Introduction
Squamous cell carcinoma of the head and neck (HNSCC)
is the sixth most common malignancy worldwide, with ~650,000 new
cases diagnosed each year (1). These
tumors may originate in the oral cavity, oropharynx, hypopharynx or
larynx (2). The incidence of HNSCC in
Sweden is ~1,200 new cases per year (2), which accounts for 3–4% of all new cancer
diagnoses in the country. Worldwide, the incidence is two times
higher in men than in women (3).
Oropharyngeal cancer develops in the base of the
tongue and palatine tonsils, the posterior pharyngeal wall and the
soft palate. Established predisposing factors include heavy tobacco
smoking and alcohol consumption, which appear to act
synergistically (4). Tonsillar cancer
is the most common form of oropharyngeal cancer in Sweden (3). Typical symptoms include swallowing
difficulties, unilateral pain in the throat and ear, and lumps in
the neck. The incidence of tonsillar cancer appears to have been
increasing during recent years, despite a decline in smoking and
alcohol consumption. One possible factor contributing to this may
be mucosal infection with human papilloma virus (HPV) (5–8).
It has been proposed that HPV-positive tonsillar
carcinomas should be considered different tumor entities from
HPV-negative tonsillar carcinomas, as HPV-positive tumors arise in
the tonsillar crypts while the non-HPV-associated form originates
from the tonsillar surface (9). The
majority of HPV-positive tonsillar cancers are associated with the
high-risk HPV type 16, and this HPV type may be present in ~95% of
the HPV-positive cases, whilst other types, such as 18, 31, 33 and
35, are relatively infrequently associated with HNSCC (10).
Radiotherapy is the optimal and standard treatment
option at present, and may be combined with chemotherapy in more
advanced cases. To date, curative surgical monotherapy of tonsillar
cancer has been unsuccessful. However, recent results using
transoral robotic surgery as single modality treatment have
demonstrated a potential therapeutic benefit (11,12).
Certain reports have suggested that HPV-related malignancies have a
more favorable prognosis, regardless of the chosen treatment
strategy (13–15).
Currently, HPV is the most common sexually
transmitted disease; however, even the most sensitive DNA tests may
fail to detect infections caused by the virus (16). The recent introduction of vaccination
against HPV 16-associated anogenital cancers in non-infected
subjects has demonstrated a promising protective effect through the
prevention of infection. This approach may have a role in the
prevention of specific HPV-subtype-positive head and neck
malignancies in the future (17). The
use of HPV vaccines as preventive and therapeutic treatments has
been discussed in the literature (18,19).
The aim of the current study was first to assess the
incidence of tonsillar cancer in northern Sweden, one of western
Europe's most sparsely populated regions. Secondly, the study aimed
to address the recent incidence in this region compared with
reported incidences in densely populated regions. It further aimed
to assess the proportion of HPV-associated tonsillar cancers and
its surrogate marker, p16, and finally, to determine whether there
were variations in the manifestation of the disease associated with
urban versus rural areas in the region.
Materials and methods
Patients
The Regional Ethical Review Board of Umeå University
(Umeå, Sweden) approved the analysis of paraffin-embedded
retrospective samples from the Biobank North (County Council of
Västerbotten, Västerbotten, Sweden; approval nos. 2012-276-32M and
2010-277-31M). In addition, study was conducted in accordance with
the Declaration of Helsinki (20).
This retrospective observational study included all
consenting patients diagnosed with tonsillar cancer between 1990
and 2013 at the University Hospital of Umeå (Umeå, Sweden). In
order to identify cases of tonsillar neoplasms, information was
extracted from the Swedish Cancer Registry database (www.socialstyrelsen.se/register/halsodataregister/cancerregistret/inenglish)
using the International Classification of Diseases (ICD)-7 code
145.0. The designation ‘northern Sweden’ was defined as the part of
Sweden consisting of the counties Västerbotten, Norrbotten,
Västernorrland and Jämtland, which include a total population of
882,563 and ~4 inhabitants per square kilometer. For comparative
purposes, data from the Swedish Cancer Registry database regarding
the whole Swedish population of ~9.5 million was used (2013).
Pre-treatment tumor samples were collected by biopsy
or surgical resection. Paraffin-embedded tumor blocks were
retrieved from the archive of the Department of Laboratory
Medicine/Pathology at the University Hospital of Umeå. The clinical
characteristics of the study population are summarized in Table I.
 | Table I.Clinical characteristics of the
patients included in the HPV DNA and p16 analysis. |
Table I.
Clinical characteristics of the
patients included in the HPV DNA and p16 analysis.
