Introduction
Inflammation is a physiological response to tissue
damage or infection, characterized by the recruitment of immune
cells, release of proinflammatory cytokines and increased blood
flow and vascular permeability (1). While acute inflammation is beneficial
for tissue repair and pathogen clearance, chronic inflammation can
lead to tissue damage and fibrosis (2).
Macrophage-inducible C-type lectin (Mincle)
recognizes pathogen-associated molecular patterns (PAMPs) and
damage-associated molecular patterns (DAMPs) and triggers immune
responses (3), primarily expressed
in macrophages (4). Once activated
by various ligands, including pathogen-derived components and
endogenous molecules released from damaged cells, Mincle triggers a
series of signaling pathways, leading to the production of
proinflammatory cytokines, which are crucial for the initiation and
progression of inflammation (5).
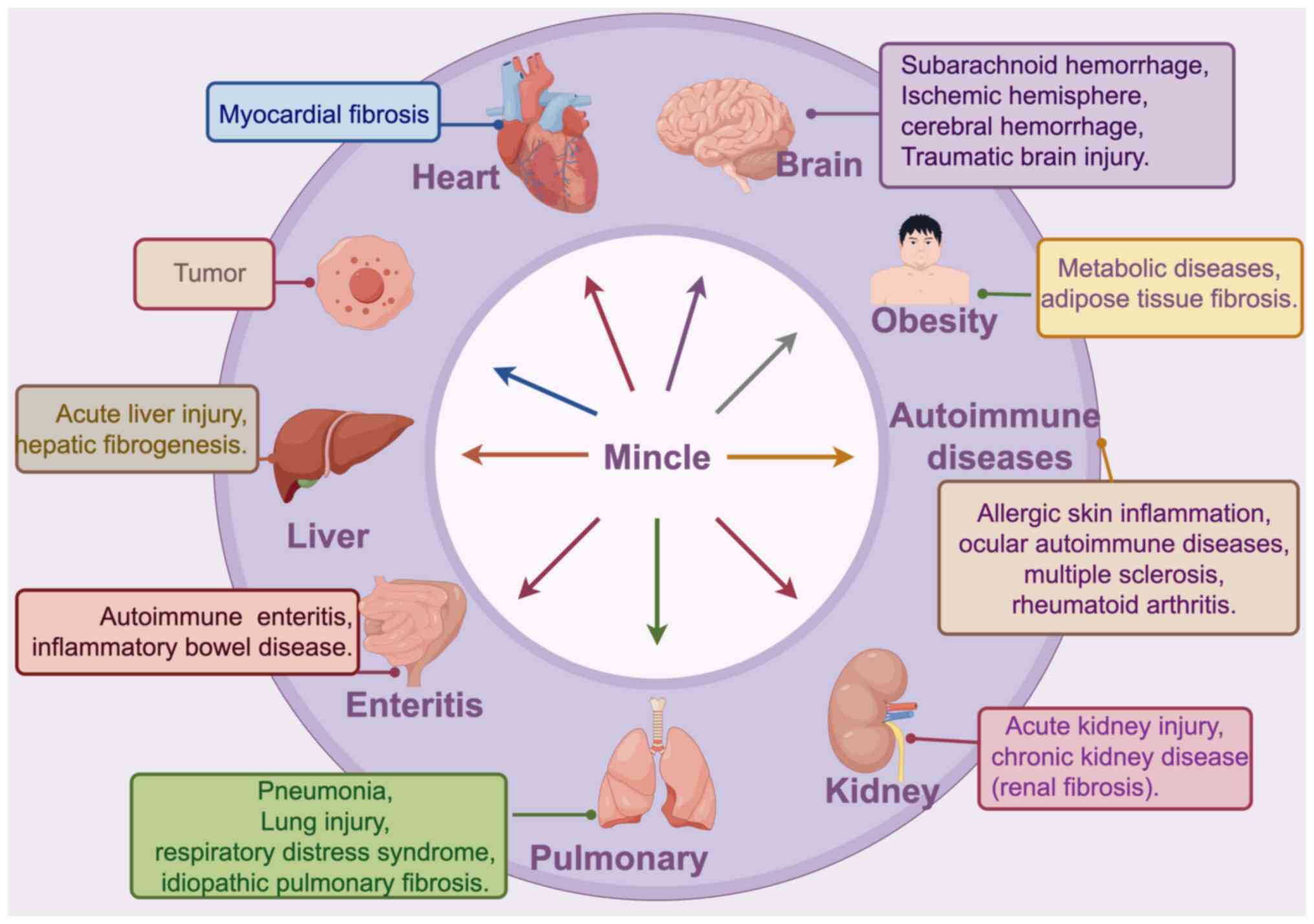
Studies have shown that Mincle is involved in the initiation of
inflammation and also in the progression to fibrosis. For example,
Mincle activation has been found to promote liver injury by
inducing the production of pro-fibrotic cytokines (6). The expression of Mincle is
upregulated in various fibrotic diseases, including liver, lung,
and kidney fibrosis, suggesting a potential role of Mincle in the
progression of inflammation to fibrosis (7). Moreover, Mincle has been shown to
interact with other receptors and signaling pathways involved in
inflammation and fibrosis. For instance, Mincle can synergize with
Toll-like receptors (TLRs) to amplify inflammatory responses and
activate the nuclear factor-κB (NF-κB) pathway, which plays a vital
role in inflammation and fibrosis. Additionally, the progression of
inflammation to fibrosis is a complex process that involves various
cellular and molecular mechanisms. The involvement of Mincle in
fibrosis is not merely a consequence of inflammation. Instead, it
appears to directly contribute to the fibrotic process. Mincle
activation has been shown to promote the differentiation of
fibroblasts into myofibroblasts (8). Therefore, Mincle may play a
significant role in the inflammatory-fibrotic axis and targeting
Mincle could be a promising strategy to alleviate inflammatory and
fibrotic diseases. The present review summarized the role of Mincle
in inflammation and fibrosis and discussed its potential as a
therapeutic target.
Mincle
The innate immune response and the adaptive immune
response make up the immunological response of the body. The innate
immune response purges the body of foreign substances and serves as
the initial line of defense against infections brought on by the
environment. The pattern recognition receptors (PRRs) of innate
immune cells interact with PAMPs derived from pathogenic bacteria,
triggering an innate immune response. The lineage encoded PRRs have
the ability to recognize various ligands, such as proteins, nucleic
acids and carbohydrates. Notably, four classes of PRRs have been
identified: TLR, RIG-I-like receptor, NOD-like receptor and C-type
lectin receptor (CLR) (9). Mincle
is a type II transmembrane CLR belonging to the same gene cluster
as dectin-2 and dectin-3 and encoded by C-type lectin domain family
4 member E (Clec4e) (10), mainly
expressed on the surface of immune cells such as macrophages,
dendritic cells and NK cells (11,12).
The structure of Mincle is characterized by the presence of an
extracellular carbohydrate recognition domain (CRD), a stalk region
and a transmembrane region with positively charged residues
(13) (Fig. 1). This allows it to recognize and
bind a wide range of pathogens and viral particles.
 | Figure 1.(A) Structure diagram of Mincle.
Mincle is encoded by six exons, including a single extracellular
CRD, a variable length stalk region, a transmembrane region and a
short cytoplasmic domain free of tyrosine residues. Among them, CRD
contains conserved residues that coordinate two Ca2+,
responsible for binding to carbohydrates. Moreover, a positively
charged arginine residue exists in the transmembrane region, which
drives Mincle to couple with FcRγ and transmits the activation
signal through ITAM in FcRγ. (B) Three dimensional structures of
human Mincle (ligand-free form) from the Protein Data Bank
(https://www.rcsb.org/structure/3WH3) are illustrated,
with the CRD EPN motif indicated in yellow, the lipophilic region
in dark orange and the CRAC-like motif in green. Mincle,
macrophage-inducible C-type lectin receptor; CRD, carbohydrate
recognition domain; FcRγ, Fc receptor γ; ITAM, immune receptor
tyrosine activation motif; EPN, glutamic acid-proline-asparagine;
CRAC, cholesterol recognition/interaction amino acid. |
In the transmembrane area of Mincle, there is a
positively charged arginine residue. Following recognition of PAMP
or DAMP by the extracellular CRD of Mincle, the positively charged
arginine residue binds to the negatively charged residue of the Fc
receptor γ (FcRγ) chain and transmits the activation signal through
the immunoreceptor tyrosine-based activation motif (ITAM). ITAM
then activates splenic tyrosine kinase (Syk) via phosphorylation
(1). According to previous
studies, caspase-recruitment domain 9 (CARD9) is considered a
crucial downstream of ITAM signaling, forming a complex with B-cell
lymphoma 10 (Bcl10) and mucosa-associated lymphoid tissue lymphoma
translocation protein 1 (Malt1). Phosphorylated Syk activates the
NF-κB signaling pathway through this complex, resulting in the
release of chemokines from antigen-presenting cells (APCs)
(Fig. 2) (3,13–16).
