Introduction
As the fifth most common type of cancer and the
third highest cause of cancer-related mortality worldwide as of
2020, gastric cancer remains a global health threat due to its high
malignancy (1). Gastric outlet
obstruction refers to a condition where food and gastric juices
cannot smoothly pass through the exit of the stomach into the
duodenum or small intestine (2).
This is a common complication of gastric cancer and can occur at
any stage of the treatment process (3). Certain patients may experience
symptoms of gastric outlet obstruction at an early stage due to the
unfavorable location of the tumor or high malignancy (4). These patients often suffer from
abdominal distention and eating difficulties, resulting in severe
malnutrition and decrease in immune function (5).
In recent years, the treatment paradigm for gastric
cancer has evolved to incorporate comprehensive strategies such as
surgical treatment, adjuvant and targeted therapy and immunotherapy
(6). However, surgery remains the
primary treatment choice for gastric cancer (7). Patients with early gastric outlet
obstruction often have poor nutritional status and treatment
tolerance and are often unable to receive surgery immediately
(8). Therefore, nutritional support
therapy serves a key role in their treatment regimen. Commonly used
clinical nutritional support strategies include enteral nutrition
(EN), parenteral nutrition (PN) and the addition of full- or
semi-liquid diets (9–11). While there are clear indications and
methods for nutritional support in gastric cancer treatment
recommendations, numerous factors such as disease state of the
patient, economic situation and personal preferences have led to
the clinical use of multiple nutritional treatment methods
(12). Further research is needed
to explore the differences in the effectiveness of these treatment
methods.
The present study aimed to assess the impact of
different preoperative nutritional treatments on postoperative
recovery and prognosis in patients with gastric cancer and early
gastric outlet obstruction by retrospectively collecting
nutritional approaches and clinical information.
Materials and methods
Patients
The present study was a retrospective study that
included 467 patients with gastric cancer and early gastric outlet
obstruction who underwent surgery at Harbin Medical University
Cancer Hospital (Harbin, China) from January 2016 to December 2018.
The inclusion criteria were as follows: i) Patients were diagnosed
with gastric cancer through pathological examination; ii) patients
underwent surgical treatment; iii) patients were confirmed to have
gastric outlet obstruction through gastroscopy, enhanced computed
tomography and patient assessment; iv) no occurrence of distant
metastasis and v) all patients received preoperative nutritional
therapy. Preoperative nutritional therapy was administered for 7–20
days, with a mean treatment duration of 8.23±2.33 days. The
inability to restore nutritional status through nutritional therapy
and lack of follow-up data were exclusion criteria. The present
study obtained approval from the Ethics Committee of Harbin Medical
University Cancer Hospital (approval no. 2019-57-IIT). Due to the
retrospective nature of this study, the Ethics Committee of Harbin
Medical University Cancer Hospital waived the requirement for
informed consent.
Data collection and follow-up
The present study screened and collected the medical
history data of all enrolled patients through the medical record
system and obtained progression-free survival (PFS), defined as the
period during which there is no tumor growth, spread or metastasis
following treatment, and overall survival (OS) defined as the
period from the initiation of treatment to the patient death or the
last follow-up. Patients received regular telephone follow-ups
every three months, with the longest follow-up duration being 80
months.
Preoperative nutritional
treatment
All patients received central venous catheter
placement on the day of admission or the following day; those
receiving EN also underwent nasoenteric nutrition tube placement.
PN solution used was Kabiven Peripheral (1,440 ml; Fresenius Kabi
AB), providing energy at a rate of 30–35 kcal/kg body weight per
day. The EN solution used was Enteral Nutritional Emulsion (TPF-T;
Fresenius Kabi AB), which was combined with PN to provide the
required energy to the patients. The full- or semi-liquid diet
primarily consisted of cereal-based foods providing carbohydrates
and Intact Protein Enteral Nutrition Powder (Milupa GmbH & Co.,
KG), with the quantity controlled based on patient tolerance.
Patients were grouped according to preoperative nutritional
treatment as follows: Group 1, 230 patients (49.3%) who received PN
only; Group 2, 162 patients (34.7%) who received PN combined with
EN and Group 3, 75 patients (16.0%) who received PN combined with a
full or semi-liquid diet. The criteria for discontinuation of
nutritional therapy included the restoration of normal peripheral
blood levels of albumin and prealbumin, resolution of fatigue
symptoms, weight gain or maintenance and meeting the standards of
the 6-min walk test (13).
Statistical analysis
Continuous and categorical variables are presented
as the mean ± standard deviation and n (%), respectively;
differences between groups were compared using an independent
sample t, χ2 or one-way ANOVA followed by Sheffe's
post-hoc test. Furthermore, when the count of cells in the
contingency table for categorical variables was ≤5 in <0% of the
cells, Fisher's exact test was used to assess the association. A
two-tailed P<0.05 was considered to indicate a statistically
significant difference.
Differences in PFS and OS between groups were
compared using Kaplan-Meier survival analysis and log-rank test.
Single- and multi-factor survival analyses were conducted to
identify independent prognostic factors. Finally, the impact of
independent prognostic factors on patient survival was validated
through nomograms and their C-indexes.
