Head and neck squamous cell carcinoma (HNSCC) is a
general term for squamous epithelial malignant tumors originating
from the nasal cavity, oral cavity, pharynx, or larynx. HNSCC
accounts for ~90% of all head and neck cancers and is characterized
by high invasiveness and a poor prognosis (1). P53, as a transcription factor, can
play its role in tumor suppression by activating the expression of
numerous target genes (2). However,
p53 is one of the most commonly mutated genes, which frequently
harbors missense mutations. These missense mutations are nucleotide
substitutions that result in the substitution of an amino acid in
the DNA binding domain. Most p53 mutations in HNSCC are missense
mutations and the mutation rate of p53 reaches 65–85% (3,4).
Mutant p53 in HNSCC can interact with proteins and have effects on
HNSCC proliferation, migration, invasion, immunosuppression and
metabolism. Studies have shown that mutant p53 can alter metabolic
pathways, including reactive oxygen species (ROS), autophagy and
lipid metabolism pathways (5,6).
Current treatments for HNSCC include more precise surgical
treatments, such as radiation therapy (RT) and chemotherapy (CT)
(7). Early-stage HNSCC can be
treated using radical RT or surgery, whereas advanced HNSCC should
be treated with RT, surgery and CT, as well as multidisciplinary
management of toxicity and side effects and follow-up (8). However, despite the extensive use of
these treatments for the management of HNSCC, the five-year overall
survival of patients with HNSCC has not changed significantly, and
the recurrence rate for advanced HNSCC has remained high at ~50%
(9). RT involves the use of α, β,
γ, and X rays [ionizing radiation (IR)] to eliminate tumor cells
and inhibit tumor cell proliferation, with the ultimate goal of
achieving radical cure of tumors and controlling tumor progression
(10). Direct transmission of
radiation through the surface of superficial tumors may lead to
irradiation of the normal tissues behind the tumor, leading to
severe damage of the normal tissues, particularly those that are
sensitive to radiation. Therefore, limiting the irradiation of
normal tissues is an important challenge in the development of
tumor RT. Currently, the developmental direction of clinical RT
technology is to improve RT technology, change the local RT mode,
maximize the accuracy of irradiation of tumor tissues, and avoid
damage to normal tissues. Notably, patients with advanced HNSCC are
radioresistant; thus, increasing their radiation dose to
therapeutic levels increases the risk of damage to the surrounding
vital organs and causes severe side effects. Therefore, protecting
organ function is important in improving the curative effects of RT
and reducing its side effects in patients with HNSCC.
Ferroptosis is a regulated form of cell death.
Unlike the traditional mode of cell death, ferroptosis is caused by
the accumulation of iron ions and ROS-induced lipid peroxidation
(11). It is closely related to the
occurrence and development of numerous human diseases, such as
cancer, viral infections and degenerative diseases. Previous
studies have shown that ferroptosis plays an important role in
RT-induced cell death and tumor suppression, and that promoting
ferroptosis in tumor cells enhances the sensitivity of the cells to
radiation and CT drugs (12).
Clinically, RT usually needs to be combined with CT, targeted
therapy, or immunotherapy to eliminate tumor cells (13–15).
In the present study, the mechanisms of ferroptosis and its
regulatory factors in HNSCC were reviewed, and the mechanism
underlying RT-induced tumor cell death in HNSCC was discussed to
provide a theoretical basis for further improving the
radiosensitivity of HNSCC.
RT is a common method of cancer therapy that
involves the use of IR to eliminate tumor cells and inhibit tumor
cell growth and metastasis (17).
The clinical applications of radiation technology include
palliative RT, conventional RT in vitro, stereotactic RT
surgery, radionuclide therapy and intensity-modulated RT (IMRT)
(18,19). IMRT is one of the most advanced and
commonly used RT techniques. IMRT can minimize the amount of
radiation normal tissues are exposed to and meet the treatment
requirements for irregularly shaped tumor targets (20). Therefore, utilization of IMRT
techniques for the treatment of patients with HNSCC can be adopted
as an organ protection strategy, especially in patients with
locally advanced disease (21).
RT-induced tumor cell death can be divided into
accidental cell death (ACD) and regulated cell death (RCD)
(22). ACD is an uncontrolled
passive cell death process, whereas RCD is a controlled cell death
process. RCD includes apoptosis, autophagic cell death, necrosis,
cornification, atypical cell death (including mitotic catastrophe),
anoikis, paraptosis, pyroptosis, entosis, excitotoxicity and
ferroptosis (23,24). IR emitted by RT can cause a variety
of DNA damage, including base damage, DNA single-strand breaks and
DNA double-strand breaks (DSBs), which can affect the integrity of
DNA or alter its chemical properties (25). Among them, DNA DSBs have been
reported to be the most deleterious effect triggering genome
stability, eliminating cancer cells, leading to genome instability,
apoptosis, altered cell cycle checkpoints or post-mitotic death,
and are the main cause of RCD in tumour cells (26).
RT and surgery can achieve similar curative effects
in patients with early-stage HNSCC. However, due to the lack of
effective biomarkers for early diagnosis of HSNCC, most patients
are diagnosed at the terminal stage of the disease. For patients
with recurrent or metastatic HNSCC (R/M HNSCC) who have lost the
opportunity for surgery and RT, long-term control of tumor growth,
distant metastasis and clinical symptoms are more important than
aiming for a cure. Systemic CT, immunotherapy and targeted therapy
can be administered as the primary treatments.
