Introduction
Retroperitoneal fibrosis (RF) is a group of
conditions characterized by abnormal growth of fibroinflammatory
tissue around the abdominal aorta, inferior vena cava, and iliac
vessels. This proliferation can affect nearby structures, often
compressing the ureters and ultimately leading to renal damage
(1).
Most cases of RF are idiopathic and associated with
IgG4; other non-malignant causes include radiation, medications,
inflammation, or trauma. The pathogenesis of RF is still unknown,
but immune responses may play an important role. Clinical symptoms
are nonspecific and may include constitutional symptoms. Laboratory
tests may show elevated erythrocyte sedimentation rate and
C-reactive protein and variable renal insufficiency as nonspecific
findings (1).
However, 10% of cases may be associated with
neoplasms, such as metastases from carcinomas, sarcomas, or
lymphomas (2,3). The distinction is clinically crucial,
as malignant RF has a poor prognosis, with a median survival of
only 3 to 6 months (4). Imaging
studies are therefore of paramount importance in this context, as
they can both detect and characterize the lesion and possible
complications and suggest the most plausible diagnosis.
We describe a case of urothelial carcinoma (UC) with
atypical radiological features resembling RF and presenting with
upper gastrointestinal symptoms. Diagnosis required image-guided
biopsy and histopathology, which allowed tailored treatment
resulting in reduced tumor burden.
Urothelial tumors are rarely associated with RF, and
only a few cases have been described in the literature (5,6), none
of which showed such extensive retroperitoneal involvement or have
demonstrated response to treatment.
Case report
A 69-year-old man, with a history of smoking and
alcoholism, presented to the Emergency Department of the University
Hospital Virgen de las Nieves in May 2023 with epigastric pain,
vomiting, and altered bowel habits of 2 weeks' evolution.
Laboratory tests revealed an elevated C-reactive
protein level of 279 mg/dl (normal range <3 mg/dl) a serum
creatinine level of 2.8 mg/dl (normal range 0.7–1.2 mg/dl),
significantly elevated from his normal baseline, and a serum urea
level of 73 mg/dl (normal range 12–54 mg/dl). Diuresis was
preserved without pollakiuria or dysuria. These findings were
consistent with acute renal failure. Urinalysis showed no
significant changes, raising doubts about the underlying nature of
the renal failure. Physical examination revealed pitting edema of
both lower extremities. The patient reported no fever or night
sweats.
An initial abdominal ultrasound showed bilateral
hydronephrosis, gastric dilation, and thickening of the duodenal
wall. A subsequent abdominal computed tomography (CT) scan was
performed and showed extensive diffuse retroperitoneal infiltration
from the periduodenal region to the pubis, causing gastric dilation
and hydronephrosis (Fig. 1).
Urological evaluation by endoscopy was
unsatisfactory due to the inability to visualize the ureteral
orifice due to the bladder floor mass and the extrinsic compression
of the lesion which prevented placement of the double J catheter.
Upper gastrointestinal endoscopy revealed congested gastric folds
and gastric biopsy was negative for neoplasia (Fig. 2).
