Introduction
The COVID-19 pandemic, which emerged in Wuhan,
China, has presented a significant global public health crisis,
affecting the majority of nations worldwide. The global effect has
been significant due to its mechanisms of transmission, the
subsequent development of severe acute respiratory syndrome, and
the global mortality rate that followed the onset of the pandemic
(1).
The World Health Organization (WHO) officially
proclaimed a state of public health emergency on January 30,
2020(2). Subsequently, the virus has
spread in >223 nations and regions, leading to a worldwide
pandemic, with >700 million cases and almost 7 million deaths,
as of October, 2023(3).
Laboratory genomic analysis demonstrated that the
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which
is responsible for COVID-19, shares a 96% overall genomic
similarity with a bat coronavirus known as CoVZXC21 (RaTG13)
(4). Additionally, there is a
significant similarity of 80% sequence identity between this newly
identified coronavirus and the SARS coronavirus (SARS-CoV-1), which
caused the previous SARS pandemic in the past (5). The angiotensin-converting enzyme 2
(ACE-2) receptor in humans serves as the primary point of viral
entrance into the human body. Notably, a single nucleotide
substitution (Arg426 to Asn426) has been found to enhance the
binding affinity of the new virus, perhaps accounting for its
heightened transmissibility (6).
SARS-CoV-2 mostly manifests as a respiratory virus (7). Consequently, the primary mode of
transmission for this pathogen is the direct contact with Flügge's
droplets from an individual infected that has symptoms, which are
transmitted via coughing, sneezing or exhaling (8). The expression of ACE-2 receptors is
higher in the small intestine, which may account for the presence
of gastrointestinal symptoms observed in some of patients. There is
a vast amount of evidence that supports the existence of viral
genetic material in the stool of patients, hence establishing the
fecal-oral pathway as a mechanism of transmission and spread of the
virus (9).
The use of infection control measures is critical
for controlling the transmission of the virus and for effectively
managing the ongoing outbreak. The risk of infection between
patients and dental practitioners may be greater in dental
environments due to their specific features (10). Considering the probable impact of
COVID-19 on dental practices and hospitals, there has been an
urgent need for the implementation of rigorous and efficient
infection control policies (11).
In combination with fever, fatigue, dry cough,
myalgias, a sore throat, breathing difficulties and respiratory
issues that can lead to severe acute respiratory syndrome, patients
with COVID-19 may experience a diverse range of additional local
and systemic complications (12).
These include acute cardiac damage, acute renal failure,
gastrointestinal complications, dysgeusia, anosmia and neurological
symptoms (13).
The oropharyngeal microbiome, which is the community
of organisms that colonizes the upper respiratory tract, can
influence the clinical progression of respiratory viral infections,
including SARS-CoV2, and the virus may be identified in saliva and
oropharyngeal secretions (14).
Aphthous-like ulcers and superficial necrosis were seen in
individuals who had been diagnosed with COVID-19, as these lesions
develop in areas that are recognized to possess ACE-2 receptors,
such as tongue epithelium and salivary gland tissue, following the
occurrence of dysgeusia (15).
Patients and methods
In the present study examined the incidence of
oropharyngeal manifestations in the context of SARS-CoV-2
infection. The differences between patients with COVID-19 group and
a control group (uninfected group) were also examined as regards
oral cavity symptoms.
Characterization of the study
groups
The present study was carried out between April,
2021 and October, 2022 at Constanța Clinical Hospital for
Infectious Diseases (Constanța, Romania). The present study
included two groups of patients and is part of a larger project
associated with the doctoral thesis entitled: ‘Oropharyngeal
manifestations in patients with compromised immunity’. The
investigated groups were the following: i) a COVID-19 group, which
included 52 patients diagnosed with SARS-CoV-2, admitted to the
Constanța Clinical Hospital for Infectious Diseases; and ii) the
control group, which consisted of 52 individuals that had
appointments for different procedures and dental evaluations. These
subjects tested negative with the nasopharyngeal swab that was
performed on their appointment date.
The vaccination status of the individuals
participating in the study was not part of the current research
aims, as the present study aimed to document the oral cavity
symptoms of those infected with the virus, without any oscillations
that a vaccine would impose. Thus, data on whether the participants
were vaccinated or not, and with which vaccine were not
recorded.
The patients in the COVID-19 group underwent an
objective examination of the oral cavity (both intraoral and
extraoral), as well as paraclinical tests, i.e., laboratory
analyzes which, in the case of some subjects, revealed the presence
of fungi and/or bacterial infections, and radiological examinations
to determine the severity of the condition.
Patients in both groups completed a questionnaire
the purpose of which was to identify oropharyngeal manifestations
before and during infection with SARS-CoV-2 for the COVID-19 group,
in comparison with the control group. Patients were only included
in the study if they had a confirmed infection and had
oropharyngeal symptoms prior to the administration of
cortisol-based therapy and the antiviral therapy. The questionnaire
addressed the following: Demographic data, information related to
oral hygiene, previous and current oropharyngeal manifestations and
interaction with the dentist. The final section of the
questionnaire addressed specific questions related to oral symptoms
associated with COVID-19 and changes in taste and smell.
The present study received ethical approval from the
Ethics Committee of Infectious Diseases Clinical Hospital in
Constanta, Romania (protocol code 2 and date of approval February
24, 2021).
Research hypotheses
The main aim of the present study was to identify
the incidence of oropharyngeal manifestations in the context of
SARS-CoV-2 infection, and to formulate specific research
hypotheses. Thus, the following hypotheses were considered: i) The
null hypothesis, where there are no significant statistical
differences between the COVID-19 group and the control group as
regards oropharyngeal manifestations. ii) Hypothesis 1, where
statistically significant differences are anticipated regarding
oropharyngeal manifestations between the group with COVID-19 and
the control group. A higher incidence of oropharyngeal
manifestations is assumed in subjects infected with SARS-COV-2.
Statistical analysis
For the statistical analysis, data management and
data visualization, the IBM Statistical Package for the Social
Sciences Statistics 25 (SPSS 25; IBM Corp.) was used. To interpret
the data, paired samples t-tests were performed, in which a
one-sided P-value of 0.500 (in the YES paired-samples test)
typically indicates that the observed data do not differ
significantly from the null hypothesis. In statistical hypothesis
testing, a P-value represents the probability of obtaining results
as extreme or more extreme than the ones observed, assuming that
the null hypothesis is true (16). A
P-value of 0.500 suggests that the data analyzed do not provide
strong evidence against the null hypothesis. In practical terms,
this means that there is a lack of sufficient evidence to reject
the null hypothesis in favor of hypothesis 1.
Results
Demographic characteristics of the
subjects in the study groups
As shown in Table I,
the age of the subjects varied from 23 to 88 years, with a mean age
of 58 years in the COVID-19 group. In the control group, the age of
the subjects ranged from 20 to 77 years, with a mean age of 47
years. The sex distribution in the COVID-19 group was 1/1, while
the control group was mainly formed by females (77%). In both
groups, the majority of the subjects were from urban areas (57.90%
in the COVID-19 group and 56.88% in the control group). In the
COVID-19 group, 28 (54%) subjects had completed secondary education
and only 24 (46%) patients had a higher education, while in the
control group, the majority of individuals had a higher education
(69%).
 | Table IDemographic characteristics of the
patients in the COVID-19 and control groups. |
Table I
Demographic characteristics of the
patients in the COVID-19 and control groups.