| Specimen
number | Year of
diagnosis | % tumor cells in
sample | p16 quickscore | HPV | Gender | Age at diagnosis,
years |
|---|
| 1 | 2012 | 25a | 6 | 1 | F | 67 |
| 2 | 2012 | 70a | 12 | 1 | M | 64 |
| 3 | 2012 | 70a | 12 | 1 | M | 53 |
| 4 | 2012 | 50a | 12 | 1 | M | 51 |
| 6 | 2011 | 80a | 12 | 1 | M | 70 |
| 8 | 2012 | 40a | 12 | 1 | M | 53 |
| 9 | 2011 | 60a | 12 | 1 | M | 55 |
| 10 | 2011 | 30a | 12 | 1 | M | 54 |
| 12 | 2011 | 30a | 12 | 1 | M | 45 |
| 13 | 2011 | 70a | 12 | 1 | F | 65 |
| 14 | 2011 | 70a | 12 | 1 | M | 72 |
| 15 | 2012 | 80a | 12 | 1 | F | 76 |
| 16 | 2012 | 60a | 12 | 1 | F | 87 |
| 17 | 2012 | 95a | 6 | 1 | F | 65 |
| 18 | 2010 | 70a | 18 | 1 | M | 58 |
| 19 | 2010 | 20a | 12 | 1 | F | 75 |
| 20 | 2010 | 75a | 12 | 1 | M | 59 |
| 21 | 2010 | 80a | 12 | 1 | M | 70 |
| 23 | 2010 | 70a | 12 | 1 | M | 71 |
| 24 | 2010 | 50a | 12 | 1 | M | 49 |
| 25 | 2006 | 60a | 18 | 1 | M | 48 |
| 26 | 2006 | 80a | 12 | 1 | M | 55 |
| 27 | 2006 | 60a | 12 | 1 | F | 76 |
| 28 | 2007 | 60a | 0 | 0 | M | 51 |
| 29 | 2007 | 95a | 12 | 1 | M | 58 |
| 30 | 2007 | 5a/10b | 12 | 1 | M | 48 |
| 31 | 2007 | 60a | 12 | 1 | M | 64 |
| 33 | 2006 | 60a | 12 | 1 | M | 59 |
| 34 | 2006 | 75a | 12 | 1 | M | 60 |
| 35 | 2005 | 80a | 12 | 1 | M | 62 |
| 36 | 2005 | 80a | 12 | 1 | M | 47 |
| 37 | 2005 | 90a | 2 | 0 | F | 64 |
| 38 | 2005 | 75a | 12 | 1 | M | 58 |
| 39 | 2003 | 80a | 12 | 1 | M | 57 |
| 40 | 2002 | 20a | 18 | 1 | M | 61 |
| 41 | 2002 | 80a | 12 | 1 | F | 64 |
| 42 | 2001 | 50a | 0 | 0 | F | 53 |
| 43 | 2001 | 70a | 12 | 1 | F | 46 |
| 44 | 2001 | 80a | 5 | 1 | M | 58 |
| 45 | 2000 | 70a | 12 | 1 | F | 69 |
| 46 | 2000 | 85a | 12 | 1 | F | 63 |
| 47 | 2000 | 30a | 18 | 1 | M | 52 |
| 48 | 2001 | 60a | 12 | 1 | F | 68 |
| 49 | 2001 |
5a | 12 | 1 | F | 45 |
| 50 | 2004 | 60a | 2 | 0 | F | 54 |
| 51 | 2009 | 95a | 12 | 1 | M | 48 |
| 52 | 2009 | 80a | 12 | 1 | M | 53 |
| 54 | 2009 | 40a | 12 | 1 | M | 51 |
| 55 | 2001 | 30a | 12 | 1 | M | 74 |
| 56 | 2004 | 40a | 4 | 0 | M | 73 |
| 57 | 2008 | 80a | 12 | 1 | M | 60 |
| 58 | 2008 | 70a | 12 | 1 | M | 49 |
| 59 | 2008 | 80a | 12 | 1 | F | 53 |
| 60 | 2009 | 70a | 18 | 1 | M | 63 |
| 61 | 2008 | 10a/50b | 12 | 1 | M | 56 |
| 62 | 2009 | 40a | 12 | 1 | M | 63 |
| 63 | 2009 | 5a/15b | 12 | 1 | M | 60 |
| 64 | 2009 | 50a | 12 | 1 | M | 49 |
| 66 | 2009 | 60a | 12 | 0 | M | 63 |
| 68 | 2008 | 60a | 18 | 1 | M | 53 |
| 69 | 2008 | 70a | 12 | 1 | M | 51 |
| 70 | 2008 | 60a | 18 | 1 | M | 53 |
| 71 | 2010 | 60a | 0 | 1 | M | 66 |
| 73 | 2012 | 70a | 12 | 1 | M | 62 |
| 74 | 2002 | 20a | 12 | 1 | M | 56 |
HPV detection by polymerase chain
reaction (PCR)
DNA was extracted from paraffin-embedded diagnostic
biopsies using a QIAamp DNA FFPE Tissue Kit or QIAamp Mini Kit
(Qiagen, Inc., Valencia, CA, USA), according to the manufacturer's
instructions. A general HPV PCR analysis was run using 100 ng of
extracted DNA from each patient and the general primers GP5+/6+, as
previously described (21). The
primers were as follows: 5′-TTT GTT ACT GTG GTA GAT ACT AC-3′ for
GP5+ and 5′-GAA AAA TAA ACT GTA AAT CAT ATT C-3′ for GP6+. The 50
µl PCR mixture consisted of 5 µl GeneAmp 10X PCR Gold Buffer, 200
µM of each dNTP (GeneAmp dNTP mix), 3.5 mM MgCl2 (all
from Applied Biosystems Life Technologies, Foster City, CA, USA),
25 pmol of each primer and 1 unit of AmpliTaq Gold DNA Polymerase
(Applied Biosystems Life Technologies).
Amplification was performed in a Biometra
Professional Thermocycler (Thermo Fisher Scientific, Waltham, MA,
USA) and was initiated with denaturation for 4 min at 94°C,
followed by 40 amplification cycles of denaturation at 94°C for 1
min, annealing at 44°C for 1 min and elongation at 72°C for 2 min.
