Introduction
Decompressive craniectomy (DC) is a surgical
procedure which as long been used with varying usefulness for the
treatment of refractory intracranial hypertension for a wide range
of pathologies (1-5).
Although the complications associated with this technique and the
functional outcomes of surviving patients have not yet been fully
determined (6,7), DC can be a lifesaving technique in the
presence of medically intractable elevations of intracranial
pressure, and may consequently increase the length of stay in
intensive care units (8).
However, the prolonged exposure of skull defects has
been associated with various neurological manifestations, including
the immediate effects of atmospheric pressure on the soft brain
tissue, obstructions and hydrodynamic changes in cerebrospinal
fluid, and modifications in cerebral blood flow and metabolism
(6,7,9,10).
Cranioplasty (CPL) is a procedure used for
reconstructing skull deficits, providing cerebral protection, and
enhancing the cosmetic effect (11).
In addition, CPL may aid in the neurological recovery of patients
due to its physiological effects on the cranial vault, allowing for
a more effective rehabilitation process (11). Nevertheless, critical clinical
questions remain, including significant post-operative morbidity,
various complications in neurological recovery and outcomes,
infections, seizures, hematomas, the influence timing has on these
factors, the selection of materials, overall cost-effectiveness and
bone graft absorption (BGA) (12,13).
Concerning the type of bone graft, above all, the
advantage of autologous as opposed to heterologous bone grafts is
that there is no rejection (14). On
the other hand, BGA is a severe complication (15). In particular, the skull bone has a
higher tendency for absorption compared with other parts of the
body. If implanted, skull graft resorption continues, and the bone
graft may break down, necessitating further surgery (15). In the literature, there are several
issues on whether early CPL, the age of the patient, or the type of
bone graft could lead to resorption (15,16).
The aim of the present retrospective study was to
confer the factors that are related to BGA and may affect the
outcomes of patients following CPL.
Patients and methods
Study design and population
The present study constitutes a single-center,
retrospective study of patients who underwent CPL. The population
of interest was defined as all patients that underwent CPL at a
local institution (University Hospital of Larissa, Larissa, Greece)
between February, 2013 and December, 2022. The Institutional Review
Board (IRB) of the University of Thessaly, Greece, and the
University Hospital of Larissa approved the study (IRB no.
2542/21-01-2021, finalized by the 28th General Assembly on January
28, 2021). Written informed consent was obtained from all included
patients or their next-of-kin before surgery, and for under-age
patients, consent was obtained from their parents or legal
guardians.
In total, of the 186 patients that underwent DC, 116
patients proceeded to the University Hospital of Larissa for CPL,
and 7 (6.0%) patients developed BGA during the follow-up. In the
final pool, 116 patients were included, and these patients were
divided into two groups. Data collection was performed, and the
data were reviewed and analyzed by two physicians (GF and CG) on
the basis of the following inclusion criteria: Patients aged >8
years old who underwent DC (for any reason) and subsequent CPL
between 2013 and 2022. Cases with incomplete medical files and
cases lost to follow-up were excluded (Fig. 1).
