Introduction
For patients with non-small cell lung cancer
(NSCLC), brain metastasis is present at diagnosis in 10–20% of
cases and develops in up to 50% of patients during the course of
their disease (1,2). Within this context, anaplastic
lymphoma kinase (ALK) rearrangement, accounting for 3–7% of all
NSCLC cases (3), is of particular
concern. Brain metastases are identified at the time of diagnosis
in 25–40% of patients with ALK-positive NSCLC, and over half can
develop brain metastases during their treatment (4,5).
According to Rangachari et al (6) retrospective study, the incidence of
brain metastases in patients with ALK-positive NSCLC was 45.5 and
58.4% at two and three years, respectively. These are notably
higher rates than in other NSCLC subtypes.
The occurrence of brain metastases is frequently
associated with a poor prognosis, with median overall survival
(mOS) times ranging from 3 to 6 months (7,8).
Crizotinib has proven to be effective in patients with ALK-positive
NSCLC, but the progression in the central nervous system (CNS)
remains the primary cause of treatment failure (9). Costa et al (2) retrospective study revealed that 72% of
patients with pre-existing brain metastases who received crizotinib
experienced secondary CNS progression. Second-generation ALK
tyrosine kinase inhibitors (TKIs)-ceritinib, brigatinib and
alectinib-were developed to overcome crizotinib resistance. These
inhibitors have shown superior CNS penetration (10–12).
In the ALEX trial, the median progression-free survival (mPFS) for
patients treated with alectinib was 27.7 months (13), and this increased to 42.3 months for
the Asian population in the ALESIA trial (14). However, the efficacy of crizotinib
and alectinib in patients with ALK-positive brain metastases has
not been established in a real-world setting.
Radiotherapy (RT) is a prevalent therapeutic
approach for NSCLC patients, especially those with brain
metastasis, and is often used in combination with systemic therapy.
In epidermal growth factor receptor-mutant NSCLC patients with
brain metastases, several retrospective studies have demonstrated
that combining targeted therapy with intracranial radiation
improves both intracranial PFS and OS compared with targeted
therapy alone (15–17). However, for patients with
ALK-positive and brain metastases, the evidence is limited,
particularly in real-world settings.
Therefore, a comparative analysis on the efficacy of
alectinib and crizotinib in patients with ALK-positive NSCLC and
CNS metastases in a real-world setting was undertaken.
Materials and methods
Patients
The present retrospective study included patients
with ALK-positive NSCLC and CNS metastases who visited the Shandong
Cancer Centre (Jinan, China) between January 2016 and December
2020. The inclusion criteria included patients with CNS metastases
at baseline as well as those without CNS metastases at initial
diagnosis but developed them as the disease progressed at the time
of ALK-TKI therapy initiation. The exclusion criteria included: i)
chemotherapy alone, ii) presence of other malignancies and iii)
predicted survival <3 months. The present study was reviewed and
approved (approval no. SDTHEC2023008007) by the Institutional
Review Board of Shandong Cancer Centre (Jinan, China).
Data collection
Clinical data, including demographic characteristics
(sex and age), smoking status, Eastern Cooperative Oncology Group
(ECOG) performance score, disease stage, histologic type, treatment
regimen and disease regression were retrieved from electronic
databases of the Shandong Cancer Centre and medical record system.
All patients underwent enhanced computed tomography scans and brain
magnetic resonance examinations at baseline, tumor assessment was
performed every 8 weeks thereafter.
Treatment
ALK-TKIs including crizotinib 250 mg twice-daily,
alectinib 600 mg twice-daily. Based on their initial ALK-TKI
treatment, patients were categorised into either the alectinib
group or crizotinib group. Whole-brain radiation therapy or
stereotactic radiosurgery was conducted at the initial of TKI
administration if radiation was applied.
Statistical analysis
The efficacy of treatment was assessed using the
Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1,
encompassing complete response (CR), partial response (PR), stable
disease (SD) and progressive disease (PD). PFS was defined as the
interval from the initiation date of the ALK-TKI therapy in
patients with CNS metastases to disease progression or death.
Intracranial PFS was determined as the period from the commencement
date of the ALK-TKI therapy in patients with CNS metastases to the
detection of CNS progression. OS was defined as the duration from
the diagnosis of lung cancer to the date of death or the last
follow-up. Survival curves were constructed using the Kaplan-Meier
method and compared with the log-rank test. For continuous
variables, differences between groups were tested with the unpaired
t-test. Categorical variables were analysed using Pearson's
chi-square test or Fisher's exact test, as appropriate. Hazard
ratios (HR) and 95% confidence intervals (CI) were calculated using
the Cox proportional hazards regression model. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were conducted by using SPSS software, version
27.0 (IBM Corp.).
Results
Patient characteristics
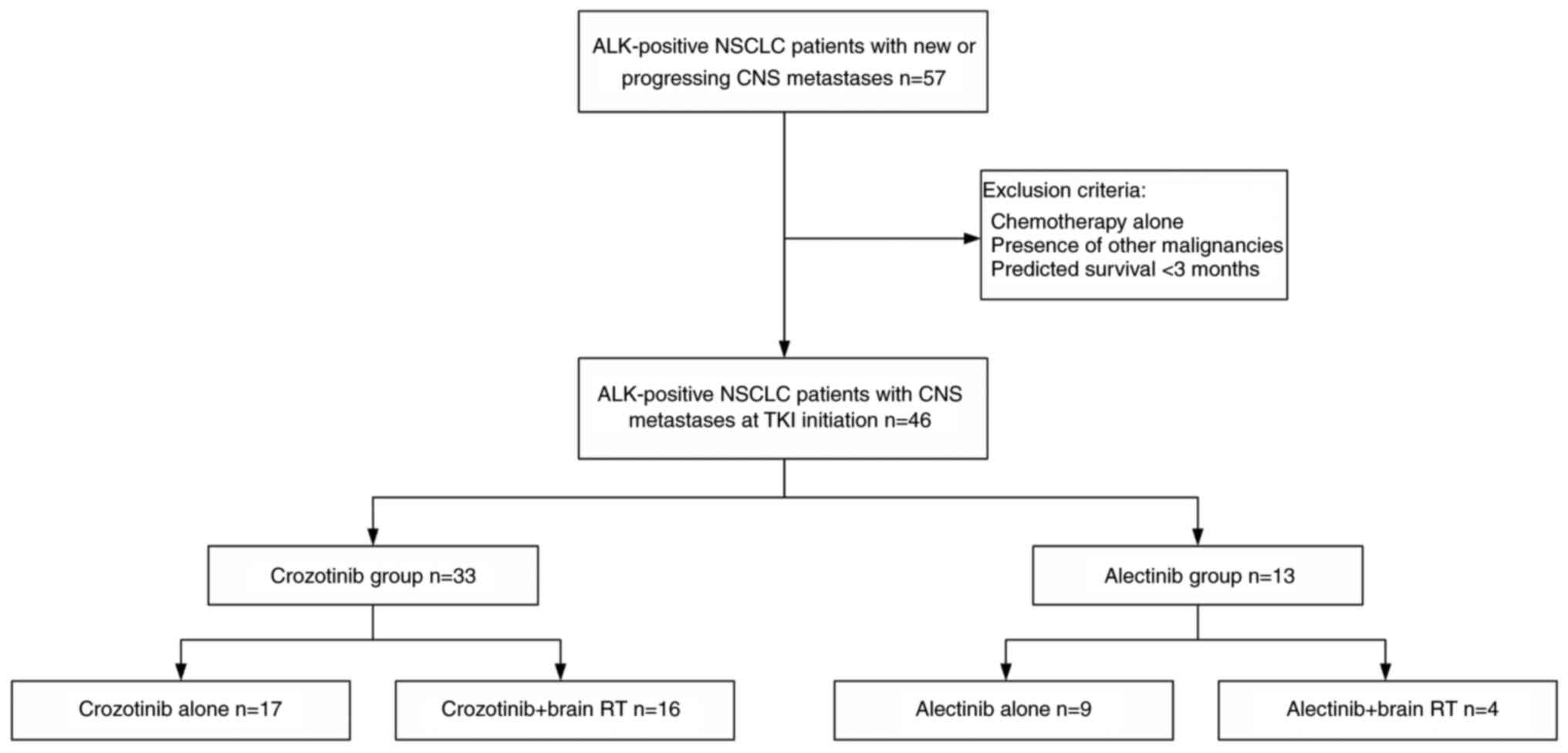
A total of 57 patients with ALK-positive NSCLC and
CNS metastases were screened at baseline or progressing CNS
metastases from January 2016 to December 2020 and 46 patients met
the eligibility criteria (Fig. 1).