The final cycle ended with a prolonged elongation step at 72°C for
10 min. PCR products were run on a 2.5% agarose gel (SeaKem® LE
Agarose; Lonza, Rockland, ME, USA) in 0.5X Tris/Borate/EDTA-buffer
(TBE; 1 L of 10X TBE: 121.1 g tris base, 46 g boric acid and 7.44 g
EDTA made up to 1 L with distilled water), stained with 0.5X GelRed
(Biotium, Hayward, CA, USA), and visualized under UV-light.
Fragments of 130–150 bp were considered positive.
To avoid false negative results due to a disrupted
L1 gene, negative samples were run with the general primers
CpI/IIG, as described in Smits et al (22). The primers were as follows: 5′-TTA TCW
TAT GCC CAY TGT ACC AT-3′ for CpI and 5′-ATG TTA ATW SAG CCW CCA
AAA TT-3′ for CpIIG. Briefly, the PCR mixture consisted of 5 µl
GeneAmp 10X PCR Gold Buffer, 200 µM of each dNTP (GeneAmp dNTP
mix), 3 mM MgCl2, 17 pmol CpI, 26 pmol CpIIG, 0.5 µl
bovine serum albumin and 1 unit of AmpliTaq Gold DNA Polymerase.
The amplification consisted of denaturation for 5 min at 94°C,
followed by 40 amplification cycles of denaturation at 95°C for 1
min, annealing at 55°C for 1 min and elongation at 72°C for 2 min.
The final cycle ended with a prolonged elongation step at 72°C for
4 min. Fragments were analyzed as described for the previous PCR
analysis, and products of 188 bp were considered positive.
PCR with the GP5+/6+ primers was run at least twice.
In cases with weak or divergent results, the PCR was repeated.
Additionally, in random cases, a new DNA preparation was used to
validate the method.
p16 immunohistochemistry and scoring
system
For the detection of p16, staining was performed in
a Ventana staining machine (BenchMark ULTRA; Ventana Medical
Systems, Tuscon, AZ, USA) according to the supplier's
recommendations. An antibody against p16 (monoclonal mouse
anti-human; cat. no. sc-56330) from Santa Cruz Biotechnology
(Dallas, TX, USA) was used at a dilution of 1:200. Prior to
staining, slides were pretreated in Tris-EDTA (10 mM Tris-HCl, 1 mM
disodium EDTA; pH 8.0). The antibody was visualized using the
Ultraview Universal DAB Detection Kit (Ventana Medical Systems) and
staining was observed using a light microscope (BX51; Olympus
Corporation, Tokyo, Japan).
The quickscore system was used for scoring the
percentage of tumor cells expressing p16 and intensity of staining
(23). The proportion of tumor cells
expressing p16 was graded from 1–6: 1, 0–4%; 2, 5–19%; 3, 20–39%;
4, 40–59%; 5, 60–79%; or 6, 80–100%. Staining intensity in turn was
divided into 4 grades: 0, negative; 1, weak; 2, intermediate; and
3, strong. By multiplying the score for percentage of tumor cells
expressing the protein by the intensity score, a quickscore ranging
from 0–18 was determined.
Classification according to place of
residence
The code numbers of the biopsy samples were used to
identify the personal identification number of each patient and,
subsequently, the residence address was extracted through the
patients' electronic records system (Systeam Cross; Eskilstuna,
Sweden). Subjects were placed into either urban or rural groups
depending on the location of their private residence. A Swedish
urban area was defined as a residential area with ≥200 inhabitants
where the distance between buildings is <200 meters (24), whilst a rural address was one where
the number of local inhabitants was <200 and the distance
between houses was ≥200 meters (25).
Analysis and statistics
The total age-standardized incidence of tonsillar
cancer over the period from 1990–2013 was determined using northern
Sweden's standard population (2000) and data obtained from the
Swedish Cancer Registry. Data regarding the cases of head and neck
cancer was extracted from the Swedish Cancer Registry for
comparative purposes (2). The
Mann-Whitney U test was used to compare patient ages between
different genders and HPV-positive and HPV-negative patients. The
χ2 test was used to investigate any differences in
HPV-status between genders. Statistical analysis was performed
using SPSS software version 22.0 (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Incidence of HNSCC
From the period between 1990 and 2012, a total of
22,640 cases of head and neck cancer were identified in the Swedish
Cancer Registry database, including tumors located in the
hypopharynx, larynx, oropharynx, oral cavity, tongue, lips,
nasopharynx, nose, paranasal sinuses and middle ear. Of these,
15,563 (68.7%) were men and 7,077 (31.3%) women. Cases from 2013
were not yet registered. The total number of cases of head and neck
cancer in northern Sweden during the same period was 1,978 (9% of
all cases in Sweden). Of these patients, 1,297 (65.6%) were men and
681 (34.4%) were women.
The number of cases of tonsillar cancer (ICD-7 code
145.0) in northern Sweden between 1990 and 2013 according to the
Swedish Cancer Registry was 214, comprising 155 (72.4%) men and 59
(27.6%) women.
