradient separation method
(Thermo Fisher Scientific, Fair Lawn, NJ, USA).
Follow-up
All the patients were followed up directly or
through family members. The follow-up was conducted up to September
30, 2013. Of the 165 patients, 65 succumbed to the disease during
the follow-up period; of the 100 surviving patients, 71 presented
with metastasis; of the 65 deceased patients, 5 had no record of
distant metastasis.
RNA extraction and complementary DNA
synthesis
Total RNA was extracted from PBMCs using TRIzol
reagent (Life Technologies, Foster City, CA, USA) according to the
manufacturer's instructions. The concentration and purity of RNA
was measured with NanoDrop 1000 (NanoDrop Technologies, Wilmington,
DE, USA) and stored at −80°C until further use. Prior to performing
reverse transcription (RT), total RNA was incubated with DNase I
(Life Technologies) to remove contaminating genomic DNA. An RT
reaction was performed using 1 µg total RNA with PrimerScript® RT
reagent (Takara Bio Inc., Dalian, China). The reaction mixture was
incubated for 1 h at 37°C, then at 90°C for 10 min, and stored at
−20°C for subsequent quantitative polymerase chain reaction
(qPCR).
qPCR
The RNA expression of CK19, 20, MMP-9 and uPA was
detected using the commercially available TaqMan Real-Time PCR kits
in the ABI 7500 Real-Time PCR system (Jiangsu BioPerfectus
Technologies, Jiangsu, China). The following thermocycling
conditions were used under the standard mode according to the
manufacturer's recommendations: 10 min at 95°C, followed by 40
cycles at 95°C for 15 sec and at 60°C for 1 min. The relative mRNA
expression was calculated as compared to the standard provided by
each kit. The median of the RNA expression was calculated and used
as a threshold to differentiate between the higher and lower
expression within the factor groups.
The primers used for RT-qPCR were MMP-9 forward,
5′-CGCTGGGCTTAGATCATTCC-3′ and reverse, 5′-TCAGGGCGAGGACCATAGAG-3′;
TaqMan Probe 5′-CAGTGCCGGAGGCGCTCATGTAG-3′; CK19 forward,
5′-CAGGAGATTGCCACCTACCG-3′ and reverse, 5′-GAGGACCTTGGAGGCAGACA-3′;
Taqman Probe 5′-CCTGCTCGAGGGACAGGAAGATCAC-3′; CK20 forward,
5′-GGACGACACCCAGCGTTTA-3′ and reverse, 5′-AGATCGCTCCCATAGTTCACC-3′;
TaqMan Probe 5′-CTGGAGTTGGAGATGCGCATCCC-3′; uPA forward,
5′-CACCCCTCGTCTGTTCCCTC-3′ and reverse,
5′-GTGAGACTCTCGTGTAGACGCC-3′; TaqMan Probe
5′-AAGGCCGCATGACTTTGACTGGAA-3′.
CRP assay
Blood samples for CRP analysis were collected in
serum separation tubes, and the serum CRP levels were measured via
immunoturbidimetry (Roche, Shanghai, China).
Statistical analysis
The relative mRNA expressions of CK19, CK20, MMP-9
and uPA and CRP protein expression are presented as means ±
standard deviation (SD) and compared using paired Students t-tests.
Two variables among the clinical data were compared using multiple
Students t-tests. Survival curves were obtained using the
Kaplan-Meier method. Overall survival (OS) was calculated as the
time from gastric surgery to death or censoring. The survival
curves were compared between the high- and low-expression groups
using the log-rank test. All the statistical calculations were
performed using SPSS 13 software (SPSS Inc., Chicago, IL, USA). A
multivariate proportional hazard Cox regression model was applied
to assess the effect of different covariates, including CK19, 20,
MMP-9, uPA and CRP, on disease-free survival and OS. P<0.05 was
considered to indicate statistically significant differences.
Results
Patient characteristics
A total of 165 gastric cancer patients were enrolled
in the study, including 124 men and 41 women. All the patients had
histologically confirmed gastric adenocarcinoma. The mean age ± SD
of the patients was 59.8±8.9 years (range, 23–85 years). According
to the TNM criteria, defined by the 2009 UICC guideline, 18
patients were classified as stage I, 33 as stage II and the
remaining 114 as stage III. The patients with stage I, II and III
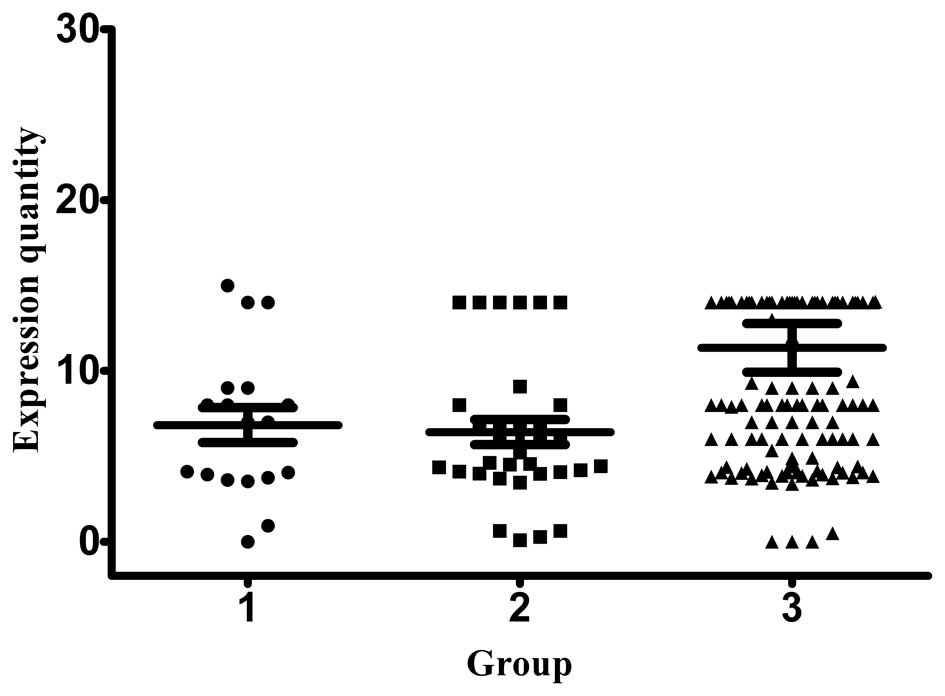
disease were designated as group 1, 2 and 3, respectively.