The patient baseline characteristics are detailed in Table I. The majority of patients were
women (61%), 39 patients (85%) had never been smokers and 38
patients (83%) had an ECOG performance status score of <2. The
major histological subtype was adenocarcinoma, accounting for 91.3%
of cases. The remaining 8.7% (4 out of 46 patients) were diagnosed
with non-adenocarcinoma subtypes, consisting of adeno-squamous
carcinoma (n=1), large-cell carcinoma (n=1), signet-ring cell
carcinoma (n=1) and squamous cell carcinoma (n=1). Based on the
initial ALK-TKI received, patients were categorised into the
crizotinib (n=33) group and the alectinib (n=13) group.
 | Table I.Baseline characteristics of
patients. |
Table I.
Baseline characteristics of
patients.
| Characteristic | Crizotinib group
(n=33) | Alectinib group
(n=13) | P-value |
|---|
| Age (mean ±
SD) | 50.7±11.65 | 54.46±10.30 | 0.318 |
| Sex, n (%) |
|
| 0.540 |
|
Male | 12 (36.4) | 6 (46.2) |
|
|
Female | 21 (63.6) | 7 (53.8) |
|
| Smoking status, n
(%) |
|
| 0.540 |
|
Never-smoker | 29 (87.9) | 10 (76.9) |
|
|
Smoker | 4 (12.1) | 3 (23.1) |
|
| ECOG at TKI
initiation, n (%) |
|
| 0.836 |
|
<2 | 28 (84.8) | 10 (76.9) |
|
| ≥2 | 5 (15.2) | 3 (23.1) |
|
| Stage at initial
diagnosis, n (%) |
|
| 0.971 |
|
III | 2 (6.1) | 0 (0.0) |
|
| IV | 31 (93.9) | 13 (100) |
|
| Histology, n
(%) |
|
| 0.275 |
|
Non-adenocarcinoma | 4 (12.1) | 0 (0.0) |
|
|
Adenocarcinoma | 29 (87.9) | 13 (100) |
|
| CNS metastasis
status at initial diagnosis, n (%) |
|
| 0.275 |
| No | 13 (39.4) | 2 (15.4) |
|
|
Yes | 20 (60.6) | 11 (84.6) |
|
| ALK testing, n
(%) |
|
| 0.002 |
|
ARMS-PCR | 22 (66.7) | 2 (15.4) |
|
|
NGS | 11 (33.3) | 11 (84.6) |
|
| Max dimension of
brain metastasis at TKI initiation, n (%) |
|
| 1.000 |
| <3
cm | 28 (84.8) | 11 (84.6) |
|
| ≥3
cm | 5 (15.2) | 2 (15.4) |
|
| Number of brain
metastasis at TKI initiation, n (%) |
|
| 0.284 |
| ≤3 | 29 (87.9) | 9 (69.2) |
|
|
>3 | 4 (12.1) | 4 (30.8) |
|
| Cranial
radiotherapy at TKI initiation, n (%) |
|
| 0.275 |
| TKI
alone | 17 (51.5) | 9 (69.2) |
|
| TKI +
radiotherapy | 16 (48.5) | 4 (30.8) |
|
| Extracranial
metastasis at ALK-TKI initiation, n (%) |
|
| 1.000 |
| No | 10 (30.3) | 4 (30.8) |
|
|
Yes | 23 (69.7) | 9 (69.2) |
|
| Treatment lines, n
(%) |
|
| 0.539 |
| First
line | 16 (48.5) | 5 (38.5) |
|
| Second
line | 17 (51.5) | 8 (61.5) |
|
Efficacy
Among patients in the crizotinib group from the
initiation date of TKI therapy, 14 exhibited PR and 5 achieved SD,
resulting in an overall response rate (ORR) of 42.4% (14/33) and a
disease control rate (DCR) of 57.6% (19/33). By contrast, the
alectinib group demonstrated superior response: The ORR and DCR
were 84.6% (11/13) and 92.3% (12/13), with 1 CR, 10 PR and 1 SD.
The difference between the two groups was statistically significant
for ORR (P=0.002), as shown in Table
II. In addition, with regard to intracranial efficacy, the
crizotinib group had 0 CR, 15 PR, 7 SD and 11 PD cases, leading to
an ORR of 45.5% (15/33) and a DCR of 66.7% (22/33). The alectinib
group, however, reported 1 CR, 11 PR, 1 SD and no PD, resulting in
an ORR of 92.3% (12/13) and a DCR of 100% (13/13). In the two
groups, there were significant differences in intracranial ORR
(P=0.004) and DCR (P=0.045), as demonstrated in Table III.
 | Table II.Overall efficacy. |
Table II.
Overall efficacy.
| Outcome | Crizotinib
(n=33) | Alectinib
(n=13) | P-value |
|---|
| CR | 0 | 1 | - |
| PR | 14 | 10 | - |
| SD | 5 | 1 | - |
| PD | 14 | 1 | - |
| ORR (%) | 42.4 | 84.6 | 0.002 |
| DCR (%) | 57.6 | 92.3 | 0.056 |
 | Table III.Intracranial efficacy. |
Table III.
