Introduction
Gastric cancer is the third most-common cancer and
the second leading cause of cancer-related death in the world
(1,2). D2 gastrectomy combined with
chemotherapy is a standard treatment for gastric cancer; however,
almost half of the patients had recurrence, even after modern
multidisciplinary treatment (3–5). To
further improve the patient's survival, approaches than
conventional chemotherapy should be considered.
The administration of eicosapentaenoic acid (EPA), a
long-chain polyunsaturated fatty acid of the omega-3 (n-3) family,
may be an attractive approach (6,7).
Many previous studies have clarified that EPA could be selectively
cytotoxic to various tumor cells in vitro and in vivo
(8–10). Moreover, EPA was reported to
suppress pro-inflammatory cytokines, such as interleukin (IL)-1 and
tumor necrosis factors (TNF), which is released by surgical stress
(11,12). Recent studies clarified that
pro-inflammatory cytokines play a critical roles in tumors (e.g.,
survival, proliferation, metastasis, and resistance to
chemotherapy) (13,14). Thus, the perioperative
administration of EPA could improve the prognosis of gastric cancer
patients.
However, there is little evidence to support that
EPA is associated with a clear survival benefit in cancer patients
(15–17). Cockbain et al examined the
efficacy of EPA in a small randomized study targeting colorectal
cancer patients with liver metastasis who had undergone liver
surgery (15). In their study, the
EPA group showed better survival than a placebo group; however, the
difference was not statistically significant. In the multivariate
analysis, they only showed that EPA supplementation was an
independent prognostic factor for overall survival (P=0.05). Thus,
an apparent clinical benefit of EPA was not demonstrated. Moreover,
the efficacy of EPA on long-term outcomes in other human
malignancies has not been examined.
Previously, we conducted a prospective multicenter
randomized trial to evaluate whether perioperative EPA-enriched
nutritional supplementation can improve the short-term and
long-term outcomes of patients with localized gastric cancer who
require total gastrectomy as curative treatment (18,19).
In the primary analysis, we evaluated whether body weight loss
caused by surgical stress and gastrectomy was prevented by EPA
supplementation. Although perioperative EPA can be safely
administered, postoperative weight loss was not prevented. We
herein report the long-term oncological outcomes, which reflect
antitumor activity, a key secondary endpoint, in this randomized
study.
Patients and methods
Patients
The present study was designed as a multicenter,
open-label, superiority, randomized trial comparing perioperative
care with or without EPA-enriched oral nutritional supplementation
for patients diagnosed with gastric cancer who required total
gastrectomy as a curative treatment (clinical trial number:
UMIN000006380, 21/September/2011). This study was approved by
Kanagawa Cancer Center Institutional Review Board committee (IRB
approval no. rinsyokenkyu:34) and we obtained written informed
consent from the patients. The primary endpoint was body weight
loss at 1 and 3 months after surgery. Key secondary endpoints were
overall survival (OS). The details of this trial were described in
a previous report. Briefly, key eligibility criteria included
histologically proven adenocarcinoma of the stomach, clinical
T1-T4a and M0, planned R0 resection by total gastrectomy. Eligible
patients were randomized to either the EPA-off group or EPA-on
group.
Perioperative care
In addition to the standard diet, an EPA-enriched
supplement (ProSure®, Abbott Japan, Tokyo, Japan) was
given to the EPA-on group but not to the EPA-off group. This
supplement included 600 kcal with 2.2 g/day of EPA. The supplement
was given from 1–7 days before surgery and 2–21 days after
surgery.
Surgery
Based on the Japanese gastric cancer treatment
guidelines published in 2010 (ver. 3), total gastrectomy with lymph
node dissection to the D1+ or D2 level was planned (20). Principally, D1+ lymphadenectomy was
selected for patients with cT1N0 tumors other than those for whom
endoscopic mucosal resection or endoscopic submucosal dissection
were recommend. D2 lymphadenectomy was indicated for patients with
potentially curable T2-T4 tumors, as well as for those with cT1N+
tumors.