CK19, CK20, MMP-9 and uPA mRNA
expression and CRP levels in different stages of gastric
cancer
In order to investigate the role of CKs in different
stages of gastric cancer, the mRNA expression of CK19 and CK20 was
measured using RT-qPCR and the relative expression was calculated
as copy numbers per ml when compared to the mRNA standard. The
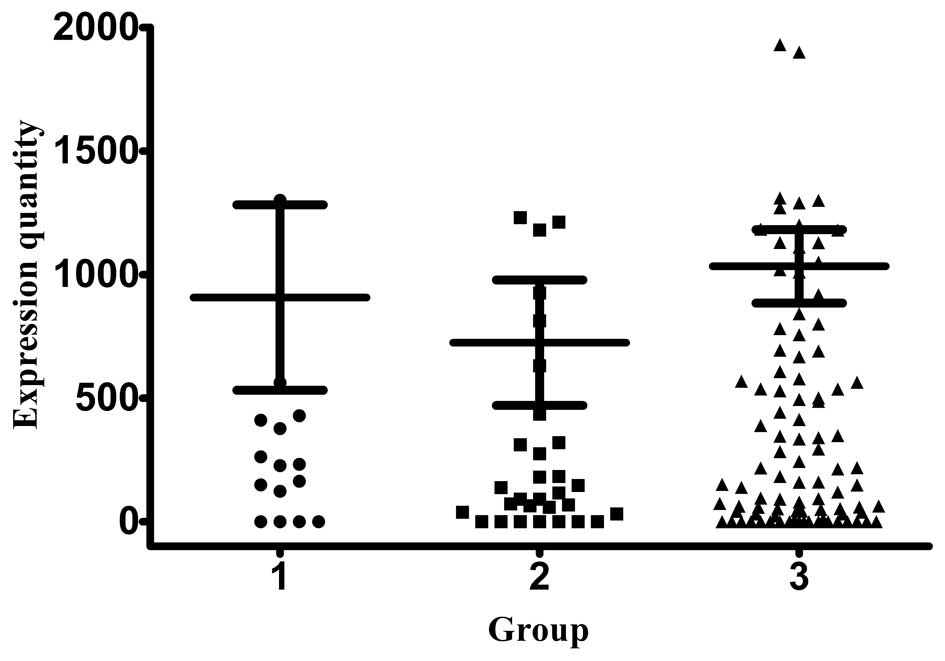
relative expression of CK19 mRNA in group 1, 2 and 3 patients was
724.42±254.14, 907.02±375.30 and 1,033.17±148.21 copies/ml,
respectively (Fig. 1). The CK20
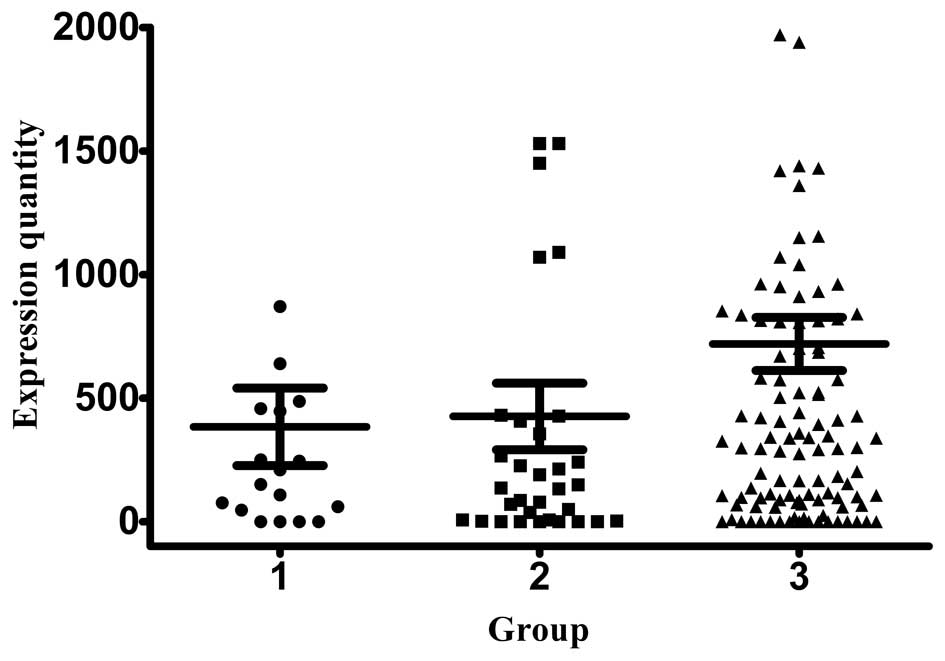
expression was 383.95±157.11, 426.19±134.77 and 719.13±107.48
copies/ml, respectively (Fig. 2).
Stage III patients exhibited the highest expression of CK19 and
CK20, while stage I and II patients exhibited similar levels of
expression for the two markers. The normal range for all the
investigated biomarkers is between 0–1000.