Clinical data
The patients were divided into two groups, namely
group A, which included patients treated with CPL who did not
develop BGA during the follow-up period, and group B, which
included those who developed BGA. These groups were identified
based on the following demographic, clinical and radiographic data
that were retrieved from the medical archives when available: Age,
sex, cause of DC [traumatic brain injury (TBI), stroke, other
neurosurgical entities that required DC, such as subarachnoid
hemorrhage, tumor, brain abscess, cerebral venous sinus thrombosis
and patients developed intracerebral hemorrhage], Glasgow Coma
Scale (GCS) and Karnofsky Performance Scale (KPS) of admission,
history of diabetes and hypertension, site of CPL [one site
fronto-temporo-parietal (FTP), bilateral FTP, bilateral frontal],
time from DC to CPL, type of bone graft (heterologous or
autologous), grafts with fractures or fragments, and peri-operative
complications such as infections and hematomas (Table I). All participants had a follow-up
period of 1 to 10 years from the day of discharge from the
hospital. Patient outcomes were evaluated using a computer
tomography (CT) scan and a complete neurological examination at 6
months, 1 year, and 3 or 6 years following discharge from the
hospital. The primary outcome was defined as neurological
deterioration, and the secondary outcomes were hospital stay and
mortality. The CPL implant material was heterologous or autologous
and cryopreserved at -83˚C and taken out to thaw at room
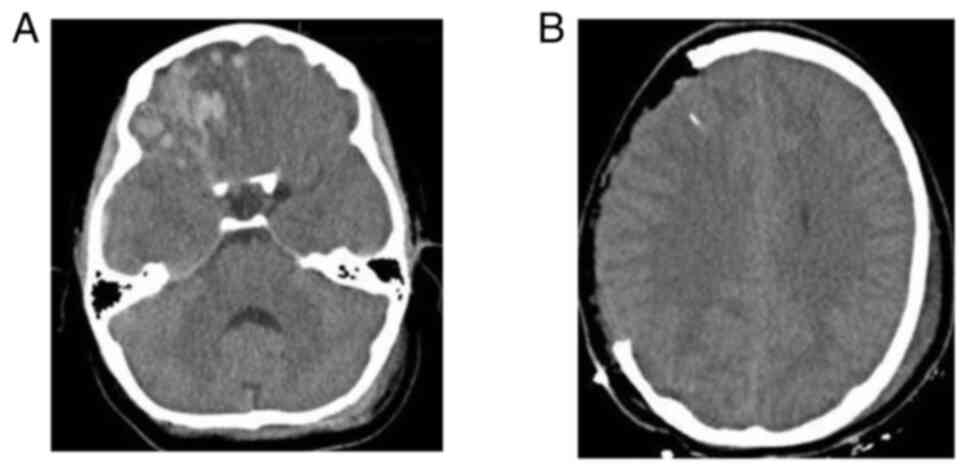
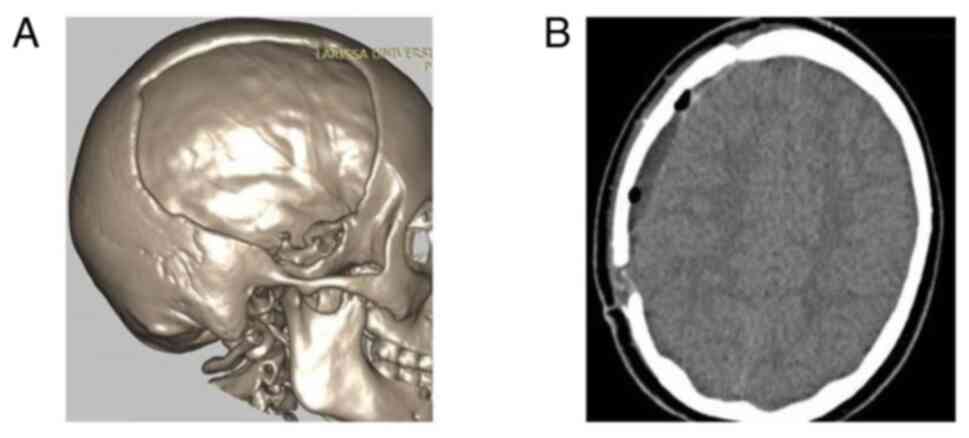
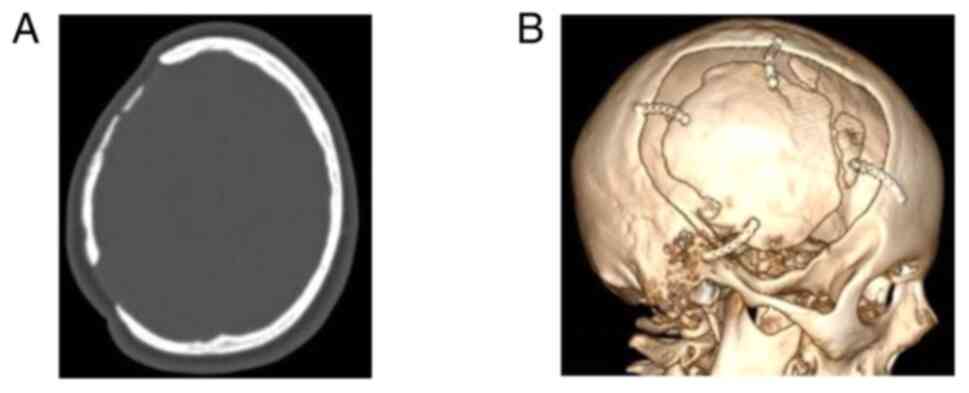
temperature 2 h before the intervention. Images of a case that was
evaluated are presented in Fig. 2,
Fig. 3 and Fig. 4.