| | COVID-19 group | Control group |
|---|
| Patient no. | Age, years | Sexa |
Urban/ruralb |
Educationc | Age, years | Sexa |
Urban/ruralb |
Educationc |
|---|
| 1 | 65 | 2 | 1 | 1 | 20 | 1 | 1 | 1 |
| 2 | 65 | 2 | 1 | 2 | 20 | 1 | 1 | 2 |
| 3 | 35 | 2 | 1 | 1 | 25 | 2 | 2 | 2 |
| 4 | 37 | 1 | 1 | 2 | 26 | 2 | 1 | 2 |
| 5 | 42 | 2 | 1 | 2 | 27 | 2 | 1 | 2 |
| 6 | 63 | 1 | 1 | 2 | 28 | 1 | 1 | 1 |
| 7 | 62 | 2 | 2 | 1 | 33 | 2 | 1 | 2 |
| 8 | 72 | 1 | 2 | 1 | 35 | 1 | 1 | 2 |
| 9 | 75 | 1 | 1 | 1 | 35 | 2 | 1 | 2 |
| 10 | 71 | 1 | 1 | 1 | 36 | 2 | 1 | 1 |
| 11 | 81 | 1 | 1 | 2 | 37 | 1 | 1 | 2 |
| 12 | 59 | 1 | 2 | 1 | 38 | 2 | 1 | 1 |
| 13 | 71 | 2 | 1 | 1 | 39 | 1 | 1 | 2 |
| 14 | 52 | 2 | 1 | 2 | 40 | 2 | 1 | 1 |
| 15 | 59 | 1 | 1 | 2 | 40 | 2 | 1 | 2 |
| 16 | 61 | 1 | 1 | 1 | 41 | 2 | 1 | 2 |
| 17 | 46 | 2 | 2 | 2 | 43 | 2 | 1 | 1 |
| 18 | 51 | 2 | 1 | 1 | 43 | 2 | 1 | 2 |
| 19 | 64 | 2 | 1 | 1 | 44 | 2 | 1 | 2 |
| 20 | 55 | 1 | 1 | 1 | 44 | 2 | 1 | 2 |
| 21 | 68 | 2 | 1 | 1 | 44 | 2 | 1 | 2 |
| 22 | 83 | 1 | 1 | 2 | 44 | 2 | 1 | 2 |
| 23 | 77 | 1 | 1 | 1 | 44 | 2 | 2 | 2 |
| 24 | 68 | 2 | 1 | 1 | 44 | 2 | 2 | 2 |
| 25 | 54 | 1 | 1 | 2 | 45 | 2 | 1 | 2 |
| 26 | 49 | 2 | 2 | 2 | 46 | 2 | 1 | 2 |
| 27 | 24 | 1 | 1 | 1 | 47 | 2 | 1 | 2 |
| 28 | 74 | 2 | 1 | 2 | 47 | 2 | 1 | 2 |
| 29 | 54 | 2 | 1 | 2 | 48 | 1 | 1 | 2 |
| 30 | 88 | 2 | 1 | 1 | 49 | 1 | 2 | 1 |
| 31 | 53 | 1 | 1 | 1 | 50 | 2 | 1 | 2 |
| 32 | 23 | 1 | 1 | 2 | 51 | 2 | 1 | 2 |
| 33 | 70 | 2 | 1 | 1 | 51 | 2 | 1 | 2 |
| 34 | 60 | 2 | 1 | 1 | 51 | 2 | 1 | 2 |
| 35 | 29 | 2 | 1 | 2 | 52 | 1 | 1 | 1 |
| 36 | 55 | 2 | 1 | 2 | 52 | 2 | 1 | 2 |
| 37 | 45 | 1 | 1 | 2 | 53 | 1 | 1 | 2 |
| 38 | 75 | 1 | 1 | 1 | 53 | 2 | 1 | 2 |
| 39 | 48 | 2 | 1 | 1 | 53 | 2 | 1 | 2 |
| 40 | 33 | 1 | 1 | 2 | 55 | 2 | 1 | 1 |
| 41 | 61 | 2 | 1 | 1 | 56 | 2 | 2 | 2 |
| 42 | 56 | 2 | 1 | 2 | 59 | 2 | 2 | 1 |
| 43 | 67 | 1 | 1 | 1 | 60 | 2 | 1 | 1 |
| 44 | 54 | 2 | 1 | 2 | 60 | 2 | 1 | 2 |
| 45 | 36 | 1 | 1 | 1 | 62 | 2 | 1 | 2 |
| 46 | 64 | 2 | 1 | 2 | 70 | 2 | 1 | 1 |
| 47 | 58 | 1 | 1 | 2 | 72 | 2 | 1 | 1 |
| 48 | 71 | 1 | 1 | 1 | 74 | 1 | 1 | 1 |
| 49 | 62 | 1 | 1 | 2 | 74 | 1 | 1 | 2 |
| 50 | 65 | 2 | 1 | 2 | 74 | 2 | 1 | 2 |
| 51 | 64 | 1 | 1 | 1 | 75 | 2 | 1 | 1 |
| 52 | 63 | 1 | 1 | 1 | 77 | 2 | 1 | 1 |
| Mean | 58.4 | | | | 47.81 | | | |
| Total | | 26 (M) | 47 (U) | 28 (S) | | 12 (M) | 46 (U) | 6 (S) |
| | | 26 (F) | 5 (R) | 24 (H) | | 40 (F) | 6 (R) | 36 (H) |
| Variance | 214.91 | | | | 201.6 | | | |
| Std. Dev. | 14.66 | | | | 14.19 | | | |
Incidence of oropharyngeal
manifestations before the SARS-CoV-2 pandemic
The oropharyngeal manifestations that were analyzed
in the group of subjects diagnosed with SARS-CoV-2, compared to the
group of uninfected subjects at the time of data collection are
presented in Table II.
 | Table IIOropharyngeal manifestations at
baseline. |
Table II
Oropharyngeal manifestations at
baseline.
| | COVID-19 group
(n=52) | Control group
(n=52) |
|---|
| Pathogens and
symptoms | No. of
patients | % | No. of
patients | % |
|---|
| Candida | 19 | 36.5 | 14 | 26.9 |
| Herpes | 21 | 40.4 | 16 | 30.8 |
| Thrush | 24 | 46.2 | 20 | 38.5 |
| Glossitis | 1 | 1.9 | 7 | 13.5 |
| Tonsillitis | 16 | 30.8 | 31 | 59.6 |
| Pharyngitis | 23 | 44.2 | 33 | 63.5 |
| Oral cavity
pain | 39 | 75.0 | 36 | 69.2 |
| Bleeding | 18 | 34.6 | 39 | 57.7 |
| Periodontal
pockets | 6 | 11.5 | 13 | 25.0 |
| Abscess | 8 | 15.4 | 14 | 26.9 |
| Facial and oral
lesions | 12 | 23.1 | 7 | 13.5 |
| Teeth color
changes | 16 | 69.2 | 28 | 53.8 |
| Recurrence | 7 | 13.5 | 6 | 11.5 |
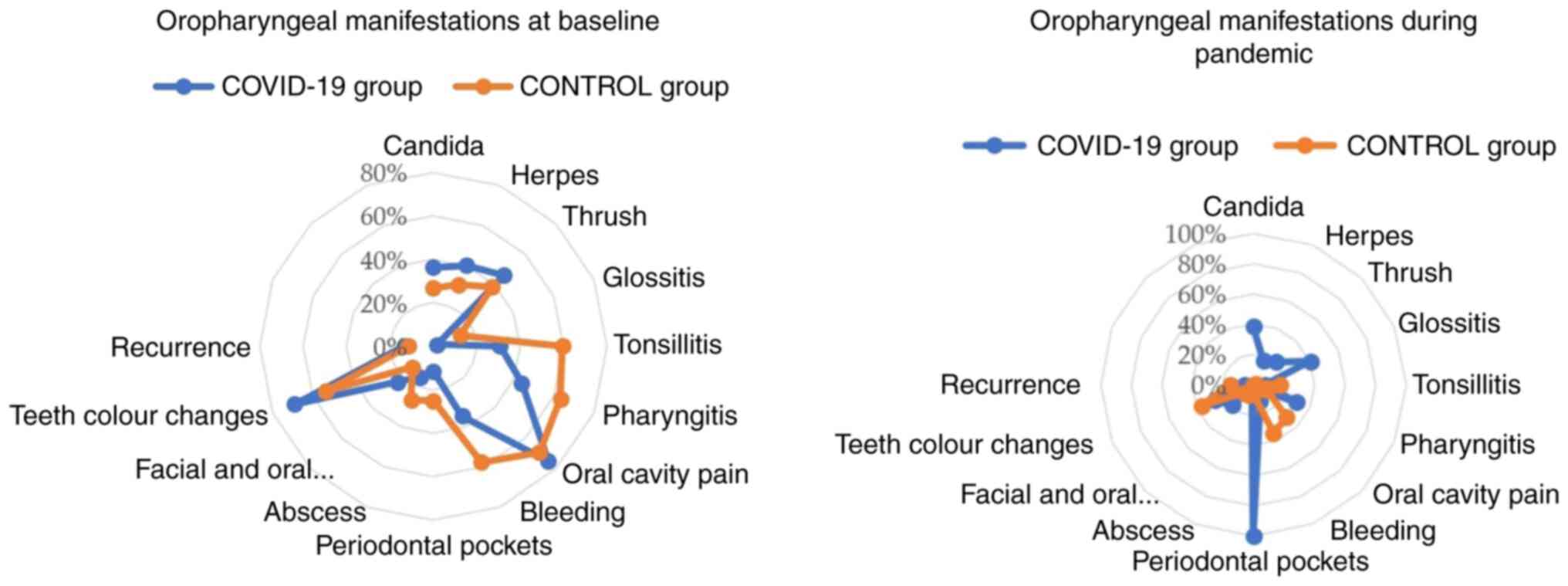
Among the cohort of patients from the COVID-19 group
examined in the present study (n=52), a proportion (mean, 16.15)
displayed oral manifestations. Specifically, 75% of the patients in
the COVID-19 group described oral cavity pain, and 69% of these
patients had changes in teeth color or dental caries. A notable
percentage of patients reported Candida (36.5%), herpes
(40.4%), thrush (46.2%), tonsillitis (30.8%), pharyngitis (44.2%)
and bleeding (34.6%). However, only 1.9% of patients in the
COVID-19 group reported glossitis, and a small proportion of
patients reported periodontal pockets (11.5%), abscesses (15.4%),
other facial and oral lesions (23.1%) and recurrence of other
oropharyngeal manifestations (13.5%) (Table II).