With the development and advancement of CT, various
platinum-based drugs have been revealed to exert certain
therapeutic effects on HNSCC. Drugs used for the treatment of HNSCC
include cisplatin, bleomycin, fluorouracil and methotrexate. These
drugs act mainly through a cytotoxic mechanism to control the
proliferation of tumor cells. The most desirable drugs from the
drug screen are platinum-based drugs, which constitute the highest
proportion of cancer drugs evaluated in clinical trials. In the TAX
323 (EORTC 24971) trials, patients with distant metastasis that
occurred during TPF therapy (docetaxel + cisplatin +
5-fluorouracil) showed significantly prolonged progression-free
survival and median overall survival (27).
Several clinical trials have shown that concurrent
chemo-RT (CRT) for advanced HNSCC to increase local tumor control
is relatively simple and can improve survival. Brizel et al
(28) compared patients with
advanced HNSCC who underwent surgery and received CRT, targeted
therapy combined with CRT, and other treatments, and found that CRT
is improved compared with RT alone for the treatment of locally
advanced metastatic HNSCC.
Immune checkpoint inhibitors (ICIs) restore the
antitumor immune response by blocking immune checkpoints.
Inhibitory immune checkpoints, including programmed death
receptor-1 (PD-1) and cytotoxic T lymphocyte-associated protein-4
(CTLA-4), are primarily expressed in T cells. Tumor-induced immune
responses mediate tumor immune escape by upregulating the
expression of immune checkpoints. ICIs can restore the antitumor
immunity function of T cells through competitive inhibition of
inhibitory receptors on T cells (29).
PD-1/PD-L1 and CTLA-4 inhibitors are the main
immunotherapy agents for the treatment of R/M HNSCC. PD-1
inhibitors include pembrolizumab, nivolumab, camrelizumab,
toripalimab, tislelizumab, avelumab and duvalumab, whereas CTLA-4
inhibitors include tremelimumab and ipilimumab. The PD-1 inhibitor
pembrolizumab is currently used as a first-line treatment for
HNSCC. The findings of the KEYNOTE-048 trial indicated that PD-L1
biomarkers are useful for selection of appropriate treatments for
HNSCC (30). In 2016, the United
States Food and Drug Administration approved the use of the ICIs
(PD-1) pembrolizumab and nivolumab for the treatment of R/M HNSCC
based on the results of the KEYNOTE-012 and CheckMate 141 trials
(31–34), which indicated that anti-PD-1
monoclonal antibodies significantly prolong and improve overall
survival in patients who show disease progression within 6 months
of receiving platinum-based therapy (35–37).
In a study on preoperative induction therapy plus postoperative
adjuvant immunization, patients in the intermediate-risk group
continued pembrolizumab monotherapy depending on whether positive
surgical margins or lymphatic tissue metastasis was present. The
patients who received pembrolizumab throughout the course of
treatment demonstrated significantly improved one-year disease-free
survival and overall.
Basic biochemical pathways and mutant proteins play
important roles in targeted antitumor therapies by blocking tumor
cell growth and survival (38). The
epidermal growth factor receptor (EGFR) is an important member of
the complex receptor tyrosine kinase family and plays a major role
in cell signaling. EGFR is involved in cell and organism growth
regulation and is highly expressed in most HNSCCs (39). Drugs currently used in the targeted
therapy for HNSCC include the monoclonal antibodies cetuximab,
panitumumab and zalutumumab; the small-molecule tyrosine kinase
inhibitors gefitinib and erlotinib; and the dual-target tyrosine
kinase inhibitor lapatinib. In 2016, the FDA approved the use of
cetuximab in combination with RT for the treatment of patients with
locally advanced HNSCC and R/M HNSCC that do not to respond to
platinum-based CT (40). Cetuximab
is a clinically effective monoclonal antibody against EGFR
(41). These monoclonal antibodies
enhance tumor antigen presentation by forming immune complexes that
enhance the induction of tumor-specific T cells (42). In addition, cetuximab promotes
natural killer cell antibody-dependent cytotoxicity and enhance the
tumor cell-killing ability of complement-dependent cytotoxicity
(43–45).