Results
Patient characteristics
There were a total of 346 (74.1%) males and 121
(25.9%) females, with a mean age of 63.95±9.66 years. Furthermore,
141 patients (30.2%) were TNM stage II, while 326 patients (69.8%)
were TNM stage III. The χ2 or Fisher's exact test
indicated that there were no statistically significant differences
in age, sex, nutritional status and pathological information
between the three groups (all P>0.05). This suggested that the
three groups of patients had good comparability (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Parameter | Group 1 | Group 2 | Group 3 | P-value |
|---|
| Age, years | 63.79±9.18 | 63.58±10.36 | 65.24±9.58 | 0.442 |
| BMI,
kg/m2 | 20.72±3.46 | 20.29±3.00 | 20.27±3.53 | 0.564 |
| TP, g/l | 61.36±7.53 | 61.70±6.70 | 60.50±7.25 | 0.324 |
| ALB, g/l | 35.75±4.56 | 35.96±4.09 | 35.38±4.44 | 0.797 |
| GLOB, g/l | 26.57±5.73 | 26.26±4.83 | 25.40±4.48 | 0.242 |
| PALB, g/l | 175.12±54.79 | 171.46±48.95 | 171.12±43.97 | 0.644 |
| Sex |
|
|
|
|
|
Male | 174 (75.7) | 118 (72.8) | 54 (72.0) | 0.743 |
|
Female | 56 (24.3) | 44 (27.2) | 21 (28.0) |
|
| Radical
resection |
|
|
|
|
|
Yes | 208 (90.4) | 149 (92.0) | 68 (90.7) | 0.428 |
| No | 22 (9.6) | 13 (8.0) | 7 (9.3) |
|
| Primary tumor
site |
|
|
|
|
| Upper
1/3 of the stomach | 16 (7.0) | 6 (3.7) | 5 (6.7) | 0.064 |
| Middle
1/3 of the stomach | 30 (13.0) | 14 (8.6) | 16 (21.3) |
|
| Lower
1/3 of the stomach | 180 (78.3) | 136 (84.0) | 54 (72.0) |
|
| Whole
stomach | 4 (1.7) | 6 (3.7) | 0 (0.0) |
|
| Borrmann type |
|
|
|
|
| I | 28 (12.2) | 36 (22.2) | 13 (17.3) | 0.467 |
| II | 174 (75.7) | 94 (58.0) | 47 (62.7) |
|
|
III | 22 (9.6) | 26 (16.0) | 12 (16.0) |
|
| IV | 6 (2.6) | 6 (3.7) | 3 (4.0) |
|
| Tumor size, mm |
|
|
|
|
|
<50 | 106 (46.1) | 56 (34.6) | 31 (41.3) | 0.053 |
|
≥50 | 124 (53.9) | 106 (65.4) | 44 (56.7) |
|
|
Differentiation |
|
|
|
|
|
Poor | 18 (7.8) | 10 (6.2) | 5 (6.6) | 0.518 |
|
Moderately | 86 (37.4) | 70 (43.2) | 28 (37.3) |
|
|
Well | 126 (54.8) | 82 (50.6) | 42 (56.1) |
|
| TNM stage |
|
|
|
|
| II | 78 (33.9) | 48 (29.6) | 15 (20.0) | 0.073 |
|
III | 152 (66.1) | 114 (70.4) | 60 (80.0) |
|
Postoperative recovery status
ANOVA demonstrated statistically significant
differences in all postoperative recovery indicators between the
three groups of patients in this study (all P<0.05, Table II). Post-hoc test results
demonstrated significant differences in nutritional treatment time
and length of hospital stay among all groups (Table III). Patients in Group 3 had a
significantly longer preoperative nutritional treatment time
compared with the other groups [Group 1 (5.75 days) vs. Group 2
(6.84 days) vs. Group 3 (12.16 days), P<0.001]. This extended
treatment time also resulted in a longer length of hospital stay
for these patients [Group 1 (18.77 days) vs. Group 2 (16.52 days)
vs. Group 3 (23.52 days), P<0.001]. Furthermore, the time to
first postoperative bowel sounds for patients in Groups 1, 2 and 3
was 2.47, 1.72 and 2.36 days, respectively (Table II). Patients in Group 2 had a
shorter time to first postoperative bowel sounds compared with the
other groups (P<0.001). However, time to first postoperative
flatus and bowel movement in Group 1 was significantly longer
compared with those in Group 2, while there was no significant
difference between Groups 2 and 3 (P=0.882 and P=0.416,
respectively; Table III).
Furthermore, there were no significant differences in removal time
of abdominal drainage tube between any groups of patients. Patients
who received preoperative PN combined with EN demonstrated
advantages in several aspects of postoperative recovery, with the
exception of no significant improvement in the removal time of the
abdominal drainage tube.
 | Table II.Postoperative recovery status. |
Table II.
Postoperative recovery status.
| Item | Group 1 | Group 2 | Group 3 | F-value | P-value |
|---|
| Mean nutritional
treatment time, days | 5.75±2.05 | 6.84±1.73 | 12.16±2.97 | 260.486 | <0.001 |
| Mean length of
hospital stay, days | 18.77±5.51 | 16.52±4.48 | 23.52±4.52 | 28.635 | <0.001 |
| Mean time to first
postoperative bowel sounds, days | 2.47±0.23 | 1.72±0.18 | 2.36±0.33 | 17.464 | 0.021 |
| Mean time to first
postoperative flatus, days | 5.88±1.10 | 5.17±0.82 | 5.24±0.77 | 29.781 | <0.001 |
| Mean time to first
postoperative bowel movement, days | 5.97±1.08 | 5.19±0.81 | 5.36±0.75 | 35.279 | <0.001 |
| Mean removal time
of abdominal drainage tube, days | 8.69±3.18 | 7.23±2.29 | 8.80±2.30 | 15.640 | 0.034 |
 | Table III.Analysis by Sheffe's post-hoc
multiple-comparisons test. |
Table III.
Analysis by Sheffe's post-hoc
multiple-comparisons test.
| Item | Mean
difference | P-value |
|---|
| Mean nutritional
treatment time, days |
|
|
| Group 1
vs. 2 | −1.092 | <0.001 |
| Group 1
vs. 3 | −6.412 | <0.001 |
| Group 2
vs. 3 | −5.320 | <0.001 |
| Mean length of
hospital stay, days |
|
|
| Group 1
vs. 2 | 2.251 | <0.001 |
| Group 1
vs. 3 | −4.754 | <0.001 |
| Group 2
vs. 3 | −7.000 | <0.001 |
| Mean time to first
postoperative bowel sounds, days |
|
|
| Group 1
vs. 2 | 0.750 | <0.001 |
| Group 1
vs. 3 | −0.113 | 0.446 |
| Group 2
vs. 3 | −0.640 | <0.001 |
| Mean time to first
postoperative flatus, days |
|
|
| Group 1
vs. 2 | 0.705 | <0.001 |
| Group 1
vs. 3 | 0.638 | <0.001 |
| Group 2
vs. 3 | −0.067 | 0.882 |
| Mean time to first
postoperative bowel movement, days |
|
|
| Group 1
vs. 2 | 0.780 | <0.001 |
| Group 1
vs. 3 | 0.605 | <0.001 |
| Group 2
vs. 3 | −0.175 | 0.416 |
| Mean removal time
of abdominal drainage tube, days |
|
|
| Group 1
vs. 2 | 0.452 | 0.282 |
| Group 1
vs. 3 | −0.113 | 0.954 |
| Group 2
vs. 3 | −0.565 | 0.344 |
Survival analysis
Uni- and multivariate survival analysis
Uni- and multivariate analyses were performed to
assess the factors associated with survival. Age, PALB, radical
resection, Borrmann type, TNM stage and nutritional treatment were
associated with PFS and OS of patients. Among these factors, age
[PFS: Hazard ratio (HR)=1.024, P=0.022; OS: HR=1.027, P=0.010],
radical resection (PFS: HR=1.635, P=0.012; OS: HR=1.674, P=0.011),
TNM stage (PFS: HR=4.046, P<0.001; OS: HR=4.198, P<0.001) and
nutritional treatment according to Group 3 (PFS: HR=2.110, P=0.001;
OS: HR=2.112, P=0.001) were independent prognostic factors in the
present study (Tables IV and
V).
 | Table IV.Uni- and multivariate analysis of
progression-free survival. |
Table IV.
