Introduction
At present, surgical resection is the only curative
treatment for patients with pancreatic cancer, whom often have poor
prognoses (1,2). Treatment plans are generally selected
based on tumor resectability status, classified according to the
National Comprehensive Cancer Network (NCCN) Clinical Practice
Guidelines (v2.2021) as either resectable (R), borderline
resectable (BR), or unresectable (3).
Occasionally, patients may experience an early
disease recurrence after surgery, even in cases diagnosed with a
resectable tumor. In fact, the feasibility of determining
resectability status by looking only at the anatomical relationship
between the tumors and surrounding major vessels, such as the
portal vein, superior mesenteric artery/vein, common hepatic
artery, and celiac artery, remains a topic of controversy (4). Recently the use of biological factors
as indicators of resectability status, such as the preoperative
serum CA19-9 levels, has become a subject of clinical research
(2,4).
CA19-9 is a sialylated antigen of the Lewis A sugar
chain of the Lewis blood group and is a major tumor marker that is
known to be elevated in pancreatic cancer. Several recent reports
have shown that preoperative serum CA19-9 levels can predict early
recurrence (5–8), and have advocated for CA19-9 to be
included as a factor indicating biological resectability. CA19-9
levels, however, can be influenced by obstructive jaundice and the
Lewis blood group [Le(a-b-)] (9).
For obstructive jaundice, appropriate bile reduction procedures,
such as endoscopic retrograde biliary drainage (ERBD), could
minimize its effects. Since the influence of Lewis blood type
cannot be completely ruled out, we believe that the use of CA19-9
alone is not sufficient for the diagnosis of tumors that are
biologically BR.
We recently reported on the usefulness of dual time
point 18F-fluorodeoxyglucose positron emission
tomography/computed tomography (FDG-PET/CT) in predicting early
postoperative recurrence in cases of pancreatic cancer (10). Diederichs et al (11) previously indicated that
hyperglycemia (fasting blood glucose >130 mg/dl) significantly
reduced the standardized uptake value (SUV) of pancreatic cancer
lesions. In a prior study, we reported that dual time point
FDG-PET/CT reduced the influence of hyperglycemia, making it useful
for the prognostication of patients with pancreatic cancer
(10). Based on these results, in
the present study, a combined evaluation of the biological factors
of CA19-9 levels and dual time point FDG-PET/CT results was
performed to enable a more accurate identification of tumors that
are biologically BR.
Materials and methods
The present study was approved by the institutional
review board of the National Defense Medical College (approval no.
4610).
Patients
We retrospectively reviewed the medical records of
patients who underwent radical (R0) resection for pancreatic ductal
adenocarcinoma (PDAC) at the National Defense Medical College
Hospital in Japan between January 2013 and August 2022. All of the
patients included in the present study provided written informed
consent for the publication of their clinical details and
images.
All PDAC diagnoses were histopathologically
confirmed prior to initiating chemotherapy via pancreatic juice
cytology, endoscopic retrograde cholangiopancreatography biopsy, or
endoscopic ultrasound-guided fine-needle aspiration biopsy. Only
patients diagnosed as having biologically R tumors based on the
NCCN Guidelines v2.2021 (3) were
included for analysis. The treatment plan for each patient was
discussed at a multidisciplinary treatment team meeting, which
included gastroenterologists, radiologists, and hepatobiliary and
pancreatic surgeons. For biologically R diseases, patients
underwent upfront surgery until 2018; however, from 2019 on,
patients received gemcitabine plus S-1 (GS) as neoadjuvant
chemotherapy (NAC) prior to surgical resection (12), the method for which was based on the
tumor location. Each patient underwent a regional lymph node
dissection, for which the pathological stage was determined based
on the tumor, node, metastasis (TNM) classification system provided
by the Union for International Cancer Control (8th edition).
Postoperatively, each patient was administered
adjuvant chemotherapy with S-1 for six months as the standard
treatment, with the follow-up consisting of blood tests performed
every three months and computed tomography (CT) every six months.
In cases in which recurrence was suspected, PET-CT was performed at
the discretion of the patient's attending physician.
Cut-off values
The standardized uptake value (SUV) percentage
change (SUVmax%) was calculated using the following formula:
SUVmax%=[(SUVmax2-SUVmax1)/SUVmax1] ×100, where
SUVmax1 and SUVmax2 represent the initial (60 min after FDG
injection) and delayed (120 min after FDG injection) scan phases,
respectively.
The cut-off values of SUVmax% and CA19-9 were 24.25%
and 500 U/ml, respectively, based on the values used in previous
studies (4,10). All FDG-PET/CT imaging was performed
prior to patients starting NAC. In patients with obstructive
jaundice, the CA19-9 values were collected after biliary drainage
was performed, and the serum total bilirubin levels decreased to
3.0 mg/dl or lower.
Grouping
Using the aforementioned cut-off values for CA19-9
(500 U/ml) and SUVmax% (24.25%), the patients were classified as
follows: i) high CA19-9 and SUVmax%, in which both CA19-9 and
SUVmax% were elevated; ii) high CA19-9 or SUVmax%, either CA19-9 or
SUVmax% was elevated; iii) low CA19-9 and SUVmax%, neither value
exceeded the cut-off. The sensitivity, specificity, positive
predictive value (PPV), negative predictive value (NPV), and
accuracy of the CA19-9 level, SUVmax%, and the combination thereof
for predicting relapse within one year of surgery were calculated,
as well as relapse-free survival (RFS) and two-year overall
survival (OS). Univariate and multivariate analyses were performed
for RFS and OS.
