Introduction
Gemcitabine plus cisplatin (GC) therapy is a
chemotherapy frequently used to treat urothelial carcinoma, biliary
tract cancer, pancreatic cancer (1) and germ cell tumors (2). Lately, immune-checkpoint inhibitors
and an antibody-drug conjugate (3)
were approved as next-generation urothelial carcinoma therapies.
However, GC therapy is currently the standard first-line
chemotherapy for urothelial carcinoma. Additionally, it provides an
improved safety profile and tolerability than the combination of
methotrexate, vinblastine, doxorubicin and cisplatin, with similar
survival benefits (4).
In a previous clinical study (4), a favorable performance status (PS)
and adequate laboratory data, including blood cell counts and renal
function, were required from included patients. These patients had
bladder cancer that was measurable and histologically proven as
locally advanced or metastatic transitional cell carcinoma of the
urothelium, excluding prior treatment with systemic therapy.
Patients received gemcitabine (1,000 mg/m2) on days 1, 8
and 15, plus cisplatin (70 mg/m2) on day 2, based on the
treatment schedule. Ultimately, the aforementioned study revealed
that GC therapy had efficacy against specific patients. However, in
clinical practice, GC therapy is widely used for numerous patients
with various backgrounds, including the primary site, histological
type, the aim of therapy, prior systemic therapy, age, PS and renal
function. It is now receiving increased attention since the results
of these clinical trials differ from real-world data, and yet both
are the most reliable sources of evidence for research (5).
As physicians treating urothelial carcinomas, the
authors have often faced the dilemma that doses defined in previous
gemcitabine and cisplatin clinical trials (4) could not be provided to the patients
they treat on schedule because of their backgrounds and various
adverse events. There has been no report of the details for when,
which and how numerous adverse events cause delays and cancelations
in GC therapy. There is also a lack of information regarding the
prognoses of patients who could not complete their scheduled GC
therapy. Nevertheless, it may be possible to predict whether the
schedule can be completed before the start of GC therapy by
clarifying which background and adverse events can affect it. The
cause of delays and cancelations for the standard 4-week GC therapy
schedule and predictive factors for completing the schedule were
assessed retrospectively as real-world evidence.
Patients and methods
Patient studies
Urothelial carcinoma patients who were assigned the
4-week GC regimen (Oita University Hospital; Yufu, Japan) between
January 2009 to December 2020 (12 years) were selected for the
present study. Cases of bellini duct carcinoma and urethra
adenocarcinoma were excluded because of the rarity of these tumors.
Additionally, prior therapy was allowed in the present study as
long as it only consisted of local intravesical therapy, radiation,
or immunotherapy completed more than 4 weeks ago, adhering to the
previous clinical trial (4).
The present study was conducted in accordance with
ethical standards of the Declaration of Helsinki (2013 revision)
and Good Clinical Practice guidelines (6). Patients received gemcitabine (Eli
Lilly) (600-1,000 mg/m2) on days 1, 8 and 15, plus
cisplatin (Pfizer, Inc.) (42-70 mg/m2) on day 2, based
on the treatment schedule. Almost all factors that were assessed
before the initiation of each GC course have been analyzed,
regardless of whether they were related to cancer treatment in
previous studies. This is because some factors such as serum
lactate dehydrogenase (7) and
C-reactive protein (8) are related
to patient prognosis even if they are not related to cancer
treatment. The Common Terminology Criteria for Adverse Events
(CTCAE) v5.0 was used to evaluate all adverse events.
Statistical analysis
Patients who completed the scheduled GC treatment
were compared with patients with a delay or canceled administration
in the middle of treatment. Pearson's chi-square test (or Fisher's
exact test) and multifactor analyses, by SPSS version 25 (IBM
Corp.), were used to analyze factors, such as blood counts and
serum chemistry, at the beginning of each cycle. The logistic
regression analysis (the forced entry method) was used for
multifactor analyses. Statistical significance was set at
P<0.05.