 | Table IBaseline demographic characteristics
of the patients. |
Table I
Baseline demographic characteristics
of the patients.
| Parameters | All patients, n=116
(100%) | Group A, n=109
(93.9%) | Group B, n=7
(6.0%) | P-value |
|---|
| Age, mean ± SD
(years) | 42.5±14 | 43.7±14 | 31.1±8.7 | 0.024 |
| Sex (male), n
(%) | 77 (66.3) | 71 (61.2) | 6 (5.1) | 0.264 |
| Cause of DC | | | | |
|
TBI, n
(%) | 66 (56.8) | 63 (54.3) | 3 (2.5) | 0.439 |
|
Stroke, n
(%) | 35 (30.1) | 33 (28.4) | 2 (1.7) | 0.924 |
|
Othera,
n (%) | 15 (12.9) | 13 (11.2) | 2 (1.7) | 0.203 |
| GCS score of
admission, mean ± SD | 10.0±2.3 | 9.1±2.1 | 77.8±6.3 | 0.310 |
| KPS score of
admission, mean ± SD | 75.9±4.6 | 19.9±7 | 18.4±6 | 0.495 |
| Diabetes mellitus, n
(%) | 9 (7.7) | 9 (7.7) | 0 (0) | 0.429 |
| Hypertension, n
(%) | 17 (14.6) | 17 (14.6) | 0 (0) | 0.258 |
| Site of
cranioplasty | | | | |
|
One-site
FTP, n (%) | 99 (85.3) | 94 (81.0) | 5 (4.3) | 0.283 |
|
Bilateral
frontal, n (%) | 7 (6.0) | 7 (6.0) | 0 (0) | 0.489 |
|
Bilateral
FTP, n (%) | 10 (8.6) | 8 (6.8) | 2 (1.7) | 0.052 |
| Time from DC to
cranioplasty, mean ± SD (months) | 6.31±3.9 | 6.13±3.8 | 9.14±4.9 | 0.034 |
| Type of graft | | | | |
|
Autologous,
n (%) | 84 (72.4) | 79 (68.1) | 5 (4.3) | 0.952 |
|
Heterologous,
n (%) | 32 (27.5) | 30 (25.8) | 2 (1.7) | 0.952 |
| Grafts with
fragments or fractures, n (%) | 9 (7.7) | 9 (7.7) | 0 (0) | 0.429 |
| Peri-operative
complications | | | | |
|
Infections,
n (%) | 6 (5.1) | 6 (5.1) | 0 (0) | 0.524 |
|
Hematoma, n
(% | 9 (7.7) | 6 (5.1) | 3 (2.5) |
<0.05 |
Statistical analysis
Statistical analyses were performed using the
Statistical Package for the Social Sciences (SPSS 11; SPSS, Inc.).
The normality of the distribution of variables was assessed using
the Shapiro-Wilk test. Categorical variables were compared between
groups using the Fisher's exact test, and continuous data were
compared using the Mann-Whitney U test. Receiver operating
characteristic (ROC) analysis was used to reveal the factors that
are related to BGA and affect the outcomes of patients following
CPL. A P-value <0.05 was considered to indicate a statistically
significant difference.
