Introduction
Chordoma is a relatively common primary malignant
bone tumor of the spine; it is mainly derived from notochord
tissues that are not completely degenerated and frequently occurs
in elderly individuals (1). In the
last decade, the incidence rate of chordoma was approximately one
to two cases per million individuals each year across all
countries, with a slightly higher frequency in males than in
females (2). Due to the special and
complex anatomical structure of the spinal cord, radical tumor
resection is difficult in most cases, and chordoma is not sensitive
to radiotherapy or chemotherapy. Therefore, the local recurrence
rate of chordoma is as high as 50–70% after surgery (3). Chordoma remains a major clinical
problem in the field of tumor surgery, which is extremely
challenging. Further research is required to further explore the
pathogenesis of chordoma to produce more effective interventions
and treatment methods to improve the clinical efficacy. With the
successful application of molecular targeted therapy in tumors,
practical ideas have been offered for the targeted therapy of
chordoma (4).
With the decrease in the cost of whole-genome
sequencing techniques, more studies have confirmed that non-coding
RNAs, especially microRNAs (miRNAs/miRs) (5–7), serve
roles in the treatment of tumors. miRNAs are a group of endogenous
short non-coding RNAs that are 21–25 nucleotides in length, which
regulate the expression of target genes by specifically degrading
or inhibiting the translation of target mRNA, thereby regulating
cell growth, proliferation, apoptosis, metastasis, cycle
distribution and differentiation (5,8).
miRNAs have abnormal expression in numerous tumors, and are closely
related to tumor development and resistance to radiotherapy and
chemotherapy (9–13). However, miRNAs are rarely studied in
chordoma. In the present study, the differential expression of
miRNAs in three pairs of chordoma and notochord tissues from the
GSE56183 dataset were studied.
The functional roles of differential expression of
miRNAs in regulating the proliferation of chordoma cell lines were
assessed in the present study, and the underlying molecular
mechanisms were explored.
Materials and methods
Microarray data
The expression levels of miRNAs in chordoma and
notochord tissues were downloaded from the GEO database (accession
no. GSE56183; http://www.ncbi.nlm.nih.gov/geo/). The GSE56183
dataset contains three pairs of chordoma and notochord tissues
(14). The data were processed
using R (version 3.2.5; R Core Team). The cut-off level was fold
change ≥1.5 and P<0.05 was used to indicate significant
differences.
Cell transfection
Human chordoma JHC7 (CRL-3267), U-CH1 (CRL-3217) and
U-CH2 (CRL-3218) cell lines and immortalized nucleus pulposus NP1
(BFN60808675) and NP2 (BFN608007213) cell lines were purchased from
the American Type Culture Collection by BFB Life Sciences [Qingqi
(Shanghai) Biotechnology Development Co., Ltd.]. The pMSCV-puro
plasmid negative control (NC), chromobox 3 (CBX3) overexpression
plasmid, CBX3 small interfering (si)RNA, non-targeting
double-stranded NC siRNA, miRNA NC mimics, miR-1224 mimics, miRNA
NC inhibitors and miR-1224 inhibitors were purchased from Guangzhou
RiboBio Co., Ltd. The cells were cultured at 37°C with 5%
CO2 and transfected with Lipofectamine 2000®
(Thermo Fisher Scientific, Inc.) for 6 h at 37°C with 5%
CO2, and the medium was then replaced with fresh culture
medium, according to the manufacturer's instructions. The
transfection efficiency was observed under a fluorescence
microscope at 24 h after transfection and was used to determine
whether subsequent experiments were to be performed. The sense and
antisense sequences of siRNA CBX3 were designed with online siRNA
design tool siRNA selection Program (https://sirna.wi.mit.edu/siRNA_search.cgi) using the
coding sequences of CBX3 transcript (NM_016587.4).
The miRNA NC mimic (cat. no. HY-R04602), miR-1224
mimic (cat. no. HY-R00114), miRNA NC inhibitor (cat. no.
HY-RI04602) and miR-1224 inhibitor (cat. no. HY-RI00114) were
originally designed by MedChemExpress (purchased by Guangzhou
RiboBio Co., Ltd.). The sense and antisense sequences of siRNA
CBX3, miR-1224 mimics and miR-1224 inhibitors, including the NCs,
are shown in Table SI.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from JHC7, U-CH1 and U-CH2
cell lines, and from nucleus pulposus NP1 and NP2 cells using
TRIzol® (Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA using the Transcriptor First Strand cDNA
Synthesis Kit (Roche Applied Science) according to the
manufacturer's instructions. RT-qPCR was performed using the SYBR
Green Realmaster Mix Kit (Tiangen Biotech Co., Ltd.) and ABI ViiA 7
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for
15 sec, 60°C for 30 sec and 2°C for 30 sec. Finally, melting curve
analysis was performed with temperature ramping from 65 to 95°C at
a rate of 0.5°C/sec with continuous fluorescence acquisition. The
expression levels of miRNAs and mRNAs were quantified using the
2−ΔΔCq method (15) and
normalized to the internal reference genes GAPDH and U6 for mRNA
and miRNA, respectively. The primer design for miR-1224 was based
on a study by Hu et al (16). All primers are shown in Table SII.
Western blotting
At 48 h after transfection, total protein was
extracted from NP1, NP2, JHC7 and U-CH1 cells with RIPA reagent
(Beyotime Institute of Biotechnology) and the protein concentration
was determined by BCA kit (Beyotime Institute of Biotechnology).
Next, 30 µg of the protein was loaded per lane and separated on 12%
gels using SDS-PAGE. The separated proteins were transferred onto a
PVDF membrane at 110 V for 2 h. The membrane was blocked with 5%
skimmed milk powder in TBST for 1 h at 25°C and incubated with
anti-CBX3 (1:2,000; cat. no. ab217999; Abcam) and anti-GAPDH
(1:5,000; cat. no. ab201822; Abcam) primary antibodies at 4°C
overnight on a shaker. After the membrane was washed with TBST
(0.1% Tween-20) three times (10 min/wash), the membrane was
incubated with the secondary anti-rabbit IgG HRP-linked antibody
(1:3,000; cat. no. 7076; Cell Signaling Technology, Inc.) at room
temperature for 1 h on a shaker. After the membrane was washed with
TBST three times (10 min/wash), the protein bands were developed
with ECL developing solution (Abcam). The expression of the target
protein was analyzed with ImageJ software (version 1.54i; National
Institutes of Health) and normalized to GAPDH.