Sites of recurrence
Tumor recurrence was diagnosed based on imaging
studies, primarily CT, although PET-CT was occasionally performed,
and was classified as local, distant, or both. Local recurrence was
defined as enlarged soft tissue shadows or lymph nodes around the
celiac, hepatic, splenic, or superior mesenteric artery in the
peripancreatic region.
Statistical analysis
The Mann-Whitney U test was used to compare
continuous variables, which were presented as the median and range,
while Fisher's exact test was used to compare categorical
variables. We utilized the Kaplan-Meier method to determine RFS and
OS. Differences in the survival curves were analyzed using log-rank
tests, and a Cox proportional hazards model was used to perform
univariate and multivariate RFS and OS analyses. EZR (Saitama
Medical Center, Jichi Medical University, Saitama, Japan), a
graphical user interface designed to add statistical functions
frequently used in biostatistics to R (The R Foundation for
Statistical Computing, Vienna, Austria), was used to perform all
statistical analyses. Statistical significance was set at
P<0.05.
Results
Patient profiles
A total of 86 patients were included in the study,
49 (57%) of which were men, with a median age at the time of
surgery of 72 years (range, 48–86 years). Of these patients, 61
(71%) had tumors located in the pancreatic head, 25 (29%) had
tumors located in the pancreatic body or tail, and 19 (22%)
received NAC with GS. Postoperatively, 67 patients (78%) received
adjuvant chemotherapy, with S-1 administered in 61 patients (91%)
and gemcitabine in the other six (9%).
Comparison of clinical and
histopathological factors based on CA19-9 and SUVmax%
Table I shows the
characteristics of each group, classified as described above, based
on each patient's CA19-9 and SUVmax% values. Of the 86 patients, 12
(14%) were classified as high CA19-9 and SUVmax%, 39 (45%) as high
CA19-9 or SUVmax%, and the rest as low CA19-9 and SUVmax%. A
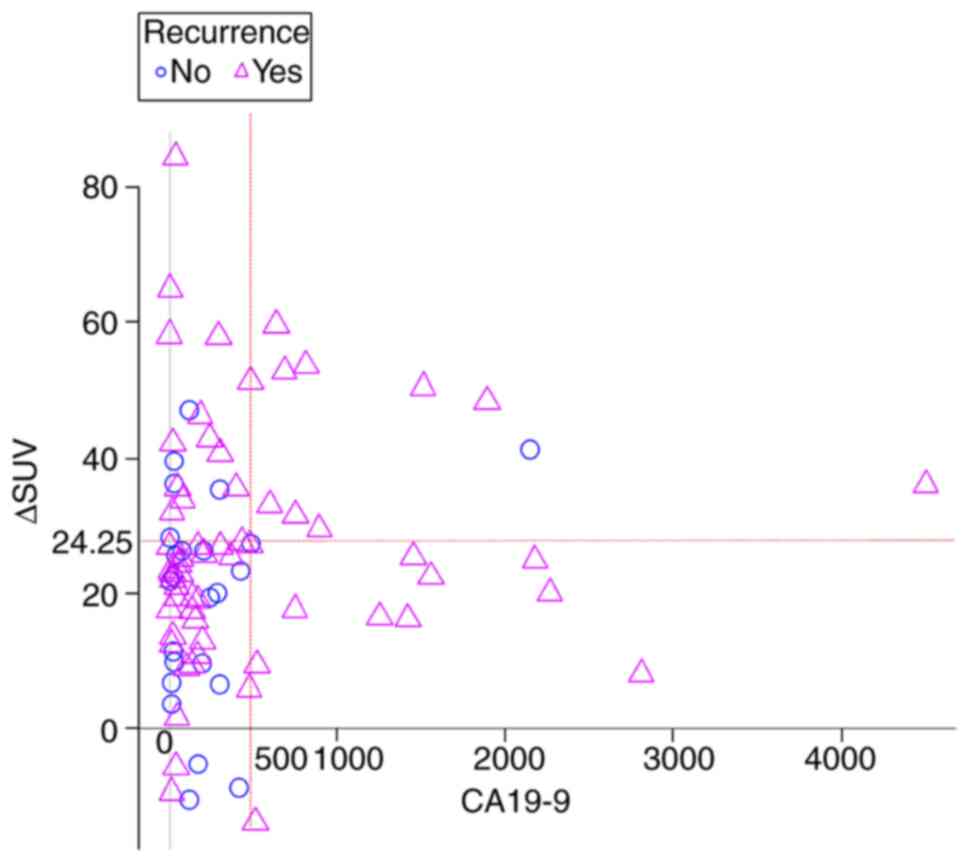
scatter plot of the patients' SUVmax% and CA19-9 levels is
presented in Fig. 1,
differentiating those patients who experienced a recurrence from
those who did not. The sensitivity and specificity of predicting
tumor recurrence within one year of surgery were 29 and 94.5%,
respectively, in the high CA19-9 and SUVmax% group (Table II).
 | Table I.Patient characteristics according to
their SUVmax% and CA19-9 values. |
Table I.