In the control group, the overall oropharyngeal
manifestations were less when compared with those in the COVID-19
group, apart from glossitis (13.5%), tonsillitis (59.6%),
pharyngitis (63.5%), bleeding (57.7%), periodontal pockets (25%)
and abscesses (26.9%) (Table
II).
The overall mean for the presence of oropharyngeal
signs and symptoms for both groups was less when compared to the
absence of these symptoms, with 16.15 (YES) vs. 35.85 (NO), and
20.31 (YES) vs. 32.38 (NO) for the COVID-19 group and CONTROL
group, respectively (Table
III).
 | Table IIIStatistical data for the
oropharyngeal manifestations before the pandemic. |
Table III
Statistical data for the
oropharyngeal manifestations before the pandemic.
| Group | Minimum | Maximum | Mean | Std. deviation |
|---|
| COVID-19 group | | | | |
|
Yes | 1 | 39 | 16.15 | 9.856 |
|
No | 13 | 51 | 35.85 | 9.856 |
| Control group | | | | |
|
Yes | 6 | 39 | 20.31 | 11.693 |
|
No | 16 | 46 | 32.38 | 10.720 |
Table IV presents
the results of the paired samples t-test, which is a statistical
procedure used to determine whether there is a significant
difference between the means of two related groups (16). In numerous statistical tests, a
common significance level (alpha) is set at 0.05. If the one-sided
f is greater than alpha (i.e., P>0.05), it is typically
considered non-significant, and it would fail to reject the null
hypothesis (17). In this case, in
the YES group, there was a P-value of 0.500, which suggests that
the observed data do not exhibit a statistically significant
deviation from the null hypothesis.
 | Table IVData from paired samples t-test:
Before the COVID-19 pandemic. |
Table IV
Data from paired samples t-test:
Before the COVID-19 pandemic.
| | Significance |
|---|
| Pairs | Test type | Difference in
proportions | One-sided
P-value | Two-sided
P-value |
|---|
| Yes for COVID-19
and control groups | Mid-P-value
adjusted binomial | 0.001 | 0.500 | 1.000 |
| No for COVID-19 and
control groups | Mid-P-value
adjusted binomial | -0.154 | 0.188 | 0.376 |
A P-value of 0.188 (in the NO paired samples test)
is a numerical value that results from the statistical hypothesis
test performed. In hypothesis testing, the P-value represents the
probability of obtaining results as extreme or more extreme than
the ones observed, assuming that the null hypothesis is true
(18). In this case, a P-value of
0.188 suggests that, if the null hypothesis were true, there is an
~18.8% chance of observing the data or results that were obtained
in the study. In the NO paired samples test, the one-sided P-value
is greater than alpha (i.e., P>0.05) also, which means that the
null-hypothesis cannot be rejected.
Oropharyngeal manifestations in the
COVID-19 group and in the control group during the pandemic
The findings of pathogens and symptoms of
individuals recruited in the present study during the pandemic are
presented in Table V. The results
will be later compared with those before the outbreak. In the
COVID-19 cohort, all patients presented periodontal pockets, a
notable proportion of patients (40.4%) reported glossitis, while
38.5% had Candida. Some of participants indicated the
presence of herpes (17.3%), thrush (21.2%), pharyngitis (30.8%),
and bleeding (11.5%). On the other hand, a mere 1.9% of patients in
the COVID-19 cohort indicated the presence of abscesses, while a
very modest percentage of patients reported tonsillitis (7.7%),
oral cavity pain (3.8%) and recurrence of oropharyngeal
manifestations (5.8%).
 | Table VOropharyngeal manifestations in the
COVID-19 group and control group during the pandemic. |
Table V
Oropharyngeal manifestations in the
COVID-19 group and control group during the pandemic.
| | COVID-19 group
(n=52) | Control group
(n=52) |
|---|
| Pathogens and
symptoms | No. of
patients | % | No. of
patients | % |
|---|
| Candida | 20 | 38.5 | 0 | 0 |
| Herpes | 9 | 17.3 | 0 | 0 |
| Thrush | 11 | 21.2 | 0 | 0 |
| Glossitis | 21 | 40.4 | 1 | 1.9 |
| Tonsillitis | 4 | 7.7 | 9 | 17.3 |
| Pharyngitis | 16 | 30.8 | 5 | 9.6 |
| Oral cavity
pain | 2 | 3.8 | 16 | 30.8 |
| Bleeding | 6 | 11.5 | 18 | 34.6 |
| Periodontal
pockets | 52 | 100 | 3 | 5.8 |
| Abscess | 1 | 1.9 | 4 | 7.7 |
| Facial and oral
lesions | 10 | 19.2 | 4 | 7.7 |
| Teeth color
changes | 14 | 26.9 | 19 | 36.5 |
| Recurrence | 3 | 5.8 | 8 | 15.4 |
As regards the control group, the prevalence of
oropharyngeal symptoms was generally lower compared with that in
the COVID-19 group, apart from oral cavity pain (30.8%),
tonsillitis (17.3%), bleeding (34.6%), teeth color changes (36.5%),
recurrence (15.4%) and abscesses (7.7%) (Table V).