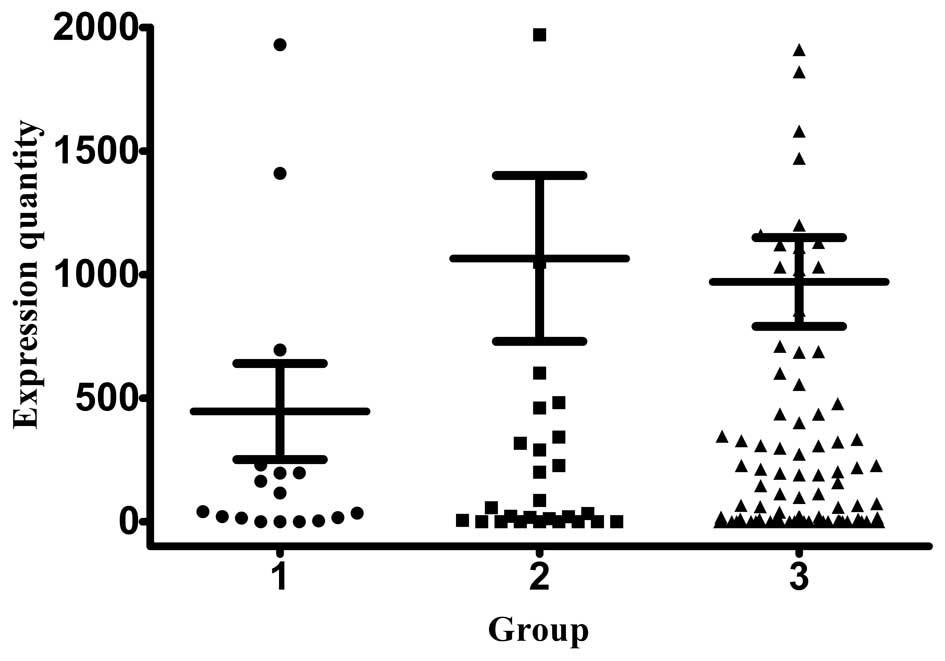
MMP-9 is a collagenase that degrades the basement
membrane and is associated with cancer metastasis. As expected, the
MMP-9 mRNA expression levels (1,065.18±335.64 copies/ml) were the
highest in stage III patients, whereas patients with stage I and II
disease exhibited similar expression levels (445.99±194.29 and
970.07±179.66 copies/ml, respectively) (Fig. 3).
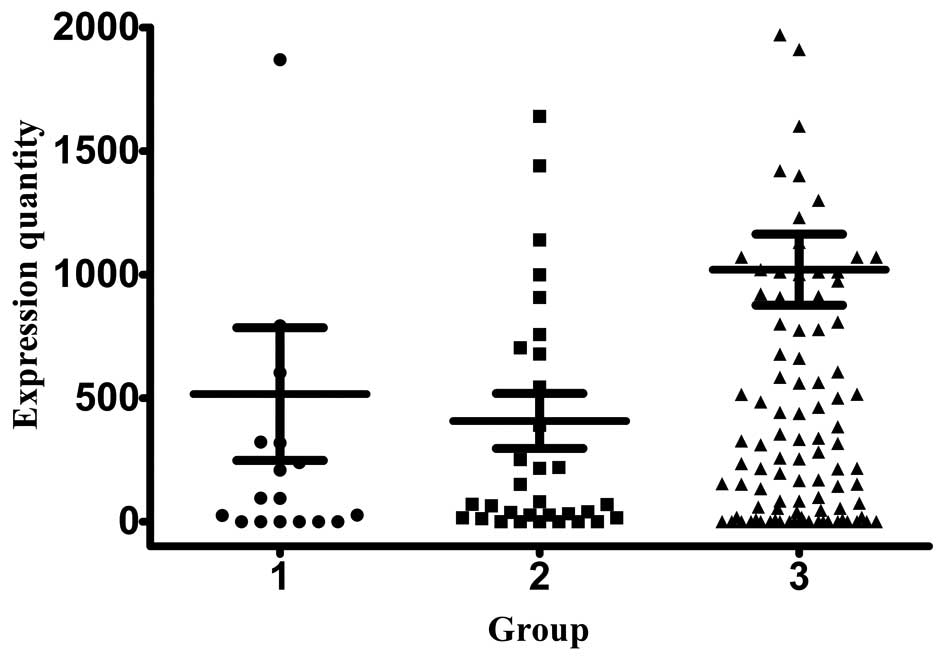
We also measured the mRNA expression of uPA, a
molecule that regulates basement membrane remodeling through its
catalyzed product. The overall uPA expression was low compared with
the three abovementioned markers in patients with stage I, II and
III disease (407.49±111.43, 516.44±268.76 and 1,019.87±143.60
copies/ml, respectively) (Fig. 4). In
contrast to what was observed for other markers, the uPA mRNA
expression was the lowest in stage III patients, without a
significant difference in its levels between stage I and II
patients.
The protein level of CRP was measured using
immunoturbidimetry in the serum samples from all the patients.
Stage III patients exhibited the highest CRP level (11.36±15.25),
which was significantly higher compared with the other two groups
(Fig. 5).
Association of CK19, CK20, MMP-9, uPA
mRNA and CRP levels with different clinicopathological factors
In order to elucidate the association of
clinicopathological factors, such as age, gender and disease stage
with the markers, the mRNA expression levels of CK19, CK20, MMP-9
and uPA in the peripheral blood were measured using qPCR and
compared by the different factors. Non-parametric tests and
Chi-square tests were used to determine the association. The
relative high and low mRNA expression level of CK19 was not
statistically significantly associated with gender, age or cancer
stage (Table I). By contrast, the
relative expression of CK20 was significantly associated with
cancer stage (P=0.037), but not with age and gender (Table II). Among patients with low CK20 mRNA
expression, 19.4% had stage I and II, whereas 29.1% had stage III
disease. In the high CK20 expression group, 11.5% of the patients
were at an early stage (I or II) and 40% were at a late stage. The
data indicated that low expression of CK20 was associated with an
earlier stage of gastric cancer, whereas high levels of CK20 were
associated with advanced stage. Similar results were observed in
uPA mRNA expression (Table III). In
the low uPA mRNA expression group, 20.6% of the patients had stage
I or II and 29.1% had stage III cancers. The percentage of the
patients with stage I or II cancer were only 10.3% in the high uPA
mRNA expression group, while 40% of the patients in that group had
stage III cancer. The difference was statistically significant
(P=0.014).
 | Table I.Association of CK19 mRNA level with
clinicopathological factors in patients with gastric cancer. |
Table I.