Patient characteristics according to
their SUVmax% and CA19-9 values.
| Parameter | Total (n=86) | High CA19-9 and
SUVmax% (n=12) | High CA19-9 or
SUVmax% (n=39) | Low CA19-9 and
SUVmax% (n=35) | P-value | P-value of high
CA19-9 and SUVmax% vs. high CA19-9 or SUVmax% |
|---|
| Sex (men/women) | 49/37 | 5/7 | 22/17 | 22/13 | 0.439 | 0.511 |
| Age, years | 72 (48–86) | 71 (58–84) | 73 (54–86) | 70 (48–83) | 0.443 | 0.417 |
| Neoadjuvant
chemotherapy | 19 (22%) | 1 (8%) | 9 (23%) | 9 (26%) | 0.447 | 0.417 |
| Preoperative diabetes
mellitus | 24 (28%) | 3 (25%) | 11 (28%) | 10 (29%) | 0.971 | 1.00 |
| Preoperative CA19-9
value | 171 | 1173 | 206.4 | 64.6 | <0.001 | <0.001 |
|
| (0.4–4510) | (598–4510) | (0.4–2812) | (2.8–474) |
|
|
| ΔSUVmax% | 23.7 | 38.6 | 27.36 | 12.99 | <0.001 | 0.059 |
|
| (−13.86–84.40) | (24.87–59.62) | (−13.86–84.4) | (−10.49–23.27) |
|
|
| Tumor location
(head/body or tail) | 61/25 | 12/0 | 28/11 | 21/14 | 0.031 | 0.048 |
| Operative
method |
|
|
|
|
|
|
| PD | 61 | 12 | 28 | 21 | 0.111 | 0.048 |
| DP or
TP | 25 | 0 | 11 | 14 |
|
|
| Pathological
T-factor, (1/2/3/4) | 3/1/80/2 | 0/0/12/0 | 1/1/36/1 | 2/0/32/1 | 0.897 | 1.00 |
| Pathological
N-factor, positive/negative | 64/22 | 10/2 | 28/11 | 26/9 | 0.725 | 0.706 |
| Residual tumor,
R0/R1 | 77/9 | 11/1 | 37/2 | 29/6 | 0.233 | 0.561 |
| Adjuvant
chemotherapy | 67 (78%) | 8 (73%) | 29 (74%) | 30 (88%) | 0.279 | 1.00 |
 | Table II.Accuracy of CA19-9, SUVmax% and the
combination thereof for predicting tumor relapse within 1 year
after surgery. |
Table II.
Accuracy of CA19-9, SUVmax% and the
combination thereof for predicting tumor relapse within 1 year
after surgery.
|
| Number of
cases |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Parameter | Total | Relapse | P-value | Sensitivity
(%) | Specificity
(%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|
| CA19-9, U/ml |
|
| 0.016 |
|
|
|
|
|
|
>500 | 20 | 12 (60%) |