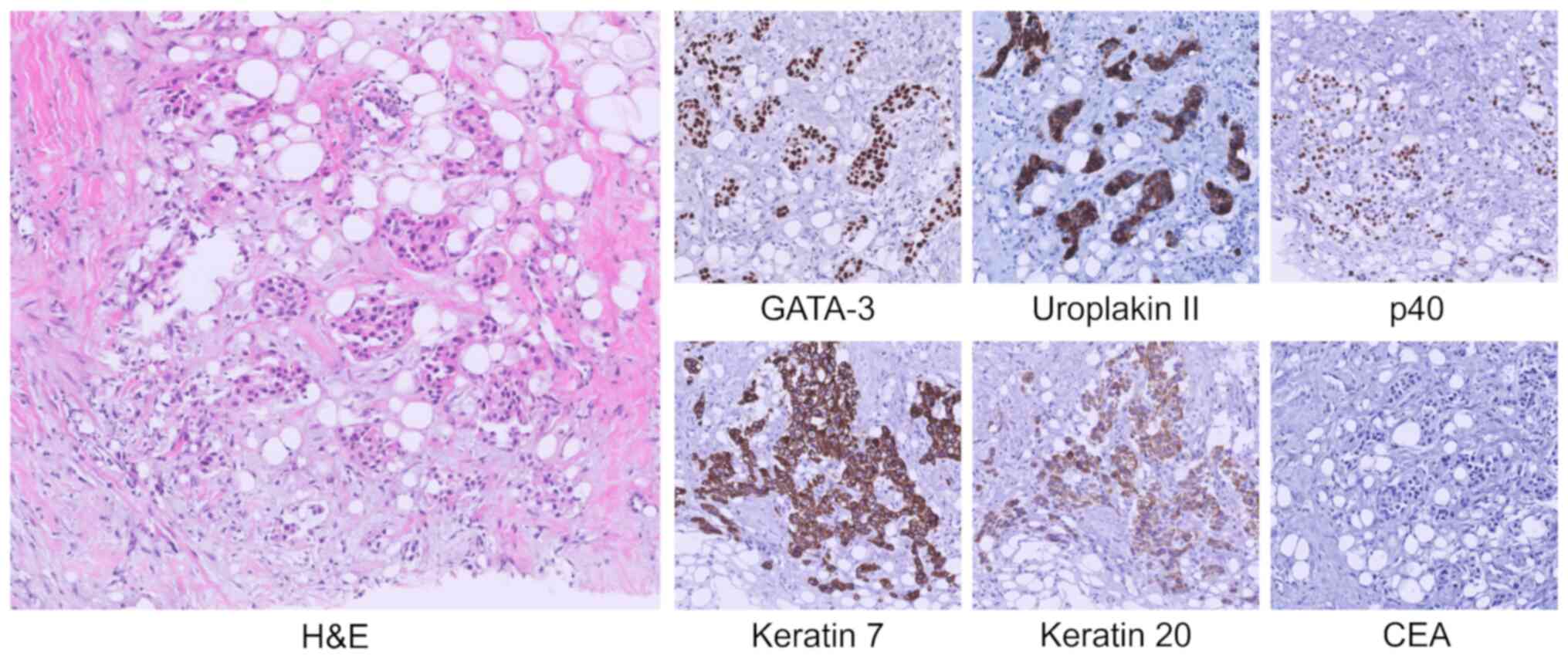
A CT-guided core-needle biopsy of the right
perirenal infiltrative lesion showed a diffuse infiltration of
neoplastic cells amidst retroperitoneal adipose fibrosis.
Immunohistochemical analysis firmly established the diagnosis of UC
and excluded differential diagnoses. Specific markers such as TTF1,
NKX3.1, CDX2, SATB2 and Hepar1 were negative, excluding pulmonary,
prostatic, intestinal, and hepatic origin. The urothelial origin of
the neoplasm was confirmed by positive staining for keratin 7,
keratin 20, p40 and especially GATA-3, together with strong
positivity for uroplakin II and absence of carcinoembryonic antigen
(CEA) expression. This profile also effectively excluded lymphoid
neoplasms due to keratin positivity, neoplasms of biliary origin
due to CEA negativity, and various types of renal cell carcinoma
due to the absence of PAX-8 (Fig.
3).