Intracranial efficacy.
| Outcome | Crizotinib
(n=33) | Alectinib
(n=13) | P-value |
|---|
| CR | 0 | 1 | - |
| PR | 15 | 11 | - |
| SD | 7 | 1 | - |
| PD | 11 | 0 | - |
| ORR, % | 45.5 | 92.3 | 0.004 |
| DCR, % | 66.7 | 100 | 0.045 |
Outcomes
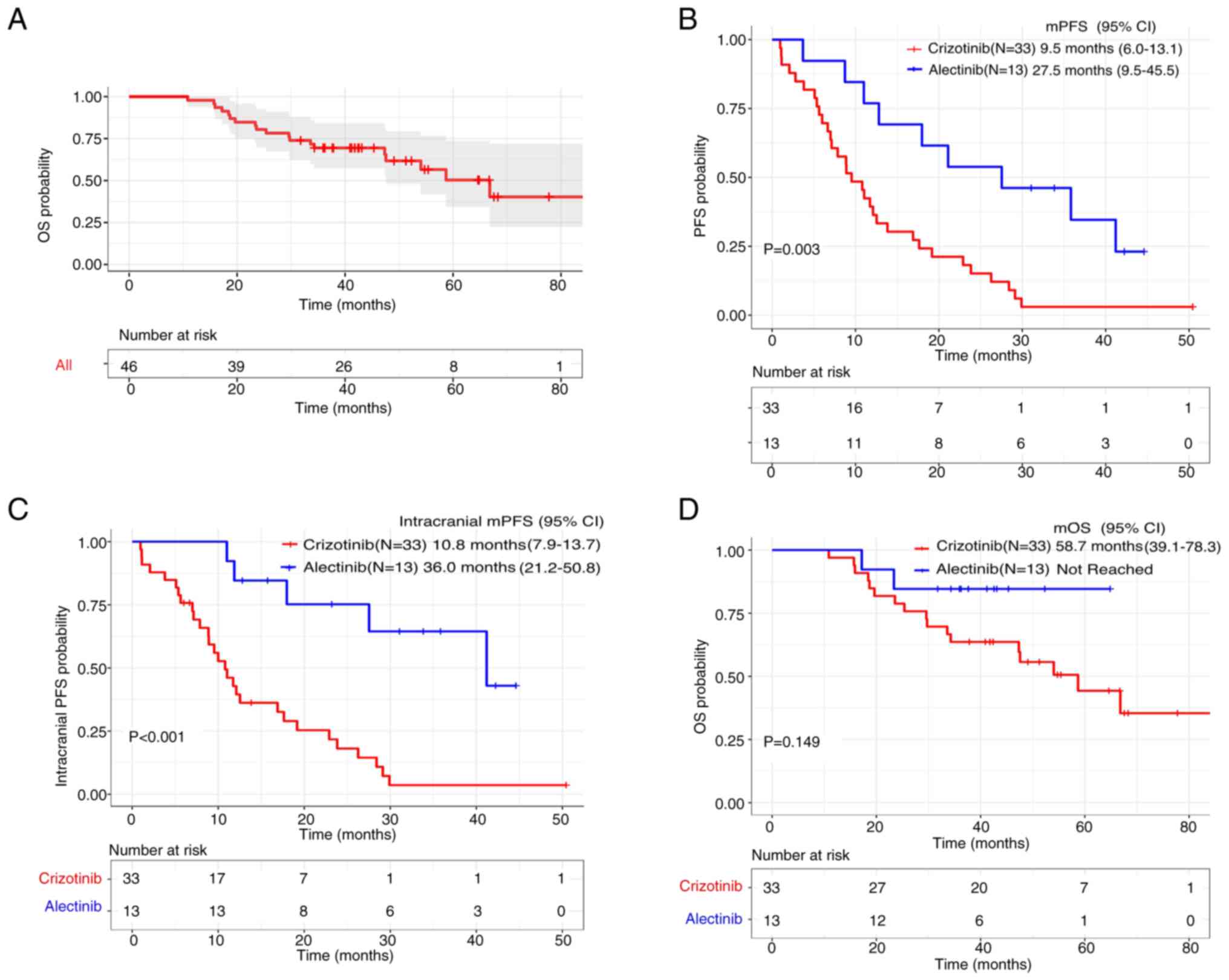
The mOS of the entire group was 66.8 months (95% CI,
48.5–85.1), as can be observed in Fig.
2A. The median duration of follow-up from the date of original
diagnosis was 41.2 months with the alectinib group and 55.4 months
with the crizotinib group. The mPFS was significantly improved in
the alectinib group (27.5 months; 95% CI, 9.5–45.5) vs. the
crizotinib group (9.5 months; 95% CI, 6.0–13.1; P=0.003) (Fig. 2B). Intracranial mPFS was also
significantly prolonged in the alectinib group (36.0 months; 95%
CI, 21.2–50.8) compared with the crizotinib group (10.8 months; 95%
CI, 7.9–13.7; P<0.001) (Fig.
2C). However, there was no significant difference in OS between
the alectinib group [not reached (NR)] and the crizotinib group
(58.7 months; 95% CI, 39.1–78.3; P=0.149) (Fig. 2D).
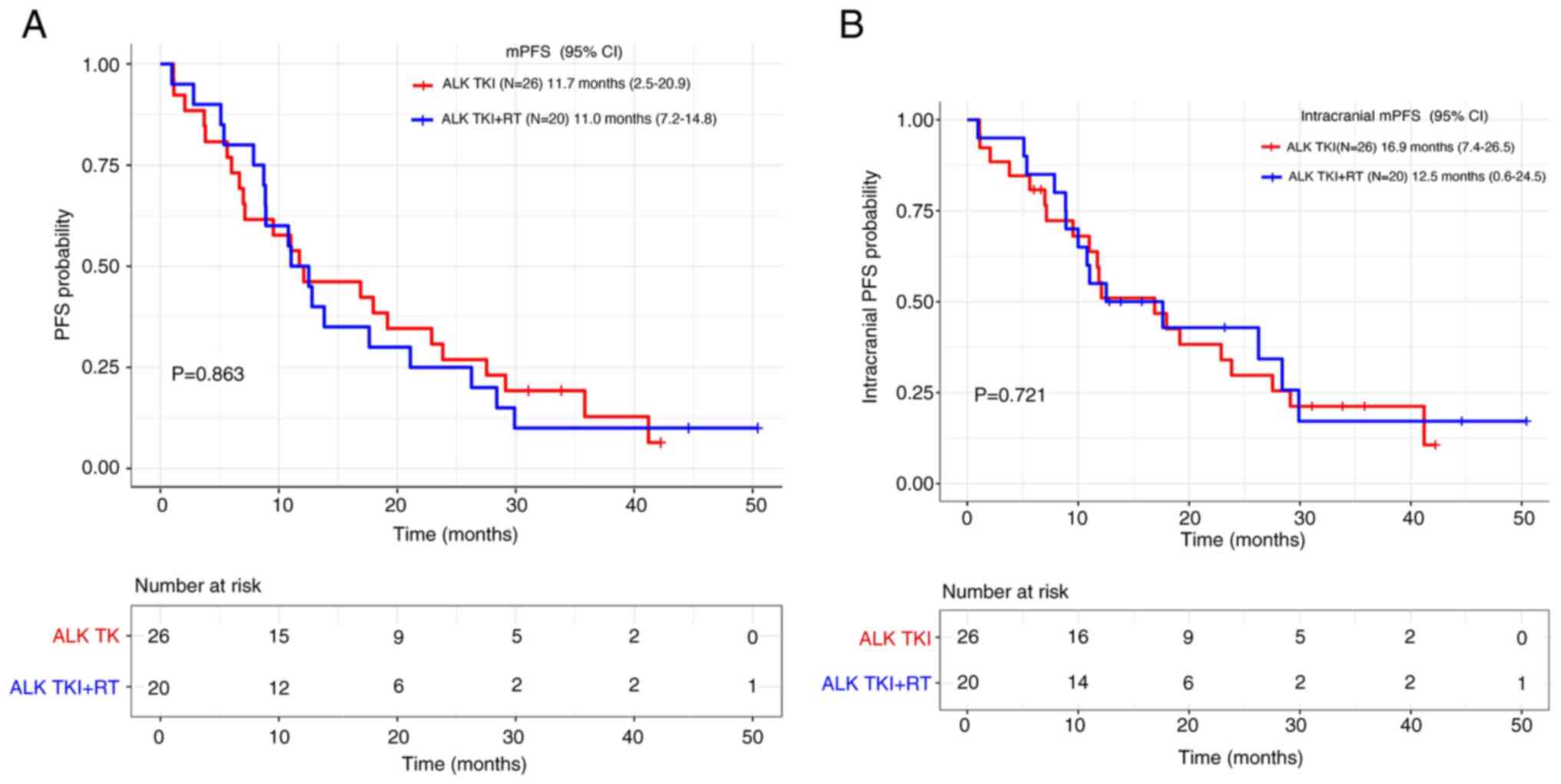
A subset analysis was conducted among patients
receiving CNS RT. The analysis indicated that there were no
significant differences between patients receiving TKI combined
with RT vs. those receiving TKI alone with respect to mPFS (11.0
vs. 11.7 months, respectively; P=0.863) as well as intracranial
mPFS (12.5 vs. 16.9 months, respectively; P=0.721) (Fig. 3A and B).