Perioperative chemotherapy
When patients had unfavorable advanced gastric
cancer (e.g., cT3) in the case of junctional cancer/scirrhous
type/giant type 3, cT4, para-aortic nodal metastasis, and/or bulky
nodal metastasis around the major branched artery, neoadjuvant
chemotherapy with S-1/CDDP or docetaxel/CDDP/S-1 (2 or 4 courses)
would have been planned as a clinical trial (21,22).
After surgery, S-1 chemotherapy for 1 year would be
planned for patients diagnosed with pathological stage II or III
(23).
Follow-up
The patients were followed at outpatient clinics.
The follow-up program of postoperative surveillance principally
consisted of a physical examination and blood chemistry assessments
(including carcinoma tumor markers) every 3 months for the first
year and every 6 months thereafter; and computed tomography of the
neck, chest, and abdomen every 6 months.
Evaluations and statistical
analyses
OS was defined as the period between random
assignment and death. The data of patients who had not experienced
an event were censored at the date of the final observation. OS
curves were calculated using the Kaplan-Meier method and were
compared by the log-rank test. The SAS software program (version
9.4; SAS Institut) was used to perform all of the statistical
analyses.
Results
Patient background
characteristics
A total of 127 patients from eight hospitals were
enrolled in the present trial between October 2011 and July 2014.
Fig. 1 shows the CONSORT diagram.
Among 127 patients who were entered, 123 patients (EPA-off group;
n=60 and EPA-on group; n=63) were finally eligible for inclusion in
the present study. Table I shows
the patient background characteristics and operative details. The
baseline characteristics and surgical procedures were well balanced
between the two groups. Median relative performance of supplement
in EPA-ON group was 100% before surgery and 54% after surgery.
There were no adverse events due to EPA-enriched supplement.
Postoperative surgical complications were observed in 8 patients in
the EPA-off group and 9 patients in the EPA-on group, and did not
differ to a statistically significant extent (Table II). In the EPA-on group, the
median relative performance of supplementation was 100% before
surgery and 54% after gastrectomy. The median dose of the EPA was
15.4 g before surgery and 23.1 g after surgery. In total, the
median cumulative dose of EPA was 38.5 g.
 | Table I.Background characteristics between the
EPA-on and EPA-off groups. |
Table I.
Background characteristics between the
EPA-on and EPA-off groups.
| Characteristic | EPA-on group
(n=63) | EPA-off group
(n=60) |
|---|
| Median age, years
(range) | 65.1 (31–79) | 65.6 (30–80) |
| Sex, male/female | 46/17 | 43/17 |
| Preoperative mean
body weight, kg | 47.1±9.8 | 47.8±8.6 |
| Mean height, cm | 160.6±8.4 | 163.7±8.0 |
| Preoperative mean
lean body mass, kg | 45.7±9.3 | 47.0±7.5 |
| Mean preoperative
serum albumin, mg/dl | 4.1±0.5 | 4.2±0.4 |
| Mean preoperative
C-reactive protein, mg/dl | 0.3±0.5 | 0.2±0.5 |
| Location of primary
tumor, upper third/middle third/lower third | 42/17/4 | 35/24/0 |
| Clinical T factor,
T1/T2/T3/T4 | 12/13/12/26 | 16/10/11/23 |
| Clinical N factor,
negative/positive | 40/23 | 39/21 |
| Surgical approach,
conventional/laparoscopic | 52/11 | 47/13 |
| Extent of lymph node
dissection, D0/D1/D2/D3 | 0/10/53/0 | 1/15/43/1 |
| Mean operation time,
min (range) | 296 (145–510) | 295 (83–523) |
| Mean blood loss, ml
(range) | 340 (0–3,560) | 320 (0–2,080) |
 | Table II.Surgical morbidity between EPA-on
group and EPA-off group. |
Table II.