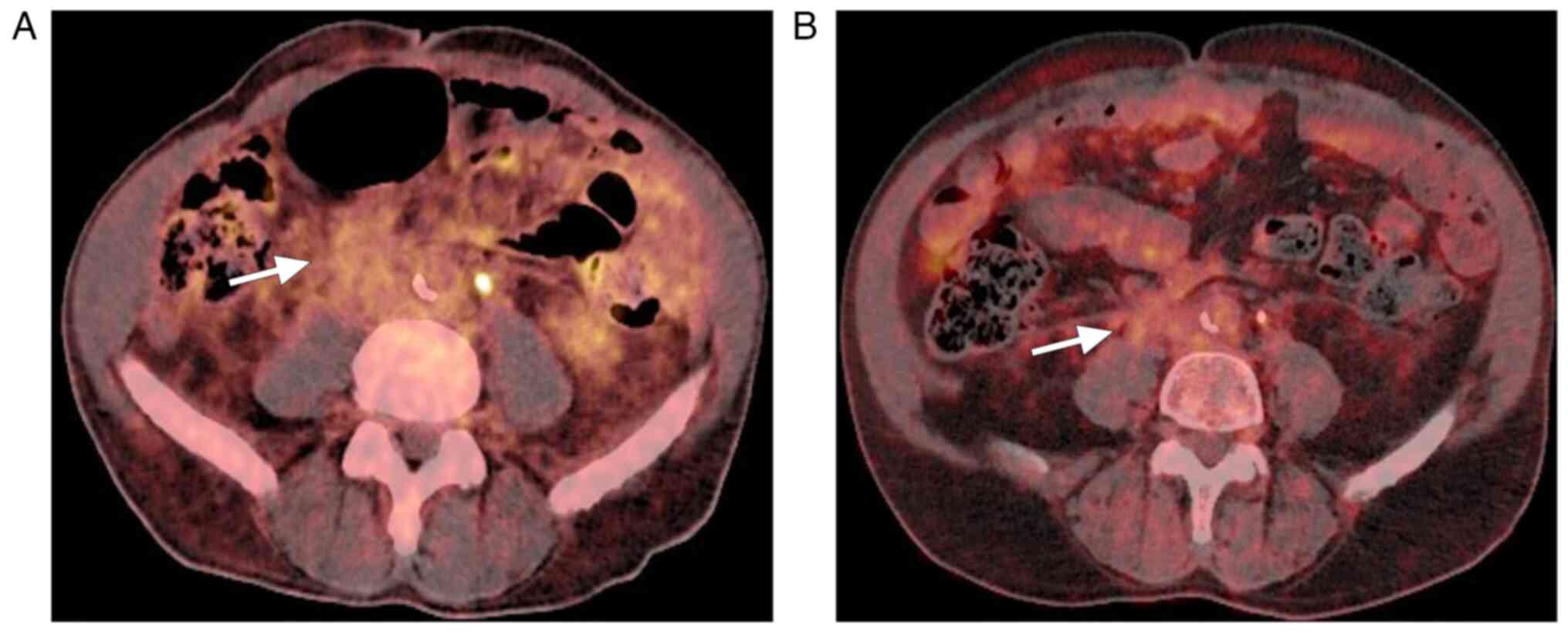
Positron emission tomography with
18F-fluorodeoxyglucose (18F-FDG PET) demonstrated moderate
metabolic activity in the described retroperitoneal mass without
evidence of other lesions or adenopathy consistent with metastasis
elsewhere (Fig. 4A). The case was
discussed in a multidisciplinary tumor board. There was a small
nodule in the bladder that was disproportionate to the
retroperitoneal infiltration and did not allow identification of
the bladder as the primary site (Fig.
1D). The inaccessibility of the lesion during cystoscopy due to
pelvic compression secondary to the retroperitoneal disease led to
the decision not to consider transurethral resection of the bladder
(TURB) in the diagnostic process.
The lesion was diagnosed as stage IV advanced
metastatic UC based on histopathologic biopsy findings in the
perirenal retroperitoneum. Periduodenal, periureteral, lateral
pelvic, and perirectal infiltrates observed on radiologic studies
were also diagnosed as retroperitoneal metastases (Fig. 1C-E).
Due to his renal insufficiency, the patient could
not receive cisplatin, so he received an individualized
chemotherapy regimen of carboplatin and gemcitabine. This regimen
consisted of a series of four 21-day cycles. Carboplatin was
administered on the first day of each cycle and the dose was
calculated to achieve an area under the curve (AUC) of 4.5. This
calculation was individualized for each cycle, taking into account
the patient's renal function according to the Calvert formula
(7). At the same time, gemcitabine
was administered on the first and eighth day of each cycle at a
dose of 1,000 mg/m2. This dose was carefully determined
according to the patient's body surface area.
A follow-up 18F-FDG PET scan performed 6 months
later showed a significant reduction in the extent of the
retroperitoneal lesion, consistent with a partial functional
response (Fig. 4B). At the most
recent follow-up visit in January 2024, the patient reported
improvement in digestive symptoms and improvement in lower
extremity edema. Creatinine improved to 2 mg/dl. The patient is
currently receiving maintenance immunotherapy with Avelumab.