Uni- and multivariate analysis of
progression-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age | 1.023
(1.004–1.042) | 0.016 | 1.024
(1.003–1.044) | 0.022 |
| TP | 0.993
(0.971–1.017) | 0.584 |
|
|
| ALB | 0.990
(0.951–1.031) | 0.633 |
|
|
| PALB | 0.994
(0.991–0.998) | 0.004 | 0.996
(0.992–1.000) | 0.084 |
| Sex (female vs.
male) | 1.032
(0.695–1.532) | 0.876 |
|
|
| Radical resection
(no vs. yes) | 2.238
(1.572–3.187) | <0.001 | 1.635
(1.112–2.405) | 0.012 |
| Borrmann type |
|
|
|
|
| II vs.
I | 0.819
(0.470–1.429) | 0.482 | 0.804
(0.456–1.418) | 0.451 |
| III vs.
I | 1.172
(0.584–2.351) | 0.655 | 0.867
(0.430–1.748) | 0.689 |
| IV vs.
I | 2.584
(1.331–5.014) | 0.005 | 1.308
(0.630–2.718) | 0.471 |
| Tumor size (≥50 vs.
<50 mm) | 1.210
(0.853–1.717) | 0.286 |
|
|
| TNM stage (III vs.
II) | 2.952
(1.918–3.503) | <0.001 | 4.046
(2.346–6.976) | <0.001 |
| Nutritional
treatment group |
|
|
|
|
| 2 vs.
1 | 1.126
(0.735–1.723) | 0.586 | 1.157
(0.747–1.792) | 0.512 |
| 3 vs.
1 | 2.818
(1.876–4.233) | <0.001 | 2.110
(1.343–3.314) | 0.001 |
 | Table V.Uni- and multivariate analysis of
overall survival. |
Table V.
Uni- and multivariate analysis of
overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age | 1.024
(1.005–1.043) | 0.012 | 1.027
(1.006–1.047) | 0.010 |
| TP | 0.994
(0.971–1.018) | 0.618 |
|
|
| ALB | 0.992
(0.953–1.033) | 0.689 |
|
|
| PALB | 0.995
(0.991–0.998) | 0.006 | 0.997
(0.993–1.001) | 0.110 |
| Sex (female vs.
male) | 1.026
(0.691–1.524) | 0.897 |
|
|
| Radical resection
(no vs. yes) | 2.261
(1.588–3.220) | <0.001 | 1.674
(1.120–2.423) | 0.011 |
| Borrmann type |
|
|
|
|
| II vs.
I | 0.799
(0.458–1.393) | 0.428 | 0.801
(0.453–1.415) | 0.444 |
| III vs.
I | 1.176
(0.586–2.358) | 0.648 | 0.894
(0.443–1.806) | 0.755 |
| IV vs.
I | 2.689
(1.386–5.219) | 0.003 | 1.457
(0.700–3.030) | 0.314 |
| Tumor size (≥50 vs.
<50 mm) | 1.167
(0.822–1.656) | 0.387 |
|
|
| TNM stage (III vs.
II) | 2.580
(1.915–3.475) | <0.001 | 4.198
(2.434–7.242) | <0.001 |
| Nutritional
treatment group |
|
|
|
|
| 2 vs.
1 | 1.121
(0.733–1.717) | 0.598 | 1.150
(0.742–1.718) | 0.532 |
| 3 vs.
1 | 2.847
(1.895–4.276) | <0.001 | 2.112
(1.344–3.312) | 0.001 |
Survival analysis of nutritional
treatments
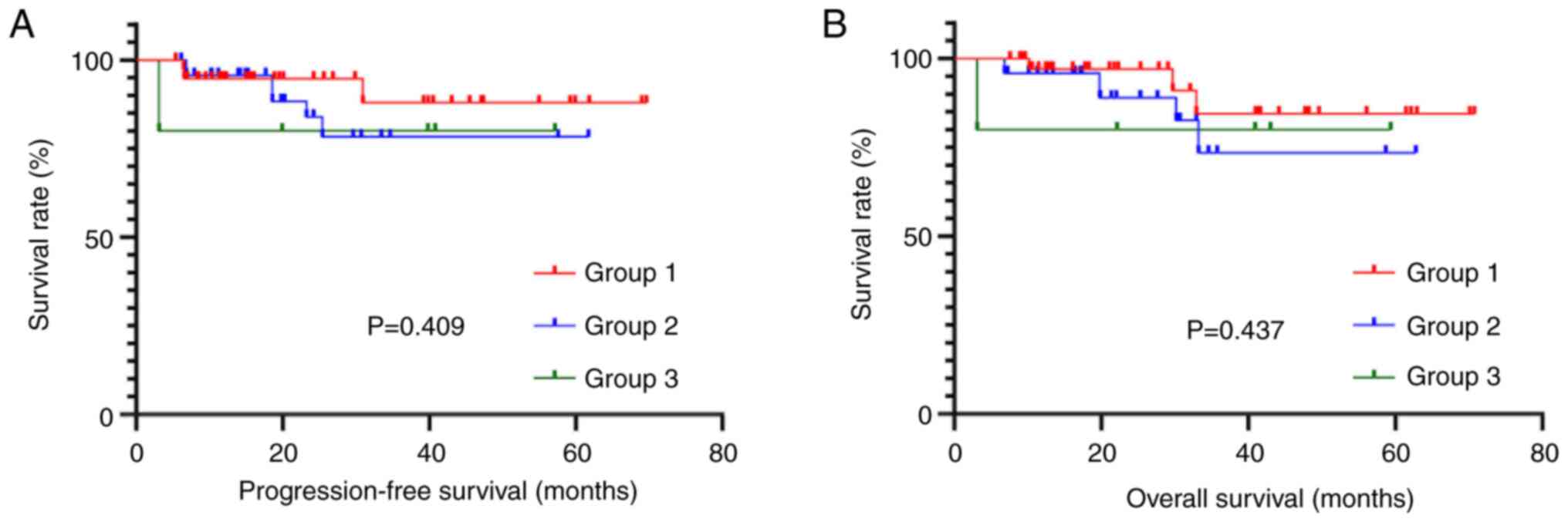
Kaplan-Meier survival curves demonstrated that
patients in Group 3 receiving PN combined with a full- or
semi-liquid diet had significantly shorter PFS
(χ2=30.485) and OS (χ2=31.249) compared with
the Group 1 receiving PN only and Group 2 receiving PN combined
with EN (Fig. 1).