Dual luciferase reporter assay
The target genes of miR-1224 were predicted using
TargetScan (http://www.targetscan.org/vert_72/), microRNAorg
(http://www.microrna.org/microrna/home.do) and
RegRNA2.0 (http://regrna2.mbc.nctu, respectively.edu.tw/detection.html)
databases. The wild-type (WT) and mutant (MUT) fluorescence
plasmids, psiCheck2-CBX3 3′-untranslated region (3′UTR)-WT and
psiCheck2-CBX3 3′UTR-MUT (Generbiol), were constructed. The miRNA
NC mimic, miR-1224 mimic and psiCheck2-CBX3 3′UTR-WT or
psiCheck2-CBX3 3′UTR-MUT vector were transfected into the JHC7
cells along with a Renilla luciferase control vector using
Lipofectamine 3000 (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The transfected cells were incubated
for 24 h to allow for protein expression and promoter activity. The
luciferase activity was measured using the Dual-Luciferase Reporter
Assay System (Promega Corporation). The ratio of firefly luciferase
activity to Renilla luciferase activity was calculated to
normalize for transfection efficiency and variations in cell
number. The firefly luciferase activity was normalize to the
internal control (Renilla luciferase) activity for each
sample. The normalized firefly luciferase activity was expressed as
relative luciferase units or fold-change compared with control
samples.
Cell Count Kit-8 (CCK-8) cell
proliferation activity assay
Cell proliferation was measured using a CCK-8 kit
(Dojindo Laboratories, Inc.). The cells were seeded at
1×104 cells/well into a 96-well plate and incubated for
24 h with 5% CO2 at 37°C. Next, 20 µl CCK-8 solution was
added and the cells were incubated at 37°C for 4 h. The absorbance
value of the two groups at 490 nm was measured using a microplate
reader at 0, 24, 48 and 72 h. The experiment was repeated three
times.
Statistical methods
SPSS 22.0 (IBM Corp.) and GraphPad Prism 6.0
software (Dotmatics) were used for data analysis. Data are
expressed as the mean ± standard deviation. The differences between
two groups were tested using an unpaired Student's t-test, and
one-way analysis of variance with Tukey's Honestly Significant
Difference post hoc test was used for the analysis of >2 groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Differential expression of miRNA in
chordoma
To assess the differential expression of miRNAs in
chordoma, the sequencing data of three pairs of chordoma and
notochord tissues from the GSE56183 dataset were analyzed. A total
of 1,109 human miRNAs were detected in GSE56183. Using cut off
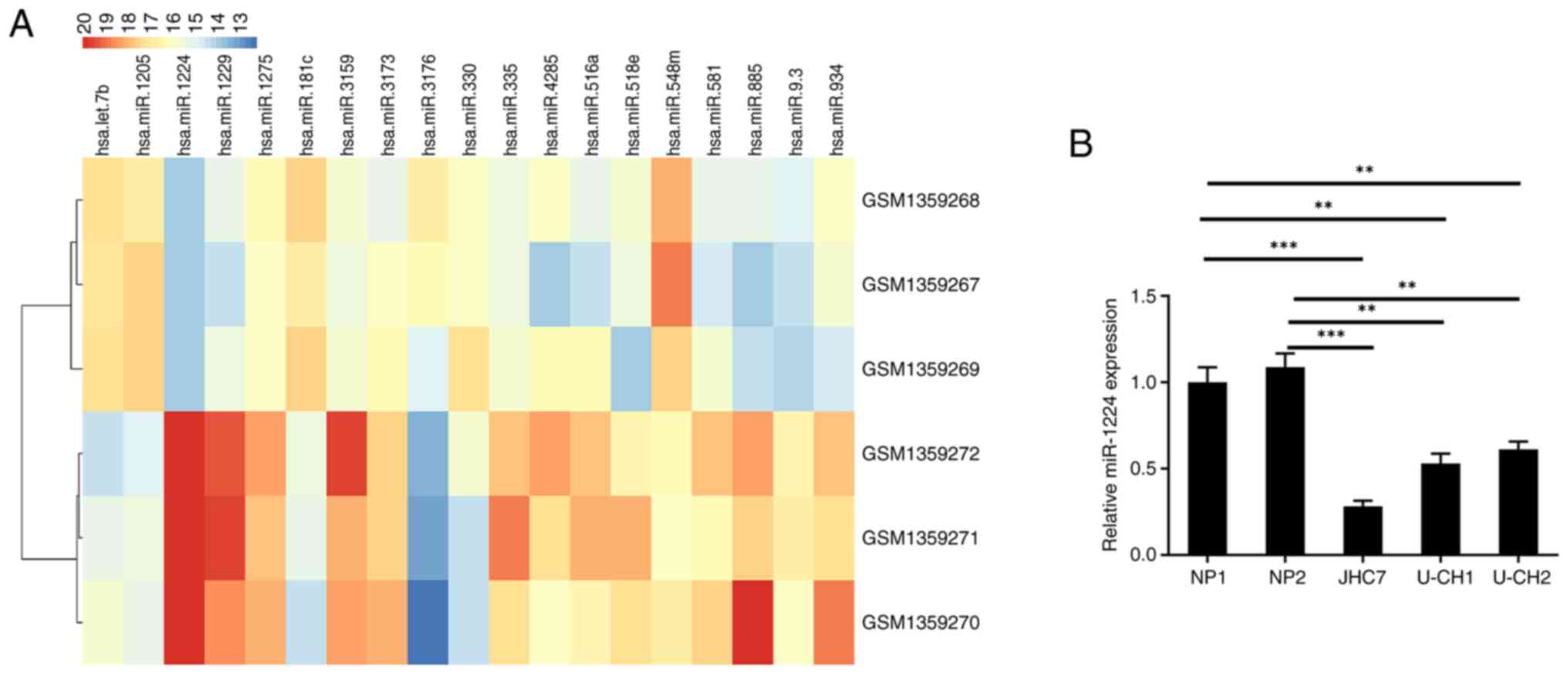
values of fold-change (FC)≥1.5 and P<0.05, 19 miRNAs were
demonstrated to be differentially expressed in chordoma. miR-1224,
miR-1229, miR-885, miR-3159, miR-934, miR-335, miR-518e, miR-516a,
miR-9.3, miR-581, miR4285, miR-3173 and miR-1275 were expressed at
low levels in chordoma compared with notochord tissues, while
miR-3176, miR-181c, miR-1205, let-7b, miR-330 and miR-548m were
highly expressed in chordoma (Fig.