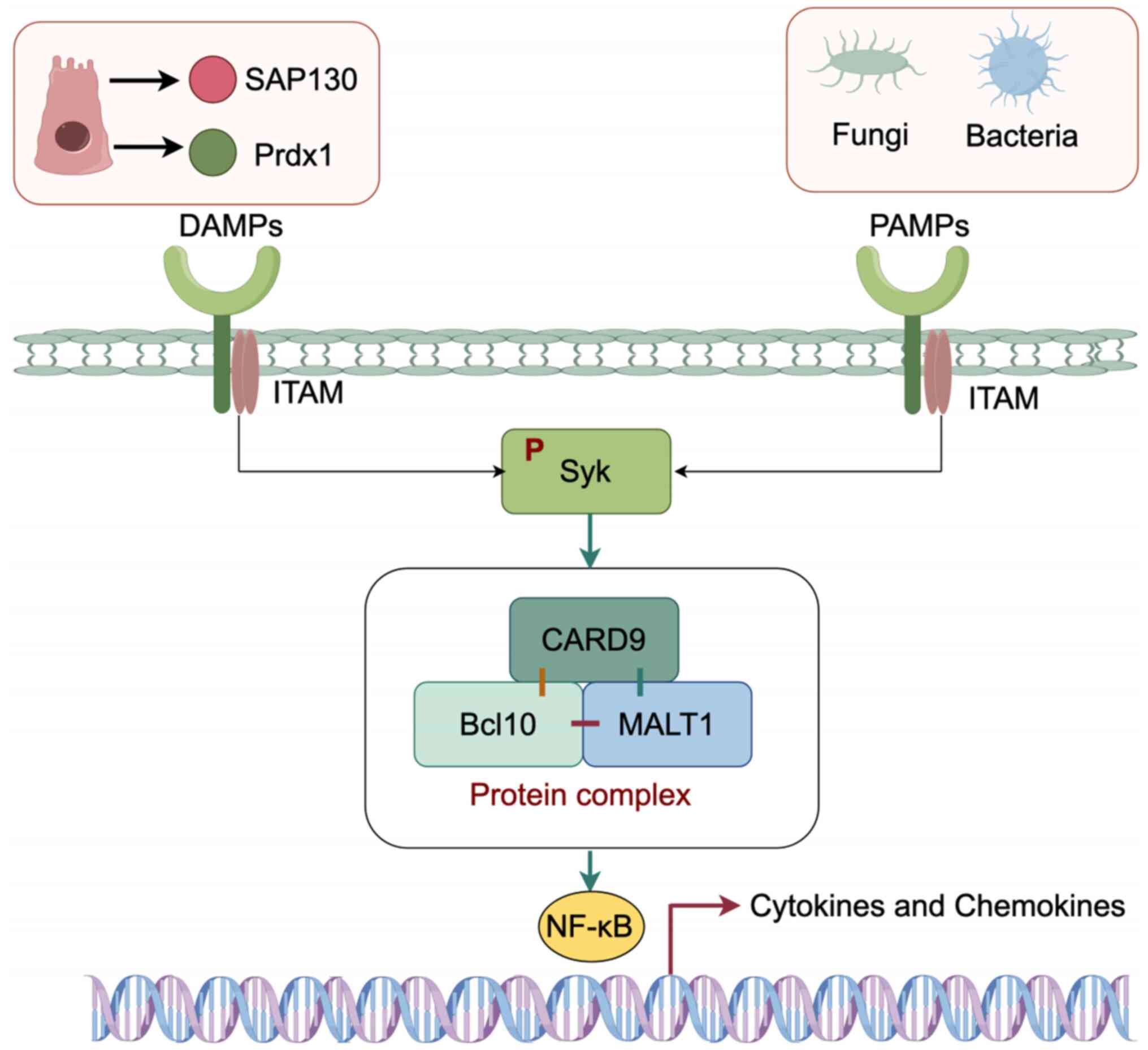 | Figure 2.Functions of Mincle. After
recognition of PAMP or DAMP by the extracellular carbohydrate
recognition domain of Mincle, the positively charged arginine
residue binds to the negatively charged residue of the FcRγ chain
and transmits the activation signal through ITAM. Then, ITAM
activates Syk via phosphorylation. According to reports, CARD9 is
considered a crucial adaptor molecule downstream of ITAM signaling,
forming a complex with Bcl10 and Malt1. Phosphorylated Syk
activates the NF-κB signaling pathway through this complex,
resulting in the release of chemokines from APCs. This figure was
created by Figdraw 2.0 (https://www.figdraw.com; Hangzhou Huikeyan Technology
Co. Ltd, China). Mincle, macrophage-inducible C-type lectin
receptor; PAMP, pathogen-associated molecular pattern; DAMP,
damage-associated molecular pattern; FcRγ, Fc receptor γ; ITAM,
immune receptor tyrosine activation motif; Syk, splenic tyrosine
kinase; CARD9, caspase-recruitment domain 9; Bcl10, B-cell lymphoma
10; Malt1, mucosa-associated lymphoid tissue lymphoma translocation
protein 1; APCs, antigen-presenting cells; SAP130,
spliceosome-associated protein 130; Prdx1, peroxiredoxin 1. |
Glycolipids make up the majority of the ligands of
Mincle. In 2008, spliceosome-associated protein 130 (SAP130), an
endogenous ligand derived from injured and necrotic cells, was
reported to be recognized by Mincle (3). The groundbreaking observation of
Wells et al (17) in the
same year established the identification of Candida albicans
by Mincle and its pivotal role in orchestrating host protective
immune responses against fungal pathogens, marking the first
documentation of the involvement of Mincle in the immune system. In
the subsequent year, Mincle was firmly recognized as a PRR for
trehalose-6,6′-dimycolate (TDM), a glycolipid predominantly located
on the cellular membranes of Mycobacterium, unravelling
further insights into its functional significance (4). Notably, an increasing number of
ligands have been discovered, such as sterols (18,19),
β-gentiobiosyl diacylglycerides (20), glucosyl-diacylglycerol (21), α-glucosyl diglyceride (22), brartemicin (23), Agrocybe aegerita lectin
(24), β-glucosylceramides
(25), α-mannose (12), glyceroglycolipid (26,27)
and peroxiredoxin 1 (Prdx1) (28).
The ligands are summarized in Table
I. The binding of Mincle with the ligands is associated with
inflammatory related diseases such as acute nephritis, hepatitis
(29,30), Crohn's disease (31), stroke (32,33),
cancer (34,35), immune diseases (36,37)
as well as numerous other diseases (Fig. 3) The relationship between these
diseases, Mincle and its ligands is still under continuous
investigation and exploration and the specific mechanisms and
regulatory processes require further in-depth research.
 | Table I.Ligands of Mincle. |
Table I.
Ligands of Mincle.
| Category | Description | Examples |
|---|
| Exogenous
ligands | Molecules derived
from microbes, fungi, plants and other external sources |
Trehalose-6,6′-dimycolate,
trehalose-6,6-dibehenate, α-mannose, gyceroglycolipid,
β-gentiobiosyl diacylglycerides, glucosyl-diacylglycerol,
α-glucosyl diglyceride, brartemicin, unique mannosyl fatty
acids-linked to mannitol. |
| Endogenous
ligands | Molecules produced
within the body, including metabolites and cellular components | Sin3-associated
protein 130, cholesterol crystal, cholesterol sulfate,
peroxiredoxin 1, |
|
|
|
β-glucosylceramides, etc. |
| Oxidized
ligands | Ligands that have
undergone oxidation modifications and can be recognized and
activate the Mincle signaling. | Oxidized LDL
(malondialdehyde-modified LDL). |
Mincle and inflammation
Inflammation is essential for the healing process of
tissue injury and wounds. Inflammation facilitates the prompt
arrival of cellular and humoral defenses to the damaged spot,
thereby impeding the process of injury and aiding in the
elimination of debris produced during the injury while promoting
repair (38). If the culpable
agent is not eliminated or controlled, it leads to the emergence of
persistent inflammation, which can have adverse effects on the
host. Tissue damage, excessive collagen buildup and fibrosis occur
due to the presence of growth factors released by neutrophils and
macrophages, proteolytic enzymes and reactive oxygen species (ROS)
(39).
Mincle and sterile inflammation
In the absence of microorganisms, inflammation
typically arises from trauma or chemical injury, which is commonly
referred to as sterile inflammation. The main features of sterile
inflammation include the recruitment of chemokines, macrophages,
neutrophils and inflammatory cytokines, with a particular emphasis
on tumor necrosis factor (TNF) and interleukin (IL)-1 (40).
The development of inflammatory diseases, such as
brain damage, liver injury and autoimmune disorders is
significantly influenced by Mincle. This is explained by the
capacity of Mincle to trigger sterile inflammation by identifying
and attaching to DAMPs. Necrotic cells which emit DAMPs such as
SAP130, are just a few of the proinflammatory cytokines that are
upregulated as a result of the interaction between Mincle and these
DAMPs. These elements contribute to neutrophil infiltration and
M1-type macrophage polarization, which exacerbate tissue injury
(41).
Mincle and renal inflammation
Mincle contributes to the promotion of M1
macrophage-mediated acute kidney injury (AKI). The TLR4/NF-κB
signaling pathway tightly controls the expression of Mincle on M1
macrophage and Mincle plays an important role in sustaining the M1
macrophage phenotype (42). Lv
et al (43) found that
Mincle contributes to the development of unilateral ureteral
obstruction (UUO) and cisplatin-induced acute kidney injury (CIAKI)
by regulating inflammatory responses mediated by macrophages,
thereby enhancing renal tubule injury. Subsequently, it was
discovered that damaged renal tubule cells release SAP130, which
controls the activation of macrophages and the occurrence of renal
inflammation by miRNA-219c-3p-dependent mechanisms in UUO and CIAKI
animal models, as well as in individuals with acute tubular
necrosis (44). miR-219c binds to
Mincle and inhibits Mincle translation, resulting in the negative
regulation of Mincle expression in macrophages. The activation of
Mincle was revealed to occur when dead cells released
β-glucosylceramide and free cholesterol. The activation of Mincle
was found to elicit a robust response in macrophages, leading to
the secretion of proinflammatory cytokines and impairing the
efficient clearance of apoptotic cells. As a consequence, this
cellular process played a pivotal role in orchestrating the
persistent inflammatory milieu following AKI, ultimately leading to
the development of renal atrophy (45). Li et al (28) identified that kidney-derived serum
Prdx1 plays a role in AKI by activating Mincle and downstream
pathways. The experimental results of these researchers revealed an
increase in serum Prdx1 levels and a decrease in Prdx1 expression
in renal tubular epithelial cells associated with AKI induced by
lipopolysaccharide and renal ischemia reperfusion injury (IRI) in
animal models. Furthermore, it was revealed that gene knockout of
Prdx1 or using Prdx1 neutralizing antibodies protected mice from
AKI, while exogenous recombinant Prdx1 (rPrdx1) introduction
weakened this protective effect. Further study of peritoneal
macrophages revealed that rPrdx1 induced M1 polarization, activated
the Mincle signaling and enhanced the production of inflammatory
cytokines. Additionally, Prdx1 interacted with Mincle, leading to
acute kidney inflammation. These findings suggested that serum
Prdx1 derived from kidneys promotes AKI by activating the Mincle
signaling and downstream pathways. Collectively, Mincle triggers
and sustains M1 macrophages via the Mincle-associated pathway and
controls macrophage-driven inflammation, which ultimately
contributes to the promotion of renal inflammation and exacerbation
of renal injury. Targeting Mincle and its associated pathways could
be a potential approach for addressing renal damage.
Mincle and liver damage
Mincle exerts a proinflammatory role in liver damage
and contributes adversely to the progression of chronic alcoholic
hepatitis. A previous study revealed that the interaction between
Mincle and its endogenous ligand SAP130 could regulate
proinflammatory reaction to enhance concanavalin A (ConA)
hepatitis. In addition, the protection against ConA hepatitis was
observed when Mincle was deleted or blocked, which resulted in
reduced expression of CAAT/enhancer-binding protein beta and
hypoxia-inducible factor 1α (29).
According to a different study, Kupffer cells (KCs) are activated
to generate and secrete proinflammatory cytokines, which in turn
promote neutrophil infiltration and liver damage in acetaminophen
(APAP)-induced liver injury (30).
The liver experienced damage when there was an overabundance of
APAP, leading to the activation of the Mincle/Syk pathway and the
subsequent release of a significant amount of proinflammatory
mediators. However, it is possible to reverse these effects and
decrease APAP-induced liver damage in case of Mincle deficiency or
KC deletion.
Mincle exacerbates chronic alcoholic liver injury
caused by alcohol abuse through the activation of the innate immune
system. The IL-1 receptor-associated kinase 3 (IRAKM)-Mincle axis
was first reported to be activated by low concentrations of
lipopolysaccharides (LPS) and SAP130 when they worked together to
cause IL-1 receptor-associated kinase injury, which was a major
factor in the emergence and progression of chronic alcohol-induced
liver injury (30).
Ethanol-induced hepatocellular damage required the activation of
the Mincle/Syk signaling pathway by SAP130 released from
ethanol-exposed hepatocytes, while low dose LPS activated IRAKM
Myddosome, significantly increasing the expression of Mincle.
Subsequently, a previous study found that Mincle interacting with
SAP130 enhanced the infiltration of inflammatory immune cells by
promoting the secretion of IL-1β in KCs. The deficiency of Mincle
or the inhibition of its downstream pathways reduces the production
of inflammatory factors and alleviates alcohol-induced liver
injury. On the contrary, the activation of Mincle signaling
produces the opposite effect (46).