Results
In total, 116 (62.3%) of the 186 patients that
underwent DC were enrolled in the present study for CPL. A total of
109 (93.9%) patients were included in group A, and 7 (6.0%)
patients were included in group B. Of the 116 patients included, 77
(66.3%) were males, and the median age was 42.5 years. The baseline
characteristics of the study participants are presented in Table I. The outcomes of the patients are
presented in Table II.
 | Table IIOutcomes of patients following
cranioplasty. |
Table II
Outcomes of patients following
cranioplasty.
| Parameters | All patients, n=116
(100%) | Group A n=109
(93.9%) | Group B n=7
(6.0%) | P-value |
|---|
| Mortality, n
(%) | 5 (4.3) | 5 (4.3) | 0 (0) | 0.562 |
| Neurological
deterioration, n (%) | 11 (9.4) | 6 (5.1) | 5 (4.3) |
<0.05 |
| Duration of
hospital stay, mean ± SD (days) | 5.9±0.9 | 5.8±0.9 | 6.4±0.9 | 0.161 |
Univariate analysis revealed that there was a
statistically significant difference in the time from DC to CPL,
infections and hematoma as peri-operative complications between the
participants who developed BGA and those who did not develop BGA
(P<0.05, Table III).
 | Table IIIUnivariate analysis for neurological
deterioration. |
Table III
Univariate analysis for neurological
deterioration.
| Parameters | No neurological
deterioration, n=105 (90.5%) | With neurological
deterioration, n=11 (9.4%) | P-value |
|---|
| Age, mean ± SD
(years) | 43.1±14 | 41.0±13 | 0.591 |
| Sex (male), n
(%) | 69 (59.4) | 8 (6.8) | 0.639 |
| Cause of DC | | | |
|
TBI, n
(%) | 62 (53.4) | 4 (3.4) | 0.148 |
|
Stroke, n
(%) | 29(25) | 6 (5.1) | 0.064 |
|
Other, n
(%) | 14 (12.0) | 1 (0.8) | 0.690 |
| GCS score of
admission, mean ± SD | 10.1±2.3 | 9.6±1.9 | 0.659 |
| KPS score of