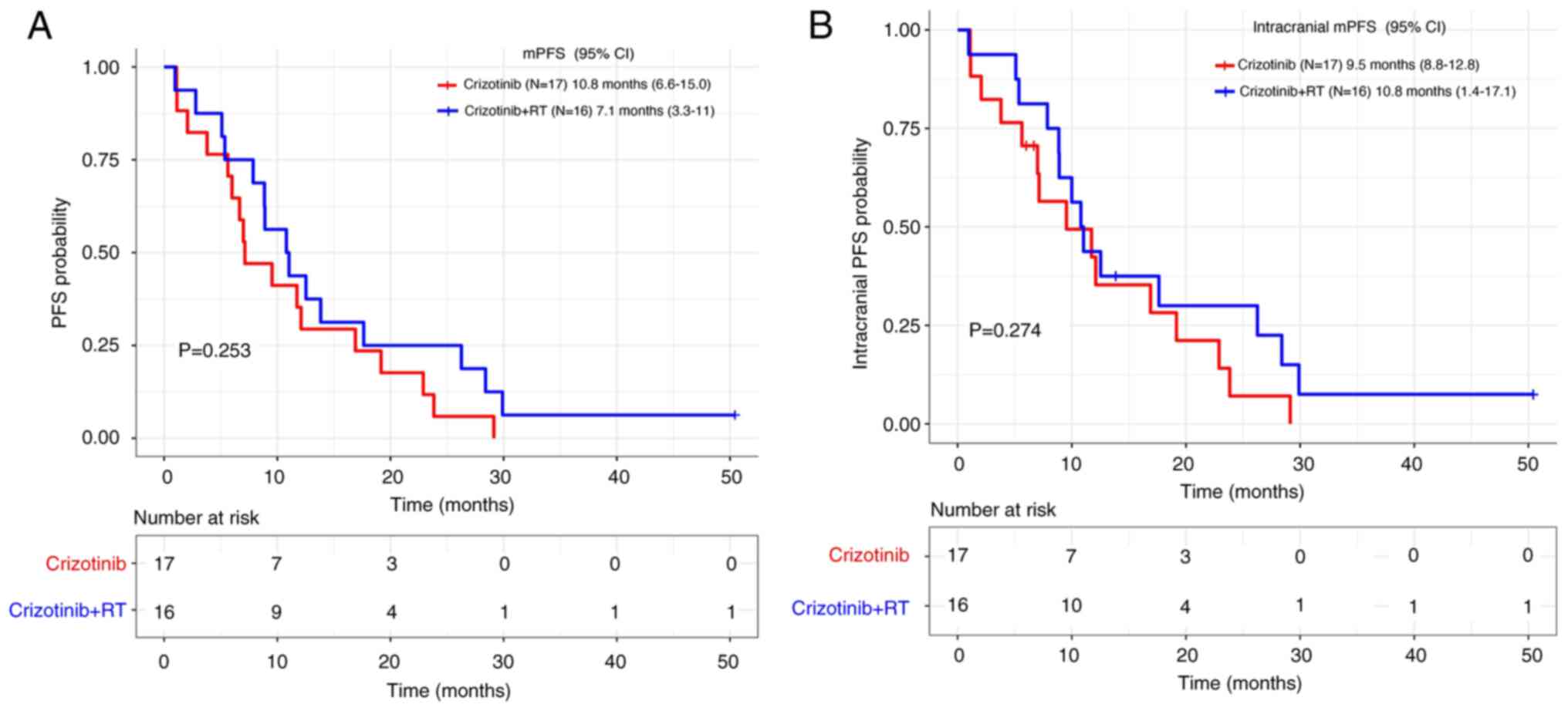
In the crizotinib group, there were no significant
differences between patients receiving TKI plus RT and those
receiving TKI alone both for mPFS (7.1 months vs. 10.8 months,
respectively; P=0.253) and intracranial mPFS (10.8 vs. 9.5 months,
respectively; P=0.274) (Fig. 4A and
B). Similarly, no significant differences were observed in the
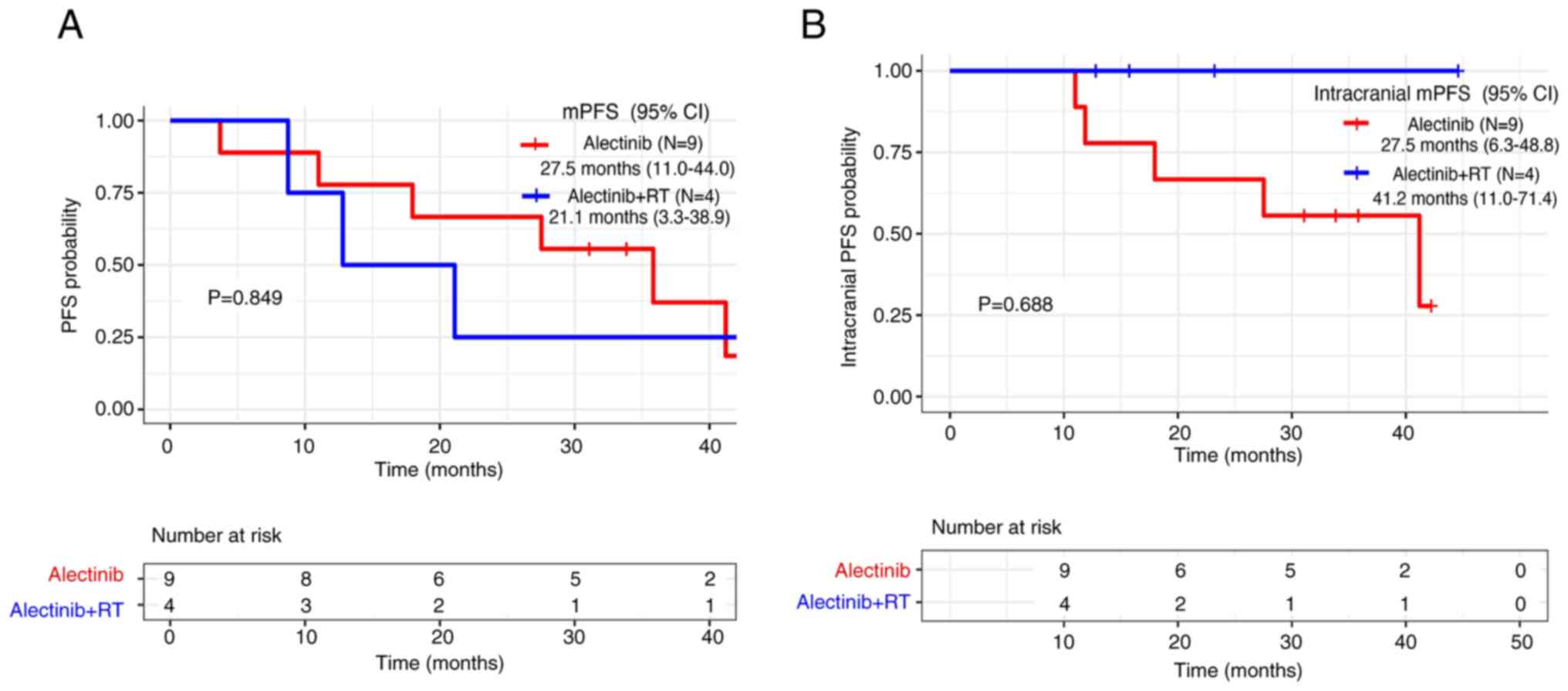
alectinib group for either mPFS (21.1 vs. 27.5 months,
respectively; P=0.849) or intracranial mPFS (alectinib plus RT,
41.2 months vs. alectinib, 27.5 months; P=0.688) (Fig. 5A and B).