| 38.7 | 85.5 | 60 | 71.2 | 68.6 |
|
≤500 | 66 | 19 (29%) |
|
|
|
|
|
|
| SUVmax%, % |
|
| 0.072 |
|
|
|
|
|
|
≥24.25 | 43 | 20 (47%) |
| 64.5 | 58.2 | 46.5 | 74.4 | 60.5 |
|
<24.25 | 43 | 11 (26%) |
|
|
|
|
|
|
| Combination of
CA19-9 | 12 | 9 (75%) | 0.007 | 29.0 | 94.5 | 75.0 | 70.3 | 70.9 |
| >500 U/ml and
SUVmax% |
|
|
|
|
|
|
|
|
| ≥24.25% |
|
|
|
|
|
|
|
|
Survival outcomes
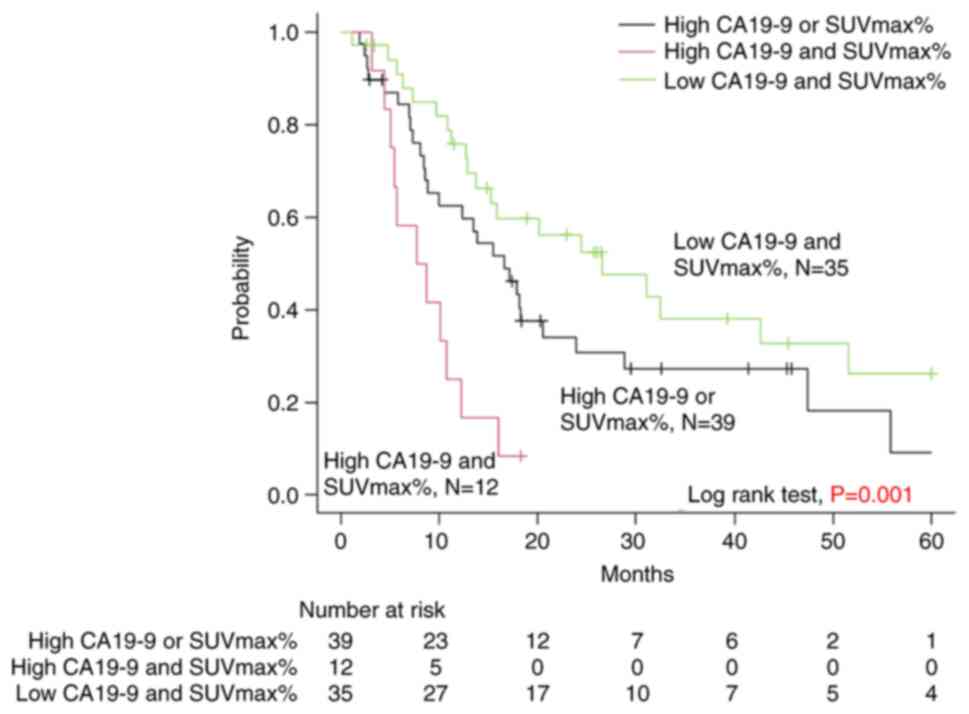
The median postoperative follow-up period was 28
months, during which a total of 30 patients (35%) were censored,
including 16 who did not experience a tumor relapse. From the high
CA19-9 and SUVmax%, high CA19-9 or SUVmax%, and low CA19-9 and
SUVmax% groups, 1 (8%), 19 (49%), and 10 (29%) patients were
censored, respectively. The median RFS was 8, 16, and 26 months in
the high CA19-9 and SUVmax%, high CA19-9 or SUVmax%, and low CA19-9
and SUVmax% groups, respectively, with a significant difference
(P<0.001) (Fig. 2). The
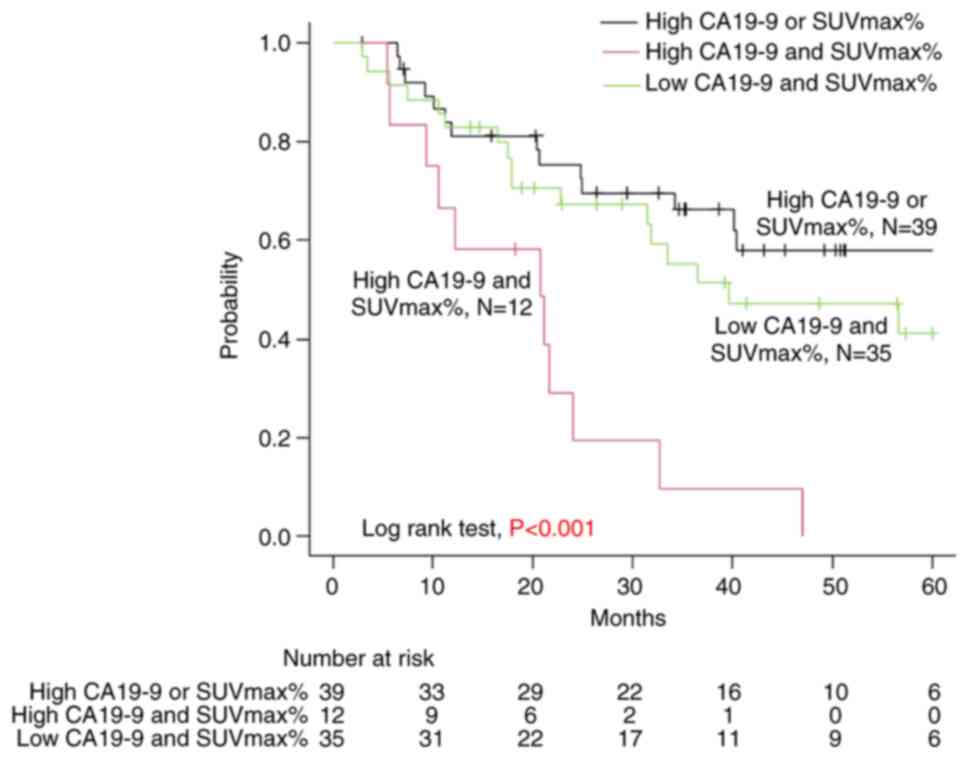
two-year OS was 19, 75, and 67% in the high CA19-9 and SUVmax%,
high CA19-9 or SUVmax%, and low CA19-9 and SUVmax% groups,
respectively, which also showed significant differences
(P<0.001) (Fig. 3).
Univariate and multivariate analyses
for the predictors of poor RFS
The univariate analysis of potential predictors
showed that lymph node metastasis [hazard ratio (HR), 2.626; 95%
confidence interval (CI), 1.289–5.350; P=0.008] and classification
as high CA19-9 and SUVmax% (HR, 4.449; 95% CI, 2.041–9.70;
P<0.001) were significant predictors of poor RFS, while high
CA19-9 or SUVmax% was not found to be a predictor of poor RFS (HR,
1.697; 95% CI, 0.949–2.997; P=0.074). The multivariate analysis of
potential predictors revealed that the pathological N-factor and
high CA19-9 and SUVmax% remained independent predictors of poor RFS
(Table III).
 | Table III.Univariate and multivariate analysis
for the predictors of poor RFS. |
Table III.