Association of CK19 mRNA level with
clinicopathological factors in patients with gastric cancer.
|
|
| CK19 |
| CK19 |
|
|---|
|
|
|
|
|
|
|
|---|
| Factors | Patients, n (%)
(n=165) | Median (mean,
5th-95th) | P-value | Low expression, n
(%) | High expression, n
(%) | P-value |
|---|
| Gender |
|
| 0.228 |
|
| 0.500 |
|
Male | 124 (75.2) | 332.93 (1,037.54,
743.55–1,331.54) |
| 59
(35.8) | 65 (39.4) |
|
|
Female | 41
(24.8) | 213.28 (716.05,
338.87–1,093.24) |
| 22
(13.3) | 19 (11.5) |
|
| Age (years) |
|
| 0.120 |
|
| 0.403 |
|
<60 | 91
(55.2) | 339.28 (1,179.42,
793.88–1,564.96) |
| 42
(25.5) | 49 (29.7) |
|
|
≥60 | 74
(44.8) | 252.98 (684.95,
444.95–924.95) |
| 39
(23.6) | 35 (21.2) |
|
| Stage |
|
| 0.170 |
|
| 0.114 |
| I | 18
(10.9) | 247.55 (907.02,
115.22–1,698.83) |
| 10 (6.1) | 8 (4.8) |
|
| II | 33
(20.0) | 138.08 (724.42,
206.76–1,242.08) |
| 21
(12.7) | 12 (7.3) |
|
|
III | 114 (69.1) | 401.10 (1,033.17,
739.54–1,326.80) |
| 50
(30.3) | 64 (38.8) |
|
 | Table II.Association of CK20 mRNA level with
clinicopathological factors in patients with gastric cancer. |
Table II.
Association of CK20 mRNA level with
clinicopathological factors in patients with gastric cancer.
|
|
| CK20 |
| CK20 |
|
|---|
|
|
|
|
|
|
|
|---|
| Factors | Patients, n (%)
(n=165) | Median (mean,
5th-95th) | P-value | Low expression, n
(%) | High expression, n
(%) | P-value |
|---|
| Gender |
|
| 0.228 |
|
| 0.444 |
|
Male | 124 (75.2) | 282.79 (686.85,
484.97–888.74) |
| 58
(35.2) | 66 (40.0) |
|
|
Female | 41
(24.8) | 213.02 (433.80,
220.40–647.20) |
| 22
(13.3) | 19 (11.5) |
|
| Age (years) |
|
| 0.329 |
|
| 0.556 |
|
<60 | 91
(55.2) | 241.0 (537.08,
332.37–741.80) |
| 46
(27.9) | 45 (27.3) |
|
|
≥60 | 74
(44.8) | 316.43 (730.83,
472.99–988.66) |
| 34
(20.6) | 40 (24.2) |
|
| Stage |
|
| 0.007 |
|
| 0.037 |
| I | 18
(10.9) | 180.34 (383.95,
52.47–715.43) |
| 10 (6.1) | 8 (4.8) |
|
| II | 33
(20.0) | 135.70 (426.19,
151.68–700.70) |
| 22
(13.3) | 11 (6.7) |
|
|
III | 114 (69.1) | 337.93 (719.13,
506.19–932.07) |
| 48
(29.1) | 66 (40.0) |
|
 | Table III.Association of uPA mRNA level with
clinicopathological factors in patients with gastric cancer. |
Table III.
Association of uPA mRNA level with
clinicopathological factors in patients with gastric cancer.
|
|
| uPA |
| uPA |
|
|---|
|
|
|
|
|
|
|
|---|
| Factors | Patients, n (%)
(n=165) | Median (mean,
5th-95th) | P-value | Low expression, n
(%) | High expression, n
(%) | P-value |
|---|
| Gender |
|
| 0.378 |
|
| 0.500 |
|
Male | 124 (75.2) | 255.66 (935.02,
667.84–1,202.20) |
| 62
(37.6) | 62 (37.6) |
|
|
Female | 41
(24.8) | 281.71 (562.58,
287.15–838.02) |
| 20
(12.1) | 21 (12.7) |
|
| Age (years) |
|
| 0.940 |
|
| 0.403 |
|
<60 | 91
(55.2) | 257.17 (865.83,
554.32–1,177.31) |
| 45
(27.3) | 46 (27.94) |
|
|
≥60 | 74
(44.8) | 286.55 (813.75,
527.84–1,099.66) |
| 37
(22.4) | 37 (22.4) |
|
| Stage |
|
| 0.019 |
|
| 0.014 |
| I | 18
(10.9) | 94.65 (516.44,
−50.60–1,083.48) |
| 12 (7.3) | 6 (3.6) |
|
| II | 33
(20.0) | 70.41 (407.49,
180.51–634.47) |
| 22
(13.3) | 11 (6.7) |
|
|
III | 114 (69.1) | 410.84 (1,019.87,
735.37–1,304.37) |
| 48
(29.1) | 66 (40.0) |
|
Similar to CK19, the relative high and low mRNA
expression level of MMP-9 was not statistically significantly
associated with gender, age or stage (Table IV).
 | Table IV.Association of MMP-9 mRNA level with
clinicopathological factors in patients with gastric cancer. |
Table IV.