Discussion
RF is a rare condition of unclear pathogenesis
characterized by the formation of a soft tissue mass around the
prevertebral area, encircling the aorta and iliac arteries. The
ureters may also be involved leading to entrapment and
hydronephrosis, as in the case presented. The signs and symptoms of
RF are variable and are not helpful in the differential diagnosis
of other conditions, such as non-specific abdominal pain or lower
extremity edema, leading to delayed diagnosis (1).
Metastatic spread of urothelial carcinoma to the
gastrointestinal tract is rare and tends to involve the rectum in
bladder cancer (8). Upper
gastrointestinal symptoms, such as vomiting and epigastric pain due
to duodenal obstruction in our specific case, are rare because
extrinsic malignancy of the duodenum in urothelial cancers of the
upper urinary tract and bladder is uncommon, with few documented
cases (9–11). Tokunaga et al (9) reported two cases of bladder cancer in
which abnormal perirectal tissue was initially identified and
classified as stage M0. Subsequently, both cases evolved with the
development of RF adjacent to the duodenal wall, although less
pronounced than in our study. On the other hand, Andersen et
al (11) described a case of UC
of the renal pelvis with retroperitoneal extension leading to
duodenal obstruction. Similarly, Iwamoto et al (10) documented a case in which
periduodenal tissue was detected and reported as inflammatory
changes unrelated to the primary tumor, with the final diagnosis
made postmortem. These reports suggest that the presence of
fibrosis or retroperitoneal inflammatory changes associated with
UC, although less obvious on imaging than in our case, deserves
detailed evaluation for its potential impact on the evolution and
clinical management of patients.
The existing literature has demonstrated the
association of RF with malignancy in tumors in a variety of sites,
including the prostate, rectum, colon, stomach, or lung, although
it is difficult to diagnose and differentiate from other secondary
conditions coexisting in the same anatomic location (12,13).
Lymphomas, sarcomas, or irregular lymph node metastases can look
very similar to RF on a CT scan (14). They are difficult to detect on CT,
and the signs that have been described to suggest a neoplastic
cause are often non-specific. These signs include anterior
displacement of the abdominal aorta and inferior vena cava or
extension into the renal hilum with lateral displacement of the
ureters (2). Idiopathic RF tends to
present as a plaque-like density, whereas neoplasms show nodularity
and peripheral lobulation (15).
Some studies have highlighted the tendency of lymphomas to have a
more cranial distribution, often involving the posterior
mediastinum, whereas benign RF occurs predominantly caudal to the
renal hilum (14). We suggest that
the extensive involvement observed in our case, particularly the
concentrated involvement of the perirectal and perivesical fat and
the inguinal canals, serves as a strong impetus to investigate the
possibility of a secondary neoplasm. This is exemplified by our
patient's condition, where such an extensive pattern of disease was
a key indicator that prompted further investigation.
Cases of RF associated with urothelial carcinoma are
rare, but present unique diagnostic challenges and insights. For
example, Murray and Woo-Ming (6)
reported a case characterized by normal cystoscopy and inability to
catheterize the ureters along with duodenal obstruction in its
third portion caused by fibrotic plaque. A biopsy from the right
fossa showed only RF and the definitive diagnosis was made post
mortem. Conversely, the case documented by Reiner et al
(5) involved a patient whose
cystoscopic biopsy failed to identify tumor cells. The suspicion
was raised by urography and subsequently confirmed by surgery.
The underlying mechanism driving the development of
RF in carcinoma is unknown. The ability to disseminate from the
original site and migrate into the surrounding stroma could be
explained by the loss of E-cadherin expression via the
epithelial-mesenchymal transition (EMT). Fibrosis may be
facilitated by an intense desmoplastic reaction that, when
occurring in the retroperitoneum, may encapsulate abdominal organs
and major blood vessels. Spread through the retromesenteric and
interfascial planes connecting the retroperitoneum from the
duodenum to the inguinal region would explain the findings seen in
this case (1,3,16).