Moreover, as independent prognostic factors, the
relationship between preoperative nutritional treatment and
prognosis in patients of different ages, TNM stages and those who
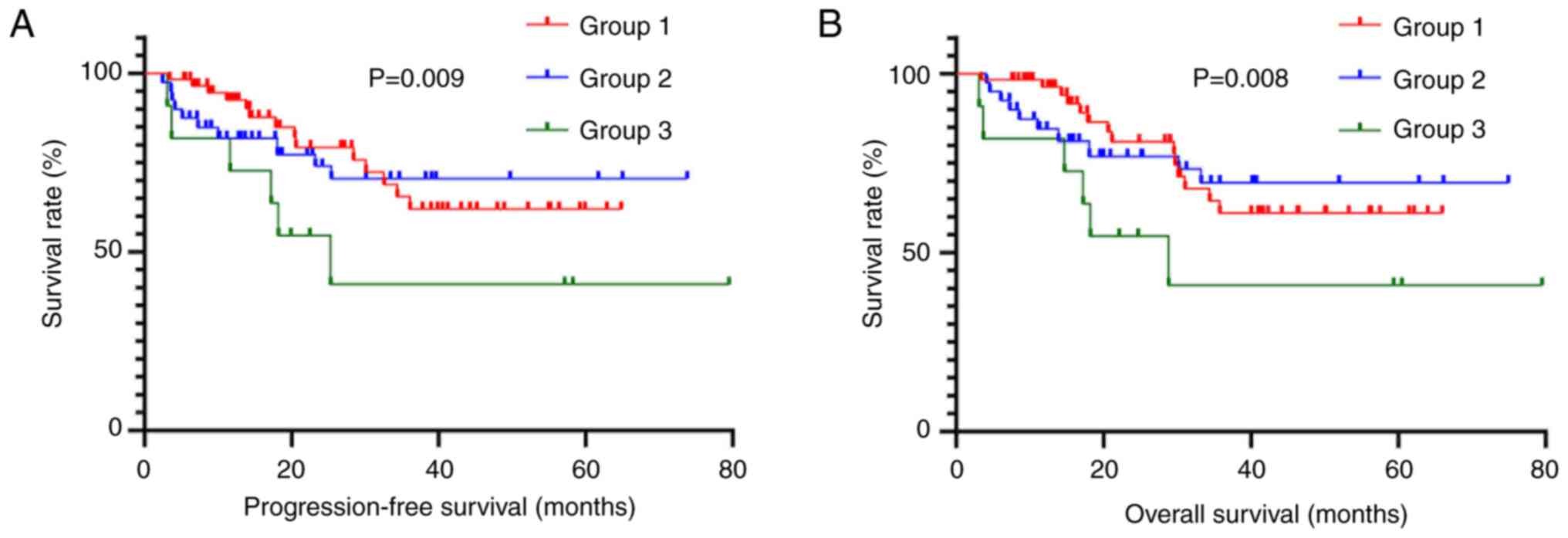
underwent radical resection was analyzed. There was a total of 141
patients in TNM stage II, with 78 in Group 1, 48 in Group 2 and 15
in Group 3. Survival curves did not show significant differences in
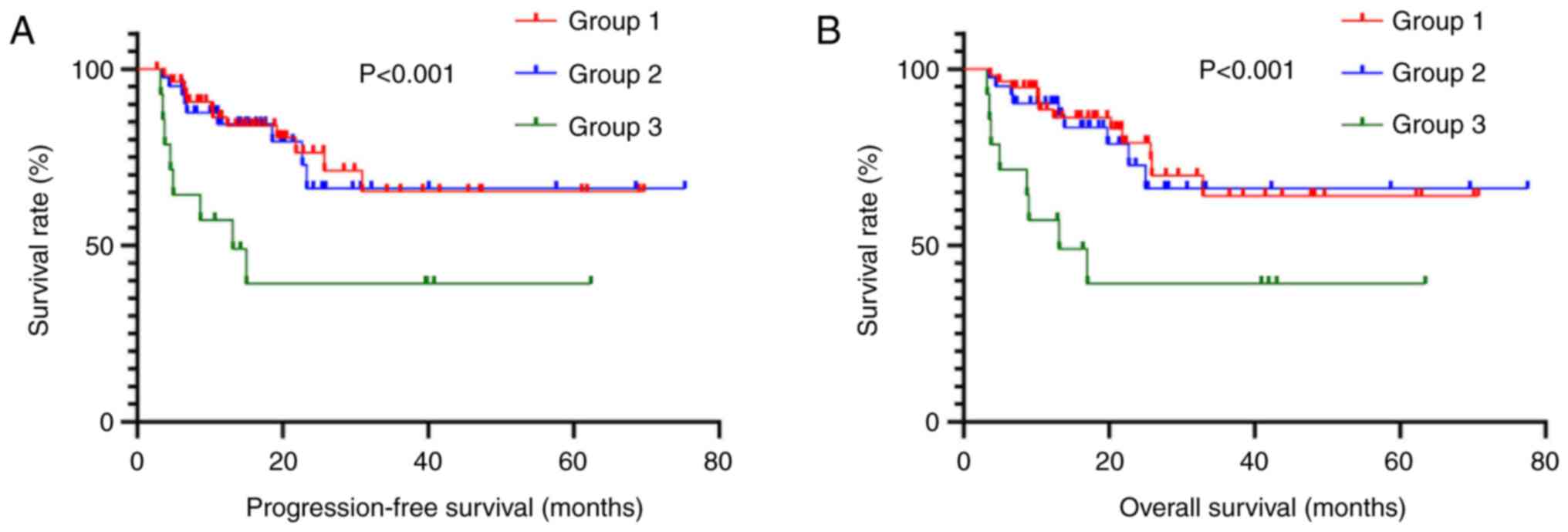
PFS (χ2=1.789) and OS (χ2=1.658; Fig. 2). There was a total of 326 patients
in TNM stage III, with 152 in Group 1, 114 in Group 2 and 60 in
Group 3. Kaplan-Meier analysis demonstrated a significantly shorter
PFS (χ2=25.350) and OS (χ2=26.535) for
patients in Group 3 compared to those in Groups 1 and 2 (Fig. 3).