Results
Patient characteristics
Patient characteristics (including sex and age
distribution; n=70) are shown in Table
I. A total of 201 courses, scheduled to 70 patients, were
analyzed. The median age of patients was 69 (29-87) years old. Most
had a PS of 0 (91.4%), but only two patients had PSs of 2-3 (2.8%).
Regarding disease breakdown, most patients had bladder cancer
(66.7%), and >50% were stage IV (58.6%). A total of 74 courses
(36.8%) were performed as neoadjuvant and adjuvant therapy, and 127
courses (63.2%) were for metastatic or locally advanced disease. In
total, four stage I patients underwent GC therapy for recurrence
with distant metastasis after primary therapy. In addition, one
patient with stage II bladder cancer underwent GC therapy as
neoadjuvant chemotherapy and the ypT classification was ypT1. The
patient was classified as stage II due to obvious muscle-invasive
cancer before neoadjuvant chemotherapy.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient
characteristics | Number | Percentage (%) |
|---|
| Sex (n=70) | | |
|
Male | 54 | 77.1 |
|
Female | 16 | 22.9 |
| Median age, years
(n=70) | 69 (range 29-87) | |
| Performance
status | | |
|
0 | 64 | 91.4 |
|
1 | 4 | 5.7 |
|
2 | 1 | 1.4 |
|
3 | 1 | 1.4 |
| Diabetes | | |
|
Yes | 12 | 17.1 |
|
No | 58 | 82.9 |
| Hypertension | | |
|
Yes | 26 | 37.1 |
|
No | 44 | 62.9 |
| Cancer type
(n=72) | | |
|
Bladder
cancer | 48 | 66.7 |
|
Ureteral
cancer | 15 | 20.8 |
|
Renal pelvic
cancer | 9 | 12.5 |
| Stage (n=70) | | |
|
I | 4 | 5.7 |
|
II | 13 | 18.6 |
|
III | 12 | 17.1 |
|
IV | 41 | 58.6 |
| Purpose (n=201) | | |
|
Neo-adjuvant
therapy | 47 | 23.4 |
|
Adjuvant
therapy | 27 | 13.4 |
|
Treatment
for metastatic or advanced tumor | 127 | 63.2 |