Univariate and multivariate analysis
for the predictors of poor RFS.
| A, Preoperative
parameters |
|---|
|
|---|
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Parameters | Group | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Sex | Men vs. Women | 1.037
(0.618–1.741) | 0.890 |
|
|
| Age, years | <70 vs. ≥70
years | 1.016
(0.604–1.708) | 0.953 |
|
|
| Tumor location | Head vs. Body or
Tail | 1.703
(0.939–3.089) | 0.079 | 1.514
(0.660–3.471) | 0.326 |
| Preoperative
diabetes | Yes vs. No | 1.026
(0.570–1.844) | 0.933 |
|
|
| NAC | Performed vs. | 1.225
(0.646–2.326) | 0.534 |
|
|
|
| Not performed |
|
|
|
|
|
| B, Biological
factors |
|
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
|
Parameters | Group | Hazard ratio
(95% CI) | P-value | Hazard ratio
(95% CI) | P-value |
|
| High CA19-9 or
SUVmax% vs. Others |
| 1.222
(0.615–2.427) | 0.565 |
|
|
| High CA19-9 and
SUVmax% vs. Others |
| 3.193
(1.450–7.032) | 0.003 | 3.041
(1.471–6.289) | 0.005 |
|
| C, Pathological
factors |
|
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
|
Parameters | Group | Hazard ratio
(95% CI) | P-value | Hazard ratio
(95% CI) | P-value |
|
| Pathological
T-factor | ≥3 vs. <3 | 1.334
(0.326–5.485) | 0.689 |
|
|
| Pathological
N-factor | ≥1 vs. <1 | 2.626
(1.289–5.350) | 0.008 | 2.071
(0.845–5.074) | 0.111 |
|
| D, Postoperative
parameters |
|
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
|
Parameters | Group | Hazard ratio
(95% CI) | P-value | Hazard ratio
(95% CI) | P-value |
|
| Adjuvant
chemotherapy | Not performed vs.
Performed | 1.521
(0.847–2.733) | 0.160 |
|
|
Univariate and multivariate analyses
for the predictors of poor OS
The univariate analysis of potential predictors
showed that pancreatic head cancer (HR, 2.638; 95% CI, 1.105–6.299;
P=0.029), no adjuvant chemotherapy (HR, 2.166; 95% CI, 1.114–4.212;
P=0.023), and high CA19-9 and SUVmax% (HR=3.193; 95% CI,
1.450–7.032; P=0.003) were predictors of poor OS, while high CA19-9
or SUVmax% was not found to be a predictor of poor OS (HR, 1.222;
95% CI, 0.615–2.427; P=0.565). The multivariate analysis of
potential predictors revealed that high CA19-9 and SUVmax% remained
independent predictors of poor OS (Table IV).
 | Table IV.Univariate and multivariate analysis
for the predictors of poor OS. |
Table IV.
Univariate and multivariate analysis
for the predictors of poor OS.
| A, Preoperative
parameter |
|---|
|
|---|
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Parameters | Group | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Sex | Men vs. Women | 1.134
(0.670–2.114) | 0.693 |
|
|
| Age, years | <70 vs. ≥70
years | 1.079
(0.579–2.013) | 0.810 |
|
|
| Tumor location | Head vs. Body or
Tail | 2.683
(1.105–6.299) | 0.029 | 1.061
(0.557–2.019) | 0.857 |
| Preoperative
diabetes | Yes vs. No | 1.051
(0.512–2.156) | 0.893 |
|
|
| NAC | Performed vs. Not
performed | 1.157
(0.485–2.762) | 0.742 |
|
|
|
| B, Biological
factors |
|
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
|
Parameters | Group | Hazard ratio
(95% CI) | P-value | Hazard ratio
(95% CI) | P-value |
|
| High CA19-9 or
SUVmax% vs. Others |
| 0.594
(0.313–1.087) | 0.092 | 1.704
(0.954–3.044) | 0.071 |
| High CA19-9 and
SUVmax% vs. Others |
| 3.655
(1.726–7.220) | <0.001 | 3.910
(1.753–8.724) | <0.001 |
|
| C, Pathological
factors |
|
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
|
Parameters | Group | Hazard ratio
(95% CI) | P-value | Hazard ratio
(95% CI) | P-value |
|
| Pathological
T-factor | ≥3 vs. <3 | 1.106
(0.150–8.121) | 0.921 |
|
|
| Pathological
N-factor | ≥1 vs. <1 | 2.555
(0.995–6.526) | 0.051 | 2.424
(1.158–5.072) | 0.018 |
|
| D, Postoperative
parameters |
|
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
|
Parameters | Group | Hazard ratio
(95% CI) | P-value | Hazard ratio
(95% CI) | P-value |
|
| Adjuvant
chemotherapy | Not performed vs.
Performed | 2.166
(1.114–4.212) | 0.023 |
|
|
Sites of tumor recurrence within one
year
Table V shows the
sites at which tumors recurred within one year of surgery for the
three groups. Tumor recurrence was observed in nine patients (75%)
categorized as high CA19-9 and SUVmax% and 14 (36%) categorized as
high CA19-9 or SUVmax% cases, with a significant difference
(P=0.023). Concurrent local and distant recurrences in the high
CA19-9 and SUVmax% and high CA19-9 or SUVmax% groups occurred in
four (33%) and three (8%) of the patients, respectively, showing
significant differences (P=0.044).
 | Table V.Recurrence status within 1 year after
surgery. |
Table V.