Subsequent therapy after
crizotinib-resistance
During the follow-up period, in the crizotinib
group, disease progression occurred in 32 out of 33 patients
(97.0%). Of those who progressed on crizotinib, six patients opted
for symptomatic supportive therapy, two chose chemotherapy and 24
received subsequent ALK-TKI therapy (alectinib, brigatinib,
ceritinib and lorlatinib). Among the patients who underwent ALK-TKI
therapy, 12 patients received alectinib as a sequential treatment
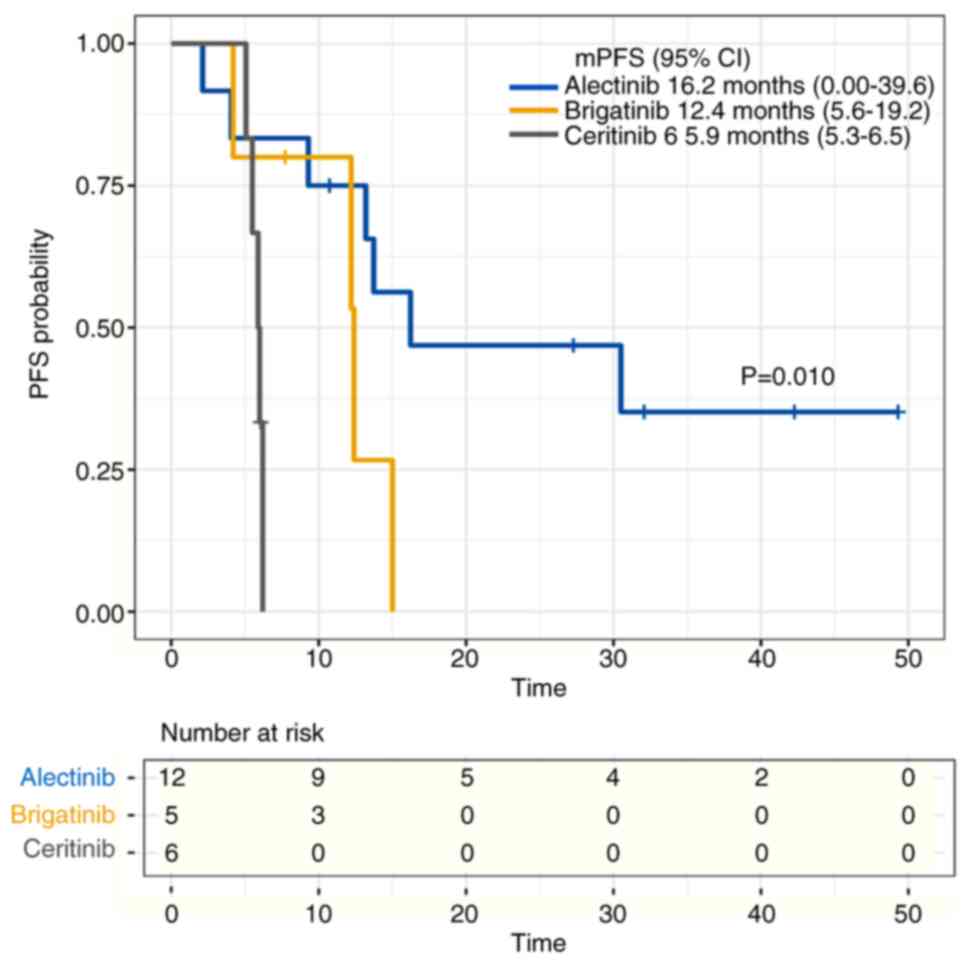
and had an mPFS time of 16.2 months (95% CI, 0.0–39.6). A total of
five patients were treated with brigatinib and had an mPFS time of
12.4 months (95% CI, 5.6–19.2). A total of six patients received
ceritinib and had an mPFS time of 5.9 months (95% CI, 5.3–6.5). The
difference in mPFS among the three groups was statistically
significant (P=0.010) (Fig. 6). In
addition, one patient was treated with the ALK-3rdG lorlatinib
after resistance to crizotinib and achieved an mPFS of 33.07 months
(data not shown).
Univariate and multivariate
analysis
In a multivariate analysis, the maximum dimension of
brain metastasis was significantly associated with an elevated risk
for intracranial PFS (HR=3.389; 95% CI, 1.249–9.915; P=0.040).
Alectinib therapy was associated with superior PFS (HR=0.292; 95%
CI, 0.131–0.650; P=0.003) and intracranial PFS (HR=0.175; 95% CI,
0.066–0.462; P<0.001), compared with crizotinib (Tables IV and V). The results from the multivariate
analysis of OS revealed that female patients (HR=4.475; 95% CI,
1.221–16.394; P=0.024) and individuals with a score of ECOG ≥2
(HR=3.860; 95% CI, 1.166–12.783; P=0.027) demonstrated a poorer
outcome compared with their counterparts. Conversely, patients with
a histologic diagnosis of adenocarcinoma exhibited superior OS
relative to those with non-adenocarcinoma (HR=0.073; 95% CI,
0.015–0.357; P=0.001) (Table
VI).
 | Table IV.Univariate and multivariate analysis
of PFS in patients. |
Table IV.