1A). The differential expression of miR-1224 in chordoma was
the most apparent. From this, the expression of miR-1224 in
chordoma JHC7, U-CH1 and U-CH2 cell lines, and normal NP1 and NP2
cell lines, was detected. These results demonstrated that miR-1224
also had significantly lower expression in chordoma cells compared
with nucleus pulposus cells (Fig.
1B).
Effects of miR-1224 on the
proliferation ability of chordoma cells
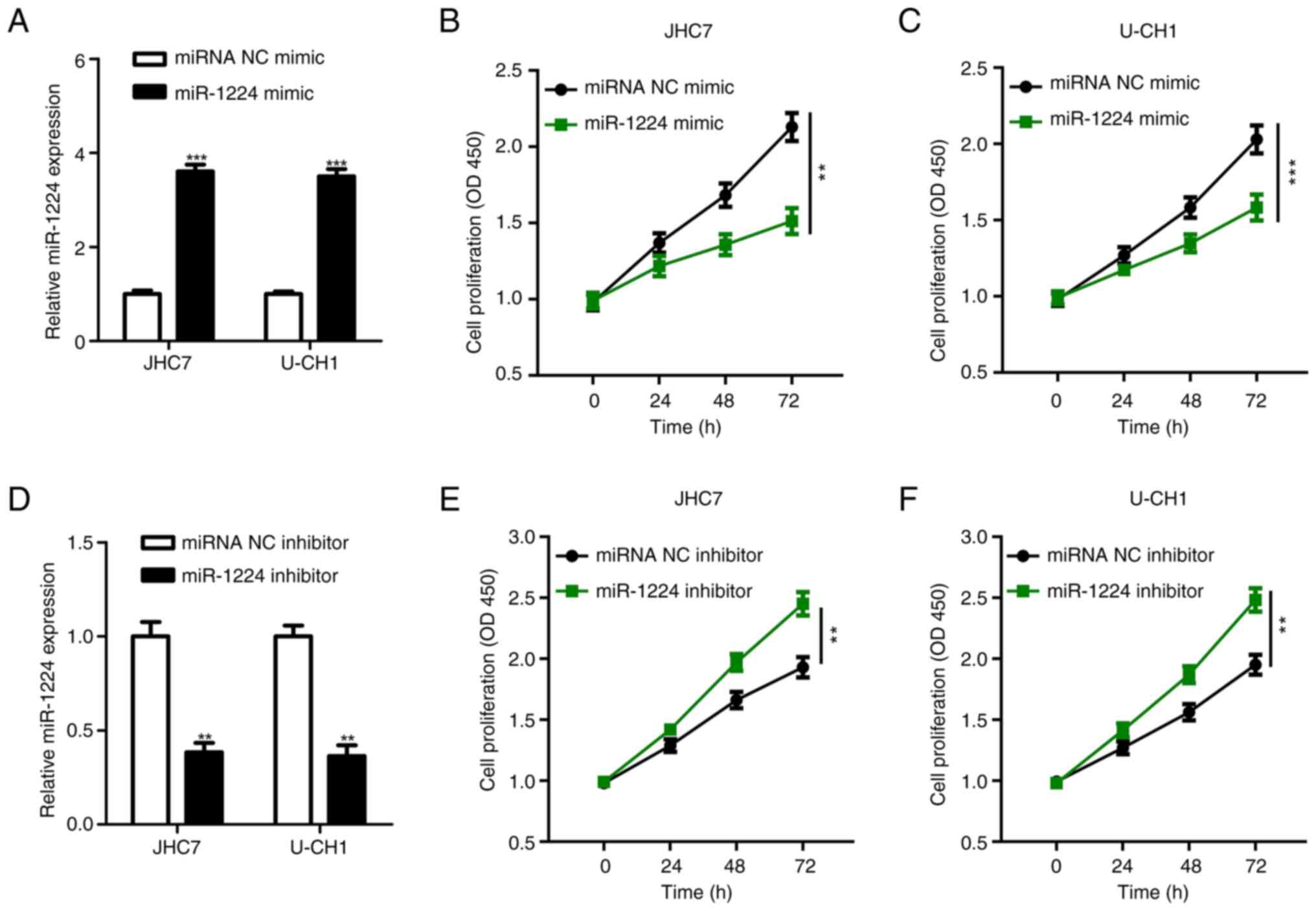
miR-1224 expression was lower in JHC7 and U-CH1
cells compared with U-CH2 cells. Therefore, JHC7 and U-CH1 were
selected for subsequent experiments (Fig. 1B). The chordoma JHC7 and U-CH1 cell
lines were transfected with the miR-1224 mimic and miRNA NC mimic,
and the expression of miR-1224 in these cells was detected using
RT-qPCR. The RT-qPCR results demonstrated that the expression
levels of miR-1224 in the miR-1224 mimic groups in both cell lines
were significantly higher compared with those in the respective
miR-NC group (Fig. 2A). Likewise,
the CCK-8 proliferation assay demonstrated that the proliferation
levels of the JHC7 (Fig. 2B) and
U-CH1 (Fig. 2C) cells with miR-1224
mimic were significantly lower compared with the levels of the
respective controls. Furthermore, the miR-1224 inhibitor and
miR-1224 inhibitor NC were transfected into the JHC7 and U-CH1
chordoma cell lines. At 48 h after transfection, the expression of
miR-1224 was detected using RT-qPCR. The expression levels of
miR-1224 in the miR-1224 inhibitor groups were significantly lower
compared with those in the respective controls (Fig. 2D). Likewise, the CCK-8 proliferation
assay demonstrated that after miR-1224 expression was
downregulated, the cell proliferation levels of JHC7 (Fig. 2E) and U-CH1 (Fig. 2F) were significantly higher compared
with those of the respective controls. Overall, this suggested that
miR-1224 could inhibit the proliferation of chordoma and serve as a
tumor suppressor.
Screening and validation of miR-1224
target genes
The target genes of miR-1224 were predicted using
TargetScan, microRNAorg and RegRNA2.0 databases, and TMEM221,
TUBB2A, LBP, CBX3 and MED20 were selected. The chordoma JHC7 and
U-CH1 cells were transfected with the miR-1224 mimic and miR-1224
mimic NC, and RT-qPCR was used to detect the mRNA expression of
candidate target genes. From these target genes, only the mRNA
expression of CBX3 was significantly decreased compared with the
control (Fig. 3A and B).