| Outcome (n=70) | | |
|
Alive | 27 | 38.6 |
|
Dead | 36 | 51.4 |
|
Unknown | 7 | 10.0 |
|
Median
number of courses/persons | 3 (1-9) | |
Laboratory data at the initiation of each cycle
showed that patients had white blood cell counts
≥2.5x109/l, platelet counts ≥8.8x109/l,
hemoglobin levels ≥8.0 g/dl and creatinine clearance levels ≥40
ml/min at the initiation of each cycle (data not shown). A total of
130 cycles were given to patients with bilateral kidneys, while
other cycles were not given due to nephrectomy or
nephroureterectomy from primary disease.
Distribution of chemotherapy
schedule
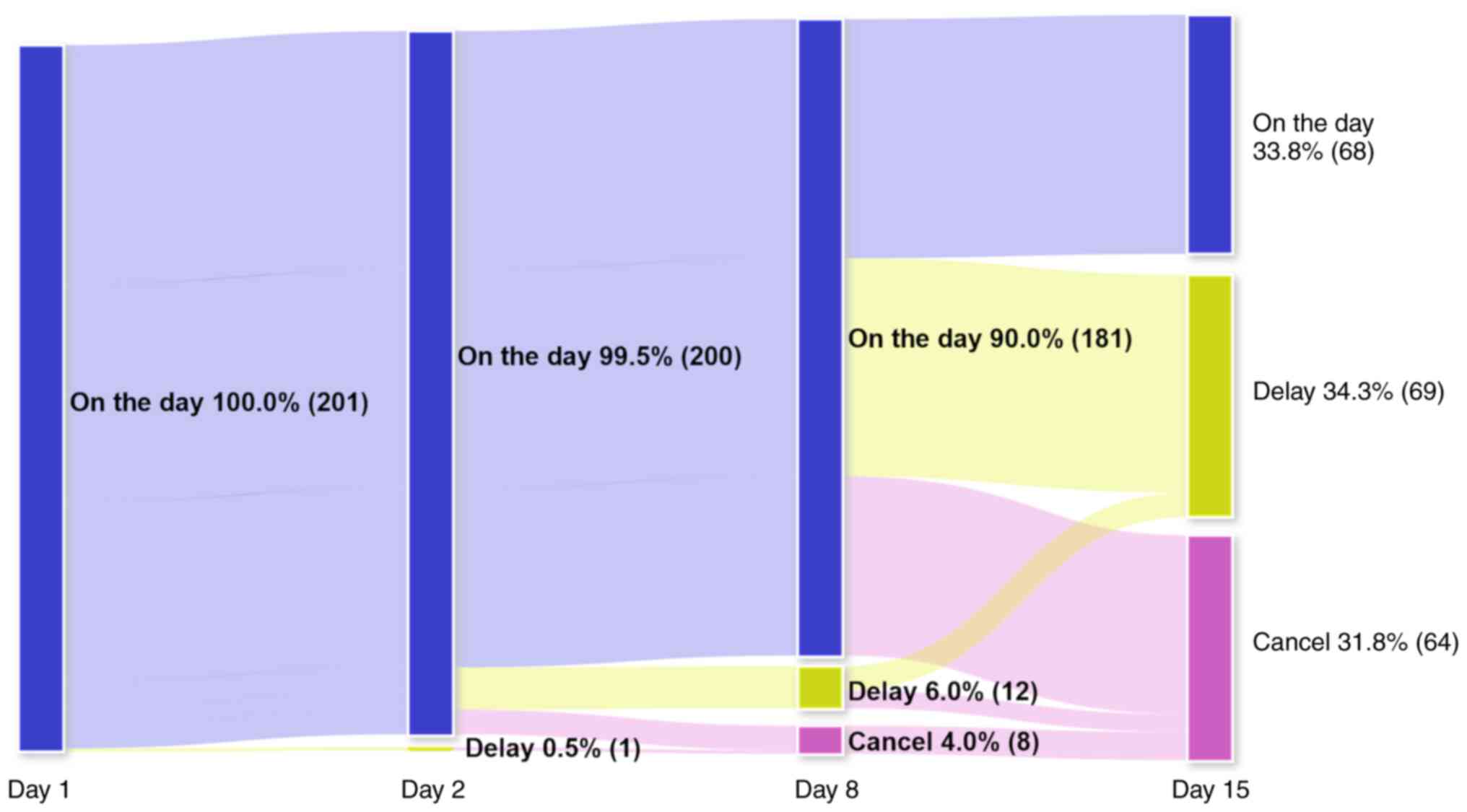
Patient distributions receiving GC therapy (Figs. 1 and S1) indicated that only 68 courses
(33.8%) were performed on schedule. The remaining were canceled or
delayed in the middle of treatment. GC therapy was delayed and/or
canceled in 1 course (0.5%) on day 2, 20 courses (10.0%) on day 8
and 133 courses (66.1%) on day 15. Day 15 had the lowest percentage
of anticancer drug scheduled administration. Only 37.6% (68/181) of
GC therapy courses scheduled on day 8 were performed on day 15.
Adverse events and chemotherapy
schedule
Myelo-suppression was the most common of all
hematologic adverse events. The focus of the present study was on
CTCAE grade 3 and 4 toxicities (Table
II); thrombocytopenia was the most common (52 cycles, 25.9%)
and neutropenia was the second (32 cycles, 15.9%) of hematologic
adverse events. Febrile neutropenia was the most common of all
non-hematologic adverse events. In clinical practice, heavier
toxicities (>grade 3) are considered to directly prevent
scheduled GC treatments.
 | Table IIGrade 3/4 adverse events by day
15. |
Table II
Grade 3/4 adverse events by day
15.