In patients with cirrhosis and rat models of
acute-on-chronic liver failure (ACLF), there was a notable
elevation in Mincle protein levels across multiple organs,
including the heart, liver, kidney and spleen, with particularly
higher expression observed in liver failure models. Surprisingly,
this upregulation did not uniformly result in improved downstream
signaling in all organs. While significant enhancement of Mincle
downstream signaling was observed in the heart, liver and kidney,
it was disrupted in the peripheral blood mononuclear cells, spleen
and small intestine. These findings suggested that Mincle may have
a significant impact on the immune paralysis observed in ACLF
(47).
Mincle plays a pivotal role in the progression of
chronic liver injury at various stages. The metabolic syndrome in
the liver is manifested as non-alcoholic steatohepatitis (NASH),
which is defined by increased hepatic lipid buildup without a
history of alcohol use. It is mostly brought on by obesity-induced
inflammation and fibrosis of adipose tissue, which causes an inflow
of free fatty acids into the liver through the portal vein.
Initially, this causes the onset of simple steatosis, which then
develops into NASH. Hepatocytes undergo death due to lipid
overload, resulting in their encapsulation by macrophages and
subsequent infiltration of inflammatory cells, ultimately
culminating in NASH (48).
In a recognized model for NASH, scientists have
observed hepatocellular crown-like structures (hCLS), reminiscent
of CLS observed in tissue under microscopic examination. hCLS
consist of CD11-positive macrophages clustering around lipid
droplets within hepatocytes (43).
The interaction between macrophages in hCLS and dying hepatocytes
leads to their phagocytosis, activating fibroblasts, promoting
fibrosis, and resulting in cirrhosis and hepatocellular carcinoma.
Upregulation of Mincle was observed in liver tissue obtained from
animal models and patients with NASH. Additionally, the expression
levels of collagen 1, a fibrosis and a Kupffer cell marker, were
found to be elevated (47). These
findings strongly indicated that the upregulation and activation of
Mincle contributes to the aggravation of hepatic fibrogenesis in
NASH.
Mincle-mediated inflammation and
immune response promote lung injury
Pneumonia
The role of Mincle in pneumonia primarily lies in
its ability to identify pathogens and control the immune response.
Mincle has the capability to recognize and bind to a variety of
pathogens, including bacteria, fungi and viruses, to initiate an
immune response that clears the pathogen. Sharma et al
(49) investigated the role of
Mincle in pneumonic sepsis induced by Klebsiella pneumoniae
(K. pneumoniae), which demonstrated that Mincle played a
protective role by coordinating bacterial clearance mechanisms of
neutrophils. A different study revealed that the Mincle-Glc-DAG
axis is a lung protective immune factor in focal pneumonia induced
by Streptococcus pneumoniae (S. pneumoniae) (50). In addition, it was revealed that
leukocytes, especially neutrophils expressing Mincle, play a
critical role in contributing to the regulation of protective
immunity against focal pneumonia-causing S. pneumoniae
(21).
Notably, Rabes et al (51) indicated that Mincle can recognize
S. pneumoniae. However, experiments with Mincle-deficient
mice revealed that this receptor does not have a significant
beneficial role in antibacterial immune response during pneumonia.
In other words, the protective effect of Mincle in focal pneumonia
may be indispensable, but it is not protective in the model of
invasive pneumococcal disease (IPD). Ishikawa et al
(4) revealed the ability of Mincle
to detect apoptotic cells and recruit inflammatory cells and that
TDM-induced pulmonary granuloma formation is entirely dependent on
Mincle. Therefore, it is possible that TDM and necrotic cells
potentially collaborate to promote the formation of pulmonary
granulomas in tuberculosis through the secretion of inflammatory
cytokines/chemokines mediated by Mincle. Thus, the role of
mincle-mediated immune responses in lung inflammatory diseases
appears to be bidirectional. The relevant published studies
according to research grouping, modeling and detection methods are
included in supplementary file Table
SI.
Lung injury
Mincle recognises and binds to DAMPs released by
deceased cells, which initiates an inflammatory response and
promotes the repair of damaged tissues. However, excessive
inflammation may lead to further aggravation of lung injury.
Yamasaki et al (3) showed
that Mincle recognises and binds to SAP130 released by dead
alveolar epithelial cells, which activates macrophages to enhance
lung injury. Fisher et al (52) established a lethal mouse model of
Orientia tsutsugamushi (O. tsutsugamushi) and
cultured macrophages and neutrophils to investigate the involvement
of immune sensors and inflammatory responses in the infection
process. In addition, a selective stimulation of Mincle expression
was observed in the lungs of infected mice by O.
tsutsugamushi, accompanied by an increase in proinflammatory
markers and markers associated with type 1 responses. Moreover, it
was found that both live and inactivated bacteria treatment of
macrophages resulted in enhanced Mincle expression, along with
elevated levels of proinflammatory markers related to type 1 and M1
responses. However, some of these markers were significantly
reduced in Mincle cells. The activation of Mincle and its
associated inflammatory profile induced by live and inactivated
O. tsutsugamushi may lead to an excessive immune response,
ultimately causing lung injury and acute respiratory distress
syndrome. Therefore, Mincle may become a new target for the
treatment of pneumonia and lung injury. Further studies are needed
on the specific mechanism of action and regulatory strategies of
Mincle in pneumonia and lung injury.
In metabolic disorders, Mincle
increases adipose tissue inflammation
Obesity is the key contributing factor to metabolic
syndrome, a common metabolic condition affecting several organs. A
distinct CLS found in obese adipose tissue is made up of
decomposing adipocytes encircled by macrophages (53). Within the CLS, adipocytes and
macrophages collaborate to create a paracrine pathway that causes
adipose tissue to be chronically inflamed. Increased adipose tissue
inflammation can promote tissue fibrosis, which in turn causes
ectopic adipose tissue buildup and insulin resistance.
As a result of the involvement of Mincle in CLS
creation, fibroblast activation and transformation, adipose tissue
inflammation and fibrosis are promoted. The expression of Mincle in
macrophages is induced partly by palmitate esters through the
TLR4/NF-κB pathway, showing significant enhancement in adipose
tissue of obese mice and humans (54). Furthermore, Mincle expression is
observed in proinflammatory M1 macrophages derived from bone marrow
cells in vitro, distinguishing them from the
anti-inflammatory M2 macrophages. Thus, Mincle could promote the
initiation and progression of inflammation of adipose tissue
brought on by obesity. Collectively, Mincle is linked with the
onset of obesity-associated metabolic disease, indicating its
potential as an intervention target for obesity-related diseases,
thus, offering promising possibilities.
Mincle promotes the initial stages and
progression of neurological inflammation in patients with brain
damage
Traumatic brain injury (TBI)
TBI refers to the brain damage caused by an external
mechanical force, leading to neuronal injury, microglial activation
and subsequent neuroinflammatory processes. This can result in
temporary or permanent impairment of brain functions (55,56).
Additionally, innate immune response and inflammatory activity
following TBI are intimately linked (57,58).
Therefore, identifying interventions to target neuroinflammatory
processes is crucial in the treatment of TBI.
According to the study published by de Rivero
Vaccari et al (59), it was
found that SAP130 can activate Mincle in cortical neurons, leading
to the synthesis of the inflammatory cytokine, TNF. Additionally,
elevated levels of Mincle and SAP130 were observed in both brain
tissue and cerebrospinal fluid samples obtained from patients and
animal models of brain injury. Subsequently, the researchers
discovered that the application of SAP130 and a Mincle neutralizing
antibody on cultured cortical neurons effectively suppressed Mincle
signaling, resulting in decreased TNF production. These findings
suggested a potential mechanism where SAP130 activates Mincle in
cortical neurons, leading to an upregulation of phosphorylated
(p)-Syk expression, thereby triggering TNF production and
initiating an inflammatory response.
He et al (60) found that BAY61-3606 inhibited the
Mincle/Syk signaling pathway and promoted the transition of
proinflammatory microglia to an anti-inflammatory phenotype. This
effectively suppressed microglial migration, contributing to
attenuation of microglia-mediated neuroinflammation and improvement
of neurological deficits after TBI. Therefore, Mincle signaling is
involved in the development of neuroinflammation following TBI and
targeting the Mincle signaling pathway could be a promising
therapeutic approach for TBI.
Non-TBI
Mincle has a detrimental effect on brain injuries
and may be a target for reducing damage to neurovascular units and
enhancing neurological function after brain injury. It was
discovered that the Mincle signaling pathway contributed to the
development of early brain damage following cerebral hemorrhage.
Rats with subarachnoid haemorrhage (SAH) showed elevated
ipsilateral cerebral hemispheres with higher IL-1β levels and
increased expression of CARD9, Mincle, Syk phosphorylation and
SAP130 (61). Altogether,
upregulation and activation of Mincle expression on microglia and
neurons during brain injury results in increased release of
inflammatory mediators, exacerbating the initiation of inflammatory
responses and promoting the progression of brain damage.
Conversely, the blocking of the Mincle pathway can suppress
inflammation, attenuate brain injury and enhance neuronal
function.
Although there is no direct evidence to suggest a
direct association between Mincle and neurodegeneration, Mincle
could serve as a promising molecular target in neurodegenerative
diseases. Neurodegeneration encompasses a group of disorders
involving the loss of neurological function, such as Parkinson's
disease, Senile dementia, Huntington's disease, and Alzheimer's
disease. In these diseases, neuronal damage and death lead to
neuroinflammation which in turn exacerbates neuronal damage,
forming a vicious cycle. Neuroinflammation is a crucial feature of
neurodegenerative diseases (62–65).
As aforementioned, inhibition of Mincle could attenuate
neuroinflammation and neurofunctional damage (60). It was hypothesized that neuronal
apoptosis may occur due to increasing age (42), drugs (66) and brain injury (67) and that Mincle recognises and binds
to neuronal death signals, triggering neuroinflammation. A previous
study identified TH17 cells as mediators of central nervous system
inflammation and suggested that they can sense danger signals
through Mincle (68). Perhaps this
inflammatory response is beneficial in the short term for the
removal of dead cells and tissue repair, but persistent and
excessive inflammation can have toxic effects on healthy neurons.
Secondly, activation of Mincle signaling may promote the production
of inflammatory factors. These inflammatory factors would further
exacerbate neuroinflammation and neuronal damage (55,56).
In addition, Mincle appears to play a role in the development of
neurodegenerative diseases by affecting neuronal autophagy and
apoptosis processes. A different study demonstrated that
neuroinflammation in demented rats can be effectively alleviated by
reducing glial cell proliferation and inhibiting neuronal
apoptosis/autophagy (69).
Therefore, Mincle may be an important molecule in mediating
neurodegenerative diseases through its involvement in
neuroinflammation. Further studies are required to confirm these
hypotheses.
Mincle and autoimmune diseases
Autoimmune diseases manifest as a breakdown of
immune tolerance towards self-antigens, resulting in persistent
inflammation and irreparable harm to multiple organ systems
(70). Numerous complex disorders,
such as uveitis, atopic dermatitis and multiple sclerosis (MS)
(71,72), are classified as autoimmune
diseases. In various autoimmune diseases, Mincle exerts a
proinflammatory effect, thereby modulating the progression of these
diseases.