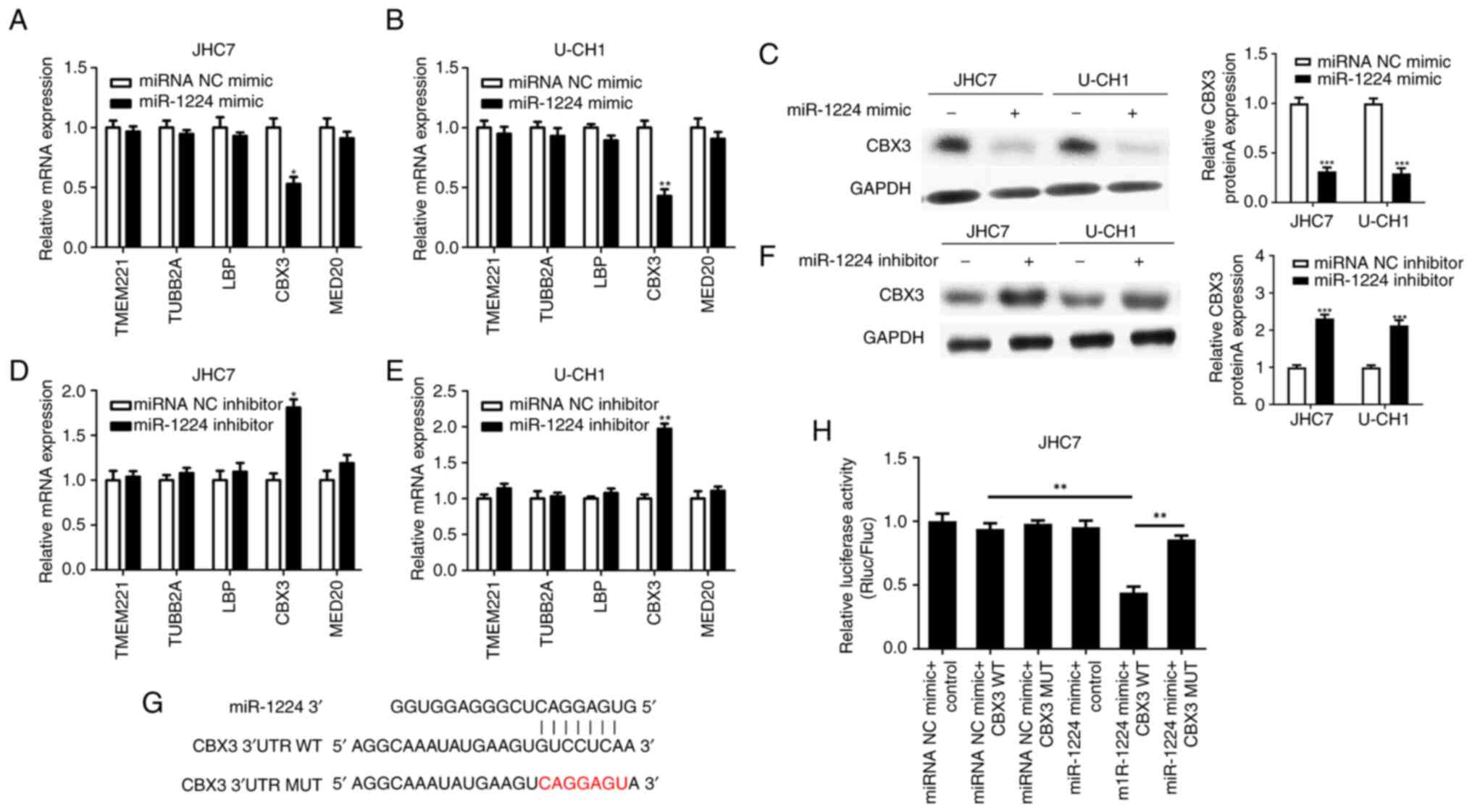 | Figure 3.mRNA expression level of candidate
target genes analyzed by RT-qPCR after the upregulation of miR-1224
expression in (A) JHC7 and (B) U-CH1 cell lines. The protein
expression of CBX3 was analyzed by western blotting after the (C)
upregulation and (F) knockdown of miR-1224 in JHC7 and U-CH1 cell
lines compared with the miRNA NC mimic or miRNA NC inhibitor. The
mRNA expression level of each candidate target genes was analyzed
by RT-qPCR after the knockdown of miR-1224 in JHC7 (D) and U-CH1
(E) cell lines. (G) Sequences used in the dual luciferase reporter
assay, showing the miR-1224, CBX3 3′UTR WT and CBX3 3′UTR MUT
sequences. (H) A luciferase reporter assay was used to verify the
direct binding between miR-1224 and CBX3. *P<0.05, **P<0.01
and ***P<0.001. WT, wild-type; MUT, mutant; UTR, untranslated
region; RT-qPCR, reverse transcription-quantitative PCR; miR,
microRNA; Rluc, Renilla luciferase; Fluc, Firefly
luciferase. |
Furthermore, western blotting demonstrated that the
protein expression of CBX3 also decreased in both cell lines
transfected with the mimic compared with the miRNA NC mimic
(Fig. 3C). Moreover, the miR-1224
inhibitor and miR-1224 inhibitor NC were transfected into JHC7
(Fig. 3D) and U-CH1 (Fig. 3E) chordoma cell lines, and RT-qPCR
demonstrated that from the target genes, only the mRNA expression
of CBX3 was significantly increased compared with that of the
control. Likewise, western blotting demonstrated that the protein
expression of CBX3 also decreased in both cell lines compared with
the miRNA NC inhibitor (Fig.
3F).
Based on the aforementioned results, it was
suggested that CBX3 could be a target of miR-1224. To further
confirm whether CBX3 is a direct target of miR-1224, CBX3 3′UTR-WT
and 3′UTR-MUT luciferase expression vectors were constructed
(Fig. 3G). Luciferase expression
vectors with WT or MUT 3′-UTRs were co-transfected with either the
miR-1224 mimic or the miR-1224 mimic NC in JHC7 cells. The results
of the dual luciferase reporter assay demonstrated that
co-transfection of miR-1224 with WT 3′-UTR significantly decreased
the luciferase signal compared with the control. However, no
significant decrease was seen with the MUT 3′-UTR (Fig. 3H). Thus, CBX3 is a direct target of
miR-1224.