As the age of 60 years is widely recognized as a
threshold in gastric cancer and considering that the median age in
the present study was close to 60 years (63.59 years), a cutoff
value of 60 years was chosen for age analysis (14,15).
There were a total of 229 patients aged <60 years, including 116
in Group 1, 80 in Group 2 and 33 in Group 3. Patients in Group 3
had a significantly shorter PFS (χ2=9.485) and OS
(χ2=9.603) compared to Groups 1 and 2 (Fig. 4A and B). Furthermore, there were 238
patients aged ≥60 years; 114 were in Group 1, 82 were in Group 2
and 42 were in Group 3. Survival curves demonstrated a
significantly shorter PFS (χ2=22.949) and OS
(χ2=23.472) in Group 3 compared to Groups 1 and 2
(Fig. 5A and B).
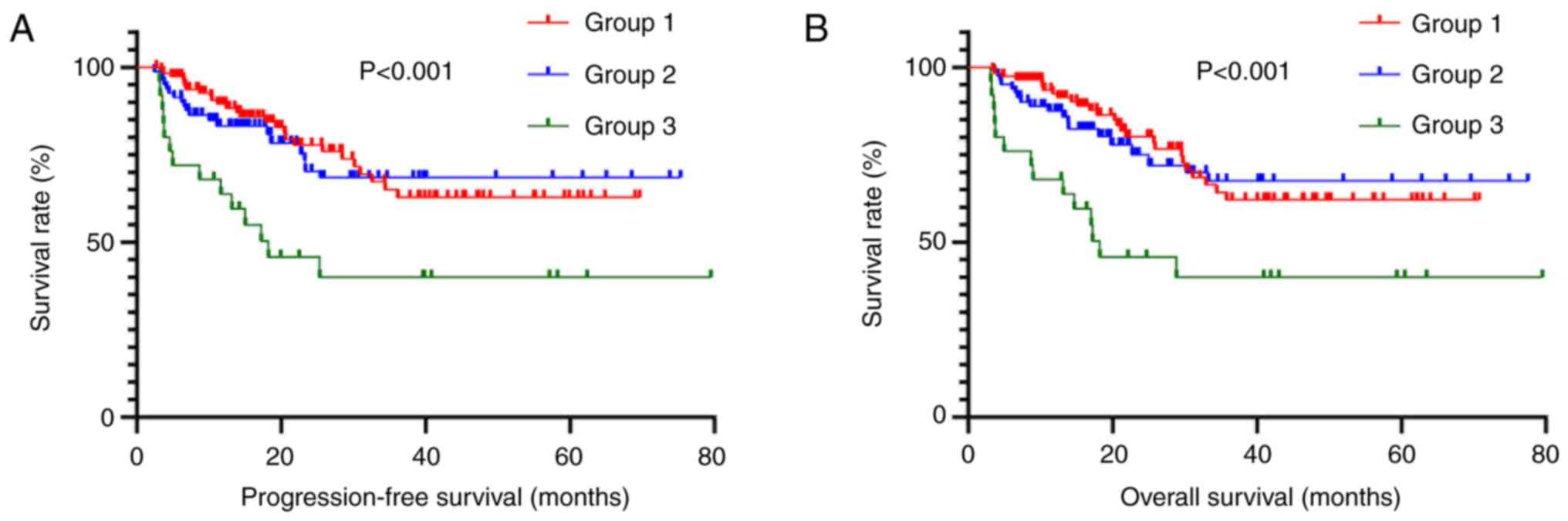
A total of 425 patients underwent R0 resection,
accounting for 91% of the total cohort. Therefore, the present
study only analyzed patients who underwent R0 resection. Among
these patients, there were 208 individuals in Group 1 (48.9%), 149
in Group 2 (35.1%) and 68 in Group 3 (16.0%). Group 3 demonstrated
a significantly shorter PFS (χ2=25.350) and OS
(χ2=26.535) compared to Groups 1 and 2 (Fig. 6).
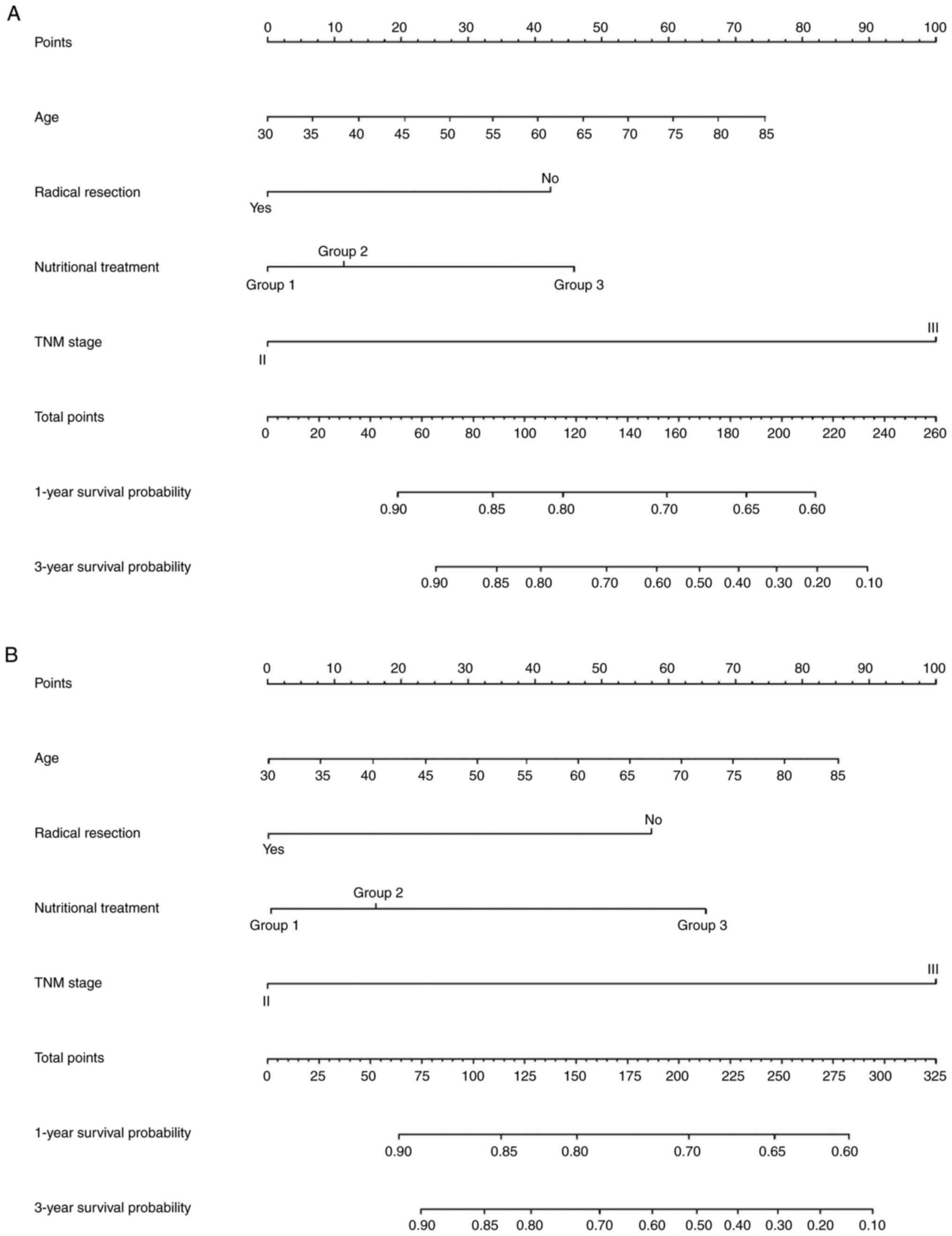
Nomograms
Finally, to assess the impact of different
preoperative nutritional treatments on prognosis of patients with
early gastric outlet obstruction, nomograms were created to predict
the 1- and 3-year survival probabilities based on the results of
the multivariate analysis. The figure shows nomograms constructed
based on independent prognostic factors for PFS and OS,
respectively (Fig. 7A and B).
C-index for the nomograms of PFS and OS was 0.819 and 0.822,
indicating the good predictive performance of nomograms that
included preoperative nutritional treatment.
Discussion
Gastric outlet obstruction is a common complication
in patients with gastric cancer (16–18).
This condition often coincides with varying degrees of
malnutrition, potentially accelerating the progression of the tumor
(19–21). To restore the nutritional and immune
status patient and enhance treatment tolerance, comprehensive
preoperative nutritional treatment is required (22,23).
However, diverse nutritional treatment approaches exhibit
differences in energy provision, side effects and patient tolerance
(24–26). The present study assessed the
effects of nutritional treatments on patients with early gastric
cancer complicated by gastric outlet obstruction, offering insights
into the development of preoperative nutritional treatment
strategies.
Gastric cancer complicated by gastric outlet
obstruction has long been a focus of researchers. In 2023, Li et
al (27) conducted comparative
analysis to investigate the unique clinical and pathological
characteristics of patients with gastric outlet obstruction. Data
were collected from 194 patients with gastric cancer accompanied by
gastric outlet obstruction and 221 patients without gastric outlet
obstruction. Patients with gastric outlet obstruction exhibited
poorer clinical features, pathological conditions and blood
parameters, which resulted in shorter survival. Another study
reported similar results: In 2021, Jiao et al (28) collected data from 343 patients with
gastric cancer who underwent radical resection. Propensity-matched
analyses were conducted to investigate clinical characteristics and
survival outcomes of patients with gastric outlet obstruction;
although gastric outlet obstruction was unrelated to postoperative
complications and mortality, it significantly decreased the OS time
of patients.
Preoperative nutritional therapy is key for the
treatment of patients with cancer and malnutrition. In 2015, Fukuda
et al (29) conducted a