Mincle is involved with skin inflammation. As a PRR
expressed on the surface of skin, Mincle is upregulated in skin
trauma and directly recognizes cholesterol sulfate, leading to
Mincle-dependent proinflammatory responses. Activation of Mincle by
cholesterol sulfate triggers the secretion of various
proinflammatory mediators, which contribute to the development of
atopic dermatitis. In an experimental model of allergic contact
dermatitis, skin inflammatory responses were significantly less
severe when Mincle was absent, indicating that Mincle promotes skin
inflammation (19). These findings
highlighted the crucial role of Mincle in driving the onset and
progression of allergic skin inflammation and suggested its
potential as a target for therapeutic interventions in related skin
disorders. Psoriasis is an immune and inflammatory disorder, while
Mincle serves as a key factor in maintaining the M1 macrophage
phenotype during the proinflammatory process. A previous study
confirmed that Mincle exhibits a marked upregulation in macrophages
derived from individuals and mouse models afflicted with psoriasis.
Furthermore, it revealed the significant role of the Mincle pathway
in macrophage-mediated psoriasis and demonstrated that inhibiting
Mincle in macrophages can suppress skin lesions and damage.
Subsequently, the study discovered that targeted treatment of
Mincle can improve symptoms in psoriasis mouse models (73). Therefore, this research suggested
that therapeutic interventions targeting Mincle may offer a novel
approach for the treatment of psoriasis.
Mincle has a fatal role in MS and promotes the
growth of neuroinflammation resembling MS. MS is a chronic
autoimmune disease affecting the central nervous system, which is
characterized by inflammation and axonal degeneration leading to
various symptoms such as muscle weakness, spasticity, fatigue,
visual and sensory disturbances, cognitive impairment and
bladder/bowel dysfunction (74–76).
N'Diaye et al (77)
demonstrated the involvement of Mincle in the development of
MS-associated inflammation. Inhibiting the MCL/Mincle signaling
pathway weakened the ability to recruit T cells in the central
nervous system (78). Furthermore,
in patients with MS, the Mincle pathway was upregulated in
peripheral blood monocytes (77).
Mincle plays a pathogenic role in intestinal mucosal
inflammation. Several studies have shown that the Mincle pathway is
obviously upregulated in human inflammatory bowel disease and
animal models. Deficiency of Mincle alleviates colonic
inflammation. Conversely, activation of Mincle with agonists
exacerbates intestinal inflammation (79). Another study has shown that the
Mincle pathway can induce the release of proinflammatory factors
through macrophage pyroptosis (2).
Mincle may be associated with the progression of
rheumatoid arthritis (RA) and considered as a biomarker for RA.
There may be a sex-specific correlation between Mincle rs10841845 G
allele and susceptibility to RA (80). Additionally, it was found that the
expression level of Mincle in the synovial tissue was significantly
higher in patients with RA compared to patients with osteoarthritis
(OA) and Mincle expression levels were higher in the macrophage
component compared to the non-macrophage component (81). Mincle could serve as a significant
biomarker and therapeutic target for these conditions.
Mincle and fibrosis
Mincle may induce the macrophage to
myofibroblast (MF) transition (MMT), promoting fibrosis
occurrence
Fibrosis, an immune-mediated disease (82), is a pathological condition marked
by excessive extracellular matrix (ECM) protein deposition that
causes organ failure. It is a common pathological outcome of
chronic inflammatory diseases, such as liver cirrhosis, pulmonary
fibrosis, and kidney fibrosis.
As aforementioned, Mincle is a crucial initiator and
maintainer of macrophage inflammation and essential factors.
Although current research on the proinflammatory role of Mincle
primarily focuses on acute diseases such as AKI, an increasing body
of evidence has suggested its critical involvement in chronic
inflammation and fibrosis. Pulmonary fibrosis is a process
characterized by abnormal remodeling of the ECM. It has been found
that the role of Mincle in pulmonary fibrosis is mediated by
activation of inflammatory and fibrosis-related signaling pathways.
Specifically, Mincle activates pathways such as NF-κB, which
promote inflammatory and fibrotic responses. In addition, Mincle
activates immune cells such as alveolar macrophages and dendritic
cells, thereby enhancing the extent of inflammatory and fibrotic
responses (83). Furthermore, it
was also found that by blocking the expression or function of
Mincle, the pathological process of pulmonary fibrosis could be
effectively attenuated (84). In
human non-alcoholic fatty liver disease, Mincle is upregulated
concomitant with increased collagen production. Similarly, in
animal models of alcoholic fatty liver, cirrhosis, and chronic
liver failure, Mincle activation significantly enhances hepatic
collagen synthesis, highlighting its significant role in chronic
liver fibrosis (47). Tanaka
(53) discovered that Mincle
promoted the formation of CLS and adipose tissue fibrosis in
adipose tissue macrophages, which could lead to reduced adipose
tissue storage capacity, insulin resistance and ectopic lipid
accumulation (85). Mincle
activation induces the fibrosis-related genes, thereby promoting
the formation of MFs. According to Watanabe et al (86), isoliquiritigenin (ISL) can reduce
the fibrosis-related genes in the interstitial blood vessels
between TLR4 and Mincle-stimulated adipose tissue and macrophages,
relieving itching. In addition, studies have reported elevated
expression of Mincle in kidneys with UUO-induced fibrosis,
predominantly expressed in macrophages and specific monomers of
traditional Chinese medicine targeting Mincle effectively suppress
its expression, thereby ameliorating kidney fibrosis (87).
Notably, a previous study suggested that MMT has a
role in the development of interstitial fibrosis in cases of
chronic renal allograft damage (88). Infiltrating macrophages exhibit a
remarkable ability to transdifferentiate into MFs, which are
pivotal cellular drivers of pathogenic fibrosis by promoting
excessive synthesis of ECM proteins. Renal tissue biopsies were
performed on patients with various forms of kidney diseases,
revealing the presence of MMT cells. It was observed that MMT cells
were virtually absent in cases of acute inflammation or sclerotic
lesions, but significantly present in cases of active fibrotic
lesions, indicating the potential role of MMT cells in progressive
renal fibrosis (89). The process
known as MMT is implicated in the pathogenesis of macular fibrosis
associated with neovascular age-related macular degeneration,
ultimately leading to the onset of retinal fibrosis (90). Therefore, it was hypothesized that
macrophage Mincle may induce MMT, thereby promoting fibrosis
occurrence.
Inhibition of Mincle with drugs alleviates
inflammation-related diseases
Numerous drugs that protect against AKI, hinder the
occurrence of inflammation by suppressing the activation of Mincle
signaling. Tan et al (91)
discovered that curcumin diminishes renal inflammation and enhances
CIAKI by blocking the Mincle pathway. Curcumin effectively inhibits
the expression of Mincle in CIAKI, thereby suppressing the
signaling pathway of Syk/NF-κB, activated and maintained by Mincle,
as well as the M1 macrophage phenotype. This leads to a reduction
in the release of proinflammatory factors and promotes the
polarization of macrophages towards the M2 phenotype, ultimately
relieving CIAKI inflammation. Consequently, they exhibited renal
protection in cisplatin-induced nephrotoxicity. Similar effects
were found in quercetin treatment of AKI. Quercetin significantly
inhibited LPS-induced expression and secretion of inflamination
including IL-6, IL-1β, and TNF-α in bone marrow-derived macrophages
(BMDMs) and reduced the activity of the Mincle/Syk/NF-κB
signalling, thereby alleviating cisplatin-induced AKI (92). Moreover, Diao et al Astragalus
mongholicus Bunge and Panax notoginseng (A&P)
formula intervened in a LPS-induced macrophage inflammatory cell
model and a cisplatin-induced mouse AKI model. The results revealed
that A&P significantly inhibited the expression level of Mincle
in vitro and in vivo, and decreased the expression
and secretion of IL-1β, IL-6, and TNFα in LPS-stimulated BMDM cells
(93). Lei et al (94) established a CIAKI mouse model and a
co-culture system of bone marrow-derived macrophages (BMDMs) and
macrophage renal tubular epithelial cells (mTECs), demonstrating
that Mincle in macrophages exacerbates LPS-induced inflammation and
necrotic apoptosis in mTECs. The study also revealed that
artemether inhibits the expression of Mincle in macrophages of AKI
mice. However, the overexpression of Mincle in BMDMs restored the
damage and necrotic apoptosis in mTECs suppressed by artemether.
This suggested that artemether may improve renal function in AKI by
inhibiting Mincle-mediated macrophage inflammation, reducing injury
and necrotic apoptosis in renal tubular cells. Additionally,
BAY61-3606 can inhibit Mincle and decrease the production of ROS
and the release of inflammatory factors, ultimately reducing renal
inflammation (95). Oridonin also
exerts its anti-inflammatory function in renal IRI through the same
mechanism (96).
Certain drugs exert protective effects on chronic
kidney disease (CKD) by mitigating kidney inflammation through
blocking the activation of Mincle signaling in macrophages. Tan
et al (96) established a
5/6 nephrectomy-induced CKD animal model as well as in vitro
and in vivo macrophage inflammatory cell models using LPS
and uremic toxins. In this study, the activation of Mincle was
observed in the kidneys and intestines of CKD mice, as well as in
toxin-stimulated macrophages, which was effectively suppressed by
the combination therapy of A&P formula with bifidobacterium.
Subsequent findings demonstrated that the inhibitory effect of
A&P combined with bifidobacterium on uremic toxin-stimulated
inflammation in RAW264.7 cells could be eliminated by Mincle gene
overexpression. These results suggested that the combination of
A&P with bifidobacterium can protect the kidneys from
CKD-induced damage by downregulating macrophage-mediated
inflammation in the kidneys and intestines through the inhibition
of Mincle signaling (97).
Moreover, A&P can suppress Mincle, resulting in a decrease in
renal inflammatory response, ultimately leading to an enhancement
in renal function in individuals with diabetic nephropathy
(98). CKD-protective drugs have
also been found to reduce the renal inflammation by targeting
Mincle, leading to a reduction in renal fibrosis and an improvement
in renal function. Liao et al (87) discovered that ISL exhibited robust
inhibition of mRNA and protein expression of Mincle in both BMDM
and UUO models, concurrently suppressing the phosphorylation of Syk
and NF-κB, which is the downstream of Mincle. Consequently,
expression of fibrotic markers was reduced. Notably, the opposite
results were observed when stimulating Mincle using its agonist.
This suggested that ISL exerts its protective effects on
UUO-induced CKD by inhibiting Mincle and its downstream
inflammatory pathways, thus suppressing renal fibrosis. In summary,
a multitude of renal protective medications achieve their
renoprotective effects by inhibiting Mincle.
A recently published study by Yang et al
(99) revealed that co-treatment
of bone marrow mesenchymal stem cells (BMSCs) with
Iron(III)-quercetin complexes (IronQ), which has excellent dual
functions with the use of an imaging probe for MRI and act as a
stimulating agent by favoring circulating proangiogenic cell
differentiation (100–102), alleviates intracerebral
hemorrhage (ICH) by ameliorating inflammation-induced neurological
deficits through inhibition of the Mincle/Syk signaling pathway.
The conditioned medium generated from BMSC combined with IronQ
reduced the LPS-induced inflammation, Mincle expression and its
downstream target activity in the BV2 cell line. Liu et al
(103) established a model of ICH
using autologous blood injection into the caudate nucleus. They
found that acupuncture at Baihui (DU20) and Qubin (GB7), with each
session lasting 30 min, every 12 h for a total of three sessions,
significantly improved neurological function and reduced brain
edema. This was achieved by inhibiting the immune reactivity and
expression of Mincle, Syk, CARD9 and IL-1β. These findings
demonstrated that acupuncture at Baihui and Qubin may be an
effective treatment for improving neurological deficits associated
with intracerebral hemorrhage. A different study (104) demonstrated that 3D
spheroid-cultured mesenchymal stem cells (MSCs) reduced the level
of microglial Mincle, thereby enhancing the anti-inflammatory
effect of MSCs on microglia and improving their homing ability.