Surgical morbidity between EPA-on
group and EPA-off group.
|
Morbiditya | EPA-on group
(n=63) | EPA-off group
(n=60) |
|---|
| Overall | 9 | 9 |
| Pancreatic
fistula | 2 | 2 |
| Abdominal
abscess | 2 | 1 |
| Anatomic
leakage | 0 | 1 |
| Bleeding | 1 | 0 |
| Others | 4 | 4 |
Survival analysis
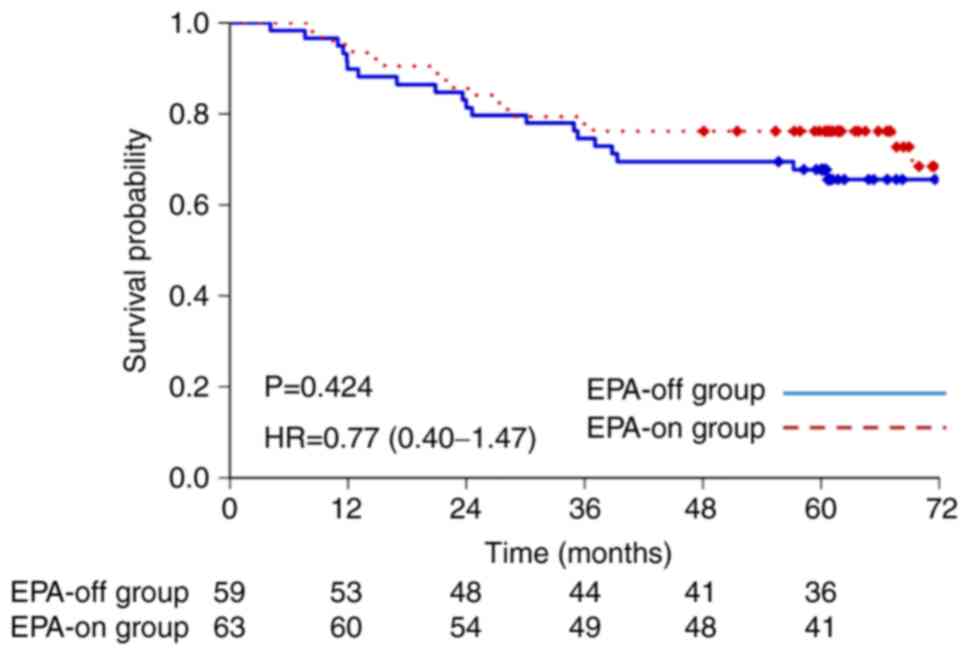
Fig. 2 shows the OS
curves. The 3-year and 5-year OS rates were 74.6 and 67.8%,
respectively in the EPA-off group, and 77.8 and 76.2% in the EPA-on
group, which did not amount to a statistically significant
difference [hazard ratio, 0.77; 95% confidence interval (CI),
0.40-1.47; P=0.424].
Subgroup analysis of overall survival
and recurrence free survival
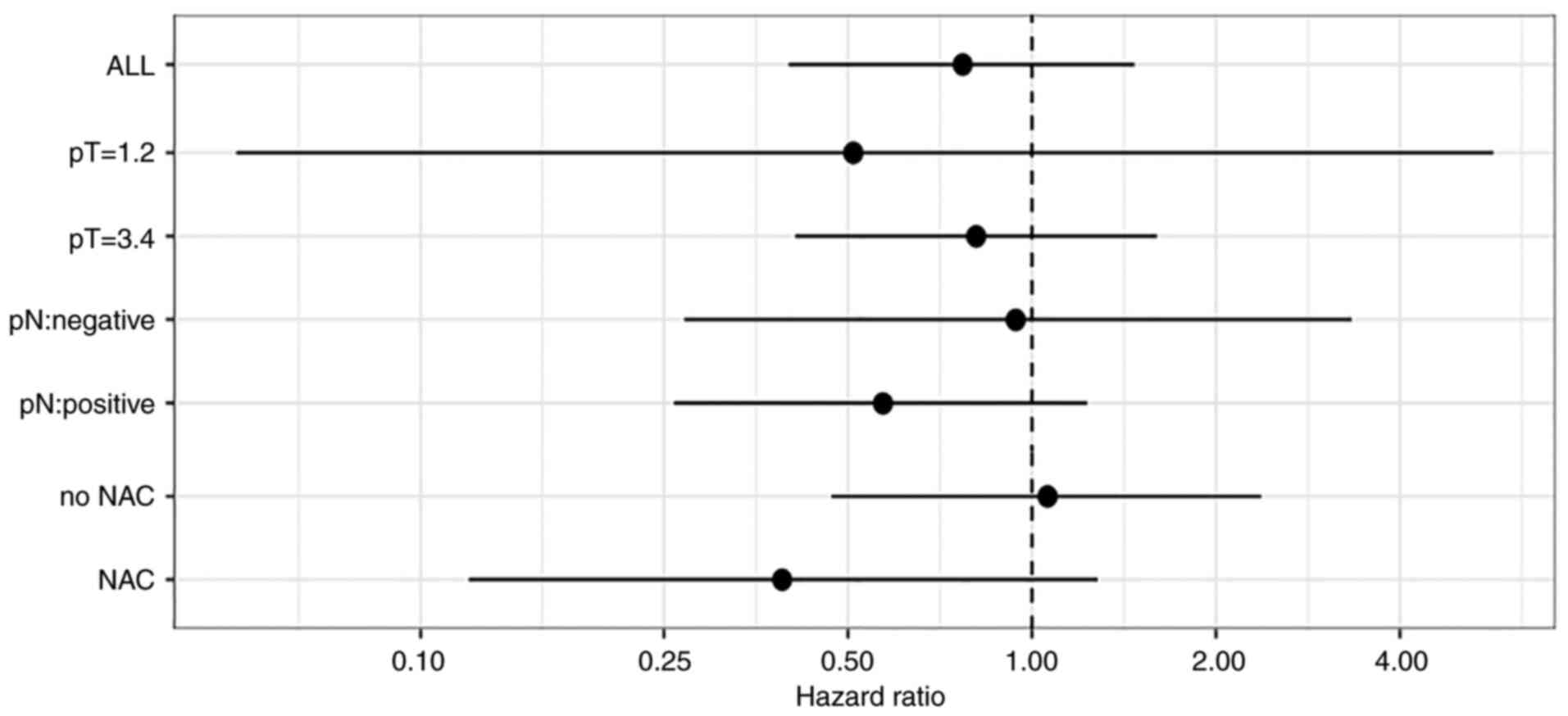
Fig. 3 shows the
subgroup analyses by neoadjuvant chemotherapy, and pathological T
and N factors for OS. Among the various sub-group factors, the
patients in the EPA-on group who received neoadjuvant chemotherapy
(NAC) and who had lymph node metastasis showed slightly better
survival. In the patients who received NAC, the 5-year OS rate was
43.8% (95% CI, 19.4-68.1%) in the EPA-off group and 71.4% (95% CI,
47.8-95.1%) in the EPA-on group (hazard ratio, 0.39; 95% CI,
0.12-1.28; P=0.108) (Fig. 4). In
patients who had lymph node metastasis, the 5-year OS rate was
52.0% (95% CI, 32.4-71.6%) in the EPA-off group and 71.8% (95% CI,
57.7-86.0%) in the EPA-on group (hazard ratio, 0.57; 95% CI,
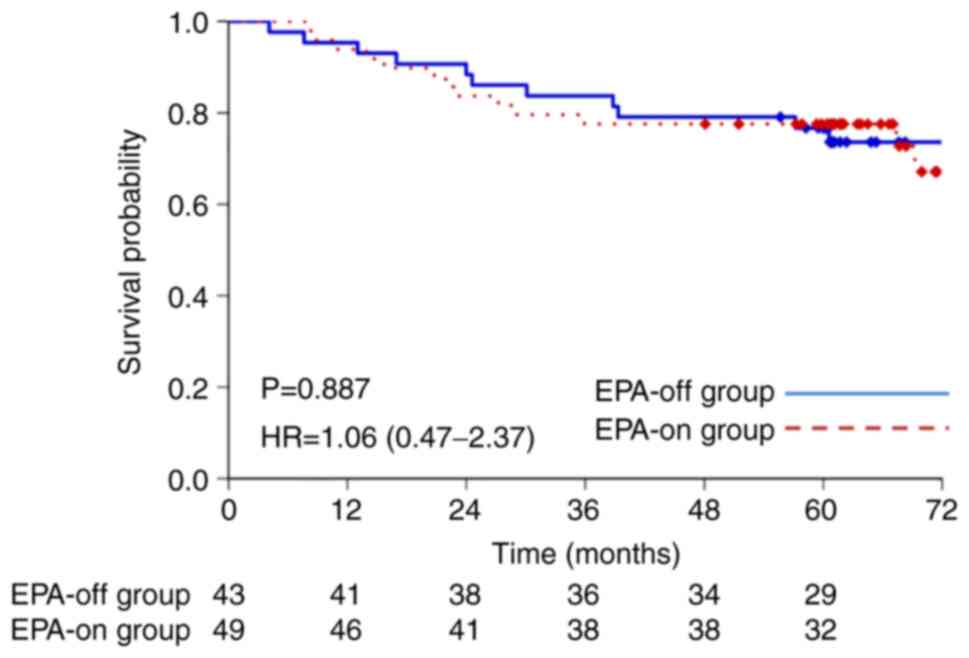