Malignant RF is refractory to pharmacologic
treatment with immunosuppressants. Therefore, the focus should be
on the diagnosis and treatment of the underlying neoplasm (17). Because recognition of a neoplastic
cause alters the therapeutic approach, management algorithms for
the diagnosis of secondary RF have been proposed, emphasizing the
need for PET to detect active fibrosis or cancer and to guide
biopsy (18,19). An optimal diagnostic strategy for
the effective detection of this type of RF should include a CT scan
to define the extent of the disease. In addition, it is essential
to perform a PET scan to identify the most hypermetabolic areas,
followed by a targeted biopsy of the most suspicious or accessible
areas for the procedure.
In this case report, the diagnosis of UC was
primarily suggested by histopathology, but the lack of a clear
origin in the bladder or ureter added complexity. What makes this
case novel is the unusually extensive RF seen on CT imaging
involving both genitourinary systems, the bladder, and beyond. This
extensive involvement, coupled with a small nodule in the bladder
that appears disproportionate to the retroperitoneal infiltration,
obscures the bladder as the primary site. In addition, the behavior
of the lesion, involving the upper urinary tract and
retroperitoneum without presenting as an expansile lesion or
showing adenopathy metastasis, mimics RF on imaging. These peculiar
and novel features make this case exceptional and demonstrate an
atypical presentation of UC.
According to the European Association of Urology
guidelines, both upper urinary tract and bladder urothelial cancer
respond to platinum-based systemic chemotherapy, with
cisplatin-based combination chemotherapy being the standard of care
for advanced or metastatic urothelial cancer. The use of
cisplatin-based chemotherapy is widely considered for patients with
an estimated GFR >45 ml/min. In patients ineligible for
cisplatin, the combination of carboplatin and gemcitabine is
recommended, as in our patient (20,21).
Maintenance immunotherapy with avelumab is the recommended standard
of care for patients whose disease has stabilized after first-line
platinum-based chemotherapy (22).
The treatment of advanced UC and malignant RF is a
major challenge in oncology due to the aggressiveness of these
diseases and the lack of effective therapeutic options.
RF-associated cancers are often diagnosed at advanced stages, which
limits the chances of successful treatment, with a median survival
of only 3 to 6 months (4). The lack
of treatment options that provide durable remissions and prolonged
survival is an ongoing unmet need for urothelial cancer patients
(23). Treatment of advanced
urothelial cancer with gemcitabine and carboplatin has shown a
limited median overall survival of approximately 9.8 months
(24). No data have been found in
the literature regarding the treatment and prognosis of cases of UC
with this unusual presentation.
Future lines of research should be directed at
further exploring the association between RF and malignancy,
improving the understanding of the biology of UC, and including the
development of treatments that specifically target molecular
pathways involved in disease progression and the development of
malignant RF. In addition, research in immunotherapy and targeted
therapy offers hope for improved outcomes in this patient
population. A multidisciplinary approach to the management of these
patients is essential.
In conclusion, the unusually extensive
retroperitoneal infiltration documented on CT in this patient
underscores the need for a comprehensive evaluation to identify the
underlying cause. Furthermore, the positive response to
chemotherapy with carboplatin and gemcitabine, as demonstrated by
PET/CT follow-up, emphasizes the importance of early diagnosis and
appropriate management of these unusual clinical presentations.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AM was responsible for the initial diagnosis and the
study design. DL and AS performed the biopsy and contributed
substantially to the conception, design and writing of the
manuscript. JP and MC performed the histopathological examination
and participated in the imaging process. DL and JP confirm the
authenticity of all the raw data All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the case information and images to be published in this
case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vaglio A, Salvarani C and Buzio C:
Retroperitoneal fibrosis. Lancet. 367:241–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amis ES Jr: Retroperitoneal fibrosis. AJR
Am J Roentgenol. 157:321–329. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caiafa RO, Vinuesa AS, Izquierdo RS,
Brufau BP, Ayuso Colella JR and Molina CN: Retroperitoneal
fibrosis: Role of imaging in diagnosis and follow-up.
Radiographics. 33:535–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koep L and Zuidema GD: The clinical
significance of retroperitoneal fibrosis. Surgery. 81:250–257.
1977.PubMed/NCBI
|
|
5
|
Reiner I, Yachia D, Nissim F and
Fishelowitz Y: Retroperitoneal fibrosis in association with
urothelial tumor. J Urol. 132:115–116. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murray SM and Woo-Ming MO: Retroperitoneal
‘fibrosis’ due to carcinoma of the ureter. Br J Urol. 38:424–427.