Univariate and multivariate analysis
of PFS in patients.
|
|
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|---|
| Variable | Univariate analysis
(P-value) | Hazard ratio | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.276 |
|
|
|
| Age (<60 vs. ≥60
years) | 0.744 |
|
|
|
| ECOG at TKI
initiation (<2 vs. ≥2) | 0.018 | 2.390 | 0.907–6.299 | 0.078 |
| Smoking status
(never-smoker vs. smoker) | 0.138 |
|
|
|
| CNS metastasis
status at diagnosis initiation (no vs. yes) | 0.543 |
|
|
|
| Extra-cranial
metastasis at TKI initiation (no vs. yes) | 0.065 | 1.823 | 0.907–6.299 | 0.124 |
| Histology
(non-adenocarcinoma vs. adenocarcinoma) | 0.257 |
|
|
|
| Max dimension of
brain metastases (<3 vs. ≥3) | 0.006 | 2.253 | 0.791–6.415 | 0.128 |
| Number of brain
metastases (≤3 vs. >3) | 0.375 |
|
|
|
| Cranial
radiotherapy at TKI initiation (no vs. yes) | 0.863 |
|
|
|
| TKI treatment
(crizotinib vs. alectinib) | 0.004 | 0.292 | 0.131–0.650 | 0.003 |
 | Table V.Univariate and multivariate analysis
of intracranial PFS in patients. |
Table V.
Univariate and multivariate analysis
of intracranial PFS in patients.
|
|
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|---|
| Variable | Univariate analysis
(P-value) | Hazard ratio | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.283 |
|
|
|
| Age (<60 vs. ≥60
years) | 0.465 |
|
|
|
| ECOG at TKI
initiation (<2 vs. ≥2) | 0.120 |
|
|
|
| Smoking Status
(never-smoker vs. smoker) | 0.215 |
|
|
|
| CNS metastasis
status at diagnosis initiation (no vs. yes) | 0.398 |
|
|
|
| Extra-cranial
metastasis at TKI initiation (no vs. yes) | 0.113 |
|
|
|
| Histology
(non-adenocarcinoma vs. adenocarcinoma) | 0.115 |
|
|
|
| Max dimension of
brain metastases (<3 vs. ≥3) | 0.040 | 3.389 | 1.249–9.195 | 0.017 |
| Number of brain
metastases (≤3 vs. >3) | 0.637 |
|
|
|
| Cranial
radiotherapy at TKI initiation (no vs. yes) | 0.721 |
|
|
|
| TKI treatment
(crizotinib vs. alectinib) | 0.001 | 0.175 | 0.066–0.462 | <0.001 |
 | Table VI.Univariate and multivariate analysis
of OS in patients. |
Table VI.
Univariate and multivariate analysis
of OS in patients.
|
|
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|---|
| Variable | Univariate analysis
(P-value) | Hazard ratio | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.071 | 4.475 | 1.221–16.394 | 0.024 |
| Age (<60 vs. ≥60
years) | 0.533 |
|
|
|
| ECOG at TKI
initiation (<2 vs. ≥2) | 0.002 | 3.860 | 1.166–12.783 | 0.027 |
| Smoking Status
(never-smoker vs. smoker) | 0.306 |
|
|
|
| CNS metastasis
status at diagnosis initiation (no vs. yes) | 0.853 |
|
|
|
| Extra-cranial
metastasis at TKI initiation (no vs. yes) | 0.048 | 2.973 | 0.683–12.941 | 0.147 |
| Histology
(non-adenocarcinoma vs. adenocarcinoma) | 0.006 | 0.073 | 0.015–0.357 | 0.001 |
| Max dimension of
brain metastases (<3 vs. ≥3) | 0.244 |
|
|
|
| Number of brain
metastases (≤3 vs. >3) | 0.323 |
|
|
|
| Cranial
radiotherapy at TKI initiation (no vs. yes) | 0.146 |
|
|
|
| TKI treatment
(crizotinib vs. alectinib) | 0.161 |
|
|
|
Discussion
The efficacy of different generations of ALK-TKIs in
patients with NSCLC exhibits considerable variation. Alectinib is
preferentially recommended in the guidelines for ALK-positive
NSCLC, considering its enhanced efficacy and safety profile
(18). In the ALEX trial (13), the mPFS of alectinib was greater
than that of crizotinib [27.7 vs. 7.4 months (HR=0.35; 95% CI,
0.22–0.56)]. However, the real-world effectiveness of alectinib in
patients with brain metastases from ALK-positive NSCLC merits
further investigation.
In the present study, alectinib significantly
prolonged the mPFS compared with crizotinib (27.5 vs. 9.5 months),
and alectinib was associated with a 70% lower risk of disease
progression (HR, 0.292; P=0.003). In a cohort of patients with CNS
metastases in the US (19), the
administration of alectinib yielded a discernible advantage in PFS
over crizotinib (24.5 vs. 5.9 months), reflecting a HR of 0.28 (95%
CI, 0.16–0.52), which is less than the PFS of the present study. In
addition, the OS advantage favouring alectinib over crizotinib was
first discerned in patients with brain metastases (HR, 0.58; 95%
CI, 0.34–1.00) in the ALEX trial (20). However, neither the WJOG9516L
(21), J-ALEX (22) trials, nor the present study
presented an OS benefit of alectinib over crizotinib. This could
potentially be attributable to limited follow-up duration or
patient crossover to a second-generation TKI post-crizotinib
progression.
In terms of intracranial efficacy, the intracranial
ORR with alectinib in the present study was 92.3%, which was in
consistency with a national multicentre retrospective study
(23). It reduced the risk of CNS
progression by 83% relative to crizotinib in the present study. The
intracranial mPFS of alectinib markedly surpassed that of
crizotinib (36.0 vs. 10.8 months). This may be related to the
blood-brain barrier penetration of alectinib and its status as a
non-substrate for P-glycoprotein (24). In addition, in the findings of the
present study, the intracranial mPFS of patients with alectinib was
extended by eight months compared with the intracranial mPFS of
patients with crizotinib. For patients with ALK-positive NSCLC and
brain metastases initially treated with alectinib, extracranial
metastases might predominantly drive disease progression. These
results further underscore the potent CNS activity of
alectinib.
In terms of the management of the intracranial
progression, RT is frequently used as a local treatment. It is
often considered to disrupt the blood-brain barrier and exert
synergistic antitumour effects when combined with TKIs (25,26).
However, the results of the present study demonstrated no PFS (11.0
vs. 11.7 months; P=0.863) or intracranial PFS (12.5 vs. 16.9
months; P=0.721) benefit from the addition of RT to TKI vs. TKI
alone. A multicentre study (27)
failed to show any benefit in either time to progression (11.4 vs.
13.4 months; P=0.98) or time to intracranial progression (18.1 vs.
21.8 months; P=0.65). One MATA analysis (28) revealed that adding RT did not result
in any PFS or OS advantage compared with an ALK-TKI alone. However,
a study by Ni et al (29)
suggested that RT in conjunction with an ALK-TKI extended survival
in patients with ALK-positive brain metastases. The effectiveness
of incorporating RT for patients with ALK brain metastases remains
to be elucidated. Given the limited evidence supporting its
efficacy, RT should perhaps not be considered the best first choice
of combination therapies in the treatment plan. It might be
beneficial to delay the initiation of RT for patients with ALK
brain metastases.