Association of MMP-9 mRNA level with
clinicopathological factors in patients with gastric cancer.
|
|
| MMP-9 |
| MMP-9 |
|
|---|
|
|
|
|
|
|
|
|---|
| Factors | Patients, n (%)
(n=165) | Median (mean,
5th-95th) | P-value | Low expression, n
(%) | High expression, n
(%) | P-value |
|---|
| Gender |
|
| 0.396 |
|
| 0.620 |
|
Male | 124 (75.2) | 112.58 (876.53,
558.57–1,194.50) |
| 63
(38.2) | 61 (37.0) |
|
|
Female | 41
(24.8) | 145.55 (1,699.45,
475.59–1,723.31) |
| 19
(11.5) | 22 (13.3) |
|
| Age (years) |
|
| 0.625 |
|
| 0.994 |
|
<60 | 91
(55.2) | 115.74 (942.00,
52.73–1,331.27) |
| 45
(27.3) | 46 (27.94) |
|
|
≥60 | 74
(44.8) | 112.03 (919.53,
502.19–1,336.86) |
| 37
(22.4) | 37 (22.4) |
|
| Stage |
|
| 0.778 |
|
| 0.998 |
| I | 18
(10.9) | 78.09 (446.00,
36.07–855.92) |
|
9 (5.45) | 9 (5.45) |
|
| II | 33
(20.0) | 200.36 (1,065.18,
381.50–1,748.86) |
| 16 (9.7) | 17 (10.3) |
|
|
III | 114 (69.1) | 129.07 (970.07,
614.13–1,326.02) |
|
57 (34.55) | 57 (34.55) |
|
The association between CRP protein expression and
the clinicopathological factors was also investigated.
Interestingly, the CRP expression was associated with gender and
cancer stage, but not with age (Table
V). A total of 33.4% of the male patients exhibited low and
41.8% high CRP expression, while 15.7 and 9.1% of the female
patients exhibited low and high CRP expression, respectively. The
association between CRP and gender was statistically significant
(P=0.034). A total of 20% of patients in the low-expression group
had stage I and II cancer, while the respective percentage in the
high-expression group was 10.9%. The percentage of patients with
stage III cancer was higher in the high CRP expression group
(40.0%), compared with that in the low-expression group (29.1%).
The association between cancer stage and CRP expression was also
statistically significant (P=0.017).
 | Table V.Association of CRP level with
clinicopathological factors in patients with gastric cancers. |
Table V.
Association of CRP level with
clinicopathological factors in patients with gastric cancers.
|
|
| CRP |
| CRP |
|---|
|
|
|
|
|
|
|---|
| Factors | Patients, n (%)
(n=165) | Median (mean,
5th-95th) | P-value | Low expression, n
(%) | High expression, n
(%) | P-value |
|---|
| Gender |
|
| 0.043 |
|
| 0.034 |
|
Male | 124 (75.2) | 8.00 (10.87,
8.25–13.49) |
| 55
(33.4) | 69
(41.8) |
|
|
Female | 41
(24.8) | 6.00 (6.87,
5.58–8.16) |
| 26
(15.7) | 15 (9.1) |
|
| Age (years) |
|
| 0.147 |
|
| 0.175 |
|
<60 | 91
(55.2) | 7.00 (8.09,
6.85–9.33) |
| 49
(29.7) | 42
(25.5) |
|
|
≥60 | 74
(44.8) | 8.00 (12.08,
7.86–16.29) |
| 32
(19.3) | 42
(25.5) |
|
| Stage |
|
| 0.013 |
|
| 0.017 |
| I | 18
(10.9) | 7.00 (6.83,
4.68–8.98) |
| 10
(6.1) | 8 (4.8) |
|
| II | 33
(20.0) | 5.23 (6.42,
4.93–7.91) |
|
23 (13.9) | 10 (6.1) |
|
|
III | 114 (69.1) | 8.00 (11.36,
8.53–14.19) |
|
48 (29.1) | 66
(40.0) |
Overall, the results demonstrated that the relative
expression of CK20 and uPA mRNA were significantly associated with
gastric cancer stage. Low expression levels of these markers were
associated with earlier stages (I and II) and high expression
levels were more predominant in patients with later stages (III).
The relative CRP protein expression was associated with gender and
cancer stage. The low CRP expression group included more cases of
early-stage gastric cancer, while the high-expression group
included more cases of late-stage cancer. Male patients tend to
exhibit high CRP expression compared with female patients. Other
markers, including CK19 and MMP-9, were not statistically
significantly associated with gender, age or cancer stage.