Effect of CBX3 on the proliferation
ability of chordoma cells
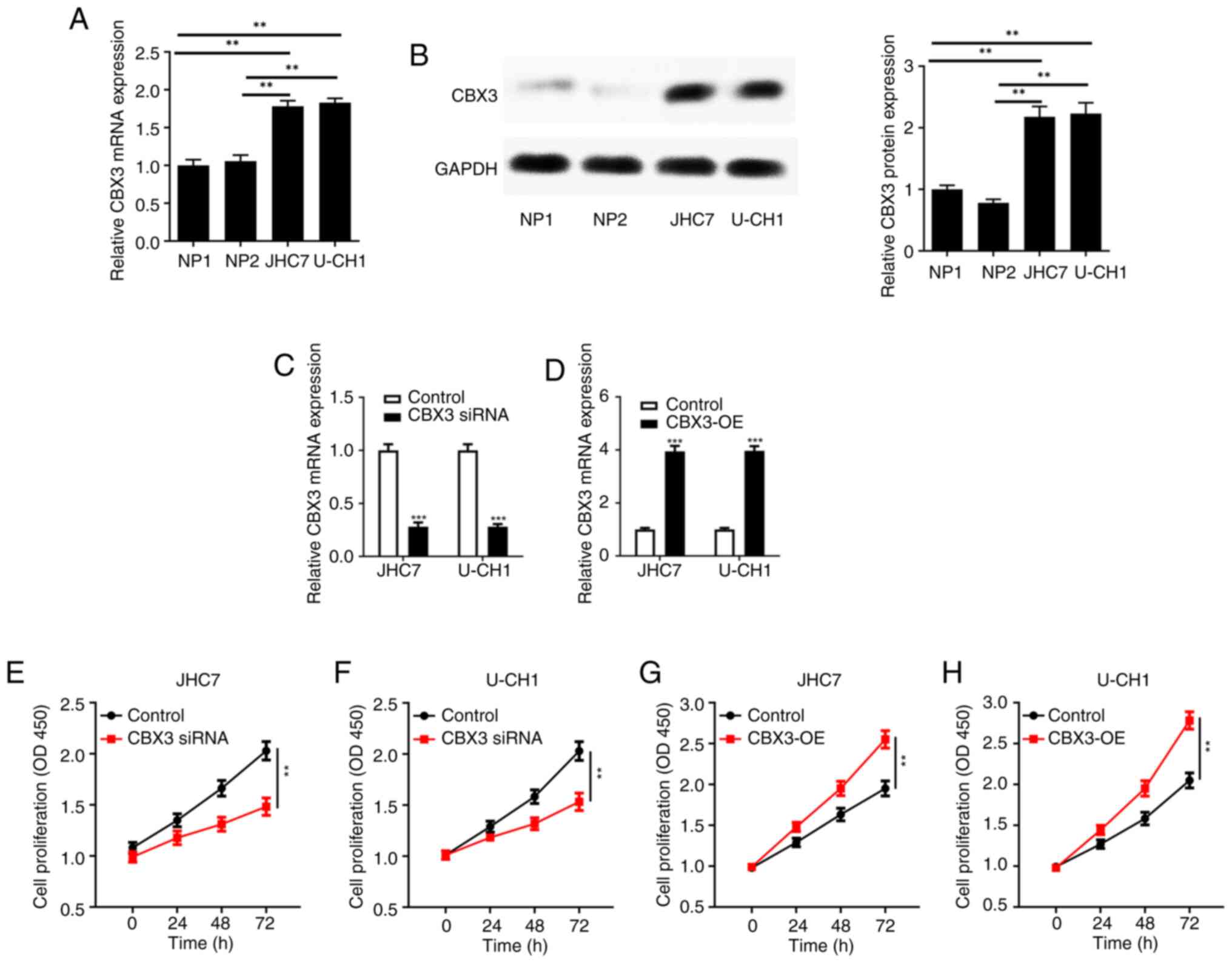
CBX3 is highly expressed in numerous tumors and can
promote tumor cell proliferation (17–20).
Therefore, the expression of CBX3 was detected in chordoma cell
lines and normal nucleus pulposus NP1 and NP2 cell lines. RT-qPCR
demonstrated that the CBX3 mRNA was significantly increased in the
JHC7 and U-CH1 cell lines compared with that in the nucleus
pulposus cells (Fig. 4A). Western
blotting showed that CBX3 protein was significantly increased in
the JHC7 and U-CH1 cell lines compared with that in the nucleus
pulposus cells (Fig. 4B).
Furthermore, the levels of CBX3 mRNA expression in
JHC7 and U-CH1 cells were assessed following the transfection of
CBX3 overexpression vector or CBX3 siRNA. The mRNA levels of CBX3
was significantly reduced in the chordoma JHC7 and U-CH1 cell lines
transfected with the CBX3 siRNA compared with the control (Fig. 4C); this significantly reduced the
proliferation ability of JHC7 (Fig.
4E) and U-CH1 (Fig. 4F)
compared with the controls. Likewise, the mRNA level of CBX3 was
significantly increased in the chordoma JHC7 and U-CH1 cell lines
transfected with the CBX3 overexpression vector compared with the
controls (Fig. 4D); this
significantly increased the proliferation of JHC7 (Fig. 4G) and U-CH1 (Fig. 4H) chordoma cell lines compared with
the controls.