admission, mean ± SD | 75.8±4.5 | 75.9±4.6 | 0.442 |
| Diabetes mellitus,
n (%) | 9 (7.7) | 0 (0) | 0.312 |
| Hypertension, n
(%) | 17 (14.6) | 0 (0) | 0.149 |
| Site of
cranioplasty | | | |
|
One-site
FTP, n (%) | 90 (77.5) | 9 (7.7) | 0.728 |
|
Bilateral
frontal, n (%) | 7 (6.0) | 0 (0) | 0.377 |
|
Bilateral
FTP, n (%) | 8 (6.8) | 2 (1.7) | 0.235 |
| Time from DC to
cranioplasty, mean ± SD (months) | 5.9±3.6 | 9.4±5.8 | 0.019 |
| Type of graft | | | |
|
Autologous,
n (%) | 75 (64.6) | 9 (7.7) | 0.463 |
|
Heterologous,
n (%) | 28 (24.1) | 4 (3.4) | 0.494 |
| Grafts with
fragments or fractures | 9 (7.7) | 0 (0) | 0.312 |
| Peri-operative
complications | | | |
|
Infections,
n (%) | 3 (2.5) | 3 (2.5) | 0.001 |
|
Hematoma, n
(%) | 7 (6.0) | 2 (1.7) | 0.034 |
| Duration of
hospital stay, mean ± SD (days) | 5.8±0.9 | 6.0±1.0 | 0.564 |
Multivariate analysis (Table IV) revealed that time from DC to
CPL, infections and hematoma as peri-operative complications were
all independent factors associated with BGA during follow-up
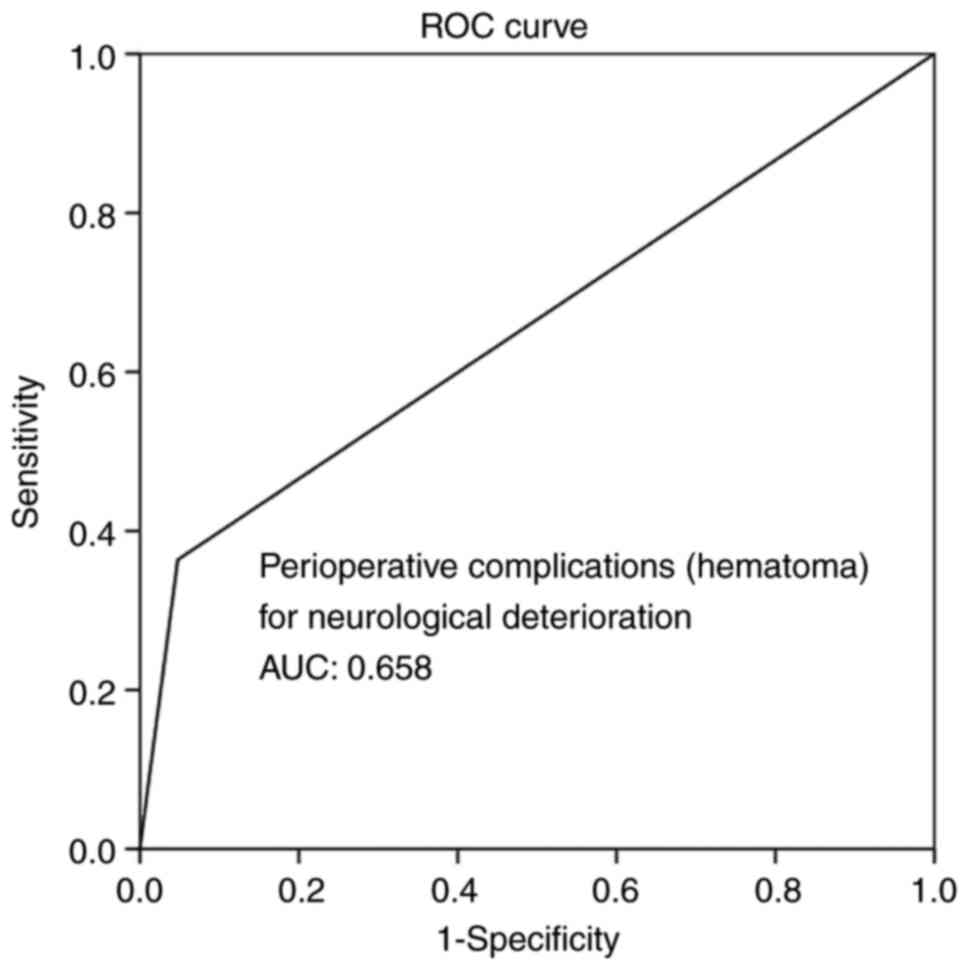
(P<0.05 for all three parameters). Overall, ROC analysis
demonstrated that infections and hematoma as peri-operative
complications exhibited the optimal performance to predict BGA, as
evaluated by an area under the curve standard error [AUC (SE)] of
[0.622 (0.10) and (P=0.184)] and [0.658 (0.10) and (P=0.085)],
respectively (Table V, and Figs. 5 and 6). In addition, ROC analysis demonstrated
that, among the variables, a time from DC to CPL of 2.5 months with
100% sensitivity and 93.3% specificity exhibited a better
dispersion to predict BGA, as evaluated by an area under the curve
standard error [AUC (SE)] of [0.714 (0.79)] and (P=0.020) (Table V and Fig.