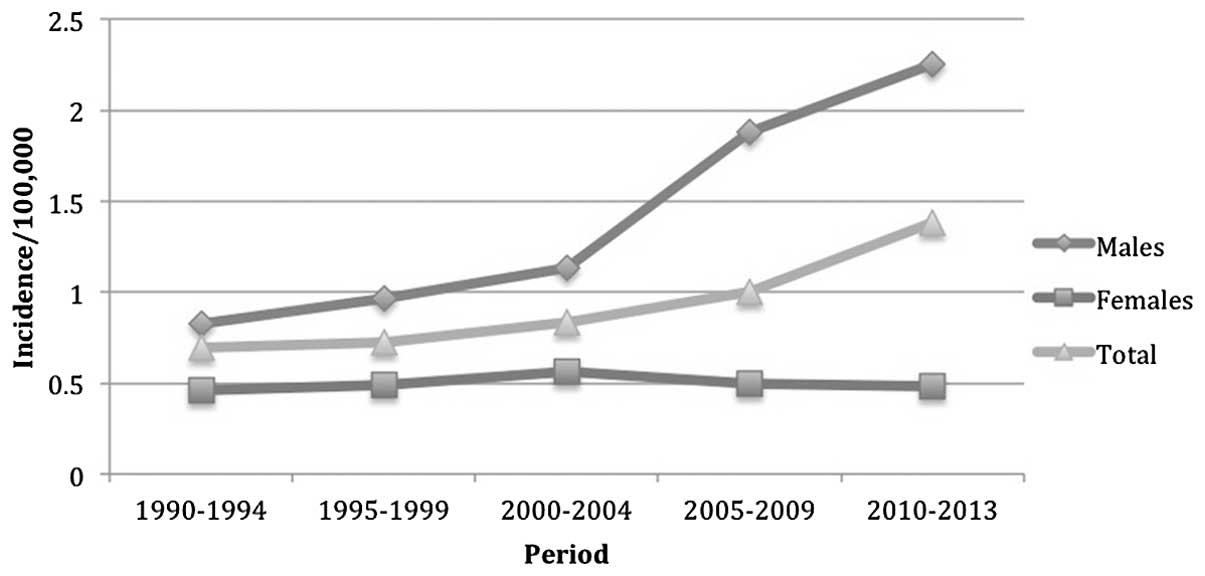
The total age-standardized incidence of tonsillar
cancer in northern Sweden doubled between 1990 and 2013, from
0.69–1.38 per 100,000 individuals. The increase in incidence in men
was 2.7-fold (0.83–2.25 per 100,000), while the female incidence
increased from 0.46 (1990) to 0.48 (2013) per 100,000 (Table II; Fig.
1).
 | Table II.Incidence of tonsillar cancer in
northern Sweden per 100,000 individuals, according to the
population of northern Sweden in 2000. |
Table II.
Incidence of tonsillar cancer in
northern Sweden per 100,000 individuals, according to the
population of northern Sweden in 2000.
|
| Period |
|---|
|
|
|
|---|
| Group | 1990–94 | 1995–99 | 2000-04 | 2005-04 | 2010-13 |
|---|
| Male | 0.826 | 0.968 | 1.13 | 1.88 | 2.25 |
| Female | 0.46 | 0.488 | 0.564 | 0.494 | 0.482 |
| Total | 0.692 | 0.724 | 0.83 | 0.998 | 1.379 |
HPV analysis
A total of 74 biopsy specimens from patients with
tonsillar cancer between 2000 and 2012 were identified and obtained
from the archives of the Department of Pathology, University
Hospital of Umeå. Of these, 4 specimens were excluded as they
contained too little or a complete lack of tumor tissue.
Additionally, 5 samples were excluded as they were duplicates of
patients already included in the database.
Of the 65 remaining specimens (median age, 58 years;
mean age, 59.3 years; range, 45–87 years), 48 (74%) were males
(median age, 57.5 years; mean, 57.6 years; range, 45–74 years) and
17 (26%) were females (median age, 65 years; mean, 64.1 years;
range, 45–87 years). Age was significantly higher among female
subjects compared with male subjects (U test, P=0.016).
HPV DNA was detected in 59 (91%) of the 65 biopsy
specimens, while the remaining 6 samples (9%) were HPV-negative.
There was no difference when comparing the variable of age between
the HPV-positive and HPV-negative samples (P=0.856, U test). A
χ2 test identified no association between HPV-status and
gender (P=0.179). In the HPV-positive cohort, there was a male
dominance [3:1; 45 males (76%) vs. 14 females (24%)], whilst an
equal proportion of males and females (1:1) was observed in the
HPV-negative cohort. When comparing gender and age in the
HPV-positive cohort, age was significantly higher in females
compared with in males (U test, P=0.006).