0.12-1.28; P=0.148). On the other hand, in the patients who did not
receive NAC, the 5-year OS rate was 76.7% (95% CI, 64.0-89.3%) in
the EPA-off group and 77.6% (95% CI, 65.9-89.2%) in the EPA-on
group (hazard ratio, 1.06; 95% CI, 0.47-2.37; P=0.887) (Fig. 5). In lymph node metastasis-negative
patients, the 5-year OS rate was 81.8% (95% CI, 68.7-95.0%) in the
EPA-off group and 83.3% (95% CI, 68.4-98.2%) in the EPA-on group
(hazard ratio, 0.94; 95% CI, 0.27-3.33; P=0.923).
Discussion
The aim of the present analysis was to explore
whether perioperative eicosapentaenoic acid (EPA) supplementation
could improve the survival of patients with localized gastric
cancer that required curative total gastrectomy. This is the first
randomized study to demonstrate a prognostic effect of EPA in
patients with gastric cancer. The major finding of this study was
that a clear survival benefit of perioperative EPA was not observed
in gastric cancer patients.
Previously, Cockbain et al showed that EPA
therapy had a marginal survival in patients with liver metastasis
of colorectal cancer (15). There
were some differences between the present study and the study by
Cockbain et al. The first difference was the median
cumulative dose of EPA, which was 38.5 g in this study but 60 g
(calculated from their results) in their study. Thus, the
cumulative EPA dose of their study was higher than that of the
present study. Second, the tumor stage was different. In the