For patients with post-crizotinib resistance,
second-generation TKIs are the standard therapies. They have
superior abilities of blood-brain barrier penetration and blocking
multiple resistance sites, including L1196M, 1151Tins, C1156Y,
G1269A, F1174L and I1171T (30–33).
In the present study, an analysis was conducted to explore the
therapeutic efficacy of sequential TKIs for patients with brain
metastases developing post-crizotinib resistance. The mPFS for
crizotinib-resistant patients was 5.9 months on ceritinib, but it
was extended to 12.4 months by brigatinib, 16.2 months by alectinib
and 33.07 months by the lorlatinib when administered sequentially.
Crizotinib followed by alectinib proved to be substantially
superior to brigatinib and ceritinib. The ALK-3rdG lorlatinib
yielded the best effect of all ALK inhibitors (34), which resulted from its potency
against all known single ALK-resistant mutations, including ALK
G1202R (32,35). However, it causes severe lipid
abnormalities and cognitive impairment (36). According to data from the CROWN
study (37), the global use of
lorlatinib did not significantly improve patients' quality of life
scores clinically. Considering the clinical safety concerns and
cost constraints associated with lorlatinib, its use in the clinic
remains restricted. Hence, establishing the optimal sequencing and
combination of ALK inhibitors for patients is crucial (38,39).
There are several limitations in the present study.
First, it was a retrospective study and the number of enrolled
patients was relatively small and they were all from a single
institution. Second, the majority of the initial TKI treatments
were crizotinib and alectinib-treated patients had shorter
follow-up time; accordingly, OS outcomes for these patients were
likely less mature.
In conclusion, the present study indicated the
superior clinical activity of alectinib in Chinese patients with
brain metastases. Furthermore, it offers preliminary indications
that pairing RT with ALK-TKIs as a starting combination treatment
may not be required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical and Health
Science and Technology Development Project of Shandong (grant no.
202103100568).
Availability of data and materials
The datasets used and/or analysed during the current
study available from the corresponding author on reasonable
request.
Authors' contributions
ZW, XH and JG were involved in the conception and
design of the present study. QL and YF collected and analysed the
data. QL drafted the manuscript. CF, CZ and NT provided advice on
research design and contributed to interpretation of the data. QL
and YF revised the manuscript. XH, ZW and CF confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
SDTHEC2023008007) by the Ethics Committee of Shandong Cancer
Hospital approved (Jinan, China). In the present retrospective
study, the privacy and personal information of all patients were
protected, and the present study was performed in accordance with
the Declaration of Helsinki (2013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Page S, Milner-Watts C, Perna M, Janzic U,
Vidal N, Kaudeer N, Ahmed M, McDonald F, Locke I, Minchom A, et al:
Systemic treatment of brain metastases in non-small cell lung
cancer. Eur J Cancer. 132:187–198. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Costa DB, Shaw AT, Ou SH, Solomon BJ,
Riely GJ, Ahn MJ, Zhou C, Shreeve SM, Selaru P, Polli A, et al:
Clinical experience with crizotinib in patients with advanced
ALK-rearranged non-small-cell lung cancer and brain metastases. J
Clin Oncol. 33:1881–1888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaw AT, Yeap BY, Mino-Kenudson M,
Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S,
McDermott U, et al: Clinical features and outcome of patients with
non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol.
27:4247–4253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JK, Park HS, Kim DW, Kulig K, Kim TM,
Lee SH, Jeon YK, Chung DH, Heo DS, Kim WH and Bang YJ: Comparative
analyses of overall survival in patients with anaplastic lymphoma
kinase-positive and matched wild-type advanced non-small cell lung
cancer. Cancer. 118:3579–3586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang HJ, Lim HJ, Park JS, Cho YJ, Yoon HI,
Chung JH, Lee JH and Lee CT: Comparison of clinical characteristics
between patients with ALK-positive and EGFR-positive lung
adenocarcinoma. Respir Med. 108:388–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rangachari D, Yamaguchi N, VanderLaan PA,
Folch E, Mahadevan A, Floyd SR, Uhlmann EJ, Wong ET, Dahlberg SE,
Huberman MS and Costa DB: Brain metastases in patients with
EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung
Cancer. 88:108–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sperduto PW, Kased N, Roberge D, Xu Z,
Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al: Summary
report on the graded prognostic assessment: An accurate and facile
diagnosis-specific tool to estimate survival for patients with
brain metastases. J Clin Oncol. 30:419–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D'Antonio C, Passaro A, Gori B, Del
Signore E, Migliorino MR, Ricciardi S, Fulvi A and de Marinis F:
Bone and brain metastasis in lung cancer: Recent advances in
therapeutic strategies. Ther Adv Med Oncol. 6:101–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Solomon BJ, Cappuzzo F, Felip E, Blackhall
FH, Costa DB, Kim DW, Nakagawa K, Wu YL, Mekhail T, Paolini J, et
al: Intracranial efficacy of crizotinib versus chemotherapy in
patients with advanced ALK-positive non-small-cell lung cancer:
Results from PROFILE 1014. J Clin Oncol. 34:2858–2865. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kodama T, Tsukaguchi T, Yoshida M, Kondoh
O and Sakamoto H: Selective ALK inhibitor alectinib with potent
antitumor activity in models of crizotinib resistance. Cancer Lett.
351:215–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gettinger SN, Bazhenova LA, Langer CJ,
Salgia R, Gold KA, Rosell R, Shaw AT, Weiss GJ, Tugnait M,
Narasimhan NI, et al: Activity and safety of brigatinib in
ALK-rearranged non-small-cell lung cancer and other malignancies: A
single-arm, open-label, phase 1/2 trial. Lancet Oncol.
17:1683–1696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marsilje TH, Pei W, Chen B, Lu W, Uno T,
Jin Y, Jiang T, Kim S, Li N, Warmuth M, et al: Synthesis,
structure-activity relationships, and in vivo efficacy of the novel
potent and selective anaplastic lymphoma kinase (ALK) inhibitor
5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine
(LDK378) currently in phase 1 and phase 2 clinical trials. J Med
Chem. 56:5675–5690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Camidge DR, Dziadziuszko R, Peters S, Mok
T, Noe J, Nowicka M, Gadgeel SM, Cheema P, Pavlakis N, de Marinis
F, et al: Updated efficacy and safety data and impact of the
EML4-ALK fusion variant on the efficacy of alectinib in untreated
ALK-positive advanced non-small cell lung cancer in the global
phase III ALEX study. J Thorac Oncol. 14:1233–1243. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou C, Kim SW, Reungwetwattana T, Zhou J,
Zhang Y, He J, Yang JJ, Cheng Y, Lee SH, Bu L, et al: Alectinib
versus crizotinib in untreated Asian patients with anaplastic
lymphoma kinase-positive non-small-cell lung cancer (ALESIA): A
randomised phase 3 study. Lancet Respir Med. 7:437–446. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng YD, Zhang L, Liao H, Liang Y, Xu F,
Liu JL, Dinglin XX and Chen LK: Gefitinib alone or with concomitant
whole brain radiotherapy for patients with brain metastasis from
non-small-cell lung cancer: A retrospective study. Asian Pac J
Cancer Prev. 13:909–914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
An N, Wang H, Li J, Zhai X, Jing W, Jia W,
Kong L, Zhu H and Yu J: Therapeutic effect of first-line EGFR-TKIs
combined with concurrent cranial radiotherapy on NSCLC patients
with EGFR activating mutation and brain metastasis: A retrospective
study. Onco Targets Ther. 12:8311–8378. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Yang J, Li X, Hao D, Wu X, Yang Y,
He C, Wang W and Wang J: First-line epidermal growth factor
receptor (EGFR)-tyrosine kinase inhibitor alone or with whole-brain
radiotherapy for brain metastases in patients with EGFR-mutated
lung adenocarcinoma. Cancer Sci. 107:1800–1805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: Non-small cell lung cancer, version 3.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:497–530. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Q, Lin JJ, Pal N, Polito L, Trinh H,
Hilton M, Smoljanović V, Kurtsikidze N, Archer V, Krebs MG, et al:
Real-world comparative effectiveness of first-line alectinib versus
crizotinib in patients with advanced ALK-positive NSCLC with or
without baseline central nervous system metastases. JTO Clin Res
Rep. 4:1004832023.PubMed/NCBI
|
|
20
|
Mok T, Camidge DR, Gadgeel SM, Rosell R,
Dziadziuszko R, Kim DW, Pérol M, Ou SI, Ahn JS, Shaw AT, et al:
Updated overall survival and final progression-free survival data
for patients with treatment-naive advanced ALK-positive
non-small-cell lung cancer in the ALEX study. Ann Oncol.