p16 expression
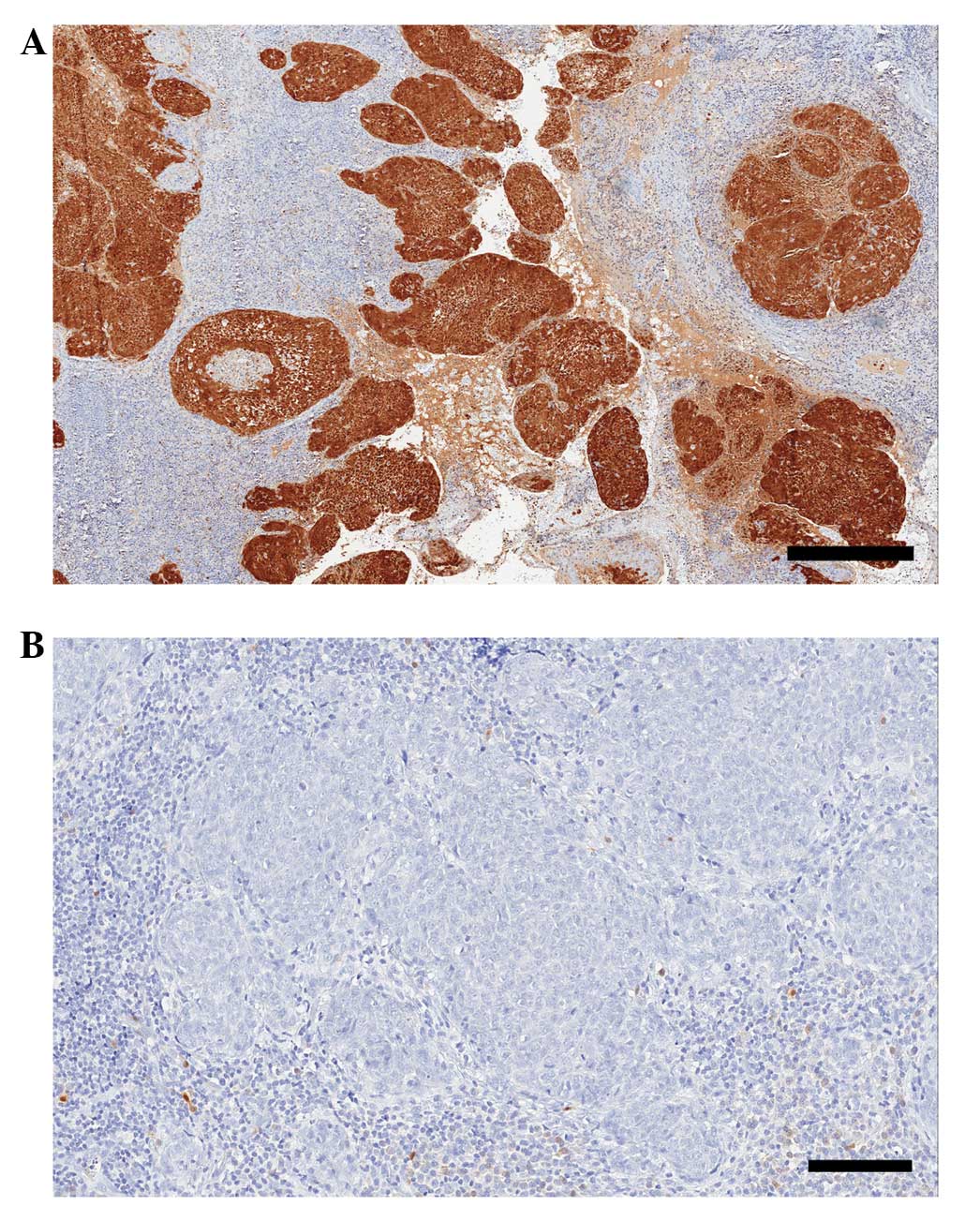
Expression of p16 was scored in all 65 samples, with
62 samples (95%) positive for the p16 marker and 3 (5%) negative
(Fig 2). Of the p16-positive samples,
7 (11%) received the highest score of 18 points, 49 (79%) a score
of 12 (intermediate intensity) and 6 samples (10%) received 2–6
points.
Classification according to
residence
The patients' residence area at the time of
diagnosis indicated that the vast majority (64 patients, 98.5%)
were living in urban areas, while only 1 patient (1.5%) resided in
a rural area. This difference is due to the overall populations of
these areas.
Discussion
The aims of the current study were to investigate
the proportion of p16-positive cases of HPV-related tonsillar
cancer, to study the incidence of tonsillar cancer in the sparsely
populated region of northern Sweden, and to examine whether this
increasing trend is parallel to the incidence observed in other,
more densely populated areas.
A 2-fold increase in cases of HPV-positive tonsillar
cancer was observed during the analyzed period of 23-years
(1990–2013), preferentially in male patients. Hammarstedt et
al (5) reported a fold increase
in tonsillar cancer incidence in the urban region of Stockholm of
2.8 (2.6 in men and 3.5 in women), while smoking had decreased in
Sweden during the same period (1970–2002) (26). In the current findings, the increased
incidence of tonsillar cancer was observed only in the male group.
The reason for this disparity is unclear, although the present
study included sampling from a more recent period of 11 years.
Another factor may be the relatively recent knowledge and treatment
patterns related to HPV 16 as a causative agent of cervical
cancer.
The findings demonstrating no recent change in the
incidence of tonsillar cancer in women in this region may be an
indication of the differences in HPV exposure and susceptibility
between sparsely populated and densely populated areas. This issue
has not been examined to date. The increased incidence of
HPV-related tonsillar cancer in males has been described in a study
examining ethnic perspectives (27),
in which a recent decline in incidence rates of tonsillar cancer
was also observed for both white and black females aged 40–64 years
in the USA. Hence, HPV infection in the oropharynx may be an
explanation for at least part of the increase in the incidence of
tonsillar cancer in men. Another possible explanation may be the
traditional risk factors of alcohol and smoking found in the male
population in northern Sweden, which could have a synergistic
effect with the HPV virus. At present, <20% of Swedish men smoke
tobacco. The significantly lower mean age of the males in the
current sample and, in particular, the lower mean age of the male
HPV-related cases, may be an indicator of an altered sexual
behavior and consequent frequent HPV contamination among men in
northern Sweden.