As demonstrated in Table
VI, within the sample of patients diagnosed with COVID-19, a
fraction of these patients, with a mean value of 13, had
oropharyngeal symptoms.
 | Table VIStatistical data for oropharyngeal
manifestations during the pandemic. |
Table VI
Statistical data for oropharyngeal
manifestations during the pandemic.
| Group | Minimum | Maximum | Mean | Std. deviation |
|---|
| COVID-19 group | | | | |
|
Yes | 1 | 52 | 13.00 | 13.441 |
|
No | 0 | 51 | 39.00 | 13.441 |
| Control group | | | | |
|
Yes | 0 | 19 | 6.69 | 6.897 |
|
No | 33 | 52 | 45.31 | 6.897 |
As aforementioned, a one-sided P-value of 0.500
generally suggests that there is no sufficient evidence to support
the claim that the observed data deviate considerably from the null
hypothesis (as shown in Table
VII). The P-value in statistical hypothesis testing is a
measure of the possibility of generating outcomes that are more
likely than the observed data, under the assumption that the null
hypothesis is valid (19). As shown
in Table VII, a P-value of 0.500
was obtained for pairs, and this result indicates that the analyzed
data do not provide substantial evidence to reject the null
hypothesis. From a practical standpoint, it could be concluded that
there is insufficient evidence to reject the null hypothesis in
favor of an alternative hypothesis.
 | Table VIIData from paired samples t-test:
During the COVID-19 pandemic. |
Table VII
Data from paired samples t-test:
During the COVID-19 pandemic.
| | Significance |
|---|
| Pairs | Test type | Difference in
proportions | One-sided
P-value | Two-sided
P-value |
|---|
| Yes for COVID-19
and control groups | Mid-P-value
adjusted binomial | 0.001 | 0.500 | 1.000 |
| No for COVID-19 and
control groups | Mid-P-value
adjusted binomial | 0.001 | 0.500 | 1.000 |
As aforementioned, in several statistical tests, it
is expected to establish a significant threshold (alpha) of
0.05(16). If the P-value for a
one-sided test exceeds the predetermined significance level (i.e.,
P>0.05), it is generally regarded as statistically
non-significant. Consequently, the null hypothesis would not be
rejected (18).
Analysis of gustatory and olfactory
alterations between the group of patients with COVID-19 and the
control group
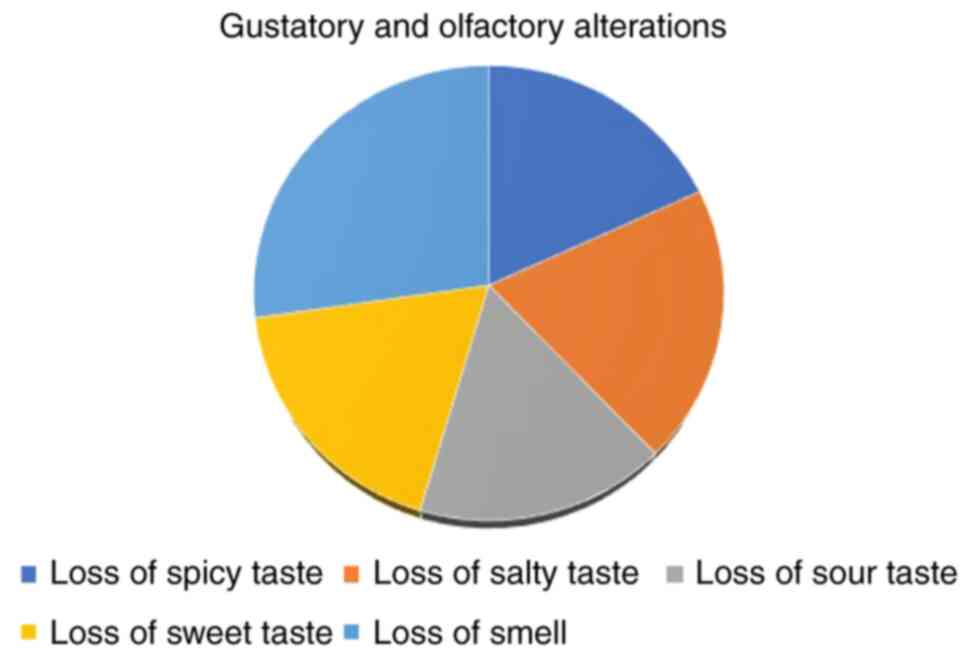
Of the 52 subjects in the COVID-19 group, 12 (23%)
indicated an altered perception for spicy taste, while no subjects
in the control group reported this change (Table VIII). A total of 13 (25%) patients
reported a change in the perception of salty taste, compared to no
subjects in the control group. In addition, 11 (21.2%) patients
reported a change in the perception for sour taste, compared to no
subjects in the control group, and 12 (23%) patients indicated an
altered perception for sweet taste, while again, no subjects in the
control group reported this change. A further 18 (34.6%) patients
from the COVID-19 group reported changes in their sense of smell,
compared to no subjects in the control group.
 | Table VIIIGustatory and olfactory alterations
observed in the present study. |
Table VIII
Gustatory and olfactory alterations
observed in the present study.
| | COVID-19 group
(n=52) | Control group
(n=52) |
|---|
| Alteration | No. of
patients | % | No. of
patients | % |
|---|
| Loss of spicy
taste | 12 | 23 | 0 | 0 |
| Loss of salty
taste | 13 | 25 | 0 | 0 |
| Loss of sour
taste | 11 | 21.2 | 0 | 0 |
| Loss of sweet
taste | 12 | 23 | 0 | 0 |
| Loss of smell | 18 | 34.6 | 0 | 0 |
As demonstrated in Table
IX, the mean number of patients that presented changes in taste
and smell is lower when compared to that patients who did not
experience these symptoms. In the present study, a decline in
olfactory and gustatory abilities was observed among around a
quarter of those diagnosed with COVID-19, in comparison to the ones
that did not report these changes, and when it comes to the control
group. Between 21 to 25% of patients reported experiencing either
olfactory or gustatory dysfunction, as per their own accounts. The
findings of the pathogens and symptoms of the individuals recruited
in the present study before and during the pandemic are illustrated
in Fig. 1.
 | Table IXStatistical data for gustatory and
olfactory alterations in the COVID-19 group. |
Table IX
Statistical data for gustatory and
olfactory alterations in the COVID-19 group.
| COVID-19 group | Minimum | Maximum | Mean | Std. deviation |
|---|
| Yes | 11 | 18 | 13.00 | 2.915 |
| No | 34 | 41 | 39.00 | 2.915 |
Between 21 to 25% of patients reported experiencing
either olfactory or gustatory dysfunction, and 34.6% reported loss
of smell. As demonstrated in Table
X, of the 52 patients with COVID-19 who were questioned and
evaluated, 32 patients presented with severe forms of infection, 16
with moderate forms and only 4 patients presented with the mild
form of COVID-19. Of these patients, 51 presented secondary
diagnoses of which: 42 patients had respiratory failure, 23
patients had hypertension, 29 patients had liver diseases, 10
patients had diabetes mellitus. Of the 52 patients, 47 (90.4%) had
cortisone-containing medications in their treatment regimen
(Table XI).
 | Table XSeverity of COVID-19 infection. |
Table X
Severity of COVID-19 infection.
| Severity | No. of
patients | % |
|---|
| Severe form | 32 | 61.5 |
| Moderate form | 16 | 30.8 |
| Mild form | 4 | 7.7 |
| Total | 52 | 100 |
 | Table XIGeneral pathological data of the
patients in the COVID-19 group. |
Table XI
General pathological data of the
patients in the COVID-19 group.
| Variable | No. of
patients | % |
|---|
| Secondary
diagnosis | | |
|
Yes | 51 | 98.1 |
|
No | 1 | 1.9 |
| Respiratory
failure | | |
|
Yes | 42 | 80.8 |
|
No | 10 | 19.2 |
| Hipertension | | |
|
Yes | 23 | 44.2 |
|
No | 29 | 55.8 |
| Chronic renal
disease | | |
|
Yes | 6 | 11.5 |
|
No | 46 | 88.5 |
| Obesity | | |
|
Yes | 8 | 15.4 |
|
No | 44 | 84.6 |
| Liver diseases | | |
|
Yes | 29 | 55.8 |
|
No | 23 | 44.2 |
| Anxiety | | |
|
Yes | 2 | 3.8 |
|
No | 50 | 96.2 |
| Autoimune
diseases | | |
|
Yes | 7 | 13.5 |
|
No | 45 | 86.5 |
| Cancers | | |
|
Yes | 5 | 9.6 |
|
No | 47 | 90.4 |
| Diabetes | | |
|
Yes | 10 | 19.2 |
|
No | 42 | 80.8 |
| Cortisone-based
treatment | | |
|
Yes | 47 | 90.4 |
|
No | 5 | 9.6 |
The gustatory and olfactory alterations of the
patients in the COVID-19 group are illustrated in Fig. 2. It can be seen that the loss of
sweet taste and loss of smell combined, comprise almost half of the
gustatory and olfactory variations.