Ferroptosis is a form of iron-dependent cell death
that was proposed in 2012 by Dixon et al (46). From a morphological and molecular
biological perspective, ferroptosis is characterized by the
presence of malformed small mitochondria, reduced mitochondrial
crista, increased mitochondrial membrane density, rupture of the
outer mitochondrial membrane, normal nuclear size, and a lack of
condensed chromatin caused by overwhelming lipid peroxidation and
oxidative disturbances in the intracellular microenvironment
(13). Ferroptosis is mainly
characterized by the presence of iron in the cell death execution
and regulatory defense systems (47). Generally, the ferroptosis defense
system can eliminate lipid peroxides and maintain a non-toxic
state; however, if the amount of iron in the cell death execution
system is higher than that in the ferroptosis defense system, the
accumulation of lipid peroxides in the cell membrane increases to
toxic levels, leading to ferroptosis (48). Ferroptosis gene regulators include
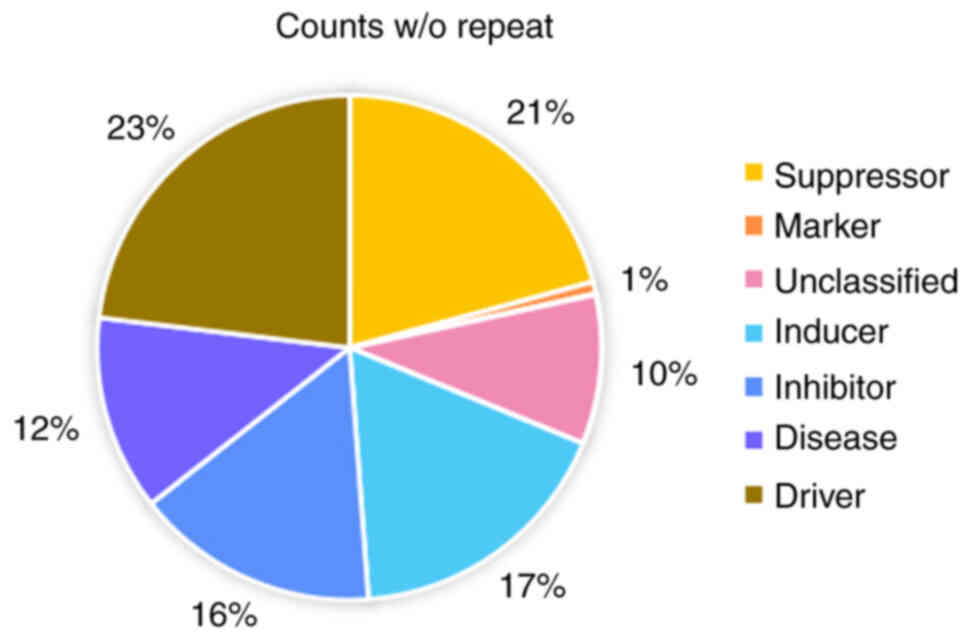
drivers, repressors, markers and unclassified regulators (FerrDb;
Fig. 1). Of these, only regulators
play an important role in the ferroptosis regulatory network. The
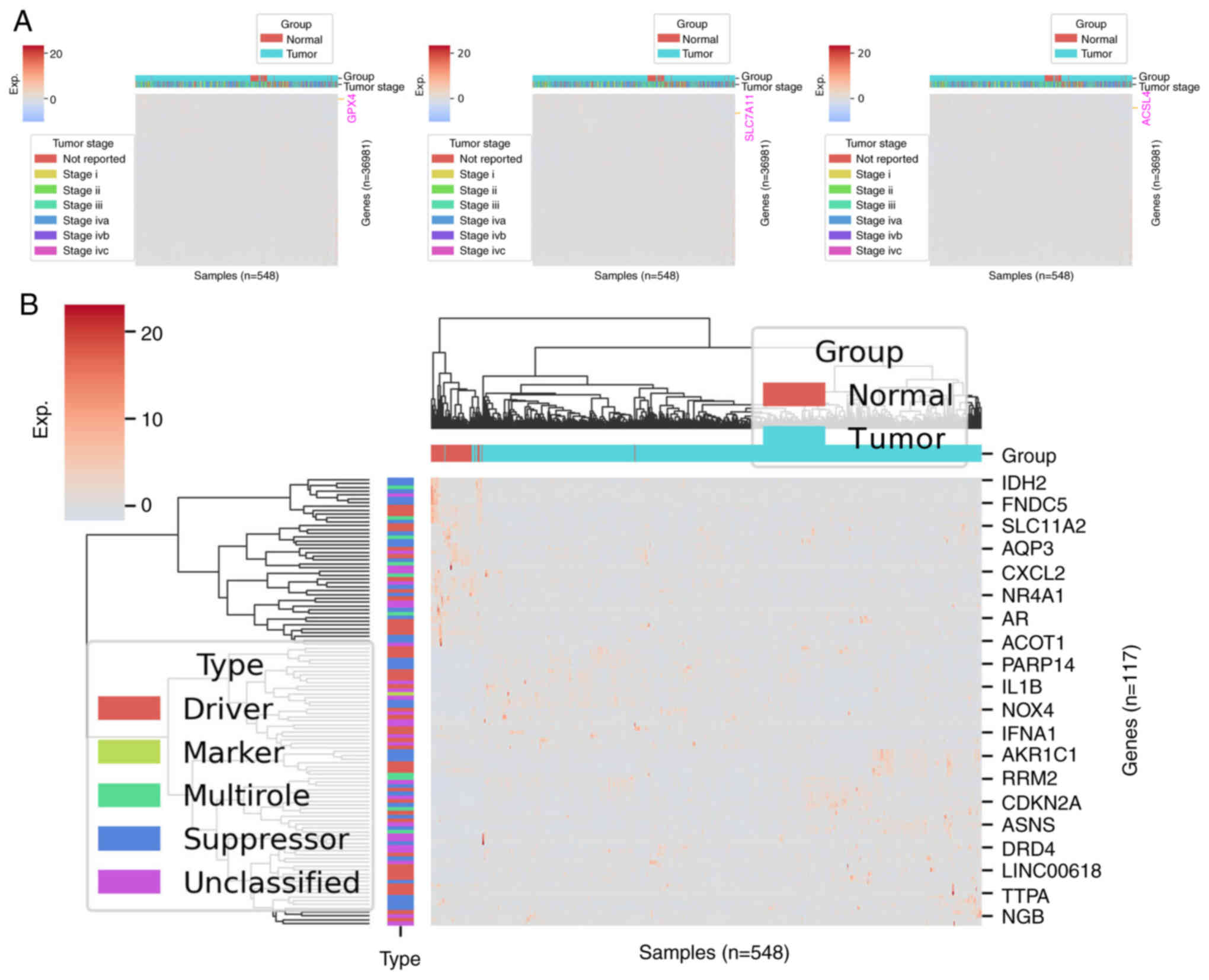
regulation network in the SLC7A11, GPX4 and acyl coenzyme A
synthetase long-chain family member 4 (ACSL4) is highly expressed
in the progression of the HNSCC cell line and reversible
ferroptosis (Fig. 2A).