Association of CK19, CK20, MMP-9, uPA
mRNA and CRP levels with cancer prognosis
All the patients in this study were followed up at
for a period of 2–3 years. A total of 65 patients succumbed to the
disease during the follow-up period; of the 100 patients who
remained alive, 71 presented with distant metastasis, while of the
65 deceased patients, 5 had no record of distal metastasis. The
association between the high and low expression of each individual
marker, including CK19, CK20, MMP-9, uPA and CRP, was determined
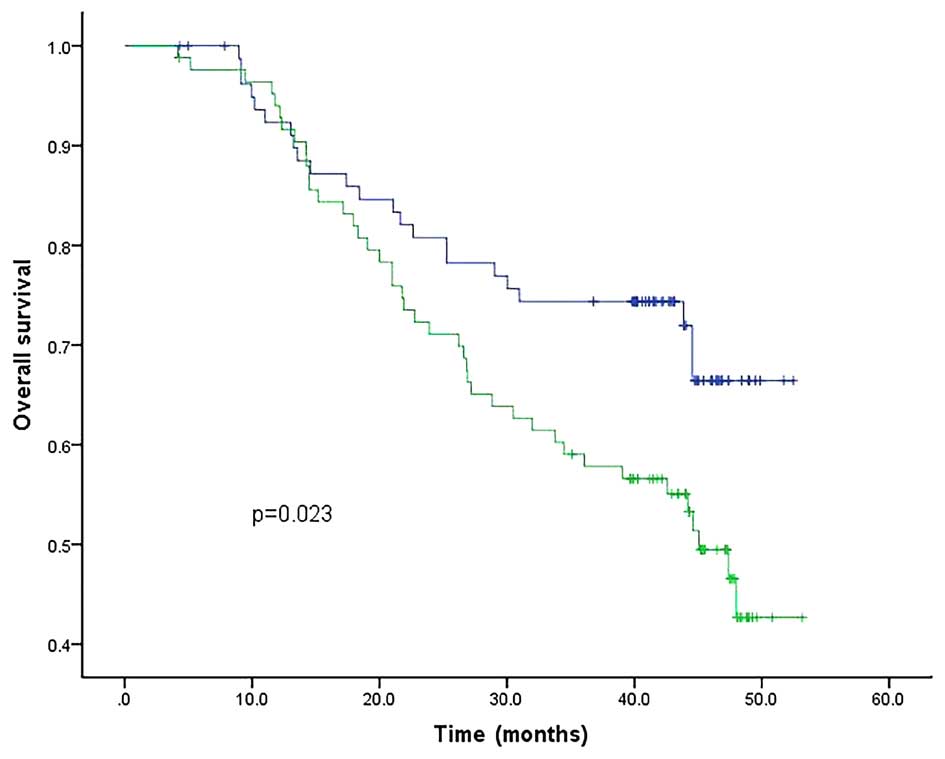
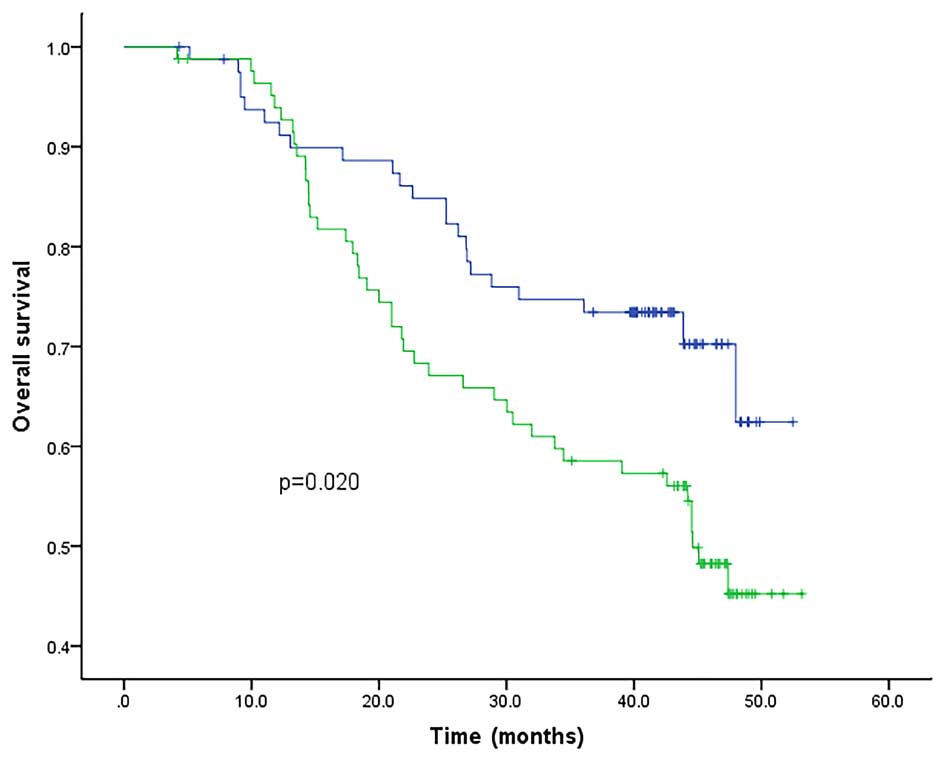
using the Kaplan-Meier method and the log-rank test. Among all the
markers, the mRNA level of CK19, CK20 and uPA, as well as that of
CRP, were all found to be associated with OS. The patients
exhibiting a high expression level of these markers had lower OS
rates compared to their peers with low expression levels; by
contrast, the expression of MMP-9 mRNA did not have a direct
association with OS (Figs. 6–10). The associations of CK19, CK20, uPA and
CRP with OS were all statistically significantly different
(P<0.05). Therefore, the upregulation of these four markers may
be considered a poor prognostic marker for gastric cancer.
Discussion
Invasion and metastasis are the most significant
factors affecting the clinical outcome of gastric cancer (29,30).
Patients with resectable tumors may undergo potentially curative
surgeries, although there is a risk of recurrence due to tumor
dissemination via the blood or lymphatic circulation. Such
micrometastasis may be detected using qPCR depending on the target
molecules (31). For gastric cancers,
several tumor-specific mRNAs, including CK19, CK20, CEACAM6,
carcinoembryonic antigen, ITGB1 and CYR61, have been used as
biomarkers for detecting tumor cells in the peripheral blood
(32–34), attracting increasing attention in the
research field to better understand the prognostic and clinical
value of the molecular detection methods.