31:1056–1064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito K, Yamanaka T, Hayashi H, Hattori Y,
Nishino K, Kobayashi H, Oya Y, Yokoyama T, Seto T, Azuma K, et al:
Sequential therapy of crizotinib followed by alectinib for
non-small cell lung cancer harbouring anaplastic lymphoma kinase
rearrangement (WJOG9516L): A multicenter retrospective cohort
study. Eur J Cancer. 145:183–193. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hotta K, Hida T, Nokihara H, Morise M, Kim
YH, Azuma K, Seto T, Takiguchi Y, Nishio M, Yoshioka H, et al:
Final overall survival analysis from the phase III J-ALEX study of
alectinib versus crizotinib in ALK inhibitor-naïve Japanese
patients with ALK-positive non-small-cell lung cancer. ESMO Open.
7:1005272022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zou Z, Gu Y, Liang L, Hao X, Fan C, Xin T,
Zhao S, Liu Z, Guo Y, Ma K, et al: Alectinib as first-line
treatment for advanced ALK-positive non-small cell lung cancer in
the real-world setting: Preliminary analysis in a Chinese cohort.
Transl Lung Cancer Res. 11:2495–2506. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Costa DB, Kobayashi S, Pandya SS, Yeo WL,
Shen Z, Tan W and Wilner KD: CSF concentration of the anaplastic
lymphoma kinase inhibitor crizotinib. J Clin Oncol. 29:e443–e445.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chinnaiyan P, Huang S, Vallabhaneni G,
Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM and Harari PM:
Mechanisms of enhanced radiation response following epidermal
growth factor receptor signaling inhibition by erlotinib (Tarceva).
Cancer Res. 65:3328–3335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Metro G, Lunardi G, Floridi P, Pascali JP,
Marcomigni L, Chiari R, Ludovini V, Crinò L and Gori S: CSF
concentration of crizotinib in two ALK-positive non-small-cell lung
cancer patients with CNS metastases deriving clinical benefit from
treatment. J Thorac Oncol. 10:e26–e27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomas NJ, Myall NJ, Sun F, Patil T,
Mushtaq R, Yu C, Sinha S, Pollom EL, Nagpal S, Camidge DR, et al:
Brain metastases in EGFR- and ALK-positive NSCLC: Outcomes of
central nervous system-penetrant tyrosine kinase inhibitors alone
versus in combination with radiation. J Thorac Oncol. 17:116–129.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh R, Lehrer EJ, Ko S, Peterson J, Lou
Y, Porter AB, Kotecha R, Brown PD, Zaorsky NG and Trifiletti DM:
Brain metastases from non-small cell lung cancer with EGFR or ALK
mutations: A systematic review and meta-analysis of
multidisciplinary approaches. Radiother Oncol. 144:165–179. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ni J, Li G, Yang X, Chu L, Wang J, Li Y,
Zou L, Li Y, Xie C and Zhu Z: Optimal timing and clinical value of
radiotherapy in advanced ALK-rearranged non-small cell lung cancer
with or without baseline brain metastases: Implications from
pattern of failure analyses. Radiat Oncol. 14:442019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doebele RC, Pilling AB, Aisner DL,
Kutateladze TG, Le AT, Weickhardt AJ, Kondo KL, Linderman DJ,
Heasley LE, Franklin WA, et al: Mechanisms of resistance to
crizotinib in patients with ALK gene rearranged non-small cell lung
cancer. Clin Cancer Res. 18:1472–1482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sakamoto H, Tsukaguchi T, Hiroshima S,
Kodama T, Kobayashi T, Fukami TA, Oikawa N, Tsukuda T, Ishii N and
Aoki Y: CH5424802, a selective ALK inhibitor capable of blocking
the resistant gatekeeper mutant. Cancer Cell. 19:679–690. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gainor JF, Dardaei L, Yoda S, Friboulet L,
Leshchiner I, Katayama R, Dagogo-Jack I, Gadgeel S, Schultz K,
Singh M, et al: Molecular mechanisms of resistance to first- and
second-generation ALK inhibitors in ALK-rearranged lung cancer.
Cancer Discov. 6:1118–1133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Friboulet L, Li N, Katayama R, Lee CC,
Gainor JF, Crystal AS, Michellys PY, Awad MM, Yanagitani N, Kim S,
et al: The ALK inhibitor ceritinib overcomes crizotinib resistance
in non-small cell lung cancer. Cancer Discov. 4:662–673. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang J, Zhao C, Zhang F, Liu Z, Zhou K,
Ren X and Wan Y: ALK inhibitors in ALK-rearranged non-small cell
lung cancer with and without brain metastases: Systematic review
and network meta-analysis. BMJ Open. 12:e0607822022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Horn L, Whisenant JG, Wakelee H, Reckamp
KL, Qiao H, Leal TA, Du L, Hernandez J, Huang V, Blumenschein GR,
et al: Monitoring therapeutic response and resistance: Analysis of
circulating tumor DNA in patients with ALK+ lung cancer. J Thorac
Oncol. 14:1901–1911. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naito T, Shiraishi H and Fujiwara Y:
Brigatinib and lorlatinib: Their effect on ALK inhibitors in NSCLC
focusing on resistant mutations and central nervous system
metastases. Jpn J Clin Oncol. 51:37–44. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shaw AT, Bauer TM, de Marinis F, Felip E,
Goto Y, Liu G, Mazieres J, Kim DW, Mok T, Polli A, et al:
First-line lorlatinib or crizotinib in advanced ALK-positive lung
cancer. N Engl J Med. 383:2018–2029. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baba K and Goto Y: Lorlatinib as a
treatment for ALK-positive lung cancer. Future Oncol. 18:2745–2766.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuang S and Leighl NB: Lorlatinib in
ALK-rearranged lung cancer. Cancer Cell. 39:25–27. 2021. View Article : Google Scholar : PubMed/NCBI
|