Ferroptosis is characterized by accumulation of iron
ions and lipid peroxides. Iron is an essential trace element
involved in redox activity in the human body. Increased levels of
iron and/or iron-binding proteins and the dysregulation of iron
metabolism contribute to the risk for cancer and promote tumor
growth (49). Tumor cells are more
dependent on iron than normal cells, and some tumor cells exhibit
iron-ion aggregation. By iron, therefore, the steady state adjusts
iron death by increasing iron intake; reduced iron can be stored
and limit loss to promote iron death, as well as through the iron
chelating agent and antioxidant to prevent death, effectively
eliminating tumor cells (50). A
recent study revealed that when iron metabolism disorder leads to
increase in the amount of free iron in cells, the iron produced by
Fenton's reaction catalyzes the production of ROS to further
promote lipid peroxidation and induce ferroptosis (51).
The underlying mechanism for ferroptosis is the
iron-dependent accumulation of lipid peroxides. The ferroptosis
defense system can inhibit lipid peroxidation under normal
conditions. However, if the amount of iron in the cell death
execution system is higher than that in the ferroptosis defense
system, lipid peroxide rapidly accumulates in the cell membrane to
toxic levels, triggering ferroptosis (48,52).
Dysregulation of ferroptosis has been associated with numerous
tumors, including HNSCC (53–56)
(Fig. 2B). In the iron metabolism
execution system, polyunsaturated fatty acids (PUFA) are produced
in cells through the catalytic formation of
PUFA-phospholipid-peroxide (PUFA-PL-OOH), causing the accumulation
of the lipid peroxide in the cell membrane (47,57,58).
This accumulation of lipid peroxides disrupts the integrity of the
membrane, thereby inducing ferroptosis.
SLC7A11/reduced glutathione (GSH)/GPX4 signaling
axis is considered to be the cell death defense system. The theory
of ferroptosis was initially based on the findings of research on
this pathway (59,60). SLC7A11, also known as the system
Xc-, is a cystine/glutamate transporter that reverses transport of
proteins by mediating the exchange of intracellular glutamate with
extracellular cystine (61).
Cystine is exchanged with glutamate in the cell in a 1:1 ratio and
is rapidly reduced to cysteine, which is involved in the synthesis
of GSH and GPX4 within the cell (62). GSH is a key cofactor in GPX4
function, and its depletion disrupts cellular redox homeostasis,
leading to accumulation of ROS and ultimately inducing the onset of
ferroptosis. Therefore, inhibiting the expression of SLC7A11 can
induce ferroptosis. Moreover, GPX4 is a key regulator of
ferroptosis. The basic function of the enzymes in the GPX family
members is to reduce H2O2 at the expense of GSH. GPX4 is the only
enzyme that reduces cholesterol, hydrogen peroxide and oxidized
fatty acids. GPX4 can convert reduced GSH to oxidized glutathione
and lipid hydrogen peroxide (L-H2O2, toxic)
to lipid alcohols (L-OH, non-toxic), thereby promoting the
decomposition of hydrogen peroxide and inhibiting ferroptosis
(63,64). This indicated that reduced GPX4
activity favors ferroptosis (65,66).
The SLC7A11/GPX4 axon-dependent system constitutes a ferroptosis
defense mechanism that maintains non-toxic lipid peroxides levels,
thus sustaining cellular viability.
(ii) The GCH1-BH4-DHFR axis inhibits ferroptosis.
Guanosine triphosphate cyclization hydrolase (GCH1) and
guanosine-5′-triphosphate are the rate-limiting enzymes involved in
the synthesis of tetrahydrobiopterin (BH4) (73). BH4, which is produced by
dihydrofolate reductase through the reduction of dihydrogen
biopterin (dihydrobiopterin, BH2), reduces the oxidation of
endogenous free radicals and protects the lipid membrane from
ferroptosis (74). The inhibition
of GCH1 expression reduces BH4, thereby oxidizing iron and
increasing GCH1 expression and BH4 synthesis, which inhibits
ferroptosis (75).
(iii) The dihydrogen orotic acid dehydrogenase
(DHODH)-CoQH 2 system inhibits ferroptosis by blocking
mitochondrial lipid peroxidation (76). DHODH is mainly located in the
mitochondrial membrane and inhibits ferroptosis through the
reduction of CoQ into CoQH2, thus reducing the production of
ROS.
DNA damage is one of the most important effects of
IR in cells. IR-induced damage may directly affect cell
proliferation and reduce the water content inside cells
(~80% of the cell is water) to produce ROS and indirectly
cause DNA damage (~60–70%) (77,78).
This is because IR causes cytoplasm damage and generates highly
active OH free radicals and other ROS, including O2 and
H2O2, which subsequently attack nucleic
acids, lipids and proteins (79,80).
Tumor cells are more susceptible to RT than normal cells owing to
the high replication rate of tumor cells and defects in the DNA
damage response (DDR) pathway (81,82).