present study, patients with localized tumors were targeted, while
their study targeted patients with metastatic tumors. In addition
to the marginal survival benefit in their study, they also
demonstrated that EPA had anti-angiogenesis effects using biopsy
specimens. Angiogenesis plays an important role in metastatic
tumors but would not play an important role in micro-metastatic
tumor cells. Several pivotal phase III studies demonstrated that
the patient survival was significantly improved by bevacizumab and
ramucirumab (vascular endothelial growth factor inhibitors) in
metastatic colorectal cancer and by ramucirumab in metastatic
gastric cancer (24–26). On the other hand, the efficacy of
bevacizumab was not confirmed in the phase III trials, not only for
resectable gastric cancer but also for colorectal cancer (27,28).
Whether the target is micrometastatic or metastatic disease might
affect the efficacy of EPA.
Although the OS rates were similar between the two
groups, the hazard ratio was slightly lower than 1.0 for OS, which
suggests some clinical efficacy of EPA in gastric cancer. In the
subgroup analyses for OS, the hazard ratio was 0.39 for OS in
patients who received NAC and 0.57 in patients who had nodal
metastasis. In the present cohort, patients who received NAC were
limited to patients with cT3 in the case of junctional cancer,
scirrhous type, or giant type 3, cT4, para-aortic nodal metastasis,
and/or bulky nodal metastasis around the major branched artery.