Recurrence status within 1 year after
surgery.
| Parameter | High CA19-9 and
SUVmax% (n=12) | High CA19-9 or
SUVmax% (n=39) | Low CA19-9 and
SUVmax% (n=35) | P-value | P-value of high
CA19-9 and SUVmax% vs. high CA19-9 or SUVmax% |
|---|
| 1 year recurrence
after surgery | 9 (75%) | 14 (36%) | 8 (23%) | 0.005 | 0.023 |
| Local and distant
recurrence | 4 (33%) | 3 (8%) | 1 (3%) | 0.016 | 0.044 |
| Local
recurrence | 2 (17%) | 3 (8%) | 1 (3%) | 0.236 | 0.580 |
| Distant
recurrence | 3 (25%) | 8 (21%) | 6 (17%) | 0.757 | 0.706 |
| Sites of distant
recurrence |
|
|
|
|
|
|
Lung | 0 | 2 | 1 |
|
|
|
Liver | 2 | 4 | 4 |
|
|
|
Para-aortic lymph node | 0 | 1 | 1 |
|
|
|
Peritoneal dissemination | 1 | 1 | 0 |
|
|
Discussion
In the present study, high CA19-9 and SUVmax% were
found to predictors of early (within a year) tumor relapse, with a
sensitivity of 29% and specificity of 94.5%. This finding suggests
that patients categorized as high CA19-9 and SUVmax% have a higher
risk of early postoperative recurrence; however, among these, a
percentage of patients categorized as high CA19-9 or SUVmax% or
high CA19-9 and SUVmax% are also at risk of early recurrence.
Moreover, the high CA19-9 and SUVmax% classification was found to
be an independent predictor of poor RFS. CA19-9 has modifiers such
as Lewis blood type, and this study suggests that ΔSUVmax% might
compensate for its limitations.
The usefulness of FDG-PET/CT as a prognostic
indicator has thus far been controversial (13–16).
Various FDG-PET/CT-based volumetric imaging parameters, such as
metabolic tumor volume and total lesion glycolysis, have been
suggested as prognostic indicators for pancreatic cancer (17–19).
Despite this, these indicators are not widely utilized because of
their complexity. Therefore, in the present study we focused on
using SUVmax as a convenient indicator to assess tumor biokinetics.
Higashi et al (20) and Sun
et al (21) reported that
FDG uptake (SUVmax) was largely dependent on the number of
activated tumor cells rather than on their proliferative activity.
Tumors with high proliferative activity express that the tumor was
prone to local invasion. It was not clear what high tumor activity
expresses clinically. Dual time point imaging used in PET/CT
imaging of 18F-FDG takes advantage of the accumulation of FDG by
tumor cells over time (22,23), suggesting that a higher SUVmax%
indicates a greater number of active tumor cells. In the present
study, SUVmax% was found to be a predictor of the one-year tumor
recurrence rate with a higher sensitivity (64.5%) than the other
indicators, suggesting that tumor activity may be a valuable
predictor of future metastatic susceptibility.
Preoperative CA19-9 levels have been previously
reported to be a useful predictor of recurrence (6,24). The
results of the present study showed it to have a specificity of
85.5% for the prediction of recurrence within one year after
surgery. On the other hand, SUVmax% ≥24.25% had the lowest
specificity, at 58.2%. The relatively good prognoses of patients
categorized as high CA19-9 or SUVmax% were affected by the
inclusion of patients with high SUVmax% and low CA19-9 values. In
contrast to its low specificity, SUVmax% ≥24.25% could predict the
one-year recurrence rate with the highest sensitivity, at 64.5%,
while being categorized as high CA19-9 and SUVmax% has the highest
accuracy, at 70.9%. We propose, therefore, that the combination of
CA19-9 and SUVmax% values, rather than each index alone, is a
reliable indicator of biological resectability. Because being
categorized as high CA19-9 and SUVmax% is an indicator of
concurrent distant and local recurrence, it is within reason to
consider surgical therapy for patients who fall into this category,
followed by strong chemotherapy, such as a combination of
oxaliplatin (L-OHP), irinotecan (CPT-11), and
5-fluorouracil/leucovorin (5-FU/l-LV), known as FOLFILINOX.
In Japan, the standard treatment for resectable
pancreatic cancer at present is NAC with GS, followed by resection.
Tajima et al (25) reported
that pancreatic cancer tissues following NAC are rich in
chemoresistance steam like-cells and epithelial-mesenchymal
transition (EMT) markers. EMT markers induced by NAC play an
important role in the aggressive behavior of tumors. This suggests
that NAC may change the biological nature of the tumor. In the
present study, FDG-PET/CT was routinely performed prior to the
start of NAC because of the impact of NAC on the tumor tissues.
Because insurance restrictions allowed only one FDG-PET/CT every
three months, most patients were only administered one round of
FDG-PET/CT. Further studies are warranted to determine whether the
change in SUVmax% before and after NAC is an indicator of early
recurrence.