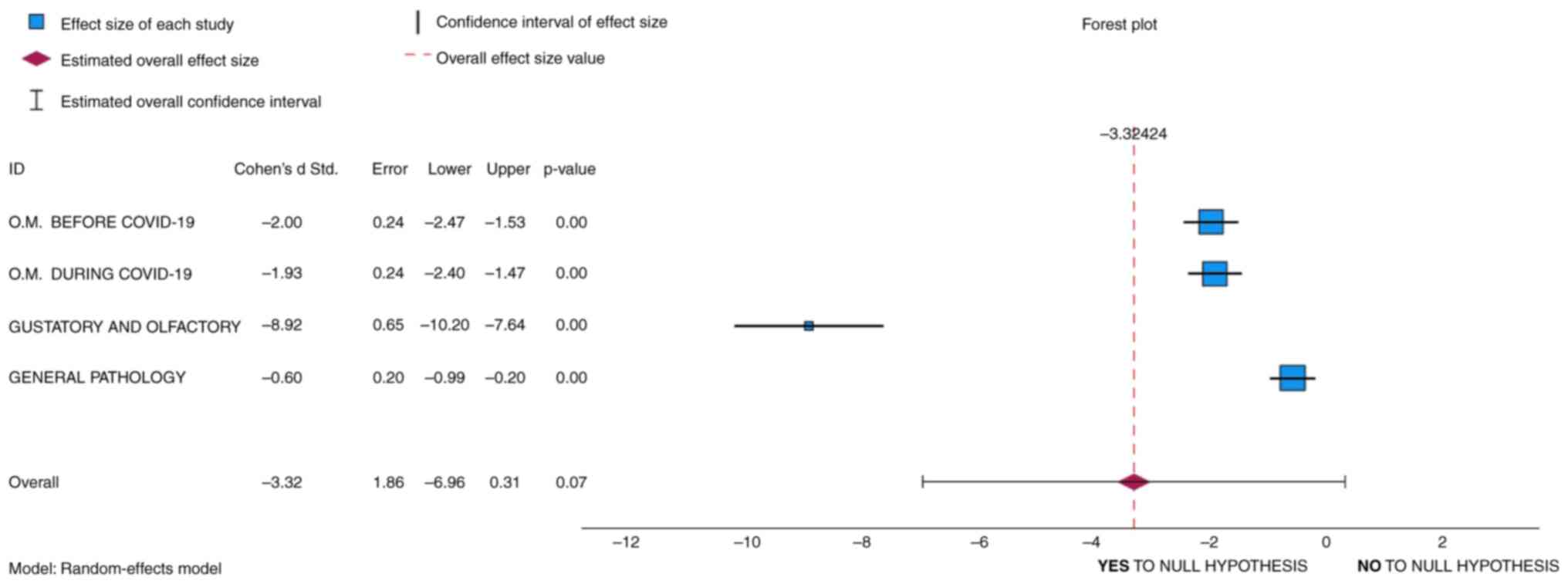
Hypothesis testing: Forest plot
The hypothesis was examined using a paired samples
t-test (Tables III and VI). Other statistical analyses were also
conducted to test the hypotheses. A we forest plot was created,
which is not typically used as a tool for directly testing
hypotheses in the same manner that statistical tests, such as like
t-tests, Chi-squared tests, or regression analyses are used
(20). However, forest plots can
indirectly inform hypothesis testing by providing a visual
representation of the individual study results and the summary
effect size (21). The forest plot
also includes a summary effect size, often represented as a
diamond. This summary effect size is calculated by pooling the
results of all included studies (22). The null hypothesis in this case may
be that the summary effect size is equal to zero or has no
practical significance.
The forest plot included in Fig. 3 demonstrates a summary effect size,
represented with a red diamond. Thus, if the summary effect size
(the diamond) includes zero within its confidence interval, it can
be concluded that there is no statistically significant effect, and
the null hypothesis that there is no effect cannot be rejected
(23). As shown in Fig. 3, although the diamond demonstrates an
overall effect of -3.31, the 0 value is indeed within the
confidence interval; thus, the null hypothesis cannot be
rejected.
In addition, when Cohen's d is negative (i.e., in
Fig. 3: -2, -1.93, -8.92, -0.6), it
means that the COVID-19 group when compared to the control group
has a lower mean or effect size. In other words, the observed
effect goes in the opposite direction of what hypothesis 1
suggests, and that is a higher incidence of oropharyngeal
manifestations is assumed in subjects infected with SARS-COV-2.
After conducting this analysis, it was noted that a higher
incidence of oropharyngeal manifestations is assumed in subjects
infected with SARS-COV-2 compared to the control group.
An overall effect size of -3.31 suggests that, on
average, the variables included in the analysis have a significant
negative effect. However, since the overall effect is not exactly
on 0, some statistical changes can be suggested in favor of
hypothesis 1, although these are not sufficient to admit it as
being correct. Since the null hypothesis often posits that there is
no significant difference between groups or no effect of an
intervention, and the result is not 0, it can then be concluded
that, although the means of the COVID-19 and control groups are not
equal, the null hypothesis can be accepted, as demonstrated.
Cortisol and antiviral treatment
Corticosteroids are often used in the treatment of
patients with COVID-19 with moderate to severe symptoms (24). Anosmia and olfactory symptoms in
patients with COVID-19 are considered to be related to inflammation
and damage to the olfactory nerves or receptors. These symptoms can
vary in severity and duration among individuals (25).
The results of the present study demonstrated that
47 individuals (90.4%) in the COVID-19 group were receiving
cortisone-based treatment (Table
XI). Although corticosteroids may have an indirect impact on
oropharyngeal symptoms by enhancing respiratory function, they are
not specifically designed to address symptoms occurring in the
throat or mouth (26).
The study by Richman and Nathanson (27) demonstrated that antiviral treatments
are designed to target the replication and spread of the virus
within the body. While they may aid in reducing the overall viral
load and symptoms associated with COVID-19, the extent to which
they can address specific symptoms, such as anosmia may vary
(4). At present, anosmia, a
condition characterized by the inability to perceive odors, mostly
attributed to COVID-19 infection, may manifest as either partial or
total and exhibit either transient or permanent effects (11,24).
According to the study by Shamsundara and Jayalakshmi (25) the occurrence of anosmia is prevalent
among the majority of individuals diagnosed with COVID-19, but
often presents as a passing symptom. The same study mentioned that
those identified with the virus had a much higher likelihood of
experiencing olfactory dysfunction, with a 27-fold increase
compared to the general population (25). In a randomized control trial, Rashid
et al (26) concluded that
symptoms, including ageusia were experienced together with anosmia
in 234 individuals, accounting for 84.8% of the total participants
of the study. The same study revealed that 83% of individuals had a
complete resolution of anosmia during a period of 30 days (26). The median duration for recovery has
been found to be 13 days (26).
Hornuss et al (28) observed
that 84% of individuals diagnosed with COVID-19 had either hyposmia
or anosmia. By contrast, among the control group consisting of
uninfected individuals, none of the participants reported anosmia,
while 27% reported hyposmia (28).
However, it is important to note that anosmia can
also arise from several other factors, such as allergies, the
common cold and marked neurological impairments (4). The olfactory function serves as a
defense mechanism for the human body against potential
environmental dangers and pathogens (29). Several hypothesized causes of anosmia
have been suggested, including the blockage of the olfactory cleft,
inflammation in the nasal epithelium, the early death of olfactory
cells, alterations in olfactory cilia, injury to the olfactory
epithelium, and damage to the olfactory neurons or stem cell
neurons (30).
Discussion
The mode of transmission refers to the mechanism by
which a disease or infection is spread from one individual to
another (31). Based on the data
derived from genetic and epidemiological studies, it is evident
that the onset of the COVID-19 epidemic may be attributed to an
initial instance of zoonotic transmission, subsequently leading to
continuous human-to-human transmission (32). It is well known that the transmission
of the virus mostly occurs via the upper respiratory tract
(33). Furthermore, it is worth
noting that there exists a potential for fecal-oral transmission,
since scientific investigations have successfully detected the
presence of SARS-CoV-2 in the feces of individuals originating from
China and the USA (34).