Hammarstedt et al (28) reported that patients with HPV-positive
tonsillar malignancies were younger (median age, 55 years) compared
with patients with HPV-negative disease, and no similar difference
regarding other oral cancers could be identified. This finding
could not be confirmed in the current data due to the limited
number of cases of HPV-negative tonsillar cancer.
The present results revealed that 91% of the cases
of tonsillar cancer were HPV-positive. A similarly high proportion
of HPV-positive cases of tonsillar cancer in Sweden has been
reported by Näsman et al (29), who identified an increase from 68%
(2000–2002) to 93% (2006–2007) (29).
In general, HPV DNA has been reported to be detected in 45–100% of
tonsillar cancer cases (30). It is
possible that the cohorts with a lower proportion of HPV-positive
cases are less recent, and this could indicate that the numbers of
HPV-related tonsillar cancer are increased in more current
published data due to the more reliable diagnostic methods
available.
Differences in HPV-related tonsillar malignancies
have been observed not only between urban and rural areas in
Sweden, but also in other countries. Blomberg et al
(7) described an increased incidence
rate of HPV related tonsillar cancer between 1978 and 2007 in
Denmark, while the incidence of non-HPV-associated HNSCC decreased
in men, but remained unchanged in the female group. Another study
of patterns of tonsillar cancer in South-eastern England, comparing
the years 1987 and 2006, revealed an incidence of tonsillar cancer
increasing from 0.600–1.45 per 100,000 among men (40–59 years), a
lower age at diagnosis and an increase in median survival time
(8). Similar studies have been
performed in the USA and Finland, demonstrating a rising incidence
of tonsillar cancer during the last decades (27,31).
Overexpression of p16 was observed in the present
tumor specimens, confirming the results of a number of previous
studies (4,32–36), as
well as demonstrating the strong association between p16 and
HPV-infection in tonsillar carcinomas. The majority of these
specimens exhibited p16 expression of medium intensity, while a
further 11% showed strong p16 expression.
In the last decade, a number of studies have
reported the prognostic value of HPV and p16 analysis in HNSCC in
general and in tonsillar cancer in particular. It has been
confirmed that patients with HPV-associated tumors in the tonsillar
region have a better prognosis compared with patients with
HPV-negative tumors in terms of locoregional control and overall
survival, regardless of treatment modality (4,14,15). Nichols et al (13) reported that patients with HPV-related
tumors are younger and have smaller primary-site tumors and a more
favorable survival rates compared with those with HPV-negative
tumors. The overexpression of p16 is highly associated with HPV
infection, and this may be used clinically as a surrogate marker
and a prognostic guide of the disease. Large and often cystic lymph
node involvement, along with clinically advanced stage (III–IV),
are also associated with HPV-positive tumors (35,37).
The favorable prognosis associated with HPV
positivity in tonsillar cancers has been studied by Lindquist et
al (35), who reported an
increased disease-specific survival rate in patients with
HPV-associated tonsillar cancer (81%) compared with patients with
HPV-negative tumors (36%), regardless of age, gender or tumor
stage. These findings have been further supported by Fischer et
al (36), who reported that the
5-year survival rate of patients with p16-positive oropharyngeal
cancer of stages III–IV was nearly as good as for those with tumors
of stages I–II. The prognostic value of p16 in the outcome of
HPV-positive tonsillar neoplasms was also addressed. Cases with
p16-positive tumors in the advanced stages (III–IV) had a 5-year
survival of 54.1%, compared to 18% for those with p16-negative
tumors. Regardless of tumor stage, p16-positive cases had a 5-year
survival rate of 59.3% compared to 24.5% for the p16-negative cases
(36). Results from the Danish
DAHANCA 5 study revealed that p16-positive HNSCCs were associated
with improved locoregional tumor control (58% vs. 28%) and
increased disease-specific (72% vs. 34%) and overall (62% vs. 26%)
survival rates compared with p16-negative tumors (38). The detection of p16 and HPV may
therefore be an important tool as part of a comprehensive strategy
to develop personalized treatment for tonsillar cancer.
Despite these findings, many current recommendations
for the treatment of HPV-positive tonsillar tumors do not differ
from those for HPV-negative tumors (39). Certain investigators have questioned
the need for aggressive treatments for patients with HPV-positive
tonsillar cancer, which has now been found to be associated with a
better prognosis and is often observed in younger and healthier
groups (4,39). The risks and benefits of aggressive
chemoradiotherapy and surgery must be considered carefully. Current
research is now focused on the de-escalation of treatment
modalities for the HPV-related cases and emphasizes the
introduction of a number of prognostic markers in order to identify
such patients in the pre-treatment stage (4,38).
At present, there is a lack of clear evidence
regarding the biological basis behind the differences in survival
and prognosis between HPV-associated and HPV-negative malignancies.
A possible explanation may be that the different molecular profile
in patients with HPV- and p16-positive tumors constitutes a
distinct subset of tonsillar malignancies (34). In addition, it is also reasonable to
speculate that a younger, non-smoking cohort is associated with a
reduced rate of co-morbidity and, therefore, improved
non-cancer-related outcomes.