Thus, patients showing a low hazard ratio were considered to have a
relatively poor prognosis. It would be interesting to investigate
whether EPA is effective for such unfavorable advanced gastric
cancer in a future study. On the other hand, the hazard ratio was
almost 1.0 in patients who did not receive the NAC and in patients
who had no nodal metastasis. EPA would not be effective for these
patients.
The present study was associated with some
limitations. First, the primary endpoint of the present randomized
study was not survival. The sample size was relatively small and
was not set to investigate differences in survival. Thus, the
results were not confirmatory. Second, EPA could be included in the
dietary supplements. The clinical trial using the dietary
supplements had bias that the patients buy the corresponding
dietary supplements outside of the clinical trial without letting
their physicians know. Thus, the negative results may be the result
of a bias that the placebo group took the dietary supplement
without permission and thereby falsified the end results. We con
not exclude that their negative outcome is the result of such a
bias. Third, we used oral nutritional supplementation including EPA
in this study. The differences between the two groups were not
limited to EPA, there were also differences in other nutrients.
Thus, we cannot deny the effects of other nutrients.
In conclusion, the present study could not
demonstrate a clear survival benefit of EPA supplementation in
patients who received curative gastrectomy for gastric cancer. The
value of EPA should be further tested in a future study by
selecting patients with unfavorable advanced gastric cancer.
Acknowledgements
The authors would like to thank Ms. Natsumi Sato
(Kanagawa Cancer Center) and Ms. Rika Takahashi (Kanagawa Cancer
Center) for their data management in this study.
Funding
This work was supported by a non-governmental organization, the
Kanagawa Standard Anti-Cancer Therapy Support System (KSATSS).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TA and TY made substantial contributions to the
conception and design. TA and TY confirm the authenticity of all
the raw data. SI, HC, KS, YI, KF, NT, YK, KN, SN and NH made
substantial contributions to the acquisition of data, or the
analysis and interpretation of data. TA and TY were involved in
drafting the manuscript or revising it critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study data and informed consent were obtained in
accordance with the Declaration of Helsinki and were approved by
Kanagawa Cancer Center Institutional Review Board committee (IRB
approval no. rinsyokenkyu:34). Written informed consent or a
substitute for it was obtained from all patients for inclusion in
the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smyth EC, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D; ESMO Guidelines Committee, : Gastric
cancer: ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 27 (Suppl 5):v38–v49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2018. ((5th edition)).
Gastric Cancer. 24:1–21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
NCCN, . NCCN clinical practice guidelines
in oncology. 2018.http://www.nccn.org
|
|
6
|
Fearon KC, Von Meyenfeldt MF, Moses AG,
Van Geenen R, Roy A, Gouma DJ, Giacosa A, Van Gossum A, Bauer J,
Barber MD, et al: Effect of a protein and energy dense N-3 fatty
acid enriched oral supplement on loss of weight and lean tissue in
cancer cachexia: A randomised double blind trial. Gut.
52:1479–1486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moses AW, Slater C, Preston T, Barber MD
and Fearon KC: Reduced total energy expenditure and physical
activity in cachectic patients with pancreatic cancer can be
modulated by an energy and protein dense oral supplement enriched
with n-3 fatty acids. Br J Cancer. 90:996–1002. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kubota H, Matsumoto H, Higashida M,
Murakami H, Nakashima H, Oka Y, Okumura H, Yamamura M, Nakamura M
and Hirai T: Eicosapentaenoic acid modifies cytokine activity and
inhibits cell proliferation in an oesophageal cancer cell line.
Anticancer Res. 33:4319–4324. 2013.PubMed/NCBI
|
|
9
|
Wigmore SJ, Fearon KC, Maingay JP and Ross
JA: Down-regulation of the acute-phase response in patients with
pancreatic cancer cachexia receiving oral eicosapentaenoic acid is
mediated via suppression of interleukin-6. Clin Sci (Lond).