In some cases, FDG-PET/CT may not be feasible, for
which it is important to use other modalities, such as MRI, for the
biological evaluation of tumors. Multiparametric MRI radiomic
nomograms have been used to differentiate early recurrent cases of
pancreatic cancer (26). If both
FDG-PET/CT and MRI are available, a combined evaluation of the
tumor biology could improve the accuracy of diagnosis in cases of
early recurrence.
This study has some limitations. First, this was a
retrospective single-center study; therefore, a multicenter study
with a larger cohort is warranted. Second, this study included a
small study population. Thus, the usefulness of these results will
need to be verified using a larger sample size. Additionally, the
follow-up period was short, with 35% of cases being excluded.
Although there was no significant difference between high CA19-9 or
SUVmax% and low CA19-9 and SUVmax% excluded cases (P=0.097),
patients should receive sufficient follow-up to monitor their
prognoses. Finally, only a few patients in the present study
received NAC, which is currently the standard treatment for
resectable pancreatic cancers. The ideal timing for FDG-PET/CT
evaluations, however, has not yet been standardized, and further
studies are needed to determine whether or not SUV fluctuations
before and after NAC play a role in tumor resectability.
To summarize, high levels of CA19-9 and SUVmax% were
found to be independent risk factors for prognosis, with a
specificity of 94.5% for the prediction of early recurrence within
one year of surgery. Additionally, the combination of CA19-9 and
SUVmax% values appear to be a feasible biological indicator of
borderline resectability in pancreatic cancers.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KK, YK, HT, and HU drafted and critically reviewed
the manuscript for important intellectual content. KK and YK made
substantial contribution to conception and design. TT, MT and TE
contributed substantially to the clinical data acquisition. NY and
YT analyzed and interpreted the patient data and contributed to the
preparation of the manuscript. HT and HU made substantial
contribution of analysis of patient data. KT and JI contributed
substantially to the interpretation of the PET/CT results, as well
as data accumulation. KK and TE confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by our institute's
committee on human research, and all patients provided their
written informed consent to retrospectively registered (approval
no. 4610; 24 June 2022).
Patient consent for publication
All patients provided their written informed consent
for the publication of their clinical details and images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BR
|
borderline resectable
|
|
FDG-PET/CT
|
8F-fluorodeoxyglucose positron
emission tomography/computed tomography
|
|
GS
|
gemcitabine plus S-1
|
|
NAC
|
neoadjuvant chemotherapy
|
|
NCCN
|
National Comprehensive Cancer
Network
|
|
NPV
|
negative predictive value
|
|
OS
|
overall survival
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
PPV
|
positive predictive value
|
|
R
|
resectable
|
|
RFS
|
relapse-free survival
|
|
SUV
|
standardized uptake value
|
References
|
1
|
Masiak-Segit W, Rawicz-Pruszyński K,
Skórzewska M and Polkowski W: Surgical treatment of pancreatic
cancer. Pol Przegl Chir. 90:45–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Einama T, Takihata Y, Aosasa S, Konno F,
Kobayashi K, Yonamine N, Fujinuma I, Tsunenari T, Nakazawa A,
Shinto E, et al: Prognosis of pancreatic cancer based on
resectability: A single center experience. Cancers (Basel).
15:11012023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
National Comprehensive Cancer Network
(NCCN), . Clinical Practice Guidelines in Oncology (NCCN
guidelines®): Pancreatic adenocarcinoma. NCCN; Plymouth
Meeting, PA: 2023, https://www.nccn.org/patients/guidelines/content/PDF/pancreatic-patient.pdf
|
|
4
|
Isaji S, Mizuno S, Windsor JA, Bassi C,
Fernández-Del Castillo C, Hackert T, Hayasaki A, Katz MHG, Kim SW,
Kishiwada M, et al: International consensus on definition and
criteria of borderline resectable pancreatic ductal adenocarcinoma
2017. Pancreatology. 18:2–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartwig W, Strobel O, Hinz U, Fritz S,
Hackert T, Roth C, Büchler MW and Werner J: CA19-9 in potentially
resectable pancreatic cancer: Perspective to adjust surgical and
perioperative therapy. Ann Surg Oncol. 20:2188–2196. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Azizian A, Rühlmann F, Krause T, Bernhardt
M, Jo P, König A, Kleiß M, Leha A, Ghadimi M and Gaedcke J: CA19-9
for detecting recurrence of pancreatic cancer. Sci Rep.
10:13322020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu H, Zenati MS, Rieser CJ, Abbas AA, Lee
KK, Singji AD, Bahary N, Hogg ME, Zeh HJ III and Zureikat AH:
CA19-9 change during neoadjuvant therapy may guide the need for
additional adjuvant therapy following resected pancreatic cancer.
Ann Surg Oncol. 27:3950–3960. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mie T, Ozaka M, Okamoto T, Takeda T,
Ushida Y, Mori C, Furukawa T, Yamada Y, Kasuga A, Matsuyama M, et
al: CA19-9 reduction after 4 months of treatment is a prognostic
factor for locally advanced pancreatic cancer. In Vivo.