The route of transmission of HPV infection in oral
carcinomas remains unclear. Much speculation has occurred regarding
possible sexual transmission. The increasing evidence of tonsillar
cancer during the last decades has been associated with a
significant change in sexual habits, and HPV is currently
considered to be the most common sexually transmitted infection
(16). This issue has been studied by
Hemminki et al (40), with
results indicating that the husbands of patients with cervical
cancer have a higher risk of tonsillar and tongue-based
malignancies. Hence, the prophylactic vaccination of women may also
lead to decreased rates of oral HPV infection in the male
population. The numbers of HPV infections in the oral cavity and,
subsequently, HPV-associated tonsillar tumors are expected to
surpass the rate of cervical malignancies by 2020, and this fact
highlights the importance of vaccinating both genders in the future
as a preventive measure against tonsillar cancer (41).
In summary, the present study identified incidences
of HPV-positive and p16-expressing tonsillar cancers in men from
northern Sweden that were similar to those already reported for
Swedish urban populations, as well as populations outside of
Sweden. This study may provide a basis for future projects
investigating the prognosis of tonsillar carcinomas. Further
investigation into the biological mechanisms of the improved
survival and prognosis associated with the HPV-positive tonsillar
cancers is warranted. Furthermore, it may be beneficial to evaluate
the role and effect of HPV vaccination as a prophylactic measure
against tonsillar and oral cancer, and the necessity for
introducing vaccination strategies to the male population.
Acknowledgements
This study was supported by grants from Lion's
Cancer Research Foundation (13-1998), at Umeå University
Sweden.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Center for Epidemiology: Cancer incidence
in Sweden 2011. National Board of Health and Welfare
(SocialStyrelsen) (Stockholm, Sweden). 2011.
|
|
3
|
Lind M, Lewin F, Lundgren J, Nathanson ALF
and Strander H: Head, neck and oesophageal cancer: Diagnosis,
treatment and follow-up of the Stockholm-Gotland region. Karolinska
University Hospital (Stockholm). Oncological Centrum. 2001.(In
Swedish) Available from. http://www.cancercentrum.se/Global/RCCSthlmGotland/Regionla%20vårdprogram/HuvudhalsEsofaguscancer2001%5B1%5D.pdfAccessed.
July 11–2014
|
|
4
|
Panwar A, Batra R, Lydiatt WM and Ganti
AK: Human papilloma virus positive oropharyngeal squamous cell
carcinoma: A growing epidemic. Cancer Treat Rev. 40:215–219. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hammarstedt L, Lindquist D, Dahlstrand H,
Romanitan M, Dahlgren LO, Joneberg J, Creson N, Lindholm J, Ye W,
Dalianis T and Munck-Wikland E: Human papillomavirus as a risk
factor for the increase in incidence of tonsillar cancer. Int J
Cancer. 119:2620–2623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gillison ML, Zhang Q, Jordan R, Xiao W,
Westra WH, Trotti A, Spencer S, Harris J, Chung CH and Ang KK:
Tobacco smoking and increased risk of death and progression for
patients with p16-positive and p16-negative oropharyngeal cancer. J
Clin Oncol. 30:2102–2111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blomberg M, Nielsen A, Munk C and Kjaer
SK: Trends in head and neck cancer incidence in Denmark, 1978-2007:
Focus on human papillomavirus associated sites. Int J Cancer.
129:733–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olaleye O, Moorthy R, Lyne O, Black M,
Mitchell D and Wiseberg J: A 20-year retrospective study of tonsil
cancer incidence and survival trends in South East England:
1987-2006. Clin Otolaryngol. 36:325–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klussmann JP, Weissenborn SJ, Wieland U,
Dries V, Eckel HE, Pfister HJ and Fuchs PG: Human
papillomavirus-positive tonsillar carcinomas: A different tumor
entity? Med Microbiol Immunol. 192:129–132. 2003.(In Swedish).
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kreimer AR, Clifford GM, Boyle P and
Franceschi S: Human papillomavirus types in head and neck squamous
cell carcinomas worldwide: A systematic review. Cancer Epidemiol
Biomarkers Prev. 14:467–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haughey BH, Hinni ML, Salassa JR, et al:
Transoral laser microsurgery as primary treatment for
advanced-stage oropharyngeal cancer: A United States multicenter
study. Head Neck. 33:1683–1694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Richmon JD, Quon H and Gourin CG: The
effect of transoral robotic surgery on short-term outcomes and cost
of care after oropharyngeal cancer surgery. Laryngoscope.
124:165–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nichols AC, Faquin WC, Westra WH, Mroz EA,
Begum S, Clark JR and Rocco JW: HPV-16 infection predicts treatment
outcome in oropharyngeal squamous cell carcinoma. Otolaryngol Head
Neck Surg. 140:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Rorke MA, Ellison MV, Murray LJ, Moran
M, James J and Anderson LA: Human papillomavirus related head and
neck cancer survival: A systematic review and meta-analysis. Oral
Oncol. 48:1191–1201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mellin H, Friesland S, Lewensohn R,
Dalianis T and Munck-Wikland E: Human papillomavirus (HPV) DNA in
tonsillar cancer: Clinical correlates, risk of relapse and
survival. Int J Cancer. 89:300–304. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schiffman M and Castle PE: Human
papillomavirus: Epidemiology and public health. Arch Pathol Lab
Med. 127:930–934. 2003.PubMed/NCBI
|
|
17
|
D'souza G and Dempsey A: The role of HPV
in head and neck cancer and review of the HPV vaccine. Prev Med.