| | Grade 3 | Grade 4 | Grade 3/4 |
|---|
| Hematologic | No. of cycles | % | No. of cycles | % | No. of cycles | % |
|---|
| Thrombocytopenia | 36 | 17.9 | 16 | 8.0 | 52 | 25.9 |
| Anemia | 9 | 4.5 | 0 | 0 | 9 | 4.5 |
| Leucopenia | 13 | 6.5 | 0 | 0 | 13 | 6.5 |
| Neutropenia | 20 | 10.0 | 12 | 6.0 | 32 | 15.9 |
| Creatinine
increased | 2 | 1.0 | 0 | 0 | 2 | 1.0 |
| eGFR decreased | 1 | 0.5 | 0 | 0 | 1 | 0.5 |
| ALT increased | 0 | 0 | 1 | 0.5 | 1 | 0.5 |
| Hyponatremia | 1 | 0.5 | 0 | 0 | 1 | 0.5 |
| Hyperamylasemia | 1 | 0.5 | 0 | 0 | 1 | 0.5 |
| | Grade 3 | Grade 4 | Grade 3/4 |
| Non-hematologic | No. of cycles | % | No. of cycles | % | No. of cycles | % |
| Febrile
neutropenia | 3 | 1.5 | 0 | 0 | 3 | 1.5 |
| Acute upper
respiratory inflammation | 1 | 0.5 | 0 | 0 | 1 | 0.5 |
| Gastric ulcer | 0 | 0 | 1 | 0.5 | 1 | 0.5 |
| Acute kidney
injury | 1 | 0.5 | 1 | 0.5 | 2 | 1.0 |
| Allergic
reaction | 1 | 0.5 | 0 | 0 | 1 | 0.5 |
| Urinary tract
infection | 1 | 0.5 | 0 | 0 | 1 | 0.5 |
| Gingivitis | 1 | 0.5 | 0 | 0 | 1 | 0.5 |
| Fever | 0 | 0 | 1 | 0.5 | 1 | 0.5 |
| Headache | 0 | 0 | 1 | 0.5 | 1 | 0.5 |
| Pelvic
infection | 1 | 0.5 | 0 | 0 | 1 | 0.5 |
| Pulmonary
infection | 1 | 0.5 | 0 | 0 | 1 | 0.5 |
In univariate analyses (Table III), male, ureteral cancer, lower
stage (stage I/II), receiving less than 90% of the gemcitabine and
cisplatin dosage, solitary kidney, high creatinine level, low
estimated glomerular filtration rate (eGFR) level, low platelet
count, and high alkaline phosphatase (ALP) level, at the initiation
of each cycle, were significantly associated with not receiving GC
chemotherapy on schedule.
 | Table IIIUnivariate analysis based on patient
characteristics. Blood exam at the initiation of each cycle and
dosage of anticancer drugs. |
Table III
Univariate analysis based on patient
characteristics. Blood exam at the initiation of each cycle and
dosage of anticancer drugs.
|
Characteristics | On schedule | Not on
schedule | P-value |
|---|
| Sex | | | 0.024 |
|
Male | 47 | 109 | |
|
Female | 21 | 23 | |
| Type of
disease | | | 0.020 |
|
Bladder
cancer | 42 | 68 | |
|
Ureteral
cancer | 9 | 41 | |
|
Renal pelvis
cancer | 17 | 23 | |
| Diabetes | | | 0.221 |
|
Yes | 12 | 31 | |
|
No | 55 | 99 | |
| Hypertension | | | 0.204 |
|
Yes | 19 | 46 | |
|
No | 49 | 86 | |
| Purpose | | | 0.009 |
|
NAC/AC | 33 | 40 | |
|
Treatment | 35 | 92 | |
| Stage | | | <0.001 |
|
I/II | 7 | 43 | |
|
III/IV | 61 | 86 | |
| Age | | | 0.297 |
|
≥70 | 39 | 69 | |
|
<70 | 29 | 63 | |
| Performance
status | | | 0.451 |
|
0 | 65 | 124 | |
|
1/2/3 | 3 | 8 | |
| Dosage of
gemcitabine | | | 0.003 |
|
≥90% | 57 | 85 | |
|
<90% | 11 | 47 | |
| Dosage of
cisplatin | | | 0.017 |
|
≥90% | 53 | 82 | |
|
<90% | 15 | 50 | |
| Kidney | | | <0.001 |
|
Bilateral | 55 | 75 | |
|
Solitary | 11 | 53 | |
| CCr (ml/min/1.73
m2) | | | 0.091 |
|
≥60 | 20 | 44 | |
|
<60 | 5 | 26 | |
| Cr | | | 0.008 |
|
Normal | 52 | 80 | |
|
High | 13 | 49 | |
| eGFR | | | 0.014 |
|
≥60 | 34 | 45 | |
|
<60 | 23 | 66 | |
| WBC | | | 0.669 |
|
Normal | 66 | 128 | |
|
High | 2 | 4 | |
| Neutrophils | | | 0.294 |
|
Normal | 58 | 111 | |
|
Low | 9 | 12 | |
| Hb | | | 0.448 |
|
Normal | 7 | 16 | |
|
Low | 61 | 116 | |
| PLT | | | 0.012 |
|