In this study, we used RT-qPCR to detect the mRNA of
CK19, CK20, MMP-9 and uPA in the peripheral blood and investigated
the association between these markers with the clinicopathological
factors. The systemic inflammation marker CRP was also included in
the study. Our data demonstrated that the circulating mRNA of CK20
and uPA were associated with gastric cancer stage: Low expression
was associated with early stages, which also indicated a better
prognosis. It was generally considered that colorectal carcinomas
consistently express CK20, whereas gastric carcinomas express CK20
less frequently (35). Our results
demonstrated that CK20 may be a reliable prognostic marker for
gastric cancer as well. CRP was the only marker tested in this
study that was associated with gender: A higher number of male
patients were included in the low-expression groups, while more
female patients were included in the high-expression groups. CRP is
synthesized in hepatocytes and has been identified as a poor
prognostic factor for several diseases, including a variety of
cancers (36–38). An increased CRP level has been
associated with local tumor invasion, more advanced pathological
stage, a higher rate of recurrence and a reduced overall survival
(37,39–41). uPA
has been associated with poor outcome of gastric cancer due to its
invasive activity and angiogenesis-promoting ability (42). Our results were consistent with those
findings and confirmed uPA as a prognostic marker. In this study,
CK19 mRNA was not found to be associated with stage, age or gender,
but it was associated with prognosis. This observation was in
agreement with other studies reporting that CK19 mRNA may be a
prognostic marker for different cancers (12–15).
Surprisingly, the MMP-9 mRNA did not exhibit an association with
clinicopathological factors or overall survival, indicating that
MMP-9 was not a good prognostic marker for gastric cancer. A
meta-analysis has demonstrated that MMP-9 may indicate a poor
prognosis in patients with gastric cancer (24). It is possible that other MMP family
members, such as MMP-2, which are important proteases in breast and
lung cancer, may be of prognostic value in gastric cancer (22). However, all these findings require
further investigation.
Tumor cells entering the peripheral blood
circulation is a small step in the complex process of tumor
micrometastasis. However, the detection of such a prognostic marker
in the peripheral blood provides quick and reliable information for
clinicians to better understand the status of the patients and
design individualized treatment plans. Our study clearly suggests
that upregulated CK19, CK20, uPA and CRP levels may function as
prognostic markers for gastric cancer patients.
Acknowledgements
This study was supported by the Natural Science
Foundation of Zhejiang Province (grant no. LQ13H160017).
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Green D, Ponce de Leon S, Leon-Rodriguez E
and Sosa-Sanchez R: Adenocarcinoma of the stomach: Univariate and
multivariate analysis of factors associated with survival. Am J
Clin Oncol. 25:84–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Msika S, Benhamiche AM, Jouve JL, Rat P
and Faivre J: Prognostic factors after curative resection for
gastric cancer. A population-based study. Eur J Cancer. 36:390–396.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yasui W, Oue N, Aung PP, Matsumura S,
Shutoh M and Nakayama H: Molecular-pathological prognostic factors
of gastric cancer: a review. Gastric cancer. 8:86–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allgayer H, Babic R, Gruetzner KU,
Tarabichi A, Schildberg FW and Heiss MM: c-erbB-2 is of independent
prognostic relevance in gastric cancer and is associated with the
expression of tumor-associated protease systems. J Clin Oncol.
18:2201–2209. 2000.PubMed/NCBI
|
|
6
|
Gaspar MJ, Arribas I, Coca MC and
Diez-Alonso M: Prognostic value of carcinoembryonic antigen, CA
19-9 and CA 72-4 in gastric carcinoma. Tumour biology. 22:318–322.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu MD, Dong L, Qi P, Weng WW, Shen XH, Ni
SJ, Huang D, Tan C, Sheng WQ, Zhou XY, et al: Pituitary
tumor-transforming gene-1 serves as an independent prognostic
biomarker for gastric cancer. Gastric cancer. Jan 28–2015.(Epub
ahead of print). View Article : Google Scholar
|
|
8
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: a new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Varadhachary GR, Abbruzzese JL and Lenzi
R: Diagnostic strategies for unknown primary cancer. Cancer.
100:1776–1785. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanaji N, Bandoh S, Fujita J, Ishii T,
Ishida T and Kubo A: Compensation of type I and type II cytokeratin
pools in lung cancer. Lung Cancer. 55:295–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moll R, Franke WW, Schiller DL, Geiger B
and Krepler R: The catalog of human cytokeratins: Patterns of
expression in normal epithelia, tumors and cultured cells. Cell.
31:11–24. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edelman MJ, Hodgson L, Rosenblatt PY,
Christenson RH, Vokes EE, Wang X and Kratzke R: CYFRA 21-1 as a
prognostic and predictive marker in advanced non-small-cell lung
cancer in a prospective trial: CALGB 150304. J Thorac Oncol.
7:649–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fahmueller YN, Nagel D, Hoffmann RT,
Tatsch K, Jakobs T, Stieber P and Holdenrieder S: Predictive and
prognostic value of circulating nucleosomes and serum biomarkers in
patients with metastasized colorectal cancer undergoing selective
internal radiation therapy. BMC Cancer. 12:52012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holdenrieder S, von Pawel J, Dankelmann E,
Duell T, Faderl B, Markus A, Siakavara M, Wagner H, Feldmann K,
Hoffmann H, et al: Nucleosomes and CYFRA 21-1 indicate tumor
response after one cycle of chemotherapy in recurrent non-small
cell lung cancer. Lung Cancer. 63:128–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakata B, Takashima T, Ogawa Y, Ishikawa T
and Hirakawa K: Serum CYFRA 21-1 (cytokeratin-19 fragments) is a
useful tumour marker for detecting disease relapse and assessing
treatment efficacy in breast cancer. Br J Cancer. 91:873–878.
2004.PubMed/NCBI
|
|
16
|
Tang L and Han X: The urokinase
plasminogen activator system in breast cancer invasion and
metastasis. Biomed Pharmacother. 67:179–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baldini E, Sorrenti S, D'Armiento E, Di
Matteo FM, Catania A and Ulisse S: The urokinase plasminogen
activating system in thyroid cancer: Clinical implications. G Chir.