55(Suppl 1): S5–S11. 2011. View Article : Google Scholar
|
|
18
|
Steinau M, Saraiya M, Goodman MT, Peters
ES, Watson M, Cleveland JL, Lynch CF, Wilkinson EJ, Hernandez BY
and Copeland G: Human papillomavirus prevalence in oropharyngeal
cancer before vaccine introduction, United States. Emerg Infect
Dis. 20:822–828. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Osazuwa-Peters N: Human papillomavirus
(HPV), HPV-associated oropharyngeal cancer and HPV vaccine in the
United States-do we need a broader vaccine policy? Vaccine.
31:5500–5505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Williams JR: The Declaration of Helsinki
and public health. Bull World Health Organ. 86:650–652. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Roda Husman AM, Walboomers JM, van den
Brule AJ, Meijer CJ and Snijders PJ: The use of general primers GP5
and GP6 elongated at their 3′ ends with adjacent highly conserved
sequences improves human papillomavirus detection by PCR. J Gen
Virol. 76:1057–1062. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smits HL, Tieben LM, Tjong-A-Hung SP,
Jebbink MF, Minnaar RP, Jansen CL and ter Schegget J: Detection and
typing of human papillomaviruses present in fixed and stained
archival cervical smears by a consensus polymerase chain reaction
and direct sequence analysis allow the identification of a broad
spectrum of human papillomavirus types. J Gen Virol. 73:3263–3268.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Detre S, Saclani Jotti G and Dowsett M: A
‘quickscore’ method for immunohistochemical semiquantitation:
Validation for oestrogen receptor in breast carcinomas. J Clin
Pathol. 48:876–878. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sweden Official Statistics: Urban areas
2010. Statistical Central Office (Stockholm, Sweden). 2012.(In
Swedish).
|
|
25
|
Sweden Official Statistics: Smaller
localities 2010. Statistical Central Office (Stockholm, Sweden).
2012.(In Swedish).
|
|
26
|
Nordlund LA: Trends in smoking habits and
lung cancer in Sweden. Eur J Cancer Prev. 7:109–116.
1998.PubMed/NCBI
|
|
27
|
Golas SM: Trends in palatine tonsillar
cancer incidence and mortality rates in the United States.
Community Dent Oral Epidemiol. 35:98–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hammarstedt L, Dahlstrand H, Lindquist D,
et al: The incidence of tonsillar cancer in Sweden is increasing.
Acta Otolaryngol. 127:988–992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Näsman A, Attner P, Hammarstedt L, et al:
Incidence of human papillomavirus (HPV) positive tonsillar
carcinoma in Stockholm, Sweden: An epidemic of viral-induced
carcinoma? Int J Cancer. 125:362–366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dahlstrand HM and Dalianis T: Presence and
influence of human papillomaviruses (HPV) in Tonsillar cancer. Adv
Cancer Res. 93:59–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Syrjänen S: HPV infections and tonsillar
carcinoma. J Clin Pathol. 57:449–455. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oguejiofor KK, Hall JS, Mani N, et al: The
Prognostic Significance of the Biomarker p16 in Oropharyngeal
Squamous Cell Carcinoma. Clin Oncol (R Coll Radiol). 25:630–638.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mellin Dahlstrand H, Lindquist D,
Björnestål L, et al: P16(INK4a) correlates to human papillomavirus
presence, response to radiotherapy and clinical outcome in
tonsillar carcinoma. Anticancer Res. 25:4375–4383. 2005.PubMed/NCBI
|
|
34
|
Weinberger PM, Yu Z, Haffty BG, et al:
Molecular classification identifies a subset of human
papillomavirus-associated oropharyngeal cancers with favorable
prognosis. J Clin Oncol. 24:736–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lindquist D, Romanitan M, Hammarstedt L,
Näsman A, Dahlstrand H, Lindholm J, Onelöv L, Ramqvist T, Ye W,
Munck-Wikland E and Dalianis T: Human papillomavirus is a
favourable prognostic factor in tonsillar cancer and its oncogenic
role is supported by the expression of E6 and E7. Mol Oncol.
1:350–355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fischer CA, Kampmann M, Zlobec I, Green E,
Tornillo L, Lugli A, Wolfensberger M and Terracciano LM: p16
expression in oropharyngeal cancer: Its impact on staging and
prognosis compared with the conventional clinical staging
parameters. Ann Oncol. 21:1961–1966. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goldenberg D, Begum S, Westra WH, Khan Z,
Sciubba J, Pai SI, Califano JA, Tufano RP and Koch WM: Cystic lymph
node metastasis in patients with head and neck cancer: An
HPV-associated phenomenon. Head Neck. 30:898–903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lassen P, Eriksen JG, Hamilton-Dutoit S,
Tramm T, Alsner J and Overgaard J: Effect of HPV-associated
p16INK4A expression on response to radiotherapy and survival in
squamous cell carcinoma of the head and neck. J Clin Oncol.
27:1992–1998. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marklund L and Hammarstedt L: Impact of
HPV in Oropharyngeal Cancer. J Oncol. 2011:5090362011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hemminki K, Dong C and Frisch M: Tonsillar
and other upper aerodigestive tract cancers among cervical cancer
patients and their husbands. Eur J Cancer Prev. 9:433–437. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gillison ML, Broutian T, Pickard RK, Tong
ZY, Xiao W, Kahle L, Graubard BI and Chaturvedi AK: Prevalence of
oral HPV infection in the United States, 2009-2010. Jama.
307:693–703. 2012. View Article : Google Scholar : PubMed/NCBI
|