A significant proportion of individuals had symptoms
such as fever and dry cough, with a subset of patients also
presenting with shortness of breath, exhaustion and other
non-typical manifestations, including muscular pain,
disorientation, headache, sore throat, diarrhea and vomiting
(35). In a previous study, in a
cohort of individuals who underwent a chest CT scan, the majority
of patients had bilateral pneumonia, with the prevailing patterns
being characterized by ground-glass opacity and bilateral patchy
shadows (36).
A wide range of signs and symptoms have been linked
to COVID-19, including dysgeusia and anosmia, even in cases when
respiratory symptoms are not present. Olfactory and gustatory
impairment are symptoms commonly observed in individuals diagnosed
with COVID-19 and may serve as early indicators throughout the
progression of the infection. The heightened knowledge of this fact
has the potential to promote early diagnosis and treatment, as well
as enhance vigilance in preventing viral transmission (37).
The present study demonstrated that 23% of the
patients with COVID-19 had an altered perception for sweet and
spicy taste, 25% experienced a change in the perception of salty
taste, 21.2% reported a change in the sour taste, and no subjects
in the control group reported any such changes. These results are
in accordance with those from the study by Cattaneo et al
(38), where ~45% of the patients in
the COVID-19 group had symptoms concerning taste and smell,
including the loss of olfactory or gustatory abilities. By
contrast, none of the individuals in the control group reported
experiencing these symptoms (38).
Salivary secretion is often compromised following
infection with SARS-CoV-2, leading to the prevalent manifestation
of xerostomia as the predominant oral symptom in individuals
afflicted with COVID-19(39). The
study conducted by Chen et al (39) demonstrated that xerostomia was
present in >46% of the patients examined. They did not find any
significant sex differences in the prevalence of xerostomia
(39). Individuals diagnosed with
xerostomia commonly exhibit a range of symptoms in addition to
their primary complaint of oral dryness (40). These accompanying manifestations
include a sensation of burning, altered taste perception
(dysgeusia), inflammation at the corners of the mouth (angular
stomatitis) and dysphagia (41).
Although xerostomia is not fatal, it can significantly affect the
quality of life and oral health of individuals (42).
As regards all the above, the present study revealed
that in the COVID-19 group, between 30 to 44% of patients indicated
tonsillitis, pharyngitis and oral cavity bleeding. On the other
spectrum, a mere 1.9% of patients indicated the presence of
glossitis, while ~11 to 15% reported periodontal pockets and
abscesses. As regards the control group, the prevalence of
oropharyngeal symptoms was generally lower compared to that in the
COVID-19 group, apart from glossitis, tonsillitis, pharyngitis,
hemorrhage, periodontal pockets and abscesses. These results are
similar to the findings reported by other researchers, whose study
revealed that glossitis was observed in both groups (positive and
negative RT-PCR test groups) with similar relative frequencies
(29).
It is worth noting that sialadenitis can also be
observed in patients. In the study conducted by Fisher et al
(43), the patient exhibited
clinical manifestations indicative of concurrent acute bacterial
suppurative parotitis and viral parotitis. Another study documented
three instances of parotitis associated with COVID-19, and patients
presented with unilateral ear pain and retromandibular edema, and
magnetic resonance imaging revealed the presence of intracarotid
lymphadenitis (44).
Parotitis refers to the inflammatory condition
affecting the parotid glands, which are the most often affected
major salivary glands (45). It has
the potential to be either a localized condition or as a symptom of
a broader systemic inflammation (46). Etiology may be attributed to several
factors, including duct blockage (such as sialolithiasis), the
presence of infectious agents, or inflammatory processes (Sjogren
syndrome, rheumatoid arthritis, systemic lupus erythematosus)
(47). Friedrich et al
(48) found out that certain
individuals diagnosed with COVID-19 have reported instances of
salivary gland enlargement that affected the parotid glands. Even
though the precise etiology of parotid gland enlargement in
individuals with COVID-19 is still not fully clarified;
nonetheless, it is suggested that this phenomenon may be associated
with inflammatory processes, viral replication inside the salivary
glands, or an immune-mediated reaction (49).
The present study demonstrated that infection was
also associated with several skin facial and oral manifestations.
Abscesses and other lesions accounted for 15 to 23% in the COVID-19
group and 13 to almost 27% in the control group. These slight
differences between the two groups are in line with the findings
reported in the literature. In their study, Nuno-Gonzalez et
al (50) analyzed 666 patients
over a 2-week period and concluded that 45% of them had various
mucocutaneous manifestations. Of these patients, >25% had oral
lesions, and swelling on the tongue was most commonly identified,
followed by inflammation, redness of the tongue and canker sores.
Several patients also reported a burning sensation in the mouth or
a loss of taste. However, the oral symptoms proved to be temporary
(50). These symptoms are not
surprising, as it is common for viruses to cause both skin rashes
and changes in the mucous membranes, such as ulcers or spots in the
oral cavity (51).
According to experts, it is likely that these cases
of tongue-COVID are not reported, as in some situations, doctors do
not ask patients to open their mouths to examine their oral cavity,
as such an examination increases the risk of infection. In
addition, patients usually keep the mask on their face, as this
protective measure is crucial to reduce the spread of the virus
(52). In the present study, a
reduction in olfactory and gustatory capacities individuals
diagnosed with COVID-19, as compared to those who did not report
such alterations. It was found that a considerable proportion of
patients, ranging from 21 to 25%, indicated the presence of either
olfactory or gustatory impairment. These findings are similar to
another literature review that analyzed 10 studies describing
olfactory dysfunction in a sample size of 1,627 individuals
(53). That study (53) revealed a prevalence rate of >52%
among patients diagnosed with COVID-19. In the same research, Tong
et al (53) further examined
nine studies to assess the occurrence of gustatory dysfunction,
with a sample size of 1,390 individuals. This analysis also
revealed a prevalence rate of almost 44% among the study group. The
researchers performed subgroup analyses on studies that assessed
olfactory dysfunction using both non-validated and validated
instruments (53).
Further research led Amorim Dos Santos et al
(54) to observe that ~21% of
individuals diagnosed with COVID-19 had developed various oral
mucosal lesions, which is a lower prevalence compared to dysgeusia
and xerostomia. In that study, a significant proportion of
individuals displayed oral mucosal lesions at ~10 days following
infection. Subsequently, these patients commonly received treatment
involving photo-biomodulation therapy and/or antiviral medication
within a timeframe of 1 to 3 weeks (54). In addition to this conclusion,
Iranmanesh et al (55)
mentioned that individuals who are elderly, or those that have been
hospitalized for an extended period of time, or exhibit poor
hygiene practices, have diabetes, or are at an increased risk of
developing oral mucosal lesions.
Likewise, these individuals often experience more
severe, persistent and extensive oral lesions. These findings
present a high prevalence of oral manifestations, including lesions
such as aphthous, herpes, Kawasaki, plaque, fungal infections such
as candidiasis and mucormycosis, mucosal petechiae, ulcers related
to herpes simplex virus reactivation, oral herpes zoster,
gingivitis and bleeding gums (56-59).
The present study had certain limitations which
should be mentioned. The younger and more digitally engaged
demographic has a greater propensity for social interaction, and
this subgroup appears to be less susceptible to the impacts of
COVID-19 in comparison to older cohorts, who have higher rates of
illness and death (60).
Cross-sectional surveys may be susceptible to bias
due to several factors, including the effect of confounding
variables, variations in the timing of patient assessments in
relation to their exposure, and potential reporting bias (61). In these investigations, such as the
present study, the researchers are primarily presenting patient
characteristics and their corresponding symptoms, rather than
focusing on treatments, interventions, and their following impacts
or outcomes (62).
This observational approach may contribute to the
reduction of observer bias. When conducting research that relies on
historical data obtained from patients, there exists a potential
for memory bias and the under-reporting or errors of symptoms,
particularly in relation to the timing and duration of symptoms
(63).