7).
 | Table IVMultivariate analysis for
neurological deterioration. |
Table IV
Multivariate analysis for
neurological deterioration.
| | | 95% CI for
Exp(B) |
|---|
| Parameter | P-value | Exp(B) | Lower | Upper |
|---|
| Time from DC to
cranioplasty, mean ± SD (months) | 0.003 | 0.245 | 0.006 | 0.030 |
| Peri-operative
complications | | | | |
|
Infections |
<0.05 | 0.359 | 0.266 | 0.682 |
|
Hematoma |
<0.05 | 0.350 | 0.211 | 0.556 |
 | Table VROC analysis for neurological
deterioration. |
Table V
ROC analysis for neurological
deterioration.
| Parameters | Area | Std. error | 95% CI
lower-upper | P-value |
|---|
| Time from DC to
cranioplasty, mean ± SD (months) | 0.714 | 0.079 | 0.560-0.868 | 0.020 |
| Peri-operative
complications | | | | |
|
Infections | 0.622 | 0.101 | 0.424-0.821 | 0.184 |
|
Hematoma | 0.658 | 0.101 | 0.461-0.855 | 0.085 |
Discussion
The results of the present study suggest that a CPL
after 2.5-7.7 months of DC increases the possibility of bone
absorption. Additionally, the presence of post-operative infections
and hematoma, not alone but in combination with the time from DC to
CPL factor, was shown to contribute decisively to the absorption of
the bone graft.
Bone graft material
The type of bone graft used for CPL can be
heterogeneous or autologous, and the material can be variable, as
there are no indications as to the ideal material which should be
used for CPL (15). Other than the
autologous bone, metal plates, hydroxyapatite (HA), poly(methyl
methacrylate, HA cement and polyethylene have been implanted in
order to perform such necessities (17). The present study did not reveal any
statistically significant differences among the types or materials
that were used for CPL.
Complications: infections and
hematoma
The rate complications associated with CPL has a
wide range of differences among several studies in the literature.
The infection rate has been reported to be 6 to 12%, which in
numerous cases leads to implant removal and, together with
hematomas, is the most frequently reported (18-22).
The findings of the present study demonstrated that the rates of
infection and hematoma were 6 and 9%, respectively, and not alone,
but in combination with the time from DC to CPL, were shown to
contribute decisively to the development of BGA.
Time from DC to CPL
As regards CPL, the time of the bone graft
re-implantation is one of the most commonly debated issues. There
are studies reporting that early bone graft implantation is related
to various complications and a poorer outcome (22,23).
Along with the complications in the early stages of CPL,
hydrocephalus was the most common due to its association with other
factors, such as size and the cause of DC. In addition, infections
constitute another severe post-CPL complication, mainly if it is
performed before 60 days have passed after DC (22). On the other hand, CPL performed at a
late stage is associated with the same complications, and there are
no indications as to the optimal time frame for performing CPL
following DC (24,25).
However, some studies have mentioned that 3-6 months
is suitable for bone graft preservation (24,25). In
the present study, the time from DC to CPL was an independent
parameter predicting BGA, and restoration after 2.5-7.7 months
increases the possibility of bone absorption. Thus, the results
presented herein suggest that in clinical practice, 2.5-7.7 months
constitute the most suitable time interval for performing CPL
following DC without the various complications related to early
bone graft implantation, such as infections, as well as with a
minimal risk of BGA, which is usually related to CPL performed at a
late stage.
Patient's age
Apart from the time interval between DC and CPL, the
age of the patients represents another parameter in the development
of BGA (24). Thus, in pediatric
research, BGA has been found at a high rate, reaching 50% of
patients with CPL at a mean follow-up of 4.8 months (26). The independent risk factors for BGA
accountably included skull fracture, underlying contusion,
post-traumatic hydrocephalus, and an age of 2.5 years (26). The present study demonstrated that
even the young age of the patients (<19 years) was not a factor
in predicting BGA during the follow-up period following CPL.
The present study had several limitations that
should be mentioned. The main limitation was that it was performed
in a single center, and its retrospective nature was related to
possible errors in collecting and interpreting the data from the
clinical history. Another limitation also was the small sample size
in group B (n=7), and thus the power to detect significant
differences is questionable. In addition, the neurological outcome
of patients following DC and subsequent CPL depends on the
underlying initial pathology.