large retrospective analysis to investigate the optimal
preoperative nutritional support for malnourished patients with
gastric cancer. Data from 800 patients with gastric cancer
undergoing gastrectomy were analyzed; adequate preoperative energy
support decreased postoperative surgical site infections in
malnourished patients. The impact of different nutritional
treatments on patients with cancer has also received attention.
Shen et al conducted a study on patients with esophageal
cancer in 2021, analyzing differences in the effectiveness of
preoperative PN and EN. Through a comparative analysis of 29
patients who received preoperative PN and 27 who received
preoperative EN, it was reported that preoperative EN had certain
advantages in postoperative recovery and occurrence of
complications (30). Another study
on short-term outcomes of patients with gastric cancer who
underwent surgery yielded similar results: In 2021, Li et al
(31) collected data from 143
patients with gastric outlet obstruction to analyze the impact of
preoperative PN and EN on postoperative recovery. Patients who
received EN had a shorter time to first postoperative flatus,
indicating faster postoperative recovery.
The present study assessed the impact of
preoperative nutritional treatment on the short- and long-term
clinical outcomes of patients with early gastric outlet obstruction
through a large sample cohort. PN combined with EN demonstrated
significant advantages in postoperative recovery status, as
evidenced by shorter lengths of hospital stay, quicker time to
first postoperative bowel sounds, earlier time to first
postoperative flatus and faster time to first postoperative bowel
movement. While PN combined with full- and semi-liquid diets
resulted in shorter times for the first postoperative flatus and
bowel movement, it was also associated with longer nutritional
treatment time and length of hospital stay. Furthermore, there was
no difference between the three groups of patients in the removal
time of the abdominal drainage tube. Removal time of the abdominal
drainage tube may be associated with surgical methods, extent and
time rather than the nutritional status. Combination of PN with
full- and semi-liquid diets was associated with poorer survival
outcomes. In subgroup analysis, except for TNM stage II patients
whose results were less accurate due to uneven distribution in
nutritional treatment groups, PN combined with full- and
semi-liquid diets also demonstrated worse clinical outcomes in
patients with TNM stage III or undergoing radical resection as well
as across all age groups. The multivariate survival analysis and
nomograms with high C-indices further supported the effect of
preoperative nutritional treatment on the clinical outcomes of
patients with early gastric outlet obstruction.
The exact mechanisms underlying the advantage of PN
combined with EN in postoperative recovery and survival require
further research and in-depth analysis. Long-term fasting may lead
to varying degrees of damage to intestinal function, including
disuse atrophy of the intestine, reduced intestinal motility,
disturbances in the intestinal microbiota and metabolic disorder
(32,33). Patients with early gastric outlet
obstruction often experience varying degrees of intake difficulty
before admission, which could result in more severe impairment of
intestinal function (34–36). Therefore, while PN could be an
effective treatment for patients with early gastric cancer and
gastric outlet obstruction to improve nutritional status, enabling
patients to undergo surgery, the addition of EN may contribute to
faster recovery of intestinal function, thereby expediting
postoperative recovery (37–40).