36:2844–2851. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yazawa S, Asao T, Izawa H, Miyamoto Y and
Matta KL: The presence of CA19-9 in serum and saliva from Lewis
blood-group negative cancer patients. Jpn J Cancer Res. 79:538–543.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Einama T, Yamagishi Y, Takihata Y, Konno
F, Kobayashi K, Yonamine N, Fujinuma I, Tsunenari T, Kouzu K,
Nakazawa A, et al: Clinical impact of dual time point
18F-fluorodeoxyglucose positron emission
tomography/computed tomography fusion imaging in pancreatic cancer.
Cancers (Basel). 14:36882022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diederichs CG, Staib L, Glatting G, Beger
HG and Reske SN: FDG PET: Elevated plasma glucose reduces both
uptake and detection rate of pancreatic malignancies. J Nucl Med.
39:1030–1033. 1998.PubMed/NCBI
|
|
12
|
Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi
S, Sho M, Honda G, Matsumoto I, Wada K, Furuse J, et al: Randomized
phase II/III trial of neoadjuvant chemotherapy with gemcitabine and
S-1 versus upfront surgery for resectable pancreatic cancer
(Prep-02/JSAP05). Jpn J Clin Oncol. 49:190–194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XY, Yang F, Jin C and Fu DL: Utility
of PET/CT in diagnosis, staging, assessment of resectability and
metabolic response of pancreatic cancer. World J Gastroenterol.
20:15580–15589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Evangelista L, Zucchetta P, Moletta L,
Serafini S, Cassarino G, Pegoraro N, Bergamo F, Sperti C and
Cecchin D: The role of FDG PET/CT or PET/MRI in assessing response
to neoadjuvant therapy for patients with borderline or resectable
pancreatic cancer: A systematic literature review. Ann Nucl Med.
35:767–776. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pu Y, Wang C, Zhao S, Xie R, Zhao L, Li K,
Yang C, Zhang R, Tian Y, Tan L, et al: The clinical application of
18F-FDG PET/CT in pancreatic cancer: A narrative review.
Transl Cancer Res. 10:3560–3575. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghidini M, Vuozzo M, Galassi B, Mapelli P,
Ceccarossi V, Caccamo L, Picchio M and Dondossola D: The role of
positron emission tomography/computed tomography (PET/CT) for
staging and disease response assessment in localized and locally
advanced pancreatic cancer. Cancers (Basel). 13:41552021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY,
Lee JH and Lee JD: Prognostic value of metabolic tumor volume and
total lesion glycolysis on preoperative 18F-FDG PET/CT
in patients with pancreatic cancer. J Nucl Med. 55:898–904. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohamed E, Needham A, Psarelli E, Carroll
M, Vinjamuri S, Sanghera B, Wong WL, Hlloran C and Ghaneh P:
Prognostic value of 18FDG PET/CT volumetric parameters
in the survival prediction of patients with pancreatic cancer. Eur
J Surg Oncol. 46:1532–1538. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fiore M, Taralli S, Trecca P, Scolozzi V,
Marinelli L, Triumbari EKA, Caputo D, Angeletti S, Ciccozzi M,
Coppola A, et al: A bio-imaging signature as a predictor of
clinical outcomes in locally advanced pancreatic cancer. Cancers
(Basel). 12:20162020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higashi K, Clavo AC and Wahl R: Does FDG
uptake measure proliferative activity of human cancer cells? In
vitro comparison with DNA flow cytometry and tritiated thymidine
uptake. J Nucl Med. 34:414–419. 1993.PubMed/NCBI
|
|
21
|
Sun Y, Duan Q, Wang S, Zeng Y and Wu R:
Diagnosis of pancreatic cancer using 18F-FDG PET/CT and
CA19-9 with SUVmax association to clinical characteristics. J BUON.
20:452–459. 2015.PubMed/NCBI
|
|
22
|
Saleh Farghaly HR, Mohamed Sayed MH, Nasr
HA and Abdelaziz Maklad AM: Dual time point fluorodeoxyglucose
positron emission tomography/computed tomography in differentiation
between malignant and benign lesions in cancer patients. Does it
always work? Indian J Nucl Med. 30:314–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xi Y, Guo R, Hu J, Zhang M, Zhang X and Li
B: 18F-fluoro-2-deoxy-D-glucose retention index as a prognostic
parameter in patients with pancreatic cancer. Nucl Med Commun.
35:1112–1118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hata T, Chiba K, Mizuma M, Masuda K,
Ohtsuka H, Nakagawa K, Morikawa T, Hayashi H, Motoi F and Unno M:
Levels of tumor markers CEA/CA 19-9 in serum and peritoneal lavage
predict postoperative recurrence in patients with pancreatic
cancer. Ann Gastroenterol Surg. 6:862–872. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tajima H, Makino I, Ohbatake Y, Nakanuma
S, Hayashi H, Nakagawara H, Miyashita T, Takamura H and Ohta T:
Neoadjuvant chemotherapy for pancreatic cancer: Effects on cancer
tissue and novel perspectives. Oncol Lett. 13:3975–3981. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang TY, Li X, Zhang Q, Guo CX, Zhang XZ,
Lao MY, Shen YN, Xiao WB, Ying SH, Sun K, et al: Development of a
novel multiparametric MRI radiomic nomogram for preoperative
evaluation of early recurrence in resectable pancreatic cancer. J
Magn Reson Imaging. 52:231–245. 2020. View Article : Google Scholar : PubMed/NCBI
|