In conclusion, although CPL is a relatively
straightforward type of surgery from a technical standpoint, it is
not come without controversies. The results of the present study
suggest that CPL performed after 2.5-7.7 months of DC increases the
possibility of bone absorption. Additionally, the presence of
post-operative infections and hematoma, not alone, but in
combination with the time from DC to CPL factor, was shown to
contribute decisively to the absorption of the bone graft. This
sequence provides a strong justification for further extensive
prospective clinical investigations into the prevention of BGA
following CPL.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CG and GF conceptualized the study. CG, VEG, TS, AK,
GF, PS, NT and KNF made a substantial contribution to data
interpretation and analysis, and wrote and prepared the draft of
the manuscript. CG and GF analyzed the data and provided critical
revisions. CG and GF confirm the authenticity of all the raw data.
All authors contributed to manuscript revision, and have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The Institutional Review Board (IRB) of the
University of Thessaly, Greece/The School of Medicine/School of
Health Sciences approved the present study (IRB approval no.
2542/21-01-2021, finalized by the 28th General Assembly on January
28, 2021). The present study was in line with the Declaration of
Helsinki (1995; as revised in Edinburgh 2000). Written informed
consent was obtained from all included patients or their
next-of-kin before surgery, and for under-age patients, consent was
obtained from their parents or legal guardians.
Patient consent for publication
Written informed consent was obtained from all
included patients or their next-of-kin, and for under-age patients,
consent was obtained from their parents or legal guardians before
surgery for the publication of the present study and any related
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fotakopoulos G, Tsianaka E, Vagkopoulos K
and Fountas KN: According to which factors in severe traumatic
brain injury craniectomy could be beneficial. Surg Neurol Int.
7(19)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arac A, Blanchard V, Lee M and Steinberg
GK: Assessment of outcome following decompressive craniectomy for
malignant middle cerebral artery infarction in patients older than
60 years of age. Neurosurg Focus. 26(E3)2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bullock MR, Chesnut R, Ghajar J, Gordon D,
Hartl R, Newell DW, Servadei F, Walters BC and Wilberger JE:
Surgical management of traumatic brain injury author group.
Surgical management of acute subdural hematomas. Neurosurgery. 58
(3 Suppl):S16–S24; discussion Si-iv. 2006.PubMed/NCBI
|
|
4
|
Hutchinson PJ, Corteen E, Czosnyka M,
Mendelow AD, Menon DK, Mitchell P, Murray G, Pickard JD, Rickels E,
Sahuquillo J, et al: Decompressive craniectomy in traumatic brain
injury: The randomized multicenter RESCUEicp study (urihttp://www.RESCUEicp.comsimplewww.RESCUEicp.com).
Acta Neurochir. (Suppl 96):17–20. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen C and Carter BS: Hemicraniectomy for
massive cerebral infarction. Top Stroke Rehabil. 11:7–11.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fotakopoulos G, Tsianaka E, Siasios G,
Vagkopoulos K and Fountas K: Posttraumatic hydrocephalus after
decompressive craniectomy in 126 patients with severe traumatic
brain injury. J Neurol Surg A Cent Eur Neurosurg. 77:88–92.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pachatouridis D, Alexiou GA, Zigouris A,
Michos E, Drosos D, Fotakopoulos G and Voulgaris S: Management of
hydrocephalus after decompressive craniectomy. Turk Neurosurg.
24:855–858. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bohman LE and Schuster JM: Decompressive
craniectomy for management of traumatic brain injury: An update.