This is also a possible reason for the significant advantage of PN
combined with EN in postoperative recovery status in the present
study compared to PN only and PN combined with a full or
semi-liquid diet.
Furthermore, while PN combined with full- or
semi-liquid diets improves the recovery of intestinal peristalsis
compared with PN only, patients often require extended duration of
nutritional treatment due to energy absorption disorder caused by
gastric outlet obstruction and decreased tolerance resulting from
symptoms such as bloating, leading to an extended hospital stay
(41,42). In the present study, PN combined
with full- or semi-liquid diet was linked to worse clinical
outcomes. As previously discussed, patients with gastric outlet
obstruction exhibit decreased capacity to absorb and tolerate full-
or semi-liquid diets, resulting in inadequate nutritional recovery.
Malnutrition can exert detrimental effects on the immune function
and treatment tolerance of patients; these are two well-documented
factors associated with tumor progression and recurrence in
numerous studies (43–47).
The present study had limitations. First, this was a
retrospective study conducted at a single medical center, which may
have introduced potential information bias. Second, due to factors
such as surgical schedules, certain patients might not have
received sufficient preoperative nutritional support. Finally,
despite using numerous statistical methods for analysis, further
well-designed prospective studies are required to validate these
findings and elucidate the underlying mechanisms.
In summary, preoperative PN combined with EN proved
advantageous for postoperative recovery of patients with gastric
cancer and early gastric outlet obstruction. Furthermore, PN
combined with full- or semi-liquid diets may not fully meet the
nutritional needs of these patients, resulting in less favorable
clinical outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CW made substantial contributions to the conception
and design of the work, and wrote and reviewed the manuscript. DY
performed experiments. CW and DY confirm the authenticity of all
the raw data. Both authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Harbin Medical University Cancer Hospital (Harbin, China; approval
no. 2019-57-IIT). Due to the retrospective nature of the study, the
Ethics Committee of Harbin Medical University Cancer Hospital
waived the requirement for informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papanikolaou IS and Siersema PD: Gastric
outlet obstruction: Current status and future directions. Gut
Liver. 16:667–675. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Troncone E, Fugazza A, Cappello A, Del
Vecchio Blanco G, Monteleone G, Repici A, Teoh AYB and Anderloni A:
Malignant gastric outlet obstruction: Which is the best therapeutic
option? World J Gastroentero. 26:1847–1860. 2020. View Article : Google Scholar
|
|
4
|
Cheung SLH and Teoh AYB: Optimal
management of gastric outlet obstruction in unresectable
malignancies. Gut Liver. 16:190–197. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koop AH, Palmer WC and Stancampiano FF:
Gastric outlet obstruction: A red flag, potentially manageable.
Clev Clin J Med. 86:345–353. 2019. View Article : Google Scholar
|
|
6
|
Lordick F, Carneiro F, Cascinu S, Fleitas
T, Haustermans K, Piessen G, Vogel A and Smyth EC; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Gastric cancer: ESMO clinical practice guideline for diagnosis,
treatment and follow-up. Ann Oncol. 33:1005–1020. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sexton RE, Al Hallak MN, Diab M and Azmi
AS: Gastric cancer: A comprehensive review of current and future
treatment strategies. Cancer Metast Rev. 39:1179–1203. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwai N, Okuda T and Kagawa K:
Gastrointestinal: Natural progression of early gastric cancer
causing pyloric stenosis. J Gastroen Hepatol. 35:92020. View Article : Google Scholar
|
|
9
|
Xu R, Chen XD and Ding Z: Perioperative
nutrition management for gastric cancer. Nutrition. 93:1114922022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng Y, Zhang J, Zhang L, Wu J and Zhan
Z: Enteral immunonutrition versus enteral nutrition for gastric
cancer patients undergoing a total gastrectomy: A systematic review
and meta-analysis. BMC Gastroenterol. 18:112018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li K, Wang D, Zhang X, Yang J and Chen X:
Efficacy of early enteral nutrition versus total parenteral
nutrition for patients with gastric cancer complicated with
diabetes mellitus: A systematic review and meta-analysis. Nutr
Diet. 79:129–139. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muscaritoli M, Arends J, Bachmann P,
Baracos V, Barthelemy N, Bertz H, Bozzetti F, Hütterer E, Isenring
E, Kaasa S, et al: ESPEN practical guideline: Clinical nutrition in
cancer. Clin Nutr. 40:2898–2913. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S, Zheng R, Arnold M, Abnet C, Zeng
H, Zhang S, Chen R, Sun K, Li L, An L, et al: Global and national
trends in the age-specific sex ratio of esophageal cancer and
gastric cancer by subtype. Int J Cancer. 151:1447–1461. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asaka M, Kobayashi M, Kudo T, Akino K,
Asaka Y, Fujimori K, Kikuchi S, Kawai S and Kato M: Gastric cancer
deaths by age group in Japan: Outlook on preventive measures for
elderly adults. Cancer Sci. 111:3845–3853. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodríguez JI, Kutscher M, Lemus M, Crovari
F, Pimentel F and Briceño E: Palliative gastrojejunostomy in
unresectable cancer and gastric outlet obstruction: A retrospective
cohort study. Ann Roy Coll Surg. 103:197–202. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen XJ, Chen GM, Wei YC, Yu H, Wang XC,
Zhao ZK, Luo TQ, Nie RC and Zhou ZW: Palliative gastrectomy versus
gastrojejunostomy for advanced Gastric cancer with outlet
obstruction: A propensity score matching analysis. BMC Cancer.
21:1882021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zu H, Wang H, Li C, Zhu W and Xue Y: The
predictive and prognostic factors in patients with gastric cancer
accompanied by gastric outlet obstruction. Gastroent Res Pract.