miR-1224 targets CBX3 and inhibit the
proliferation of chordoma cells
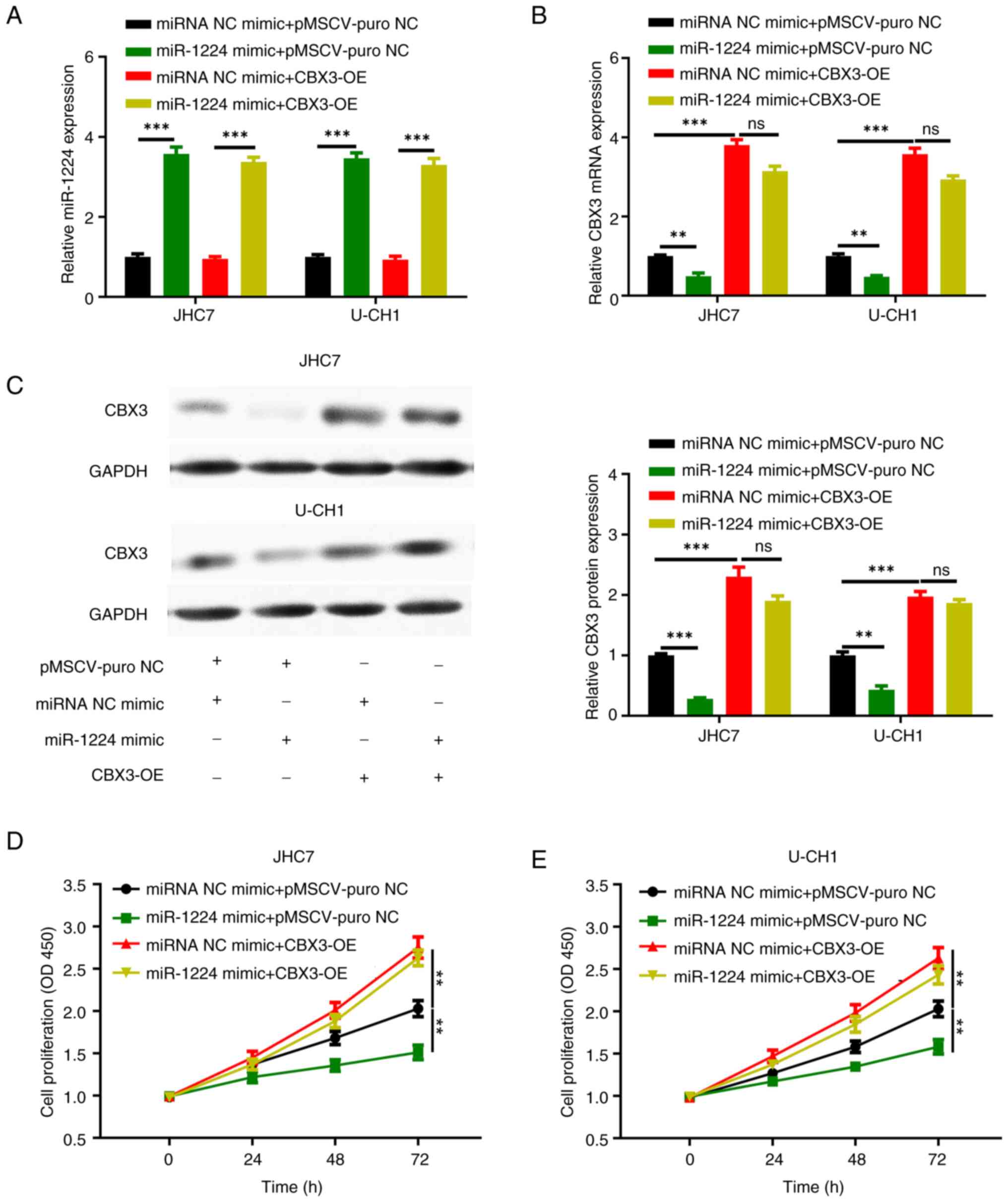
To determine the functional relationship between
miR-1224 and its potential target CBX3, and whether it is a
functional target gene of miR-1224, a functional recovery
experiment was further designed. The miR-1224 mimic and CBX3
overexpression plasmid were transfected into chordoma JHC7 and
U-CH1 cells and divided into the following four groups: miRNA NC
mimic + pMSCV-puro NC, miR-1224 mimic + pMSCV-puro NC, miRNA NC
mimic + CBX3 overexpression (CBX3-OE) and miR-1224 mimic + CBX3-OE.
RT-qPCR showed that the expression levels of miR-1224 was
significantly increased in miR-1224 mimic + control group and
miR-1224 mimic + CBX3 overexpression group compared with that in
the other groups in the JHC7 and U-CH1 cells (Fig. 5A). RT-qPCR also showed that the
expression of CBX3 mRNA was significantly increased in miRNA NC
mimic + CBX3 overexpression group and the miR-1224 mimic + CBX3
overexpression group compared with that in the other groups in the
JHC7 and U-CH1 cells, and that the expression of CBX3 mRNA was
significantly decreased in the miR-1224 mimic + control group
compared with that in the other groups (Fig. 5B). Western blotting demonstrated
that the expression of CBX3 protein was significantly increased in
the miRNA NC mimic + CBX3 overexpression group and the miR-1224
mimic + CBX3 overexpression group compared with that in the other
groups in the JHC7 and U-CH1 cells, and the expression of CBX3
protein was significantly decreased in the miR-1224 mimic + control
group compared with that in the other groups (Fig. 5C).
Furthermore, the CCK-8 proliferation assay
demonstrated that the cell proliferation was significantly
increased in the miR-1224 mimic + CBX3 overexpression group
compared with that in the miRNA NC mimic + control group, and that
the cell proliferation was significantly decreased in the miR-1224
mimic + control group compared with that in the miRNA NC mimic +
control group in the JHC7 (Fig. 5D)
and U-CH1 (Fig. 5E) cells. Although
the mRNA and protein levels of CBX3 detected in the miR-1224 mimic
+ CBX3 cell group were lower compared with the miRNA NC mimic +
CBX3 group, there was no statistically significant difference
(Fig. 5B). Likewise, there was no
significant difference in the proliferation ability between the two
groups (Fig. 5D and E). It was
demonstrated that CBX3 overexpression can reverse the decrease in
the proliferation caused by miR-1224.
Discussion
Chordoma originates from the residual chordoma
tissues of the embryo and is a moderate- to low-grade malignant
bone tumor, accounting for 1–4% of primary malignant bone tumors
(1). Chordoma has no obvious
clinical symptoms in the early stages of onset, grows slowly and
has a long course of disease. The tumor is often large and often
accompanied by bone destruction or invasion of adjacent soft-tissue
structures when detected (21).
However, chordoma has a complex local anatomy near important neural
vasculature, so surgical removal is difficult. Therefore, chordoma
has a poor prognosis, with a postoperative recurrence rate as high
as 30–85% (22). It is estimated
that ~20% of chordomas will relapse within 1 year after surgery and
40–60% of patients will have distant metastasis. The 5-year
survival rate of patients is 47–80% (3,23). The
molecular mechanisms of proliferation and invasion in chordoma
remain unclear. The biological role of miRNAs in tumors has
gradually become a popular research topic. miRNAs can inhibit mRNA
translation, directly degrade mRNA, inhibit target gene expression
at the post-transcriptional level and serve a role in the
proliferation, invasion and metastasis of malignant tumors. miRNAs
can simultaneously regulate multiple target genes and serve key
roles in the signal regulation network of tumorigenesis and tumor
development, making them promising novel targets for molecular
targeted therapy of malignant tumors (9,24–27).
Previous studies have reported that numerous miRNAs, including
miR-31, miR-185-5p, miR-125b-5p, miR-1260a, miR-1290 and miR-637
(28–30), are abnormally expressed in chordoma.