33:305–310. 2012.PubMed/NCBI
|
|
18
|
Andreasen PA, Kjoller L, Christensen L and
Duffy MJ: The urokinase-type plasminogen activator system in cancer
metastasis: a review. Int J Cancer. 72:1–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: Biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000.PubMed/NCBI
|
|
20
|
Pellikainen JM, Ropponen KM, Kataja VV,
Kellokoski JK, Eskelinen MJ and Kosma VM: Expression of matrix
metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special
reference to activator protein-2, HER2, and prognosis. Clin Cancer
Res. 10:7621–7628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmalfeldt B, Prechtel D, Härting K,
Späthe K, Rutke S, Konik E, Fridman R, Berger U, Schmitt M, Kuhn W,
et al: Increased expression of matrix metalloproteinases (MMP)-2,
MMP-9, and the urokinase-type plasminogen activator is associated
with progression from benign to advanced ovarian cancer. Clin
Cancer Res. 7:2396–2404. 2001.PubMed/NCBI
|
|
22
|
Sullu Y, Demirag GG, Yildirim A, Karagoz F
and Kandemir B: Matrix metalloproteinase-2 (MMP-2) and MMP-9
expression in invasive ductal carcinoma of the breast. Pathol Res
Pract. 207:747–753. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arii S, Mise M, Harada T, Furutani M,
Ishigami S, Niwano M, Mizumoto M, Fukumoto M and Imamura M:
Overexpression of matrix metalloproteinase 9 gene in hepatocellular
carcinoma with invasive potential. Hepatology. 24:316–322. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang QW, Liu L, Chen R, Wei YQ, Li P, Shi
HS and Zhao YW: Matrix metalloproteinase-9 as a prognostic factor
in gastric cancer: A meta-analysis. Asian Pac J Cancer Prev.
13:2903–2908. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roxburgh CS and McMillan DC: Role of
systemic inflammatory response in predicting survival in patients
with primary operable cancer. Future Oncol. 6:149–163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saito K and Kihara K: Role of C-reactive
protein in urological cancers: a useful biomarker for predicting
outcomes. Int J Urol. 20:161–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kleihues P and Sobin LH: World Health
Organization classification of tumors. Cancer. 88:2887. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours (7th). Wiley-Blackwell.
New York, NY: 2009.
|
|
29
|
Adachi Y, Yasuda K, Inomata M, Sato K,
Shiraishi N and Kitano S: Pathology and prognosis of gastric
carcinoma: Well versus poorly differentiated type. Cancer.
89:1418–1424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siewert JR, Böttcher K, Stein HJ and Roder
JD: Relevant prognostic factors in gastric cancer: Ten-year results
of the German Gastric Cancer Study. Ann Surg. 228:449–461. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujita Y, Terashima M, Hoshino Y, Ohtani
S, Kashimura S, Kanzaki N, Osuka F, Kogure M and Gotoh M: Detection
of cancer cells disseminated in bone marrow using real-time
quantitative RT-PCR of CEA, CK19, and CK20 mRNA in patients with
gastric cancer. Gastric cancer. 9:308–314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo J, Yao F, Lou Y, Xu C, Xiao B, Zhou W,
Chen J, Hu Y and Liu Z: Detecting carcinoma cells in peripheral
blood of patients with hepatocellular carcinoma by immunomagnetic
beads and rt-PCR. J Clin Gastroenterol. 41:783–788. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koga T, Tokunaga E, Sumiyoshi Y, Oki E,
Oda S, Takahashi I, Kakeji Y, Baba H and Maehara Y: Detection of
circulating gastric cancer cells in peripheral blood using real
time quantitative RT-PCR. Hepatogastroenterology. 55:1131–1135.
2008.PubMed/NCBI
|
|
34
|
Zhao ZS, Li L, Wang HJ and Wang YY:
Expression and prognostic significance of CEACAM6, ITGB1, and CYR61
in peripheral blood of patients with gastric cancer. J Surg Oncol.
104:525–529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moll R, Löwe A, Laufer J and Franke WW:
Cytokeratin 20 in human carcinomas. A new histodiagnostic marker
detected by monoclonal antibodies. Am J Pathol. 140:427–447.
1992.PubMed/NCBI
|
|
36
|
Dossus L, Jimenez-Corona A, Romieu I,
Boutron-Ruault MC, Boutten A, Dupré T, Fagherazzi G,
Clavel-Chapelon F and Mesrine S: C-reactive protein and
postmenopausal breast cancer risk: Results from the E3N cohort
study. Cancer Causes Control. 25:533–539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shimura T, Kitagawa M, Yamada T, Ebi M,
Mizoshita T, Tanida S, Kataoka H, Kamiya T and Joh T: C-reactive
protein is a potential prognostic factor for metastatic gastric
cancer. Anticancer Res. 32:491–496. 2012.PubMed/NCBI
|
|
38
|
Zhang SM, Lin J, Cook NR, Lee IM, Manson
JE, Buring JE and Ridker PM: C-reactive protein and risk of breast
cancer. J Natl Cancer Inst. 99:890–894. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kitagawa M, Shimura T, Yamada T, Itoh K,
Hasegawa C and Kawai T: C-reactive protein as an independent
prognostic factor for metastatic gastric cancer. J Clin Oncol (ASCO
Meeting Abstracts). 31:1102013.
|
|
40
|
Nozoe T, Iguchi T, Adachi E, Matsukuma A
and Ezaki T: Preoperative elevation of serum C-reactive protein as
an independent prognostic indicator for gastric cancer. Surg Today.
41:510–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Woo Y, Hyung WJ, Obama K, Kim HI, Pak KH,
Son T and Noh SH: Elevated high-sensitivity C-reactive protein, a
marker of advanced stage gastric cancer and postgastrectomy disease
recurrence. J Surg Oncol. 105:405–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaneko T, Konno H, Baba M, Tanaka T and
Nakamura S: Urokinase-type plasminogen activator expression
correlates with tumor angiogenesis and poor outcome in gastric
cancer. Cancer Sci. 94:43–49. 2003. View Article : Google Scholar : PubMed/NCBI
|