Normal | 65 | 111 | |
|
Low | 3 | 21 | |
| Alb | | | 0.067 |
|
Normal | 7 | 28 | |
|
Low | 47 | 88 | |
| AST | | | 0.131 |
|
Normal | 63 | 116 | |
|
High | 4 | 16 | |
| ALT | | | 0.344 |
|
Normal | 65 | 126 | |
|
High | 1 | 5 | |
| ALP | | | <0.05 |
|
Normal | 52 | 99 | |
|
High | 4 | 21 | |
| LDH | | | 0.148 |
|
Normal | 42 | 98 | |
|
High | 14 | 20 | |
| Na | | | 0.512 |
|
Normal | 55 | 107 | |
|
Low | 12 | 25 | |
| K | | | 0.389 |
|
Normal | 53 | 108 | |
|
Low/high | 14 | 24 | |
| CRP | | | 0.403 |
|
Normal | 21 | 40 | |
|
High | 39 | 85 | |
| Ca | | | 0.339 |
|
Normal | 50 | 98 | |
|
High | 3 | 3 | |
| P | | | 0.406 |
|
Normal | 27 | 55 | |
|
Low/high | 2 | 7 | |
In the multivariate analysis, receiving more than
90% of the cisplatin dosage and having bilateral kidneys were
significant and independent factors for receiving GC chemotherapy
on schedule. Age and PS were included in the analysis because they
are clinically important factors in chemotherapy decision making.
eGFR level is highly associated with serum creatinine, therefore,
creatinine levels were adopted for this assessment, which had a
higher association in Table II
and is easier to use clinically. The type of cancer is highly
associated with the purpose of treatment, and most
neoadjuvant/adjuvant chemotherapy treatments were performed for
bladder cancer. Therefore, the purpose of treatment, considered a
potentially more generalizable factor (as to other cancers), was
included in the analysis.
Discussion
The present study revealed several risk factors that
interfered with scheduled GC therapy for urothelial carcinomas by
using real-world data rather than controlled clinical trials. The
data of the present study demonstrated that receiving more than 90%
of the scheduled cisplatin dosage and having bilateral kidneys are
the most important predictive factors. Renal function was one of
the most important factors, as other renal factors such as serum
creatinine and eGFR were also associated with whether scheduled GC
therapy was completed. Patient conditions that allow tolerance of
sufficient amounts of cisplatin may contribute to the success of
receiving the complete schedule of GC therapy. The present study
focused on the completion rate for each cycle of GC therapy.
Patients with UC typically require 3-4 cycles of chemotherapy and
the median cycles per patient of GC therapy was also 3 cycles in
the present study.
In a phase III study of GC therapy reported by von
der Maase et al (4), prior
systemic chemotherapy was excluded. However, clinicians often
encounter patients with myelosuppression caused by primary diseases
and prior chemotherapy before scheduling GC therapy in the real
world. Therefore, it would be necessary for clinicians to explain
why patients with these risk factors may not complete their
scheduled cycles on schedule. Regarding adverse events that might
delay and/or lead to cancelled GC therapy, neutropenia was the most
common hematologic toxicity in the previous phase III study
(4), but in the present study,
thrombocytopenia was the most common hematologic toxicity. In
non-hematologic adverse events, nausea was the most common in the
previous study (9), but febrile
neutropenia was the most common in the present study.