In the present study, miR-1224 had the largest differential
expression in chordoma tissues compared with notochord tissues, and
its expression was significantly downregulated in chordoma tissues
(GEO database) and cell lines. Knockdown of miR-1224 increased the
proliferation of chordoma cells, while the overexpression of
miR-1224 reduced the proliferation of chordoma cells. The
aforementioned findings suggest that miR-1224 serves a role as a
tumor suppressor gene in chordoma. Several previous studies have
reported that miR-1224 also serves a role as a tumor suppressor
gene in a variety of tumors. For example, Mosakhani et al
(31) studied the differential
miRNA expression in 99 patients with metastatic colorectal cancer
and reported that the downregulation of miR-1224 was correlated
with poor patient survival. Scarpati et al (32) performed a microarray analysis of 38
patients with rectal cancer who underwent surgery and reported 14
abnormally expressed miRNAs, of which miR-1224 was significantly
upregulated. Qian et al (33) studied 198 glioma samples and the
Chinese Genome Map, and reported that miR-1224 has a lower
expression level compared with other miRNAs in low-grade gliomas,
and that miR-1224 can reduce the proliferative capacity of
malignant gliomas by targeting CREB1 (33). Furthermore, in gastric cancer,
miR-1224 inhibits the metastasis of gastric cancer cells by
inhibiting the FAK-mediated STAT3 and NF-κB signaling pathways
(34).
In the present study, bioinformatics and dual
reporter luciferase assays confirmed that miR-1224 directly
targeted CBX3 mRNA, and miR-1224 inhibited CBX3 to inhibit the
proliferation of chordoma cells. CBX3 is a member of the
heterochromatin protein 1 family and its chromatin binding domain
can recognize the methylated histone H3K9 with the assistance of
the histone methyltransferase uv339H1, thereby regulating gene
expression (35–37). The combination of CBX3 and
methylated H3K9 can recruit various cofactors to participate in a
variety of biological processes of cells, including telomere
metabolism, DNA damage repair, RNA splicing, transcription
extension, transcription inhibition and activation (38). Previous studies reported that CBX3
is closely related to numerous human cancer types, including lung
cancer, colon cancer, osteosarcoma and prostate cancer (17–20).
For example, Alam et al (17) reported that CBX3 is the most
commonly overexpressed and amplified histone reader protein in
human lung adenocarcinoma, and that high CBX3 mRNA levels are
associated with a poor prognosis in patients with lung
adenocarcinoma. The study also reported that CBX3 binding to
methylated histone H3K9 is necessary for the proliferation and
migration of lung adenocarcinoma cells. Liu et al (18) reported that CBX3 expression is
significantly upregulated in human colorectal cancer and can
promote colorectal cancer cell proliferation in vitro and
in vivo. Likewise, Ma et al (19) reported that CBX3 is highly expressed
in human osteosarcoma tissues. Furthermore, high CBX3 mRNA
expression is a predictor of a poor prognosis in patients with
osteosarcoma (19). Moreover,
downregulating the expression of CBX can significantly reduce the
proliferation capacity of osteosarcoma cells and lead to increased
apoptosis and cell cycle arrest in the G0 and
G1 phases in osteosarcoma cells (19). Chang et al (20) reported that CBX3 is upregulated in
prostate cancer, and the elevated CBX3 level in prostate cancer
indicates a poor patient prognosis, while downregulating the
expression of CBX3 in prostate cancer cells can significantly
inhibit their proliferation and induce apoptosis. The
aforementioned results, and the results of the present study,
indicate that CBX3 is an oncogene in tumors.
Due to the difficulty in constructing a chordoma
animal model, no animal experiments were performed. This is a
limitation of the present study. To the best of our knowledge, the
present study was the first to have verified the role of miR-1224
in chordoma and confirmed the mechanism of miR-1224-suppressed
chordoma cell proliferation by inhibiting the target gene CBX3. The
results of the present study provide a novel therapeutic target and
theoretical basis for the treatment of chordoma.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WX and KY conceived and designed the study, and
drafted the manuscript. JH, CS and FS performed the experiments. WX
and KY confirm the authenticity of all the raw data. JH and CS
performed the statistical analysis. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walcott BP, Nahed BV, Mohyeldin A, Coumans
JV, Kahle KT and Ferreira MJ: Chordoma: Current concepts,
management, and future directions. Lancet Oncol. 13:e69–e76. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan Y, Lu L, Chen J, Zhong Y and Dai Z:
Analysis of prognostic factors for survival in patients with
primary spinal chordoma using the SEER Registry from 1973 to 2014.
J Orthop Surg Res. 13:762018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kayani B, Hanna SA, Sewell MD, Saifuddin
A, Molloy S and Briggs TW: A review of the surgical management of
sacral chordoma. Eur J Surg Oncol. 40:1412–1420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Le Cesne A, Chevreau C, Perrin C, Italiano
A, Hervieu A, Blay JY, Piperno-Neumann S, Saada-Bouzid E, Bertucci
F, Firmin N, et al: Regorafenib in patients with relapsed advanced
or metastatic chordoma: Results of a non-comparative, randomised,
double-blind, placebo-controlled, multicentre phase II study. ESMO
Open. 8:1015692023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu H, Lei C, He Q, Pan Z, Xiao D and Tao
Y: Nuclear functions of mammalian MicroRNAs in gene regulation,
immunity and cancer. Mol Cancer. 17:642018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Breulmann FL, Hatt LP, Schmitz B, Wehrle
E, Richards RG, Bella ED and Stoddart MJ: Prognostic and
therapeutic potential of microRNAs for fracture healing processes
and non-union fractures: A systematic review. Clin Transl Med.
13:e11612023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shao Y, Song X, Jiang W, Chen Y, Ning Z,
Gu W and Jiang J: MicroRNA-621 acts as a tumor radiosensitizer by
directly targeting SETDB1 in hepatocellular carcinoma. Mol Ther.
27:355–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao Y, Zhang D, Li X, Yang J, Chen L,
Ning Z, Xu Y, Deng G, Tao M, Zhu Y and Jiang J: MicroRNA-203
increases cell radiosensitivity via directly targeting Bmi-1 in
hepatocellular carcinoma. Mol Pharm. 15:3205–3215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi L, Zhu W, Huang Y, Zhuo L, Wang S,
Chen S, Zhang B and Ke B: Cancer-associated fibroblast-derived
exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to
promote the progression and chemoresistance of non-small cell lung
cancer. Clin Transl Med. 12:e9892022. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Zhang N, Jiao X, Wang C, Sun W, He
Y, Ren G, Huang S, Li M, Chang Y, et al: Downregulation of
microRNA-6125 promotes colorectal cancer growth through
YTHDF2-dependent recognition of N6-methyladenosine-modified GSK3β.