These effects were associated with improved therapeutic outcomes in
the ischemic hemisphere of rats. Xie et al (33) found that human albumin attenuates
SAP130-induced Mincle upregulation and subsequent microglial
inflammatory response after SAH by inhibiting Mincle/Syk/IL-1β
signaling. Collectively, several neuroprotective therapeutic
approaches alleviate brain damage by inhibiting the Mincle
signaling pathway.
In Table II,
studies on treatment through Mincle-related pathways are
summarized, including Chinese herbal extracts (curcumin,
artesunate, oridonin, ISL), natural monomers (quercetin), Chinese
herbal compounds (A&P), a pair of acupuncture points (Baihui
and Qubin), Mincle inhibitors (BAY61-3606), MSCs and
Mincle-neutralizing antibodies. These treatments act mainly through
the Mincle/Syk pathway to relieve inflammation and thus serve a
therapeutic role. However, more studies are needed to confirm the
role of Mincle in these diseases.
 | Table II.Summary of research on disease
treatment through Mincle-related pathways. |
Table II.
Summary of research on disease
treatment through Mincle-related pathways.
|
| Intervention
drug | Intervened
pathway | Alleviated disease
model | (Refs.) |
|---|
| Kidney | Curcumin | Mincle/Syk/NF-κB
signaling | Cisplatin-induced
AKI | (91) |
|
| Quercetin | Mincle/Syk/NF-κB
signaling | Cisplatin-induced
AKI | (92) |
|
| A&P | Mincle/Syk/NF-κB
signaling | Cisplatin-induced
AKI | (93) |
|
| Artesunate |
Mincle/RIPK1/RIPK3/MLKL signaling | Cisplatin-induced
AKI | (94) |
|
| BAY61-3606 | Mincle/Syk/NF-κB
signaling | IRI-induced
AKI | (95) |
|
| Oridonin | Mincle signaling
pathway | Renal IRI | (96) |
|
| A&P combined
with | Mincle/NF-κB
signaling | Chronic renal
failure | (97) |
|
|
Bifidobacterium |
|
|
|
|
| A&P | Mincle/CARD9/NFκB
signaling | Diabetic
nephropathy | (98) |
|
|
Isoliquiritigenin | Mincle/Syk/NF-kappa
B signaling | UUO-induced CKD
with renal fibrosis | (87) |
| Brain | BMSCs pretreated
with IronQ | Mincle/Syk
signaling pathway | Inflammation and
neurological deficits after ICH | (99) |
|
| Acupuncture (Baihui
and Qubin points) |
Mincle/Syk/CARD9/IL-1β signaling | Neurological
deficits and brain edema after ICH | (103) |
|
| 3D spheroid
cultured MSCs | Mincle signaling
pathway | Ischemia injury in
the brain | (104) |
|
| Human albumin | Mincle/Syk/IL-1β
signaling pathway | Inflammatory
response after SAH | (33) |
|
| BAY61-3606
(BAY) | Mincle/Syk
signaling | Neuroinflammation
after TBI | (60) |
| Autoimmune
diseases | Mincle-neutralizing
antibody | Mincle-Syk
pathway | Psoriasis | (73) |
Inflammatory and fibrotic axis
Inflammation is a normal physiological response,
which is a protective response of the body to external stimuli
(39). The inflammatory response
usually includes processes such as the release of inflammatory
mediators, infiltration of leukocytes and tissue repair. However,
when the inflammatory response lasts too long or occurs frequently,
it leads to tissue damage and persistence of the inflammatory
response, which promotes the transition from inflammation to
fibrosis (105).
Fibrosis is a pathological process, a common stage
in the progression of inflammatory lesions in a variety of organs,
which usually occurs after prolonged or repetitive tissue injury
and is typically characterized by the deposition of collagen and
ECM (106). The pathological
process of fibrosis is the activation of macrophages and the
release of numerous cytokines such as transforming growth factor-β
and IL-1β after endogenous/exogenous factors damage the organ
(107). These cytokines converge
the intrinsic cells in the organ to fibroblasts through receptors
on the surface of the cell membrane, leading to activation of the
intrinsic cells, production of large amounts of collagen and ECM
and formation of fibrosis in the organ (39) (Fig.
4). This process usually leads to changes in tissue structure
and loss of function, which affects the normal physiological
functions of the organism.
The inflammatory-to-fibrotic transition plays an
important role in numerous diseases such as liver fibrosis, lung
fibrosis and cardiac fibrosis, which are all closely related to
each other (108–110). Therefore, it is important to
study the mechanism of inflammation-to-fibrosis transition to
prevent the occurrence of fibrosis.
It is well known that molecules on the immune
pathway such as programmed cell death protein 1/programmed cell
death ligand 1 (PD-1/PD-L1) (111) or NY-ESO-1/MAGEA (112) play an important role in disease
development and intervention. Similar to Mincle, PD-1/PD-L1
mediates inflammation and fibrosis-related diseases to a certain
extent. A previous study demonstrated that the activation of the
PD-1/PD-L1 signaling pathway by MSCs effectively attenuated the
inflammatory response in lung tissue, leading to a reduction in
fibrosis (113). The PD-1
signaling pathway holds significant value as a biomarker for
assessing immune status in patients diagnosed with sepsis or septic
shock, playing a pivotal role in risk stratification and prognostic
prediction of patients with sepsis (114). This observation also implies the
potential feasibility of activating the PD-1/PD-L1 signaling
pathway as a means of attenuating the inflammatory response for the
therapeutic management of pulmonary fibrosis. Mincle senses dead
cells through its primary endogenous ligand SAP130, the expression
of which is involved in the presentation of cancer cell antigens to
cells of the immune system (115). SAP130 is a histone deacetylase
protein involved in the regulation of gene transcription and
chromatin structure and is closely related to biological processes
such as cell proliferation, differentiation and apoptosis. There
are no clear conclusions about the role and value of SAP130 in
fibroma therapy and it may be a potential topic to be investigated.
NY-ESO-1/MAGEA belongs to a group of cancer-testis antigens,
primarily expressed in germ cells and placental cells and
aberrantly expressed in malignant tumor cells. Moreover, these
antigens have been explored as potential targets for tumor
immunotherapy (111,116). However, it is unclear whether the
immune system of NY-ESO-1 and MAGEA-4 is effective during
inflammation and fibrogenesis. There is no clear evidence for their
role in the fibrotic process.
Altogether, Mincle is closely associated with
inflammation and fibrosis, including the progression of
inflammation to fibrosis. It promotes inflammatory responses and
fibrosis by activating inflammatory signaling pathways and inducing
the production of inflammatory cytokines. In addition, Mincle can
interact with other molecules involved in the regulation of
inflammation and fibrosis. These findings provide an important
theoretical basis for further exploration of the mechanisms
underlying the excessive progression of inflammation to fibrosis
and for the development of related therapies. In the future, Mincle
may offer new insights into halting the progression of inflammation
to fibrosis.
Conclusion
Mincle is known as a multifunctional receptor. It
plays a crucial role in eliminating pathogens and preserving immune
balance by recognizing various pathogens such as bacteria, fungi,
parasites, and even endogenous ligands. As research has advanced,
the importance and adaptability of Mincle in immune response have
become more apparent, sparking greater interest in its potential
use in treating different diseases. Mincle ligands are being
increasingly recognized as natural lipids produced by pathogens.
Mincle agonists have been deliberately designed to attain
comparable or enhanced levels of immune activation compared to
natural core factors, considering the arrangement of these ligands.
Additionally, the process of simplifying structures has already
begun. Researchers have shown significant interest in trehalose
dibehenate (TDB), due to its minimal toxicity and aim to utilize
TDB as an adjuvant in subunit vaccines for tumors and some
infectious diseases (27). CAF01,
a mixture of TDB and liposome, has shown promise in stimulating
both humoral and cellular immunity, rendering it suitable as an
adjuvant in vaccines for infectious diseases and potentially for
cancer immunotherapy, including adjuvant vaccines targeting
tumor-associated carbohydrate antigens (71).
Nevertheless, the investigation and revelation of
inhibitors for Mincle signaling in sterile inflammatory
circumstances have not been extensively explored. Due to its
ability to cause inflammation in sterile inflammatory conditions,
Mincle signaling is associated with various diseases, promoting the
progression of inflammation to fibrosis, ultimately resulting in
organ failure. The discovery of small compound antagonists for
Mincle signal transmission could hold immense importance in
reducing inflammation, prolonging the advancement of inflammation
into fibrosis and halting the progression of organ fibrosis.
Moreover, Mincle has been shown to interact with other receptors
and signaling pathways involved in inflammation and fibrosis. These
findings suggest that Mincle is a central player in the
inflammatory-fibrotic axis and targeting Mincle could be a
promising strategy to treat inflammatory and fibrotic diseases.
However, the exact mechanisms by which Mincle promotes inflammation
and fibrosis are still not fully understood. Further in-depth
research is necessary to elucidate the downstream signaling
pathways of Mincle and to identify the cell types and contexts in
which Mincle exerts its proinflammatory and fibrotic effects.
Additionally, the development of Mincle inhibitors or antagonists
requires careful consideration of potential side effects.
In conclusion, Mincle is an important mediator of
inflammation and fibrosis and represents a potential therapeutic
target. Understanding the molecular and cellular mechanisms of
Mincle will not only shed light on the pathogenesis of inflammatory
and fibrotic diseases, but also pave the way for the development of
novel anti-inflammatory and anti-fibrotic therapies.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr RuiZhi Tan
(Research Center for Integrated Traditional Chinese and Western
Medicine, The Affiliated Traditional Chinese Medicine Hospital of
Southwest Medical University, Luzhou, China), for his guidance in
the writing of this manuscript. The authors would also like to
thank Dr Maryam Mazhar (Institute of Integrated Chinese and Western
Medicine, Southwest Medical University, Luzhou, China), for
polishing the language of the manuscript.
Funding
The present study was funded by The National Traditional Chinese
Medicine Inheritance and Innovation Team (grant no.
ZYYCXTDC-202207), the Sichuan Science and Technology Program (grant
nos. 2022YFS0621, 21ZDYF0348 and 2022YFH0118), the National Natural
Science Foundation of China (grant no. 82205002), the
Luzhou-Southwest Medical University Science and Technology
Strategic Cooperation Project (grant. nos. 2021LZXNYD-P04 and
2021LZXNYD-D13) and the Southwest Medical University Natural
Science Youth Project (grant no. 2019ZQN179).
Availability of data and materials
Not applicable.