DNA DSB is the most serious type of DNA damage. Cell death may
occur if a DSB is not repaired in a timely manner. In RT, the
absorption of IR by water leads to the generation of ROS, which
subsequently act on PUFA, leading to lipid peroxidation,
peroxidation of membrane phospholipid lipids, and ultimately,
ferroptosis (83). Therefore, RT
can inhibit tumour progression by inducing iron death in tumour
cells. It has been found that a variety of morphological features
associated with iron death, such as shrunken mitochondria,
increased mitochondrial membrane density and reduced mitochondrial
cristae, were observed in tumour cells eliminated by radiation,
including lung, breast, esophageal and ovarian cancers (84). Herrera et al (85) discussed the existing treatment of
ovarian tumours with RT and the mechanisms by which RT mobilizes
anticancer immunity. Lang et al (86) described iron death as a previously
unappreciated mechanism of action of RT. Finally, the study named
SLC7A11, a key regulator of iron mutations, as a mechanistic
determinant of the synergistic effect of RT and immunotherapy
(86). Furthermore, IR-induced
ACSL4 expression increases PUFA-PL biosynthesis, which together
with ROS, drives PUFA-PL peroxidation (PUFA-PL-OOH) and ferroptosis
(87). SLC7A11 reduces ferroptosis
by promoting the synthesis of GSH and reducing the production of
L-OOH (88). RT increases the
production of ROS, which can induce the activation the nuclear
factor erythrocyte 2 related factor 2 (Nrf2)-heme oxygenase 1
(HO-1) pathway. The role of the Nrf2/HO-1 pathway in ferroptosis is
bidirectional. A previous study showed that Nrf2 can activate
SLC7A11, inhibit ferroptosis, and reduce the radiosensitivity of
esophageal squamous cell carcinomas (89). Moreover, Wei et al (90) found that activation of the Nrf2/HO-1
pathway can increase Fe2+ levels in colorectal cancer
cells and induce ferroptosis. Induction of ferroptosis in tumor
cells is one way to enhance radiosensitivity. For example,
pancreatic and renal cell carcinomas are sensitive to RT (91), which may be related to their
dependence on cystine uptake (92).
Inhibition of SLC7A11 and promotion of ferroptosis can increase the
radiosensitivity of esophageal squamous cell carcinomas (93). A previous study found that IR may
induce ferroptosis in tumor cells. This is because RT induces an
increase in siderophiles, which can increase the sensitivity of
tumor cells to RT.
RT for DSB is the most effective method of damaging
and eliminating cancer cells; however, the intrinsic efficiency of
tumor cells in DNA damage repair may lead to cellular resistance
and impair therapeutic outcomes. Genes and proteins involved in DSB
repair are targets of cancer therapy because their alteration,
interaction, translocation and regulation can affect the repair
process and render tumor cells more sensitive to RT. Therefore,
targeting DNA damage repair as a means of sensitizing cancer cells
to RT is a promising strategy for precise and effective treatment
of patients with cancer.
RT can eliminate tumor cells to a certain extent and
remains one of the most effective non-surgical treatments for
numerous tumors. However, reduced effects of RT on tumor cells is
usually unavoidable because of RT resistance (RR), which is the
reduction in the effectiveness of antitumor treatment (94). Tumor cells may show increased
expression of antioxidant defense system-related proteins and
ferroptosis to control the RT-induced abnormal increase in lipid
peroxides, which leads to ferroptosis and RR (95,96).
RR can lead to tumor recurrence, poor treatment response, poor
prognosis, decreased quality of life and an increased treatment
burden. Therefore, increased radiation sensitivity helps to reduce
the incidence of adverse reactions through RT-induced tumor cell
death.
IR-induced radiobiological effects are closely
associated with ferroptosis. IR can induce ferroptosis in tumor
cells, and the ROS produced by ferroptosis are involved in the
regulation of tumor cell radiosensitivity and the tumor
microenvironment (TME) (97). Local
hypoxia and inherent or adaptive RR of tumor cells may lead to
decreased radiosensitivity. Some KEAP1 mutant tumors rely on
ferroptosis defense mechanisms, such as adaptive upregulation of
FSP1/CoQ and inhibition of PUFA-PL synthesis, to avoid IR-induced
ferroptosis, and thus develop RR (98). Hypoxia has long been recognized as a
key regulator of RR. Due to impaired ribonucleotide reductase
activity at low oxygen concentrations, reduced nucleotide levels
lead to accumulation of single-stranded DNA at stalled replication
forks and under replication stress. At the same time, hypoxic
environments can also lead to a DDR that activates ATR-mediated and
ATM-mediated downstream targets such as p53, H2AX and CHK-1/2
(99,100). Subsequently, this downstream
signalling can shift cells to a less radiosensitive phase by
inducing cell cycle arrest (101,102). Besides, RT-induced expression of
SLC7A11 and GPX4 contributes to RR as an adaptive response that
protects cells from ferroptosis. Therefore, depletion of SCL7A11
and GPX4 can induce ferroptosis and achieve radiosensitization
(103,104). Chen et al (105) reported that suppressor of cytokine
signaling 2 (SOCS2), a potential prognostic predictor of RT,
promotes ferroptosis and increases the radiosensitivity of tumor
cells by increasing the ubiquitination and degradation of SLC7A11.
Thus, SOCS2 can promote the radiosensitization of tumor cells in
vivo and in vitro (105).
Inactivation of ACSL4 impairs the biosynthesis of
PUFA-PL, which in turn causes RR. Ferroptosis inducers (FINs), such
as erastin, FIN and sulfasalazine, block the activation of the
ferroptosis defense system, increase total intracellular iron
content, promote ROS production, reduce glutathione concentration,
and increase lipid peroxidation in radioresistant tumor cells,
thereby enhancing the radiosensitivity of the cells by inducing
ferroptosis (106–108). A previous study demonstrated that
FINs have a synergistic effect in tumor treatment (109). Class I FINs targeting SLC7A11,
such as erastin and sulfasalazine (SAS); class II FINs targeting
GPX 4, such as RSL3 and ML162; and class III FINs depleting CoQ and
GPX 4, such as FIN 56, can induce tumor sensitivity to RT in
vitro (110).