In conclusion, impairment in olfactory and gustatory
functions is often observed in individuals diagnosed with COVID-19,
perhaps serving as early markers of disease progression. These
manifestations were more frequent in the COVID-19 group, during
SARS-CoV-2 infection compared to the control group. Compared to the
control group, the COVID-19 group typically had a decreased
prevalence of oropharyngeal symptoms, apart from oral cavity
discomfort (30.8%), tonsillitis (17.3%), bleeding (34.6%), tooth
colour changes (36.5%), recurrence (15.4%), and abscesses (7.7%).
These symptoms were not associated with the severity of the
disease, nor with the administration of cortisone therapy or
antiviral therapy.
These manifestations may be an early sign of the
disease and, taken into account, could lead to an early diagnosis,
which would limit the transmission of infection in dental
offices.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ATC and CSC were involved in the conceptualization
of the study. MR, CI and CSC were involved in data curation. AC,
ANT and IMD were involved in formal analysis. ATC, SI, AH and MN
were involved in the investigative aspects of the study. ATC, MR
and GMB were involved in the study methodology. CI, GMB and IMD
were involved in project administration. MR, ATC, MR, AC, MN and
IMD supervised the study. ATC, CI, GMB and CSC were involved in
data validation. MR, SI, AH, CSC and ANT were involved in
visualization (the creation of the figures). ATC, SI, MN and IMD
were involved in the writing of the original draft. ATC, AH, CI,
ANT and IMD were involved in the writing, reviewing and editing of
the manuscript. All authors have read and approved the final
manuscript. ATC and ANT confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and was approved by the Ethics
Committee of Hospital for Infectious Diseases, 100 Ferdinand
Boulevard, Constanta, Romania 900178 (protocol code 2 and date of
approval February 24, 2021). Informed consent for publication of
their data was obtained from all individual participants included
in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Muralidar S, Ambi SV, Sekaran S and
Krishnan UM: The emergence of COVID-19 as a global pandemic:
Understanding the epidemiology, immune response and potential
therapeutic targets of SARS-CoV-2. Biochimie. 179:85–100.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
World Health Organization (WHO):
Coronavirus disease (COVID-19). WHO, Geneva, 2023. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
|
|
3
|
Zhang B, Wu Q, Yin L, Zhang J, Gao W, Chen
H and Ni H: Current diagnostic and therapeutic approaches for
severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and
the role of nanomaterial-based theragnosis in combating the
pandemic. Nanotechnol Rev. 12(20230155)2023.
|
|
4
|
Rastogi M, Pandey N, Shukla A and Singh
SK: SARS coronavirus 2: From genome to infectome. Respir Res.
21(318)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dhama K, Khan S, Tiwari R, Sircar S, Bhat
S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK and
Rodriguez-Morales AJ: Coronavirus disease 2019-COVID-19. Clin
Microbiol Rev. 33:e00028–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y,
Hou C, Wang H, Liu J, Yang D, et al: Role of angiotensin-converting
enzyme 2 (ACE2) in COVID-19. Crit Care. 24(422)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang H, Penninger JM, Li Y, Zhong N and
Slutsky AS: Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2
receptor: Molecular mechanisms and potential therapeutic target.
Intensive Care Med. 46:586–590. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
El Hassan M, Assoum H, Bukharin N, Al
Otaibi H, Mofijur M and Sakout A: A review on the transmission of
COVID-19 based on cough/sneeze/breath flows. Eur Phys J Plus.
137(1)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vuille-Dit-Bille RN, Liechty KW, Verrey F
and Guglielmetti LC: SARS-CoV-2 receptor ACE2 gene expression in
small intestine correlates with age. Amino Acids. 52:1063–1065.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Reich P and Elward A: Infection prevention
during the coronavirus disease 2019 pandemic. Infect Dis Clin North
Am. 36:15–37. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Popa MF, Deacu S, Neculai-Cândea L, Radu
S, Pricop Ș, Mocanu L, Gheju A and Tăbîrcă DD: Virus-associated
hemophagocytic lymphohistiocystosis-the severe course expression in
SARS-COV-2 infection? Rom J Leg Med. 28:1–7. 2020.
|
|
12
|
Centers for Disease Control and Prevention
(CDC): Symptoms of COVID-19. CDC, Atlanta, GA, 2020. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
|
|
13
|
Nicolae M, Mihai CM, Chisnoiu T, Balasa
AL, Frecus CE, Mihai L, Lupu VV, Ion I, Pantazi AC, Nelson Twakor
A, et al: Immunomodulatory effects of vitamin D in respiratory
tract infections and COVID-19 in children. Nutrients.
15(3430)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mocanu A, Lazureanu VE, Marinescu AR, Cut
TG, Laza R, Rusu LC, Marza AM, Nelson-Twakor A, Negrean RA, Popescu
IM and Mederle AO: A retrospective assessment of laboratory
findings and cytokine markers in severe SARS-CoV-2 infection among
patients of roma population. J Clin Med. 11(6777)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lin W, Gao F, Wang X, Qin N, Chen X, Tam
KY, Zhang C, Zhang M and Sha O: The oral manifestations and related
mechanisms of COVID-19 caused by SARS-CoV-2 infection. Front Cell
Neurosci. 16(1006977)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu M, Fralick D, Zheng JZ, Wang B, Tu XM
and Feng C: The differences and similarities between two-sample
T-test and paired T-test. Shanghai Arch Psychiatry. 29:184–188.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dahiru T: P-value, a true test of
statistical significance? A cautionary note. Ann Ib Postgrad Med.
6:21–26. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tanha K, Mohammadi N and Janani L:
P-value: What is and what is not. Med J Islam Repub Iran.
31(65)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bonovas S and Piovani D: On P-values and
statistical significance. J Clin Med. 12(900)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Einav S and O'Connor M: P-values and
significance: The null hypothesis that they are not related is
correct. J Crit Care. 54:159–162. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dettori JR, Norvell DC and Chapman JR:
Seeing the forest by looking at the trees: How to interpret a
meta-analysis forest plot. Global Spine J. 11:614–616.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Verhagen AP and Ferreira ML: Forest plots.
J Physiother. 60:170–173. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Meena S: Difference between Z-test and
T-test. Analytics Vidhya, 2024. https://www.analyticsvidhya.com/blog/2020/06/statistics-analytics-hypothesis-testing-z-test-t-test/.
|
|
24
|
El-Saber Batiha G, Al-Gareeb AI, Saad HM
and Al-Kuraishy HM: COVID-19 and corticosteroids: A narrative
review. Inflammopharmacology. 30:1189–1205. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shamsundara M and Jayalakshmi L:
Anosmia-an effect of COVID-19 infection-review. Indian J
Otolaryngol Head Neck Surg. 75 (Suppl 1):S815–S821. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rashid RA, Zgair A and Al-Ani RM: Effect
of nasal corticosteroid in the treatment of anosmia due to
COVID-19: A randomised double-blind placebo-controlled study. Am J
Otolaryngol. 42(103033)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Richman DD and Nathanson N: Antiviral
therapy. Viral Pathogenesis. 271–87. 2016.
|
|
28
|
Hornuss D, Lange B, Schröter N, Rieg S,
Kern WV and Wagner D: Anosmia in COVID-19 patients. Clin Microbiol
Infect. 26:1426–1427. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Deacu M, Enciu M, Nicolau AA, Bălţătescu
GI, Neculai-Cândea LS, Deacu S and Popa MF: Morphopathological
features induced by SARS-CoV-2 infection-a series of 57 autopsies.
Histol Histopathol. 38:513–524. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yale Medicine: Loss of Smell (Anosmia).