Authors' contributions
ND and LW conceived the review. YZ and JCL wrote the
original draft preparation. YZ and HS wrote, reviewed and edited
the manuscript. JL and HS helped to draw figures/generate tables
included in the article and revised the manuscript. All authors
read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Mincle
|
macrophage-inducible C-type
lectin
|
|
PRR
|
pattern recognition receptor
|
|
DAMPs
|
damage-associated molecular
patterns
|
|
PAMPs
|
pathogen-associated molecular
patterns
|
|
CRD
|
carbohydrate recognition domain
|
|
ITAM
|
immunoreceptor tyrosine-based
activation motif
|
|
ITIM
|
immunoreceptor tyrosine-based
inhibitory motif
|
|
CARD9
|
caspase-recruitment domain 9
|
|
Syk
|
splenic tyrosine kinase
|
|
APCs
|
antigen-presenting cells
|
|
ECM
|
extracellular matrix
|
|
UUO
|
unilateral ureteral obstruction
|
|
CIAKI
|
cisplatin-induced acute kidney
injury
|
|
IRI
|
ischemia reperfusion injury
|
|
CKD
|
chronic kidney disease
|
|
TBI
|
traumatic brain injury
|
|
A&P
|
Astragalus mongholicus Bunge
and Panax notoginseng formula
|
|
IronQ
|
iron(III)-quercetin complexes
|
|
MS
|
multiple sclerosis
|
|
KCs
|
Kupffer cells
|
|
ROS
|
reactive oxygen species
|
|
ACLF
|
acute-on-chronic liver failure
|
|
CLS
|
crown-like structures
|
|
NASH
|
non-alcoholic steatohepatitis
|
|
BMDMs
|
bone marrow-derived macrophages
|
|
mTECs
|
macrophages and renal tubular
epithelial cells
|
|
MF
|
myofibroblast
|
|
RA
|
rheumatoid arthritis
|
|
MMT
|
macrophage to MF transition
|
|
SAP130
|
spliceosome-associated protein
130
|
References
|
1
|
Oishi Y and Manabe I: Macrophages in
inflammation, repair and regeneration. Int Immunol. 30:511–528.
2018. View Article : Google Scholar
|
|
2
|
Gao Q, Mok HP and Zhuang J: Secreted
modular calcium-binding proteins in pathophysiological processes
and embryonic development. Chin Med J (Engl). 132:2476–2484. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamasaki S, Ishikawa E, Sakuma M, Hara H,
Ogata K and Saito T: Mincle is an ITAM-coupled activating receptor
that senses damaged cells. Nat Immunol. 9:1179–1188. 2008.
View Article : Google Scholar
|
|
4
|
Ishikawa E, Ishikawa T, Morita YS,
Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y
and Yamasaki S: Direct recognition of the mycobacterial glycolipid,
trehalose dimycolate, by C-type lectin Mincle. J Exp Med.
206:2879–2888. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Werninghaus K, Babiak A, Gross O, Hölscher
C, Dietrich H, Agger EM, Mages J, Mocsai A, Schoenen H, Finger K,
et al: Adjuvanticity of a synthetic cord factor analogue for
subunit Mycobacterium tuberculosis vaccination requires
FcRgamma-Syk-Card9-dependent innate immune activation. J Exp Med.
206:89–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lefèvre L, Lugo-Villarino G, Meunier E,
Valentin A, Olagnier D, Authier H, Duval C, Dardenne C, Bernad J,
Lemesre JL, et al: The C-type lectin receptors dectin-1, MR, and
SIGNR3 contribute both positively and negatively to the macrophage
response to Leishmania infantum. Immunity. 38:1038–1049. 2013.
View Article : Google Scholar
|
|
7
|
Seifert L, Werba G, Tiwari S, Giao Ly NN,
Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH,
et al: The necrosome promotes pancreatic oncogenesis via CXCL1 and
Mincle-induced immune suppression. Nature. 532:245–249. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee WB, Kang JS, Choi WY, Zhang Q, Kim CH,
Choi UY, Kim-Ha J and Kim YJ: Mincle-mediated translational
regulation is required for strong nitric oxide production and
inflammation resolution. Nat Commun. 7:113222016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar H, Kawai T and Akira S: Pathogen
recognition by the innate immune system. Int Rev Immunol. 30:16–34.
2011. View Article : Google Scholar
|
|
10
|
Matsumoto M, Tanaka T, Kaisho T, Sanjo H,
Copeland NG, Gilbert DJ, Jenkins NA and Akira S: A novel
LPS-inducible C-type lectin is a transcriptional target of NF-IL6
in macrophages. J Immunol. 163:5039–5048. 1999. View Article : Google Scholar
|
|
11
|
Honjoh C, Chihara K, Yoshiki H, Yamauchi
S, Takeuchi K, Kato Y, Hida Y, Ishizuka T and Sada K: Association
of C-type lectin Mincle with FcεRIβγ subunits leads to functional
activation of RBL-2H3 cells through Syk. Sci Rep. 7:460642017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamasaki S, Matsumoto M, Takeuchi O,
Matsuzawa T, Ishikawa E, Sakuma M, Tateno H, Uno J, Hirabayashi J,
Mikami Y, et al: C-type lectin Mincle is an activating receptor for
pathogenic fungus, Malassezia. Proc Natl Acad Sci USA.
106:1897–1902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saijo S, Ikeda S, Yamabe K, Kakuta S,
Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, et
al: Dectin-2 recognition of alpha-mannans and induction of Th17
cell differentiation is essential for host defense against
Candida albicans. Immunity. 32:681–691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gross O, Gewies A, Finger K, Schäfer M,
Sparwasser T, Peschel C, Förster I and Ruland J: Card9 controls a
non-TLR signalling pathway for innate anti-fungal immunity. Nature.
442:651–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hara H, Ishihara C, Takeuchi A, Imanishi
T, Xue L, Morris SW, Inui M, Takai T, Shibuya A, Saijo S, et al:
The adaptor protein CARD9 is essential for the activation of
myeloid cells through ITAM-associated and Toll-like receptors. Nat
Immunol. 8:619–629. 2007. View
Article : Google Scholar
|
|
16
|
Schoenen H, Bodendorfer B, Hitchens K,
Manzanero S, Werninghaus K, Nimmerjahn F, Agger EM, Stenger S,
Andersen P, Ruland J, et al: Cutting edge: Mincle is essential for
recognition and adjuvanticity of the mycobacterial cord factor and
its synthetic analog trehalose-dibehenate. J Immunol.
184:2756–2760. 2010. View Article : Google Scholar
|
|
17
|
Wells CA, Salvage-Jones JA, Li X, Hitchens
K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold
C, et al: The macrophage-inducible C-type lectin, mincle, is an
essential component of the innate immune response to Candida
albicans. J Immunol. 180:7404–7413. 2008. View Article : Google Scholar
|
|
18
|
Kiyotake R, Oh-Hora M, Ishikawa E,
Miyamoto T, Ishibashi T and Yamasaki S: Human Mincle binds to
cholesterol crystals and triggers innate immune responses. J Biol
Chem. 290:25322–25332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kostarnoy AV, Gancheva PG, Lepenies B,
Tukhvatulin AI, Dzharullaeva AS, Polyakov NB, Grumov DA, Egorova
DA, Kulibin AY, Bobrov MA, et al: Receptor Mincle promotes skin
allergies and is capable of recognizing cholesterol sulfate. Proc
Natl Acad Sci USA. 114:E2758–E2765. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Richardson MB, Torigoe S, Yamasaki S and
Williams SJ: Mycobacterium tuberculosis β-gentiobiosyl
diacylglycerides signal through the pattern recognition receptor
Mincle: Total synthesis and structure activity relationships. Chem
Commun (Camb). 51:15027–15030. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Behler-Janbeck F, Takano T, Maus R,
Stolper J, Jonigk D, Tort Tarrés M, Fuehner T, Prasse A, Welte T,
Timmer MS, et al: C-type lectin mincle recognizes
glucosyl-diacylglycerol of Streptococcus pneumoniae and
plays a protective role in pneumococcal pneumonia. PLoS Pathog.
12:e10060382016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shah S, Nagata M, Yamasaki S and Williams
SJ: Total synthesis of a cyclopropane-fatty acid α-glucosyl
diglyceride from Lactobacillus plantarum and identification of its
ability to signal through Mincle. Chem Commun (Camb).
52:10902–10905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jacobsen KM, Keiding UB, Clement LL,
Schaffert ES, Rambaruth ND, Johannsen M, Drickamer K and Poulsen
TB: The natural product brartemicin is a high affinity ligand for
the carbohydrate-recognition domain of the macrophage receptor
mincle. Medchemcomm. 6:647–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, He L, Hu S, Wang Y, Lai Q, Yang
P, Yu Q, Zhang S, Xiong F, Simsekyilmaz S, et al: AAL exacerbates
pro-inflammatory response in macrophages by regulating
Mincle/Syk/Card9 signaling along with the Nlrp3 inflammasome
assembly. Am J Transl Res. 7:1812–1825. 2015.PubMed/NCBI
|
|
25
|
Nagata M, Izumi Y, Ishikawa E, Kiyotake R,
Doi R, Iwai S, Omahdi Z, Yamaji T, Miyamoto T, Bamba T and Yamasaki
S: Intracellular metabolite β-glucosylceramide is an endogenous
Mincle ligand possessing immunostimulatory activity. Proc Natl Acad
Sci USA. 114:E3285–E3294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishikawa T, Itoh F, Yoshida S, Saijo S,
Matsuzawa T, Gonoi T, Saito T, Okawa Y, Shibata N, Miyamoto T and
Yamasaki S: Identification of distinct ligands for the C-type
lectin receptors Mincle and dectin-2 in the pathogenic fungus
Malassezia. Cell Host Microbe. 13:477–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Williams SJ: Sensing lipids with Mincle:
Structure and function. Front Immunol. 8:16622017. View Article : Google Scholar
|
|
28
|
Li S, Zhang Y, Lu R, Lv X, Lei Q, Tang D,
Dai Q, Deng Z, Liao X, Tu S, et al: Peroxiredoxin 1 aggravates
acute kidney injury by promoting inflammation through
Mincle/Syk/NF-κB signaling. Kidney Int. 104:305–323. 2023.
View Article : Google Scholar
|
|
29
|
Greco SH, Torres-Hernandez A, Kalabin A,
Whiteman C, Rokosh R, Ravirala S, Ochi A, Gutierrez J, Salyana MA,
Mani VR, et al: Mincle signaling promotes Con A hepatitis. J
Immunol. 197:2816–2827. 2016. View Article : Google Scholar
|
|
30
|
Zhou H, Yu M, Zhao J, Martin BN,
Roychowdhury S, McMullen MR, Wang E, Fox PL, Yamasaki S, Nagy LE
and Li X: IRAKM-Mincle axis links cell death to inflammation:
Pathophysiological implications for chronic alcoholic liver
disease. Hepatology. 64:1978–1993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Te Velde AA: The C-type lectin Mincle:
Clues for a role in Crohn's disease adjuvant reaction. Front
Immunol. 8:13042017. View Article : Google Scholar
|
|
32
|
Arumugam TV, Manzanero S, Furtado M,
Biggins PJ, Hsieh YH, Gelderblom M, MacDonald KP, Salimova E, Li
YI, Korn O, et al: An atypical role for the myeloid receptor Mincle
in central nervous system injury. J Cereb Blood Flow Metab.
37:2098–2111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie Y, Guo H, Wang L, Xu L, Zhang X, Yu L,
Liu Q, Li Y, Zhao N, Zhao N, et al: Human albumin attenuates
excessive innate immunity via inhibition of microglial Mincle/Syk
signaling in subarachnoid hemorrhage. Brain Behav Immun.