In radioresistant tumor cells, activation of the
ferroptosis execution system or inhibition of the ferroptosis
defense system can further enhance ferroptosis and inhibit the
development of RR. Therefore, further research on the mechanisms
related to ferroptosis are needed to clarify how to maximize the
antitumor effects of targeted ferroptosis combined with radiation
while minimizing damage to normal tissues.
RT can eliminate tumor cells and activate antitumor
immunity. The antitumor immune system activated by RT can further
induce ferroptosis in tumor cells and inhibit tumor development.
Jhunjhunwala et al found that dendritic cells are the most
important antigen-presenting cells that can ingest, process and
present antigens and activate CD8+ T cells (111). Activation of CD4+ T
cells releases interferon gamma (IFN gamma) and activates the
system Xc-, thereby promoting tumor lipid peroxidation and cell
death (111,112). The combination of RT and some
drugs can significantly induce ferroptosis in tumor cells compared
with single drug therapy, leading to a considerable increase in the
number of immune cells in the tumor tissue (113).
Cisplatin is a platinum-based drug commonly used as
a first-line chemotherapeutic agent for the treatment of solid
tumors. Owing to its wide antitumor spectrum and high curative
effect, cisplatin is recommended by the World Health Organization
for the treatment of cancer. Cisplatin can bind with guanine
residues induced between multiple chain and chain stated, a
crosslinking that leads to rapid cell death (114). In addition, Ma et al
(115) found that iron-oxide
nanocarriers enhance the anticancer efficacy of cisplatin and
simultaneously reduce toxicity caused by generation of ROS.
Cisplatin can produce H2O2 through a cascade
in the cytoplasm of the cells in the TME.
H2O2 can be further catalyzed by ferric iron
ions into toxic hydroxyl free radicals generated by Fenton's
reaction, leading to tumor cell apoptosis and ferroptosis (115).
Ferroptosis induced by the tumor suppressor p53
inhibits tumor development. The induction of p53 in the presence of
lipid peroxidation may eliminate cells stressed by ferroptosis.
Ferroptosis can induce cell death by releasing PUFA into the
extracellular environment or by driving the expression of enzymes
that stimulate PUFA-PL synthesis, such as LPCAT3 and ACSL4
(116). Sulfasalazine can inhibit
the expression of glutathione by downregulating SLC7A11, thereby
inactivating GPX4, causing ROS accumulation, and inducing
ferroptosis to play a role as a tumor suppressor (117).
Ferroptosis is a newly discovered mode of programmed
cell death that is closely related to RT and combined
immunotherapy. Drugs can play a role in tumor suppression by
inducing ferroptosis in tumor cells. The underlying mechanism
involves inducing ferroptosis in tumor cells in a variety of ways
and eventually inhibiting tumor progression.
HNSCC is the most common malignant head and neck
tumor, with recurrence and metastasis rates of >65% and a
five-year survival rate of <50% (118). The combination of surgery and RT
has increased the survival rate for HNSCC over the past 20 years.
However, the first-line therapeutic agents used for HNSCC, such as
platinum-based agents, 5-fluorouracil, polyene paclitaxel, and
cetuximab, have little effect on most patients. Therefore,
effective treatment of patients with HNSCC to improve their quality
of life and prognosis remains a challenge.
RT currently plays an important role in the
treatment of HNSCC. Continuous improvement in RT technology will
allow for more accurate dosing and mapping of the radiation area in
patients with HNSCC. However, there are still certain limitations:
How to make the radiation dose received by HNSCC more precise and
reduce the damage to the surrounding normal tissues needs further
exploration. In addition, RT resistance is a major barrier to
improving the survival benefit of HNSCC treatment; therefore,
methods to reduce RT resistance need to be explored. Ferroptosis
plays a key role in radiation-induced cell death. Induction of
ferroptosis can enhance the radiosensitivity of tumor cells by
inducing iron overload and lipid peroxidation, thereby maintaining
the efficacy of RT.
Research on ferroptosis will help solve the major
problems associated with HNSCC treatment and identify novel
therapeutic targets and strategies for the diagnosis and clinical
treatment of HNSCC. In the future, continuous research shall be
conducted by the authors and improved experimental protocols will
be utilized to further explore the effects of RT and other combined
immunotherapies to improve the prognosis of patients with HNSCC and
minimize treatment-related side effects.
Not applicable.
Funding: No funding was received.
Not applicable.
YF and XL wrote the manuscript. BY and ML were
responsible for gathering the associated research and designing the
review. YD, JW and SL collected and analyzed data. LGa, LGo and LL
contributed to the study design, interpretation of the research
articles, editing and critical revision of the manuscript. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare they have no competing
interests.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vousden KH and Lane DP: p53 in health and
disease. Nat Rev Mol Cell Biol. 8:275–283. 2007. View Article : Google Scholar
|
|
3
|
Gleber-Netto FO, Zhao M, Trivedi S, Wang
J, Jasser S, McDowell C, Kadara H, Zhang J, Wang J, William WN Jr,
et al: Distinct pattern of TP53 mutations in human immunodeficiency
virus-related head and neck squamous cell carcinoma. Cancer.
124:84–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kandoth C, McLellan MD, Vandin F, Ye K,
Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al:
Mutational landscape and significance across 12 major cancer types.