1966. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calvert AH, Newell DR, Gumbrell LA,
O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME and
Wiltshaw E: Carboplatin dosage: Prospective evaluation of a simple
formula based on renal function. J Clin Oncol. 7:1748–1756. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stillwell TJ, Rife CC and Lieber MM:
Bladder carcinoma presenting with rectal obstruction. Urology.
34:238–240. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tokunaga K, Furuta A, Arizono S, Teramoto
Y, Negoro H, Kido A, Isoda H and Togashi K: Duodenal obstruction
induced by retroperitoneal progression of bladder cancer: A report
of two cases. Abdom Radiol (NY). 44:1223–1229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwamoto N, Oikawa M, Kukimoto T, Ito J,
Murakami K and Kaiho Y: Renal pelvis cancer with initial symptoms
of malignant gastric outlet obstruction. IJU Case Rep. 6:475–478.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andersen K, Burroughs S, Munis A, Hoff RT
and Shapiro A: Gastric outlet obstruction as the initial
presentation of upper tract urothelial carcinoma. Case Rep
Gastrointest Med. 2020:88500622020.PubMed/NCBI
|
|
12
|
Brandt AS, Kamper L, Kukuk S, Haage P and
Roth S: Associated findings and complications of retroperitoneal
fibrosis in 204 patients: Results of a urological registry. J Urol.
185:526–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SJ, Eun JS, Kim MJ, Song YW and Kang
Y: Association of retroperitoneal fibrosis with malignancy and its
outcomes. Arthritis Res Ther. 23:2492021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brun B, Laursen K, Sørensen IN, Lorentzen
JE and Kristensen JK: CT in retroperitoneal fibrosis. AJR Am J
Roentgenol. 137:535–538. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fagan CJ, Larrieu AJ and Amparo EG:
Retroperitoneal fibrosis: Ultrasound and CT features. AJR Am J
Roentgenol. 133:239–243. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeung KT and Yang J:
Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol.
11:28–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brandt AS, Dreger NM, Müller E, Kukuk S
and Roth S: New (and old) aspects of retroperitoneal fibrosis.
Urologe A. 56:887–894. 2017.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Łoń I, Wieliczko M, Lewandowski J and
Małyszko J: Retroperitoneal fibrosis is still an underdiagnosed
entity with poor prognosis. Kidney Blood Press Res. 47:151–162.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cronin CG, Lohan DG, Blake MA, Roche C,
McCarthy P and Murphy JM: Retroperitoneal fibrosis: A review of
clinical features and imaging findings. AJR Am J Roentgenol.
191:423–431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alfred Witjes J, Max Bruins H, Carrión A,
Cathomas R, Compérat E, Efstathiou JA, Fietkau R, Gakis G, Lorch A,
Martini A, et al: European association of urology guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2023
guidelines. Eur Urol. 85:17–31. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rouprêt M, Seisen T, Birtle AJ, Capoun O,
Compérat EM, Dominguez-Escrig JL, Gürses Andersson I, Liedberg F,
Mariappan P, Hugh Mostafid A, et al: European association of
urology guidelines on upper urinary tract urothelial carcinoma:
2023 Update. Eur Urol. 84:49–64. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cathomas R, Lorch A, Bruins HM, Compérat
EM, Cowan NC, Efstathiou JA, Fietkau R, Gakis G, Hernández V,
Espinós EL, et al: The 2021 updated European association of urology
guidelines on metastatic urothelial carcinoma. Eur Urol. 81:95–103.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Geynisman DM, Broughton E, Hao Y, Zhang Y,
Le T and Huo S: Real-world treatment patterns and clinical outcomes
among patients with advanced urothelial carcinoma in the United
States. Urol Oncol. 40:195.e1–195.e11. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dogliotti L, Cartenì G, Siena S, Bertetto
O, Martoni A, Bono A, Amadori D, Onat H and Marini L: Gemcitabine
plus cisplatin versus gemcitabine plus carboplatin as first-line
chemotherapy in advanced transitional cell carcinoma of the
urothelium: Results of a Randomized phase 2 trial. Eur Urol.
52:134–141. 2007. View Article : Google Scholar : PubMed/NCBI
|