60:346–360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li C, Xue VW, Wang QM, Lian GY, Huang XR,
Lee TL, To KF, Tang PM and Lan HY: The Mincle/Syk/NF-κB signaling
circuit is essential for maintaining the protumoral activities of
tumor-associated macrophages. Cancer Immunol Res. 8:1004–1017.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sethi G, Sung B and Aggarwal BB: TNF: A
master switch for inflammation to cancer. Front Biosci.
13:5094–5107. 2008. View
Article : Google Scholar
|
|
36
|
Shapiro H, Lutaty A and Ariel A:
Macrophages, meta-inflammation, and immuno-metabolism.
ScientificWorldJournal. 11:2509–2529. 2011. View Article : Google Scholar
|
|
37
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View Article : Google Scholar
|
|
38
|
Luyendyk JP, Schoenecker JG and Flick MJ:
The multifaceted role of fibrinogen in tissue injury and
inflammation. Blood. 133:511–520. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mack M: Inflammation and fibrosis. Matrix
Biol. 68–69. 106–121. 2018.
|
|
40
|
Chen GY and Nuñez G: Sterile inflammation:
Sensing and reacting to damage. Nat Rev Immunol. 10:826–837. 2010.
View Article : Google Scholar
|
|
41
|
Drouin M, Saenz J and Chiffoleau E: C-type
lectin-like receptors: Head or tail in cell death immunity. Front
Immunol. 11:2512020. View Article : Google Scholar
|
|
42
|
Zhang J, Jiang J, Wang B, Wang Y, Qian Y,
Suo J, Li Y and Peng Z: SAP130 released by ferroptosis tubular
epithelial cells promotes macrophage polarization via Mincle
signaling in sepsis acute kidney injury. Int Immunopharmacol.
129:1115642024. View Article : Google Scholar
|
|
43
|
Lv LL, Tang PMK, Li CJ, You YK, Li J,
Huang XR, Ni J, Feng M, Liu BC and Lan HY: The pattern recognition
receptor, Mincle, is essential for maintaining the M1 macrophage
phenotype in acute renal inflammation. Kidney Int. 91:587–602.
2017. View Article : Google Scholar
|
|
44
|
Lv LL, Wang C, Li ZL, Cao JY, Zhong X,
Feng Y, Chen J, Tang TT, Ni HF, Wu QL, et al: SAP130 released by
damaged tubule drives necroinflammation via miRNA-219c/Mincle
signaling in acute kidney injury. Cell Death Dis. 12:8662021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tanaka M, Saka-Tanaka M, Ochi K, Fujieda
K, Sugiura Y, Miyamoto T, Kohda H, Ito A, Miyazawa T, Matsumoto A,
et al: C-type lectin Mincle mediates cell death-triggered
inflammation in acute kidney injury. J Exp Med. 217:e201922302020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim JW, Roh YS, Jeong H, Yi HK, Lee MH,
Lim CW and Kim B: Spliceosome-associated protein 130 exacerbates
alcohol-induced liver injury by inducing NLRP3
inflammasome-mediated IL-1β in mice. Am J Pathol. 188:967–980.
2018. View Article : Google Scholar
|
|
47
|
Schierwagen R, Uschner FE, Ortiz C, Torres
S, Brol MJ, Tyc O, Gu W, Grimm C, Zeuzem S, Plamper A, et al: The
role of macrophage-inducible C-type lectin in different stages of
chronic liver disease. Front Immunol. 11:13522020. View Article : Google Scholar
|
|
48
|
Nati M, Haddad D, Birkenfeld AL, Koch CA,
Chavakis T and Chatzigeorgiou A: The role of immune cells in
metabolism-related liver inflammation and development of
non-alcoholic steatohepatitis (NASH). Rev Endocr Metab Disord.
17:29–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sharma A, Steichen AL, Jondle CN, Mishra
BB and Sharma J: Protective role of Mincle in bacterial pneumonia
by regulation of neutrophil mediated phagocytosis and extracellular
trap formation. J Infect Dis. 209:1837–1846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hollwedel FD, Maus R, Stolper J, Khan A,
Stocker BL, Timmer MSM, Lu X, Pich A, Welte T, Yamasaki S and Maus
UA: Overexpression of macrophage-inducible C-Type lectin mincle
aggravates proinflammatory responses to Streptococcus
pneumoniae with fatal outcome in mice. J Immunol.
205:3390–3399. 2020. View Article : Google Scholar
|
|
51
|
Rabes A, Zimmermann S, Reppe K, Lang R,
Seeberger PH, Suttorp N, Witzenrath M, Lepenies B and Opitz B: The
C-type lectin receptor Mincle binds to Streptococcus
pneumoniae but plays a limited role in the anti-pneumococcal
innate immune response. PLoS One. 10:e01170222015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fisher J, Card G, Liang Y, Trent B,
Rosenzweig H and Soong L: Orientia tsutsugamushi selectively
stimulates the C-type lectin receptor Mincle and type 1-skewed
proinflammatory immune responses. PLoS Pathog. 17:e10097822021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tanaka M: Molecular mechanism of
obesity-induced adipose tissue inflammation; the role of Mincle in
adipose tissue fibrosis and ectopic lipid accumulation. Endocr J.
67:107–111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ichioka M, Suganami T, Tsuda N, Shirakawa
I, Hirata Y, Satoh-Asahara N, Shimoda Y, Tanaka M, Kim-Saijo M,
Miyamoto Y, et al: Increased expression of macrophage-inducible
C-type lectin in adipose tissue of obese mice and humans. Diabetes.
60:819–826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Capizzi A, Woo J and Verduzco-Gutierrez M:
Traumatic brain injury: An overview of epidemiology,
pathophysiology, and medical management. Med Clin North Am.
104:213–238. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Khellaf A, Khan DZ and Helmy A: Recent
advances in traumatic brain injury. J Neurol. 266:2878–2889. 2019.
View Article : Google Scholar
|
|
57
|
Corrigan F, Mander KA, Leonard AV and Vink
R: Neurogenic inflammation after traumatic brain injury and its
potentiation of classical inflammation. J Neuroinflammation.
13:2642016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Postolache TT, Wadhawan A, Can A, Lowry
CA, Woodbury M, Makkar H, Hoisington AJ, Scott AJ, Potocki E,
Benros ME and Stiller JW: Inflammation in traumatic brain injury. J
Alzheimers Dis. 74:1–28. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
de Rivero Vaccari JC, Brand FJ III, Berti
AF, Alonso OF, Bullock MR and de Rivero Vaccari JP: Mincle
signaling in the innate immune response after traumatic brain
injury. J Neurotrauma. 32:228–236. 2015. View Article : Google Scholar
|
|
60
|
He X, Huang Y, Liu Y, Zhang X, Yue P, Ma
X, Miao Z, Long X, Yang Y, Wan X, et al: BAY61-3606 attenuates
neuroinflammation and neurofunctional damage by inhibiting
microglial Mincle/Syk signaling response after traumatic brain
injury. Int J Mol Med. 49:52022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
He Y, Xu L, Li B, Guo ZN, Hu Q, Guo Z,
Tang J, Chen Y, Zhang Y, Tang J and Zhang JH: Macrophage-inducible
C-Type lectin/spleen tyrosine kinase signaling pathway contributes
to neuroinflammation after subarachnoid hemorrhage in rats. Stroke.
46:2277–2286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Calsolaro V and Edison P:
Neuroinflammation in Alzheimer's disease: Current evidence and
future directions. Alzheimers Dement. 12:719–732. 2016. View Article : Google Scholar
|
|
63
|
Leng F and Edison P: Neuroinflammation and
microglial activation in Alzheimer disease: Where do we go from
here? Nat Rev Neurol. 17:157–172. 2021. View Article : Google Scholar
|
|
64
|
Marogianni C, Sokratous M, Dardiotis E,
Hadjigeorgiou GM, Bogdanos D and Xiromerisiou G: Neurodegeneration
and inflammation-an interesting interplay in Parkinson's disease.
Int J Mol Sci. 21:84212020. View Article : Google Scholar
|
|
65
|
Subhramanyam CS, Wang C, Hu Q and Dheen
ST: Microglia-mediated neuroinflammation in neurodegenerative
diseases. Semin Cell Dev Biol. 94:112–120. 2019. View Article : Google Scholar
|
|
66
|
Thadathil N, Nicklas EH, Mohammed S, Lewis
TL Jr, Richardson A and Deepa SS: Necroptosis increases with age in
the brain and contributes to age-related neuroinflammation.
Geroscience. 43:2345–2361. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Brett BL, Gardner RC, Godbout J,
Dams-O'Connor K and Keene CD: Traumatic brain injury and risk of
neurodegenerative disorder. Biol Psychiatry. 91:498–507. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhao Q, Bai J, Chen Y, Liu X, Zhao S, Ling
G, Jia S, Zhai F and Xiang R: An optimized herbal combination for
the treatment of liver fibrosis: Hub genes, bioactive ingredients,
and molecular mechanisms. J Ethnopharmacol. 297:1155672022.
View Article : Google Scholar
|
|
69
|
Zhang J, Xiao Y, Liu H, Xu L, Guo X, Gao
Y, Li M, Xu J, Qi Q and Lv P: Edaravone dexborneol alleviates
neuroinflammation by reducing neuroglial cell proliferation and
suppresses neuronal apoptosis/autophagy in vascular dementia rats.
Neurochem Res. 48:3113–3128. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang XL, Zhang L, Duan Y, Wang YJ and
Wang J: Association of pentraxin 3 with autoimmune diseases: A
systematic review and meta-analysis. Arch Med Res. 47:223–231.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Davidson A and Diamond B: Autoimmune
diseases. N Engl J Med. 345:340–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Xiao ZX, Miller JS and Zheng SG: An
updated advance of autoantibodies in autoimmune diseases. Autoimmun
Rev. 20:1027432021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tan RZ, Zhong X, Han RY, Xie KH, Jia J,
Yang Y, Cheng M, Yang CY, Lan HY and Wang L: Macrophages mediate
psoriasis via Mincle-dependent mechanism in mice. Cell Death
Discov. 9:1402023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Oh J, Vidal-Jordana A and Montalban X:
Multiple sclerosis: Clinical aspects. Curr Opin Neurol. 31:752–759.
2018. View Article : Google Scholar
|
|
75
|
Doshi A and Chataway J: Multiple
sclerosis, a treatable disease. Clin Med (Lond). 16 (Suppl
6):s53–s59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Dobson R and Giovannoni G: Multiple
sclerosis-a review. Eur J Neurol. 26:27–40. 2019. View Article : Google Scholar
|
|
77
|
N'Diaye M, Brauner S, Flytzani S, Kular L,
Warnecke A, Adzemovic MZ, Piket E, Min JH, Edwards W, Mela F, et
al: C-type lectin receptors Mcl and Mincle control development of
multiple sclerosis-like neuroinflammation. J Clin Invest.
130:838–852. 2020. View Article : Google Scholar
|
|
78
|
Jadidi-Niaragh F and Mirshafiey A: Th17
cell, the new player of neuroinflammatory process in multiple
sclerosis. Scand J Immunol. 74:1–13. 2011. View Article : Google Scholar
|
|
79
|
Gong W, Zheng T, Guo K, Fang M, Xie H, Li
W, Tang Q, Hong Z, Ren H, Gu G, et al: Corrigendum to: Mincle/Syk
signalling promotes intestinal mucosal inflammation through
induction of macrophage pyroptosis in Crohn's disease. J Crohns
Colitis. 16:10082022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wu XY, Guo JP, Yin FR, Lu XL, Li R, He J,
Liu X and Li ZG: Macrophage-inducible C-type lectin is associated
with anti-cyclic citrullinated peptide antibodies-positive
rheumatoid arthritis in men. Chin Med J (Engl). 125:3115–3119.