Nature. 502:333–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berkers CR, Maddocks OD, Cheung EC, Mor I
and Vousden KH: Metabolic regulation by p53 family members. Cell
Metab. 18:617–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldstein I and Rotter V: Regulation of
lipid metabolism by p53-fighting two villains with one sword.
Trends Endocrinol Metab. 23:567–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saraniti C, Speciale R, Santangelo M,
Massaro N, Maniaci A, Gallina S, Serra A and Cocuzza S: Functional
outcomes after supracricoid modified partial laryngectomy. J Biol
Regul Homeost Agents. 33:1903–1907. 2019.PubMed/NCBI
|
|
9
|
Nissi L, Suilamo S, Kytö E, Vaittinen S,
Irjala H and Minn H: Recurrence of head and neck squamous cell
carcinoma in relation to high-risk treatment volume. Clin Transl
Radiat Oncol. 27:139–146. 2021.
|
|
10
|
Ma L, Men Y, Feng L, Kang J, Sun X, Yuan
M, Jiang W and Hui Z: A current review of dose-escalated
radiotherapy in locally advanced non-small cell lung cancer. Radiol
Oncol. 53:6–14. 2019. View Article : Google Scholar
|
|
11
|
Chen X, Comish PB, Tang D and Kang R:
Characteristics and biomarkers of ferroptosis. Front Cell Dev Biol.
9:6371622021. View Article : Google Scholar
|
|
12
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma S, Henson ES, Chen Y and Gibson SB:
Ferroptosis is induced following siramesine and lapatinib treatment
of breast cancer cells. Cell Death Dis. 7:e23072016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun X, Niu X, Chen R, He W, Chen D, Kang R
and Tang D: Metallothionein-1G facilitates sorafenib resistance
through inhibition of ferroptosis. Hepatology. 64:488–500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C,
Dai X, Li Z and Wu G: Ferroptosis: A novel anti-tumor action for
cisplatin. Cancer Res Treat. 50:445–460. 2018. View Article : Google Scholar
|
|
16
|
Shi Y, Wei W, Li L, Wei Q, Jiang F, Xia G
and Yu H: The global status of research in breast cancer liver
metastasis: A bibliometric and visualized analysis. Bioengineered.
12:12246–12262. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schaue D and McBride WH: Opportunities and
challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol.
12:527–540. 2015. View Article : Google Scholar
|
|
18
|
Sierko E, Hempel D, Zuzda K and
Wojtukiewicz MZ: Personalized radiation therapy in cancer pain
management. Cancers (Basel). 11:3902019. View Article : Google Scholar
|
|
19
|
Stevens S, Moloney S, Blackmore A, Hart C,
Rixham P, Bangiri A, Pooler A and Doolan P: IPEM topical report:
Guidance for the clinical implementation of online treatment
monitoring solutions for IMRT/VMAT. Phys Med Biol.
68:10.1088/1361–6560/acecd0. 2023. View Article : Google Scholar
|
|
20
|
Gao S, Xu Q, Lan Y and He L: Recurrent
trichilemmal carcinoma of the periorbital region treated with IMRT
radiotherapy: A case report and a review of literature. Medicine
(Baltimore). 102:e340382023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Machiels JP, René Leemans C, Golusinski W,
Grau C, Licitra L and Gregoire V; EHNS Executive Board: ESMO
Guidelines Committee, : ESTRO Executive Board: Reprint of ‘Squamous
cell carcinoma of the oral cavity, larynx, oropharynx and
hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for
diagnosis, treatment and follow-up’. Oral Oncol. 113:1050422021.
View Article : Google Scholar
|
|
22
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the Nomenclature Committee on Cell Death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang D, Kang R, Berghe TV, Vandenabeele P
and Kroemer G: The molecular machinery of regulated cell death.
Cell Res. 29:347–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al: Classification of cell death:
Recommendations of the Nomenclature committee on cell death 2009.
Cell Death Differ. 16:3–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Povirk LF: Biochemical mechanisms of
chromosomal translocations resulting from DNA double-strand breaks.
DNA Repair (Amst). 5:1199–1212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mladenov E, Magin S, Soni A and Iliakis G:
DNA double-strand break repair as determinant of cellular
radiosensitivity to killing and target in radiation therapy. Front
Oncol. 3:1132013. View Article : Google Scholar
|
|
27
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brizel DM and Esclamado R: Concurrent
chemoradiotherapy for locally advanced, nonmetastatic, squamous
carcinoma of the head and neck: Consensus, controversy, and
conundrum. J Clin Oncol. 24:2612–2617. 2006. View Article : Google Scholar
|
|
29
|
Ghosh C, Luong G and Sun Y: A snapshot of
the PD-1/PD-L1 pathway. J Cancer. 12:2735–2746. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferris RL and Licitra L: PD-1
immunotherapy for recurrent or metastatic HNSCC. Lancet.
394:1882–1884. 2019. View Article : Google Scholar
|
|
31
|
Seiwert TY, Burtness B, Mehra R, Weiss J,
Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et
al: Safety and clinical activity of pembrolizumab for treatment of
recurrent or metastatic squamous cell carcinoma of the head and
neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial.
Lancet Oncol. 17:956–965. 2016. View Article : Google Scholar
|
|
32
|
Cohen EEW, Soulières D, Le Tourneau C,
Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R,
et al: Pembrolizumab versus methotrexate, docetaxel, or cetuximab
for recurrent or metastatic head-and-neck squamous cell carcinoma
(KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet.