Clin Transl Med. 11:e6022021. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Wu K, Gu S, Wang W, Xie S, Lu T, Li
L, Dong C, Wang X and Zhou Y: A methyltransferase-like
14/miR-99a-5p/tribble 2 positive feedback circuit promotes cancer
stem cell persistence and radioresistance via histone deacetylase
2-mediated epigenetic modulation in esophageal squamous cell
carcinoma. Clin Transl Med. 11:e5452021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Long C, Jiang L, Wei F, Ma C, Zhou H, Yang
S, Liu X and Liu Z: Integrated miRNA-mRNA analysis revealing the
potential roles of miRNAs in chordomas. PLoS One. 8:e666762013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu L, Xie X, Xue H, Wang T, Panayi AC, Lin
Z, Xiong Y, Cao F, Yan C, Chen L, et al: MiR-1224-5p modulates
osteogenesis by coordinating osteoblast/osteoclast differentiation
via the Rap1 signaling target ADCY2. Exp Mol Med. 54:961–972. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alam H, Li N, Dhar SS, Wu SJ, Lv J, Chen
K, Flores ER, Baseler L and Lee MG: HP1γ promotes lung
adenocarcinoma by downregulating the transcription-repressive
regulators NCOR2 and ZBTB7A. Cancer Res. 78:3834–3848. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu M, Huang F, Zhang D, Ju J, Wu XB, Wang
Y, Wang Y, Wu Y, Nie M, Li Z, et al: Heterochromatin protein
HP1gamma promotes colorectal cancer progression and is regulated by
miR-30a. Cancer Res. 75:4593–4604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma C, Nie XG, Wang YL, Liu XH, Liang X,
Zhou QL and Wu DP: CBX3 predicts an unfavorable prognosis and
promotes tumorigenesis in osteosarcoma. Mol Med Rep. 19:4205–4212.
2019.PubMed/NCBI
|
|
20
|
Chang C, Liu J, He W, Qu M, Huang X, Deng
Y, Shen L, Zhao X, Guo H, Jiang J, et al: A regulatory circuit
HP1γ/miR-451a/c-Myc promotes prostate cancer progression. Oncogene.
37:415–426. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pamir MN and Ozduman K: Tumor-biology and
current treatment of skull-base chordomas. Adv Tech Stand
Neurosurg. 33:35–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huo X, Ma S, Wang C, Song L, Yao B, Zhu S,
Li P, Wang L, Wu Z and Wang K: Unravelling the role of immune cells
and FN1 in the recurrence and therapeutic process of skull base
chordoma. Clin Transl Med. 13:e14292023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanna SA, Aston WJ, Briggs TW, Cannon SR
and Saifuddin A: Sacral chordoma: Can local recurrence after
sacrectomy be predicted? Clin Orthop Relat Res. 466:2217–2223.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iorio MV and Croce CM: microRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma YS, Yu F, Zhong XM, Lu GX, Cong XL, Xue
SB, Xie WT, Hou LK, Pang LJ, Wu W, et al: miR-30 family reduction
maintains self-renewal and promotes tumorigenesis in
NSCLC-initiating cells by targeting oncogene TM4SF1. Mol Ther.
26:2751–2765. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Slater SC, Jover E, Martello A, Mitić T,
Rodriguez-Arabaolaza I, Vono R, Alvino VV, Satchell SC, Spinetti G,
Caporali A and Madeddu P: MicroRNA-532-5p regulates pericyte
function by targeting the transcription regulator BACH1 and
angiopoietin-1. Mol Ther. 26:2823–2837. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bayrak OF, Gulluoglu S, Aydemir E, Ture U,
Acar H, Atalay B, Demir Z, Sevli S, Creighton CJ, Ittmann M, et al:
MicroRNA expression profiling reveals the potential function of
microRNA-31 in chordomas. J Neurooncol. 115:143–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen K, Chen H, Zhang K, Sun S, Mo J, Lu
J, Qian Z and Yang H: MicroRNA profiling and bioinformatics
analyses reveal the potential roles of microRNAs in chordoma. Oncol
Lett. 14:5533–5539. 2017.PubMed/NCBI
|
|
30
|
Huo X, Wang K, Yao B, Song L, Li Z, He W,
Li Y, Ma J, Wang L and Wu Z: Function and regulation of miR-186-5p,
miR-125b-5p and miR-1260a in chordoma. BMC Cancer. 23:11522023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mosakhani N, Lahti L, Borze I,
Karjalainen-Lindsberg ML, Sundström J, Ristamäki R, Osterlund P,
Knuutila S and Sarhadi VK: MicroRNA profiling predicts survival in
anti-EGFR treated chemorefractory metastatic colorectal cancer
patients with wild-type KRAS and BRAF. Cancer Genet. 205:545–551.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Scarpati GD, Falcetta F, Carlomagno C,
Ubezio P, Marchini S, De Stefano A, Singh VK, D'Incalci M, De
Placido S and Pepe S: A specific miRNA signature correlates with
complete pathological response to neoadjuvant chemoradiotherapy in
locally advanced rectal cancer. Int J Radiat Oncol Biol Phys.
83:1113–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qian J, Li R, Wang YY, Shi Y, Luan WK, Tao
T, Zhang JX, Xu YC and You YP: MiR-1224-5p acts as a tumor
suppressor by targeting CREB1 in malignant gliomas. Mol Cell
Biochem. 403:33–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Wen T, Li Z, Che X, Gong L, Yang
X, Zhang J, Tang H, He L, Qu X and Liu Y: MicroRNA-1224 inhibits
tumor metastasis in intestinal-type gastric cancer by directly
targeting FAK. Front Oncol. 9:2222019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Canzio D, Larson A and Narlikar GJ:
Mechanisms of functional promiscuity by HP1 proteins. Trends Cell
Biol. 24:377–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mishima Y, Jayasinghe CD, Lu K, Otani J,
Shirakawa M, Kawakami T, Kimura H, Hojo H, Carlton P, Tajima S and
Suetake I: Nucleosome compaction facilitates HP1γ binding to
methylated H3K9. Nucleic Acids Res. 43:10200–10212. 2015.PubMed/NCBI
|
|
37
|
Bannister AJ, Zegerman P, Partridge JF,
Miska EA, Thomas JO, Allshire RC and Kouzarides T: Selective
recognition of methylated lysine 9 on histone H3 by the HP1 chromo
domain. Nature. 410:120–124. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheutin T, McNairn AJ, Jenuwein T, Gilbert
DM, Singh PB and Misteli T: Maintenance of stable heterochromatin
domains by dynamic HP1 binding. Science. 299:721–725. 2003.
View Article : Google Scholar : PubMed/NCBI
|