92:215–221. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beck SA, Smith KL and Tisdale MJ:
Anticachectic and antitumor effect of eicosapentaenoic acid and its
effect on protein turnover. Cancer Res. 51:6089–6093.
1991.PubMed/NCBI
|
|
11
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zubair A and Frieri M: Role of nuclear
factor-ĸB in breast and colorectal cancer. Curr Allergy Asthma Rep.
13:44–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Falconer JS, Fearon KC, Ross JA, Elton R,
Wigmore SJ, Garden OJ and Carter DC: Acute-phase protein response
and survival duration of patients with pancreatic cancer. Cancer.
75:2077–2082. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McNamara MJ, Alexander HR and Norton JA:
Cytokines and their role in the pathophysiology of cancer cachexia.
JPEN J Parenter Enteral Nutr. 16 (Suppl 6):50S–55S. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cockbain AJ, Volpato M, Race AD, Munarini
A, Fazio C, Belluzzi A, Loadman PM, Toogood GJ and Hull MA:
Anticolorectal cancer activity of the omega-3 polyunsaturated fatty
acid eicosapentaenoic acid. Gut. 63:1760–1768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
West NJ, Clark SK, Phillips RK, Hutchinson
JM, Leicester RJ, Belluzzi A and Hull MA: Eicosapentaenoic acid
reduces rectal polyp number and size in familial adenomatous
polyposis. Gut. 59:918–925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ueno M, Sugimori K, Taguri M, Ohkawa S,
Kobayashi S, Miwa H, Kaneko T, Morimoto M and Yamanaka T:
Randomized phase II study of gemcitabine monotherapy vs gemcitabine
with an EPA-enriched oral supplement in advanced pancreatic cancer.
Nutr Cancer. 74:122–130. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshikawa T, Hiki N, Taguri M, Sano T,
Nunobe S, Taniguchi H, Fukushima R, Cho H, Morita S and Tsuburaya
A: A Phase III trial to evaluate the effect of perioperative
nutrition enriched with eicosapentaenoic acid on body weight loss
after total gastrectomy for T2-T4a gastric cancer. Jpn J Clin
Oncol. 42:459–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aoyama T, Yoshikawa T, Ida S, Cho H,
Sakamaki K, Ito Y, Fujitani K, Takiguchi N, Kawashima Y, Nishikawa
K, et al: Effects of perioperative eicosapentaenoic acid-enriched
oral nutritional supplement on lean body mass after total
gastrectomy for gastric cancer. J Cancer. 10:1070–1076. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aoyama T, Nishikawa K, Fujitani K, Tanabe
K, Ito S, Matsui T, Miki A, Nemoto H, Sakamaki K, Fukunaga T, et
al: Early results of a randomized two-by-two factorial phase II
trial comparing neoadjuvant chemotherapy with two and four courses
of cisplatin/S-1 and docetaxel/cisplatin/S-1 as neoadjuvant
chemotherapy for locally advanced gastric cancer. Ann Oncol.
28:1876–1881. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwasaki Y, Terashima M, Mizusawa J,
Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito S, Kaji M, Kimura
Y, et al: Gastrectomy with or without neoadjuvant S-1 plus
cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): An
open-label, phase 3, randomized controlled trial. Gastric Cancer.
24:492–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Gramont A, Van Cutsem E, Schmoll HJ,
Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht
JR, Rivera F, et al: Bevacizumab plus oxaliplatin-based
chemotherapy as adjuvant treatment for colon cancer (AVANT): A
phase 3 randomised controlled trial. Lancet Oncol. 13:1225–1233.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van Cutsem E, de Haas S, Kang YK, Ohtsu A,
Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ
and Shah MA: Bevacizumab in combination with chemotherapy as
first-line therapy in advanced gastric cancer: A biomarker
evaluation from the AVAGAST randomized phase III trial. J Clin
Oncol. 30:2119–2127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clavien PA and Strasberg SM: Severity
grading of surgical complications. Ann Surg. 250:197–198. 2009.
View Article : Google Scholar : PubMed/NCBI
|