Yale University, 2022. https://www.yalemedicine.org/conditions/smell-and-taste-disorders#:~:text=What%20is%20anosmia%3F,a%20sinus%20infection%2C%20for%20example.
|
|
31
|
Rahmah L, Abarikwu SO, Arero AG, Essouma
M, Jibril AT, Fal A, Flisiak R, Makuku R, Marquez L, Mohamed K, et
al: Oral antiviral treatments for COVID-19: Opportunities and
challenges. Pharmacol Rep. 74:1255–1278. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ghanbari R, Teimoori A, Sadeghi A,
Mohamadkhani A, Rezasoltani S, Asadi E, Jouyban A and Sumner SC:
Existing antiviral options against SARS-CoV-2 replication in
COVID-19 patients. Future Microbiol. 15:1747–1758. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mocanu A, Noja GG, Istodor AV, Moise G,
Leretter M, Rusu LC, Marza AM and Mederle AO: Individual
characteristics as prognostic factors of the evolution of
hospitalized COVID-19 romanian patients: A comparative
observational study between the first and second waves based on
gaussian graphical models and structural equation modeling. J Clin
Med. 10(1958)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Termansen MB and Frische S: Fecal-oral
transmission of SARS-CoV-2: A systematic review of evidence from
epidemiological and experimental studies. Am J Infect Control.
51:1430–1437. 2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mocanu A, Lazureanu V, Cut T, Laza R,
Musta V, Nicolescu N, Marinescu A, Nelson-Twakor A, Dumache R and
Mederle O: Angiocatheter decompression on a Covid-19 patient with
severe pneumonia, pneumothorax, and subcutaneous emphysema. Clin
Lab. 68:2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Carotti M, Salaffi F, Sarzi-Puttini P,
Agostini A, Borgheresi A, Minorati D, Galli M, Marotto D and
Giovagnoni A: Chest CT features of coronavirus disease 2019
(COVID-19) pneumonia: Key points for radiologists. Radiol Med.
125:636–646. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gori A, Leone F, Loffredo L, Cinicola BL,
Brindisi G, De Castro G, Spalice A, Duse M and Zicari AM:
COVID-19-related anosmia: The olfactory pathway hypothesis and
early intervention. Front Neurol. 11(956)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cattaneo C, Pagliarini E, Mambrini SP,
Tortorici E, Mené R, Torlasco C, Perger E, Parati G and Bertoli S:
Changes in smell and taste perception related to COVID-19
infection: A case-control study. Sci Rep. 12(8192)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen L, Zhao J, Peng J, Li X, Deng X, Geng
Z, Shen Z, Guo F, Zhang Q, Jin Y, et al: Detection of SARS-CoV-2 in
saliva and characterization of oral symptoms in COVID-19 patients.
Cell Prolif. 53(e12923)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ziuzia-Januszewska L and Januszewski M:
Pathogenesis of olfactory disorders in COVID-19. Brain Sci.
12(449)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mulabbi EN, Tweyongyere R and Byarugaba
DK: The history of the emergence and transmission of human
coronaviruses. Onderstepoort J Vet Res. 88:e1–e8. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
de Oliveira WQ, De Sousa PHM and Pastore
GM: Olfactory and gustatory disorders caused by COVID-19: How to
regain the pleasure of eating? Trends Food Sci Technol.
122:104–109. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fisher J, Monette DL, Patel KR, Kelley BP
and Kennedy M: COVID-19 associated parotitis. Am J Emerg Med.
39:254.e1–254.e3. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lechien JR, Chetrit A, Chekkoury-Idrissi
Y, Distinguin L, Circiu M, Saussez S, Berradja N, Edjlali M, Hans S
and Carlier R: Parotitis-like symptoms associated with COVID-19,
France, March-April 2020. Emerg Infect Dis. 26:2270–2271.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Templer JW: Parotitis: Practice
Essentials, Background, Pathophysiology. Medscape, 2022. https://emedicine.medscape.com/article/882461-overview?form=fpf.
Accessed April 11, 2022.
|
|
46
|
Gebretsadik HG: An update on oral clinical
courses among patients with severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) infection: A clinical follow-up (a
prospective prevalent cohort) study. PLoS One.
17(e0275817)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wilson M and Pandey S: Parotitis. (Updated
2023 Jun 25). In: StatPearls (Internet). StatPearls Publishing,
Treasure Island, FL, 2024.
|
|
48
|
Friedrich RE, Droste TL, Angerer F, Popa
B, Koehnke R, Gosau M and Knipfer C: COVID-19-associated parotid
gland abscess. In Vivo. 36:1349–1353. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
V'kovski P, Kratzel A, Steiner S, Stalder
H and Thiel V: Coronavirus biology and replication: Implications
for SARS-CoV-2. Nat Rev Microbiol. 19:155–170. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nuno-Gonzalez A, Martin-Carrillo P,
Magaletsky K, Martin Rios MD, Herranz Mañas C, Artigas Almazan J,
García Casasola G, Perez Castro E, Gallego Arenas A, Mayor
Ibarguren A, et al: Prevalence of mucocutaneous manifestations in
666 patients with COVID-19 in a field hospital in Spain: oral and
palmoplantar findings. Br J Dermatol. 184:184–185. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chern A, Famuyide AO, Moonis G and Lalwani
AK: Sialadenitis: A possible early manifestation of COVID-19.
Laryngoscope. 130:2595–2597. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mocanu A, Lazureanu VE, Laza R, Marinescu
AR, Cut TG, Sincaru SV, Marza AM, Popescu IM, Herlo LF,
Nelson-Twakor A, et al: Laboratory findings and clinical outcomes
of ICU-admitted COVID-19 patients: A retrospective assessment of
particularities identified among Romanian minorities. J Pers Med.
13(195)2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tong JY, Wong A, Zhu D, Fastenberg JH and
Tham T: The prevalence of olfactory and gustatory dysfunction in
COVID-19 patients: A systematic review and meta-analysis.
Otolaryngol Head Neck Surg. 163:3–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Amorim Dos Santos J, Normando AGC,
Carvalho da Silva RL, Acevedo AC, De Luca Canto G, Sugaya N,
Santos-Silva AR and Guerra ENS: Oral manifestations in patients
with COVID-19: A living systematic review. J Dent Res. 100:141–154.
2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Iranmanesh B, Khalili M, Amiri R, Zartab H
and Aflatoonian M: Oral manifestations of COVID-19 disease: A
review article. Dermatol Ther. 34(e14578)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Orilisi G, Mascitti M, Togni L,
Monterubbianesi R, Tosco V, Vitiello F, Santarelli A, Putignano A
and Orsini G: Oral manifestations of COVID-19 in hospitalized
patients: A systematic review. Int J Environ Res Public Health.
18(12511)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Drozdzik A and Drozdzik M: Oral pathology
in COVID-19 and SARS-CoV-2 infection-molecular aspects. Int J Mol
Sci. 23(1431)2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Jacobs M and Ellis C: Social connectivity
during the COVID-19 pandemic: Disparities among medicare
beneficiaries. J Prim Care Community Health.
12(21501327211030135)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Tahmasebi E, Keshvad A, Alam M, Abbasi K,
Rahimi S, Nouri F, Yazdanian M, Tebyaniyan H, Heboyan A and
Fernandes GVO: Current infections of the orofacial region:
Treatment, diagnosis, and epidemiology. Life (Basel).
13(269)2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Faculty of Public Health: Biases and
Confounding. Health Knowledge. Faculty of Public Health, London,
2018. https://www.healthknowledge.org.uk/public-health-textbook/research-methods/1a-epidemiology/biases.
|
|
61
|
Althubaiti A: Information bias in health
research: Definition, pitfalls, and adjustment methods. J
Multidiscip Healthc. 9:211–217. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Muthyam AK, Reddy MP, Kulkarni S, Srilatha
A, Sahithi K and Satyanarayana D: Oral manifestations in COVID-19
patients: An observational study. J Family Med Prim Care.
11:1000–1005. 2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Pérez-Jardón A, Pérez-Sayáns M,
Peñamaría-Mallón M, Otero-Rey E, Velasco-Ortega E, López-López J,
Martínez-González JM and Blanco-Carrión A: Xerostomia, the
perception of general and oral health and health risk behaviours in
people over 65 years of age. BMC Geriatr. 22(982)2022.PubMed/NCBI View Article : Google Scholar
|