This meant that adverse events varied between
patients with the same background and good laboratory data, and
patients with various backgrounds in clinical practice. Therefore,
the differences between real-world data and controlled clinical
trials is important information for patients and clinicians.
It was expected that patients with higher stages
(stage III/IV) would not complete the scheduled GC treatment. In
fact, patients with lower stages (stage I/II) were those who were
unable to complete it. Stage I/II was significantly correlated with
solitary kidney and abnormal renal function (serum creatinine,
creatinine clearance rate and eGFR) in the present study. Patients
with stage I/II tended to receive a lower dose of both cisplatin
and gemcitabine compared with stage III/IV. This may have been
because surgery is the main therapy for stage I/II patients and
chemotherapy is the primary treatment for stage III/IV.
Myelosuppression is a very common adverse event in
patients receiving chemotherapy, and thrombocytopenia was the most
common hematologic adverse event in the present study (Table III). This result indicated that
having a normal platelet count before initiating GC chemotherapy
could enhance the likelihood of completing all scheduled
cycles.
Problems related to the kidneys negatively affected
GC therapy scheduling (Tables
III and IV). Urothelial
tumors are more likely to be associated with having a solitary
kidney and high serum creatinine levels, due to the primary disease
and resulting surgery, compared with other tumor regions (10). Having a solitary kidney and low
eGFR-related renal dysfunction could lead to a reduced dose of
cisplatin.
 | Table IVMultivariate analysis based on
patient characteristics. Blood exam at the initiation of each cycle
and dosage of anticancer drugs. |
Table IV
Multivariate analysis based on
patient characteristics. Blood exam at the initiation of each cycle
and dosage of anticancer drugs.
| | 95% Confidence
interval | |
|---|
|
Characteristics | Hazard ratio | Lower bound | Upper bound | P-value |
|---|
| Age | 1.134 | 0.512 | 2.512 | 0.756 |
| Sex | 0.766 | 0.303 | 1.94 | 0.574 |
| Performance
status | 1.694 | 0.263 | 10.916 | 0.579 |
| Purpose | 2.193 | 0.93 | 5.17 | 0.073 |
| Stage | 0.577 | 0.205 | 1.624 | 0.298 |
| Dosage of
gemcitabine | 4.171 | 0.977 | 17.802 | 0.054 |
| Dosage of
cisplatin | 4.651 | 21.27 | 1.003 | 0.049 |
| Kidney | 3.512 | 1.212 | 10.176 | 0.021 |
| Creatinine | 1.746 | 0.637 | 4.783 | 0.279 |
| Platelet | 2.407 | 0.596 | 9.721 | 0.218 |
| Alkaline
phosphatase | 2.478 | 0.716 | 8.577 | 0.152 |
The present study is also consistent with another
previous study that reported an association between elevated ALP
levels and adverse pathologic features of upper tract urothelial
carcinomas (11). The present
study also suggested that elevated ALP levels may be associated
with the completion of chemotherapy for urothelial carcinomas.
The current study has certain limitations. First, as
a retrospective study, data was collected from patient medical
records and blood test results were lacking in a few patients.
Hence, it is possible that not all adverse events were accounted
for, particularly with low-grade non-hemorrhagic events. A second
limitation is related to the focus on GC treatment schedules in
each course, and that only initial laboratory data before each
cycle were used for the analysis. Therefore, survival curves could
not be created since most patients received multiple GC courses.
Imaging tests were usually performed between every few courses;
therefore, the effectiveness of each GC course individually could
not be evaluated. Additionally, the present study could not show
efficacy in terms of patient survival and therapy evaluations based
on response criteria in solid tumors. Third, the current study
focused on whether all anticancer drugs were administered on
schedule during one 15-day cycle, therefore, adverse events after
day 16 were not evaluated. While the anticancer drugs in the
current study could be provided to patients with low-grade adverse
events before day 15, any severe adverse events after day 16 could
have influenced the next course of chemotherapy. Finally, the
present study had a small sample size and was not randomized.