2020:65295632020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen ZH, Lin SY, Dai QB, Hua J and Chen
SQ: The effects of pre-operative enteral nutrition from nasal
feeding tubes on gastric outlet obstruction. Nutrients. 9:3732017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramos MFKP, Barchi LC, de Oliveira RJ,
Pereira MA, Mucerino DR, Ribeiro U Jr, Zilberstein B and Cecconello
I: Gastric partitioning for the treatment of malignant gastric
outlet obstruction. World J Gastrointest Oncol. 11:1161–1171. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang B, Sunde B, Tsekrekos A, Hayami M,
Rouvelas I, Nilsson M, Lindblad M and Klevebro F: Partial
stomach-partitioning gastrojejunostomy for gastric outlet
obstruction: A cohort study based on consecutive case series from a
single center. Asian J Surg. 45:326–331. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gillis C and Wischmeyer PE: Pre-operative
nutrition and the elective surgical patient: Why, how and what?
Anaesthesia. 74 (Suppl 1):S27–S35. 2019. View Article : Google Scholar
|
|
23
|
Weimann A, Braga M, Carli F, Higashiguchi
T, Hübner M, Klek S, Laviano A, Ljungqvist O, Lobo DN, Martindale
R, et al: ESPEN guideline: Clinical nutrition in surgery. Clin
Nutr. 36:623–650. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amano K, Maeda I, Ishiki H, Miura T,
Hatano Y, Tsukuura H, Taniyama T, Matsumoto Y, Matsuda Y, Kohara H,
et al: Effects of enteral nutrition and parenteral nutrition on
survival in patients with advanced cancer cachexia: Analysis of a
multicenter prospective cohort study. Clin Nutr. 40:1168–1175.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oshima T, Singer P and Pichard C:
Parenteral or enteral nutrition: Do you have the choice? Curr Opin
Crit Care. 22:292–298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abunnaja S, Cuviello A and Sanchez JA:
Enteral and parenteral nutrition in the perioperative period: State
of the art. Nutrients. 5:608–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li G, He L and Sun H: Nutritional risk
index predicts the prognosis of gastric cancer patients with
pyloric stenosis who received preoperative parenteral nutrition.
Oncol Lett. 26:4012023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiao X, Wang Y, Qu X, Qu J and Wang X:
Effects of preoperative pyloric stenosis on outcomes and
nutritional status in 73 patients following curative gastrectomy
for gastric cancer: A retrospective study from a single center. Med
Sci Monit. 27:e9309742021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fukuda Y, Yamamoto K, Hirao M, Nishikawa
K, Maeda S, Haraguchi N, Miyake M, Hama N, Miyamoto A, Ikeda M, et
al: Prevalence of malnutrition among gastric cancer patients
undergoing gastrectomy and optimal preoperative nutritional support
for preventing surgical site infections. Ann Surg Oncol. 22 (Suppl
3):S778–S785. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen Y, Zhou Y, He T and Zhuang X: Effect
of preoperative nutritional risk screening and enteral nutrition
support in accelerated recovery after resection for esophageal
cancer. Nutr Cancer. 73:596–601. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Li S, Xi H, Liu P, Liang W, Gao Y,
Wang C, Wei B, Chen L, Tang Y and Qiao Z: Effect of preoperative
nutrition therapy type and duration on short-time outcomes in
gastric cancer patient with gastric outlet obstruction. Chin J
Cancer Res. 33:232–242. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lappas BM, Patel D, Kumpf V, Adams DW and
Seidner DL: Parenteral nutrition: Indications, access, and
complications. Gastroenterol Clin North Am. 47:39–59. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mattson MP, Longo VD and Harvie M: Impact
of intermittent fasting on health and disease processes. Ageing Res
Rev. 39:46–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hom J, Lam SHF, Delaney KM, Koos JA and
Kunkov S: Vomiting, pyloric mass, and point-of-care ultrasound:
Diagnostic test accuracy for hypertrophic pyloric stenosis-a
meta-analysis. J Emerg Med. 65:e427–e431. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gilna GP, Saberi RA, Huerta CT, O'Neil CF,
Ramsey WA, Parreco JP, Thorson CM, Sola JE and Perez EA:
Laparoscopic versus open pyloromyotomies: Outcomes and disparities
in pyloric stenosis. J Pediatr Surg. 57:932–936. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Danko ME, Evans PT and Upperman JS:
Current management of pyloric stenosis. Semin Pediatr Surg.
31:1511452022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cadena AJ, Habib S, Rincon F and Dobak S:
The benefits of parenteral nutrition (PN) versus enteral nutrition
(EN) among adult critically Ill patients: What is the evidence? A
literature review. J Intensive Care Med. 35:615–626. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elke G, van Zanten AR, Lemieux M, McCall
M, Jeejeebhoy KN, Kott M, Jiang X, Day AG and Heyland DK: Enteral
versus parenteral nutrition in critically ill patients: An updated
systematic review and meta-analysis of randomized controlled
trials. Crit Care. 20:1172016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pash E: Enteral nutrition: Options for
short-term access. Nutr Clin Pract. 33:170–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baiu I and Spain DA: Enteral nutrition.
JAMA. 321:20402019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ye BW, Lee KC and Hou MC: Endoscopic
management of malignant gastric outlet obstruction. J Chin Med
Assoc. 84:346–353. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qu C, Yu X, Duan Z, Zhou J, Mao W, Wei M,
Cao L, Zhang J, Zhang H, Ke L, et al: Clinical characteristics and
management of gastric outlet obstruction in acute pancreatitis.
Pancreatology. 21:64–68. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun H, Chen L, Huang R, Pan H, Zuo Y, Zhao
R, Xue Y and Song H: Prognostic nutritional index for predicting
the clinical outcomes of patients with gastric cancer who received
immune checkpoint inhibitors. Front Nutr. 9:10381182022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Salas S, Cottet V, Dossus L, Fassier P,
Ginhac J, Latino-Martel P, Romieu I, Schneider S, Srour B,
Touillaud M, et al: Nutritional factors during and after cancer:
Impacts on survival and quality of life. Nutrients. 14:29582022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mizukami T and Piao Y: Role of nutritional
care and general guidance for patients with advanced or metastatic
gastric cancer. Future Oncol. 17:3101–3109. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu LB, Shi MM, Huang ZX, Zhang WT, Zhang
HH, Shen X and Chen XD: Impact of malnutrition diagnosed using
global leadership initiative on malnutrition criteria on clinical
outcomes of patients with gastric cancer. JPEN J Parenter Enteral
Nutr. 46:385–394. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park JH, Kim E, Seol EM, Kong SH, Park DJ,
Yang HK, Choi JH, Park SH, Choe HN, Kweon M, et al: Prediction
model for screening patients at risk of malnutrition after gastric
cancer surgery. Ann Surg Oncol. 28:4471–4481. 2021. View Article : Google Scholar : PubMed/NCBI
|