Curr Neurol Neurosci Rep. 13(392)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Honeybul S: Neurological susceptibility to
a skull defect. Surg Neurol Int. 5(83)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Andrabi SM, Sarmast AH, Kirmani AR and
Bhat AR: Cranioplasty: Indications, procedures, and outcome-An
institutional experience. Surg Neurol Int. 8(91)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mee H, Anwar F, Timofeev I, Owens N,
Grieve K, Whiting G, Alexander K, Kendrick K, Helmy A, Hutchinson P
and Kolias A: Cranioplasty: A multidisciplinary approach. Front
Surg. 9(864385)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Robles LA and Cuevas-Solórzano A: Massive
brain swelling and death after cranioplasty: A systematic review.
World Neurosurg. 111:99–108. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brommeland T, Rydning PN, Pripp AH and
Helseth E: Cranioplasty complications and risk factors associated
with bone flap resorption. Scand J Trauma Resusc Emerg Med.
23(75)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sundseth J, Sundseth A, Berg-Johnsen J,
Sorteberg W and Lindegaard KF: Cranioplasty with autologous
cryopreserved bone after decompressive craniectomy: Complications
and risk factors for developing surgical site infection. Acta
Neurochir (Wien). 156:805–811; discussion 811. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee SH, Yoo CJ, Lee U, Park CW, Lee SG and
Kim WK: Resorption of autogenous bone graft in cranioplasty:
Resorption and reintegration failure. Korean J Neurotrauma.
10:10–14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
de Monaco BA, Fonoff ET and Teixeira MJ:
Early resorption of an artificial bone graft made of calcium
phosphate for cranioplasty: Case report. Neuropsychiatr Dis Treat.
9:1801–1802. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee BS, Min KS, Lee MS, Kim YG and Kim DH:
Comparison with subcutaneous abdominal preservation and
cryoconservation using autologous bone flap after decompressive
craniectomy. Korean J Neurotrauma. 8:21–25. 2012.
|
|
18
|
Alkhaibary A, Alharbi A, Alnefaie N,
Oqalaa Almubarak A, Aloraidi A and Khairy S: Cranioplasty: A
comprehensive review of the history, materials, surgical aspects,
and complications. World Neurosurg. 139:445–452. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Piitulainen JM, Kauko T, Aitasalo KM,
Vuorinen V, Vallittu PK and Posti JP: Outcomes of cranioplasty with
synthetic materials and autologous bone grafts. World Neurosurg.
83:708–714. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cheng YK, Weng HH, Yang JT, Lee MH, Wang
TC and Chang CN: Factors affecting graft infection after
cranioplasty. J Clin Neurosci. 15:1115–1119. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee CH, Chung YS, Lee SH, Yang HJ and Son
YJ: Analysis of the factors influencing bone graft infection after
cranioplasty. J Trauma Acute Care Surg. 73:255–260. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim YM, Park T, Lee SP, Baek JW, Ryou KS
and Kim SH: Optimal Timing and Complications of Cranioplasty: A
single-center retrospective review of 109 cases. J Neurointensive
Care. 3:48–57. 2020.
|
|
23
|
Zanaty M, Chalouhi N, Starke RM, Clark SW,
Bovenzi CD, Saigh M, Schwartz E, Kunkel ES, Efthimiadis-Budike AS,
Jabbour P, et al: Complications following cranioplasty: Incidence
and predictors in 348 cases. J Neurosurg. 123:182–188.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fan MC, Wang QL, Sun P, Zhan SH, Guo P,
Deng WS and Dong Q: Cryopreservation of autologous cranial bone
flaps for cranioplasty: A large sample retrospective study. World
Neurosurg. 109:e853–e859. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Klinger DR, Madden C, Beshay J, White J,
Gambrell K and Rickert K: Autologous and acrylic cranioplasty: A
review of 10 years and 258 cases. World Neurosurg. 82:e525–e530.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bowers CA, Riva-Cambrin J, Hertzler DA 2nd
and Walker ML: Risk factors and rates of bone flap resorption in
pediatric patients after decompressive craniectomy for traumatic
brain injury. J Neurosurg Pediatr. 11:526–532. 2013.PubMed/NCBI View Article : Google Scholar
|