Besides these limitations, there have been no other studies of
real-world data on GC therapy in a scale comparable to that of the
present study.
In conclusion, the present study reported how cycle
percentages are completed on scheduled GC therapy, the type of
adverse events that prevented it, and predictors that aided in the
completion of the schedule against various patient backgrounds.
This evidence has been disseminated to clinicians in this field to
recognize the difference between clinical trials and real-world
situations. In addition, based on real-world data, predictive
factors for patients with various backgrounds who completed the
scheduled 4-week GC therapy have been identified. This information
can be productive for clinical physicians to decide the course of
treatment.
Supplementary Material
Flowchart of the distribution of the
gemcitabine plus cisplatin therapy schedule. The anticancer drugs
were administrated on days 1, 2, 8 and 15. This shows the number of
cycles those could be given anticancer drug on schedule or
not.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MS, SH, HM and ToS contributed to the concept and
design of the present study. MS, TI, TaS and TA contributed in the
data collection. MS and SH contributed to the analysis and
interpretation of data and all authors confirm the authenticity of
all the raw data. MS, SH and ToS drafted the article. All authors
critically revised the article for important intellectual content.
HM and ToS provided administrative, technical and material support.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the of Oita University (approval no. 2358; Oita,
Japan). All study participants provided informed consent (or a
formal waiver of consent). Informed consent was obtained in the
form of opt-out on the website (https://oita-urol.jp/exam/).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kayahan N, Karaca M, Satış H, Yapar D and
Özet A: Folfirinox versus gemcitabine-cisplatin combination as
first-line therapy in treatment of pancreaticobiliary cancer. Turk
J Med Sci. 51:1727–1732. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fujiwara M, Hayashi T, Takeda H, Yuasa T,
Komai Y, Numao N, Yamamoto S, Fukui I, Kouno T and Yonese J:
Cisplatin, gemcitabine, and paclitaxel as a salvage second-line
therapy for metastatic germ-cell cancer. Clin Genitourin Cancer.
19:e6–e11. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hanna KS, Campbell M, Kolling A, Husak A,
Sturm S, Bello D and Blake K: Updates in the management and future
landscape of urothelial carcinoma. J Oncol Pharm Pract. 27:435–444.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim HS, Lee S and Kim JH: Real-world
evidence versus randomized controlled trial: Clinical research
based on electronic medical records. J Korean Med Sci.
33(e213)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
International Council for Harmonisation of
Technical Requirements for Pharmaceuticals for Human Use (ICH): ICH
Harmonised Guideline: Integrated Addendum to ICH E6(R1): Guideline
for Good Clinical Practice E6(R2). Version 4. https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf.
Accessed November 9, 2016.
|
|
7
|
Wu M, Lin P, Xu L, Yu Z, Chen Q, Gu H and
Liu C: Prognostic role of serum lactate dehydrogenase in patients
with urothelial carcinoma: A systematic review and meta-analysis.
Front Oncol. 10(677)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Saito K, Urakami S, Komai Y, Yasuda Y,
Kubo Y, Kitsukawa S, Okubo Y, Yamamoto S, Yonese J and Fukui I:
Impact of C-reactive protein kinetics on survival of patients with
advanced urothelial carcinoma treated by second-line chemotherapy
with gemcitabine, etoposide and cisplatin. BJU Int. 110:1478–1484.
2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xylinas E, Rink M, Margulis V, Clozel T,
Lee RK, Comploj E, Novara G, Raman JD, Lotan Y, Weizer A, et al:
Impact of renal function on eligibility for chemotherapy and
survival in patients who have undergone radical
nephro-ureterectomy. BJU Int. 112:453–461. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sheth KR, Haddad AQ, Ashorobi OS, Meissner
MA, Sagalowsky AI, Lotan Y and Margulis V: Prognostic serum markers
in patients with high-grade upper tract urothelial carcinoma. Urol
Oncol. 34:418.e419–418.e16. 2016.PubMed/NCBI View Article : Google Scholar
|