2012.PubMed/NCBI
|
|
81
|
Takata K, Takano S, Miyagi M, Mukai M,
Iwase D, Aikawa J, Ohashi Y, Inoue G, Takaso M and Uchida K:
Elevated macrophage-inducible C-type lectin expression in the
synovial tissue of patients with rheumatoid arthritis. Cent Eur J
Immunol. 46:470–473. 2021. View Article : Google Scholar
|
|
82
|
Wick G, Backovic A, Rabensteiner E, Plank
N, Schwentner C and Sgonc R: The immunology of fibrosis: Innate and
adaptive responses. Trends Immunol. 31:110–119. 2010. View Article : Google Scholar
|
|
83
|
Tao C, Xian H, Nian-Yu Z, Jia-Cui S, Dong
W and Hui-Ping L: C-type lectin Mincle initiates IL-17-mediated
inflammation in acute exacerbations of idiopathic pulmonary
fibrosis. Biomed Pharmacother. 159:1142532023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen T, Qiu H, Zhao MM, Chen SS, Wu Q,
Zhou NY, Lu LQ, Song JC, Tang DL, Weng D and Li HP: IL-17A
contributes to HSV1 infection-induced acute lung injury in a mouse
model of pulmonary fibrosis. J Cell Mol Med. 23:908–919. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tanaka M, Ikeda K, Suganami T, Komiya C,
Ochi K, Shirakawa I, Hamaguchi M, Nishimura S, Manabe I, Matsuda T,
et al: Macrophage-inducible C-type lectin underlies obesity-induced
adipose tissue fibrosis. Nat Commun. 5:49822014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Watanabe Y, Nagai Y, Honda H, Okamoto N,
Yamamoto S, Hamashima T, Ishii Y, Tanaka M, Suganami T, Sasahara M,
et al: Isoliquiritigenin attenuates adipose tissue inflammation in
vitro and adipose tissue fibrosis through inhibition of innate
immune responses in mice. Sci Rep. 6:230972016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liao Y, Tan RZ, Li JC, Liu TT, Zhong X,
Yan Y, Yang JK, Lin X, Fan JM and Wang L: Isoliquiritigenin
attenuates UUO-Induced renal inflammation and fibrosis by
inhibiting Mincle/Syk/NF-kappa B signaling pathway. Drug Des Devel
Ther. 14:1455–1468. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang YY, Jiang H, Pan J, Huang XR, Wang
YC, Huang HF, To KF, Nikolic-Paterson DJ, Lan HY and Chen JH:
Macrophage-to-myofibroblast transition contributes to interstitial
fibrosis in chronic renal allograft injury. J Am Soc Nephrol.
28:2053–2067. 2017. View Article : Google Scholar
|
|
89
|
Meng XM, Wang S, Huang XR, Yang C, Xiao J,
Zhang Y, To KF, Nikolic-Paterson DJ and Lan HY: Inflammatory
macrophages can transdifferentiate into myofibroblasts during renal
fibrosis. Cell Death Dis. 7:e24952016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Little K, Llorián-Salvador M, Tang M, Du
X, Marry S, Chen M and Xu H: Macrophage to myofibroblast transition
contributes to subretinal fibrosis secondary to neovascular
age-related macular degeneration. J Neuroinflammation. 17:3552020.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tan RZ, Liu J, Zhang YY, Wang HL, Li JC,
Liu YH, Zhong X, Zhang YW, Yan Y, Lan HY and Wang L: Curcumin
relieved cisplatin-induced kidney inflammation through inhibiting
Mincle-maintained M1 macrophage phenotype. Phytomedicine.
52:284–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tan RZ, Wang C, Deng C, Zhong X, Yan Y,
Luo Y, Lan HY, He T and Wang L: Quercetin protects against
cisplatin-induced acute kidney injury by inhibiting
Mincle/Syk/NF-κB signaling maintained macrophage inflammation.
Phytother Res. 34:139–152. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hui D, Rui-Zhi T, Jian-Chun L, Xia Z, Dan
W, Jun-Ming F and Li W: Astragalus propinquus Schischkin and
Panax notoginseng (A&P) compound relieved
cisplatin-induced acute kidney injury through inhibiting the mincle
maintained macrophage inflammation. J Ethnopharmacol.
252:1126372020. View Article : Google Scholar
|
|
94
|
Lei XY, Tan RZ, Jia J, Wu SL, Wen CL, Lin
X, Wang H, Shi ZJ, Li B, Kang Y and Wang L: Artesunate relieves
acute kidney injury through inhibiting macrophagic Mincle-mediated
necroptosis and inflammation to tubular epithelial cell. J Cell Mol
Med. 25:8775–8788. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tan RZ, Li JC, Liu J, Lei XY, Zhong X,
Wang C, Yan Y, Linda Ye L, Darrel Duan D, Lan HY and Wang L:
BAY61-3606 protects kidney from acute ischemia/reperfusion injury
through inhibiting spleen tyrosine kinase and suppressing
inflammatory macrophage response. FASEB J. Sep 15–2020.(Epub ahead
of print). View Article : Google Scholar
|
|
96
|
Tan RZ, Yan Y, Yu Y, Diao H, Zhong X, Lin
X, Liao YY and Wang L: Renoprotective effect of oridonin in a mouse
model of acute kidney injury via suppression of macrophage involved
inflammation. Biol Pharm Bull. 44:714–723. 2021. View Article : Google Scholar
|
|
97
|
Rui-Zhi T, Hui D, Jian-Chun L, Xia Z,
Xiao-Jia W, Dan W, Jun-Ming F and Li W: Astragalus
mongholicus bunge and Panax notoginseng formula
(A&P) combined with bifidobacterium contribute a renoprotective
effect in chronic kidney disease through inhibiting macrophage
inflammatory response in kidney and intestine. Front Physiol.
11:5836682020. View Article : Google Scholar
|
|
98
|
Lin X, Lei XQ, Yang JK, Jia J, Zhong X,
Tan RZ and Wang L: Astragalus mongholicus bunge and Panax
notoginseng formula (A&P) improves renal mesangial cell
damage in diabetic nephropathy by inhibiting the inflammatory
response of infiltrated macrophages. BMC Complement Med Ther.
22:172022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yang G, Kantapan J, Mazhar M, Bai X, Zou
Y, Wang H, Huang B, Yang S, Dechsupa N and Wang L: Mesenchymal stem
cells transplantation combined with IronQ attenuates ICH-induced
inflammation response via Mincle/syk signaling pathway. Stem Cell
Res Ther. 14:1312023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Papan P, Kantapan J, Sangthong P,
Meepowpan P and Dechsupa N: Iron (III)-quercetin complex:
Synthesis, physicochemical characterization, and MRI cell tracking
toward potential applications in regenerative medicine. Contrast
Media Mol Imaging. 2020:88778622020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kantapan J, Anukul N, Leetrakool N, Rolin
G, Vergote J and Dechsupa N: Iron-quercetin complex preconditioning
of human peripheral blood mononuclear cells accelerates angiogenic
and fibroblast migration: Implications for wound healing. Int J Mol
Sci. 22:88512021. View Article : Google Scholar
|
|
102
|
Dechsupa N, Kosintarajit P, Kamkan K,
Khanjina T, Sirikul C, Innuan P, Suwan A, Anukul N and Kantapan J:
Iron(III)-quercetin complexes' safety for MRI cell tracking in cell
therapy applications: Cytotoxic and genotoxic assessment.
Nanomaterials (Basel). 12:27762022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Liu XY, Dai XH, Zou W, Yu XP, Teng W, Wang
Y, Yu WW, Ma HH, Chen QX, Liu P, et al: Acupuncture through Baihui
(DU20) to Qubin (GB7) mitigates neurological impairment after
intracerebral hemorrhage. Neural Regen Res. 13:1425–1232. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Li Y, Dong Y, Ran Y, Zhang Y, Wu B, Xie J,
Cao Y, Mo M, Li S, Deng H, et al: Three-dimensional cultured
mesenchymal stem cells enhance repair of ischemic stroke through
inhibition of microglia. Stem Cell Res Ther. 12:3582021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Antar SA, Ashour NA, Marawan ME and
Al-Karmalawy AA: Fibrosis: Types, effects, markers, mechanisms for
disease progression, and its relation with oxidative stress,
immunity, and inflammation. Int J Mol Sci. 24:40042023. View Article : Google Scholar
|
|
106
|
Wynn TA and Vannella KM: Macrophages in
tissue repair, regeneration, and fibrosis. Immunity. 44:450–462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pellicoro A, Ramachandran P, Iredale JP
and Fallowfield JA: Liver fibrosis and repair: Immune regulation of
wound healing in a solid organ. Nat Rev Immunol. 14:181–194. 2014.
View Article : Google Scholar
|
|
108
|
Savin IA, Zenkova MA and Sen'kova AV:
Pulmonary fibrosis as a result of acute lung inflammation:
Molecular mechanisms, relevant in vivo models, prognostic and
therapeutic approaches. Int J Mol Sci. 23:149592022. View Article : Google Scholar
|
|
109
|
Mohammed S, Thadathil N, Selvarani R,
Nicklas EH, Wang D, Miller BF, Richardson A and Deepa SS:
Necroptosis contributes to chronic inflammation and fibrosis in
aging liver. Aging Cell. 20:e135122021. View Article : Google Scholar
|
|
110
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067. 2011.
View Article : Google Scholar
|
|
111
|
Hashimoto K, Nishimura S, Shinyashiki Y,
Ito T, Kakinoki R and Akagi M: Clinicopathological assessment of
PD-1/PD-L1 immune checkpoint expression in desmoid tumors. Eur J
Histochem. 67:36882023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hashimoto K, Nishimura S, Shinyashiki Y,
Ito T, Kakinoki R and Akagi M: Involvement of NY-ESO-1 and MAGE-A4
in the pathogenesis of desmoid tumors. Medicine (Baltimore).
102:e339082023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ni K, Liu M, Zheng J, Wen L, Chen Q, Xiang
Z, Lam KT, Liu Y, Chan GC, Lau YL and Tu W: PD-1/PD-L1 pathway
mediates the alleviation of pulmonary fibrosis by human mesenchymal
stem cells in humanized mice. Am J Respir Cell Mol Biol.
58:684–695. 2018. View Article : Google Scholar
|
|
114
|
Nakamori Y, Park EJ and Shimaoka M: Immune
deregulation in sepsis and septic shock: Reversing immune paralysis
by targeting PD-1/PD-L1 pathway. Front Immunol. 11:6242792021.
View Article : Google Scholar
|
|
115
|
Roperto S, Russo V, Esposito I, Ceccarelli
DM, Paciello O, Avallone L, Capparelli R and Roperto F: Mincle, an
innate immune receptor, is expressed in urothelial cancer cells of
papillomavirus-associated urothelial tumors of cattle. PLoS One.
10:e01416242015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Thomas R, Al-Khadairi G, Roelands J,
Hendrickx W, Dermime S, Bedognetti D and Decock J: NY-ESO-1 based
immunotherapy of cancer: Current perspectives. Front Immunol.
9:9472018. View Article : Google Scholar
|