393:156–167. 2019. View Article : Google Scholar
|
|
33
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar
|
|
34
|
Haddad RI, Harrington K, Tahara M, Ferris
RL, Gillison M, Fayette J, Daste A, Koralewski P, Zurawski B,
Taberna M, et al: Nivolumab plus ipilimumab versus EXTREME Regimen
as First-Line treatment for Recurrent/Metastatic squamous cell
carcinoma of the head and neck: The final results of CheckMate 651.
J Clin Oncol. 41:2166–2180. 2023. View Article : Google Scholar
|
|
35
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE,
Even C, et al: Nivolumab vs investigator's choice in recurrent or
metastatic squamous cell carcinoma of the head and neck: 2-year
long-term survival update of CheckMate 141 with analyses by tumor
PD-L1 expression. Oral Oncol. 81:45–51. 2018. View Article : Google Scholar
|
|
37
|
Wise-Draper TM, Gulati S, Palackdharry S,
Hinrichs BH, Worden FP, Old MO, Dunlap NE, Kaczmar JM, Patil Y,
Riaz MK, et al: Phase II clinical trial of neoadjuvant and adjuvant
pembrolizumab in resectable local-regionally advanced head and neck
squamous cell carcinoma. Clin Cancer Res. 28:1345–1352. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Druker BJ: David A. Karnofsky Award
lecture. Imatinib as a paradigm of targeted therapies. J Clin
Oncol. 21 (23 Suppl):239S–245S. 2003. View Article : Google Scholar
|
|
39
|
Xu MJ, Johnson DE and Grandis JR:
EGFR-targeted therapies in the post-genomic era. Cancer Metastasis
Rev. 36:463–473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Parmar K, Mohamed A, Vaish E, Thawani R,
Cetnar J and Thein KZ: Immunotherapy in head and neck squamous cell
carcinoma: An updated review. Cancer Treat Res Commun.
33:1006492022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dougan M and Dranoff G: Immune therapy for
cancer. Annu Rev Immunol. 27:83–117. 2009. View Article : Google Scholar
|
|
42
|
Correale P, Botta C, Cusi MG, Del Vecchio
MT, De Santi MM, Gori Savellini G, Bestoso E, Apollinari S,
Mannucci S, Marra M, et al: Cetuximab ± chemotherapy enhances
dendritic cell-mediated phagocytosis of colon cancer cells and
ignites a highly efficient colon cancer antigen-specific cytotoxic
T-cell response in vitro. Int J Cancer. 130:1577–1589. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maréchal R, De Schutter J, Nagy N,
Demetter P, Lemmers A, Devière J, Salmon I, Tejpar S and Van
Laethem JL: Putative contribution of CD56 positive cells in
cetuximab treatment efficacy in first-line metastatic colorectal
cancer patients. BMC Cancer. 10:3402010. View Article : Google Scholar
|
|
44
|
Dechant M, Weisner W, Berger S, Peipp M,
Beyer T, Schneider-Merck T, Lammerts van Bueren JJ, Bleeker WK,
Parren PW, van de Winkel JG and Valerius T: Complement-dependent
tumor cell lysis triggered by combinations of epidermal growth
factor receptor antibodies. Cancer Res. 68:4998–5003. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hsu YF, Ajona D, Corrales L, Lopez-Picazo
JM, Gurpide A, Montuenga LM and Pio R: Complement activation
mediates cetuximab inhibition of non-small cell lung cancer tumor
growth in vivo. Mol Cancer. 9:1392010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar
|
|
47
|
Wang Y, Wang Y, Pan J, Gan L and Xue J:
Ferroptosis, necroptosis, and pyroptosis in cancer: Crucial cell
death types in radiotherapy and post-radiotherapy immune
activation. Radiother Oncol. 184:1096892023. View Article : Google Scholar
|
|
48
|
Stockwell BR, Jiang X and Gu W: Emerging
mechanisms and disease relevance of ferroptosis. Trends Cell Biol.
30:478–490. 2020. View Article : Google Scholar
|
|
49
|
Liang W and Ferrara N: Iron metabolism in
the tumor microenvironment: Contributions of innate immune cells.
Front Immunol. 11:6268122021. View Article : Google Scholar
|
|
50
|
Galaris D, Barbouti A and Pantopoulos K:
Iron homeostasis and oxidative stress: An intimate relationship.
Biochim Biophys Acta Mol Cell Res. 1866:1185352019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
He YJ, Liu XY, Xing L, Wan X, Chang X and
Jiang HL: Fenton reaction-independent ferroptosis therapy via
glutathione and iron redox couple sequentially triggered lipid
peroxide generator. Biomaterials. 241:1199112020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zheng J and Conrad M: The metabolic
underpinnings of ferroptosis. Cell Metab. 32:920–937. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roh JL, Kim EH, Jang HJ, Park JY and Shin
D: Induction of ferroptotic cell death for overcoming cisplatin
resistance of head and neck cancer. Cancer Lett. 381:96–103. 2016.
View Article : Google Scholar
|
|
54
|
Roh JL, Kim EH, Jang H and Shin D: Nrf2
inhibition reverses the resistance of cisplatin-resistant head and
neck cancer cells to artesunate-induced ferroptosis. Redox Biol.
11:254–262. 2017. View Article : Google Scholar
|
|
55
|
Kim EH, Shin D, Lee J, Jung AR and Roh JL:
CISD2 inhibition overcomes resistance to sulfasalazine-induced
ferroptotic cell death in head and neck cancer. Cancer Lett.
432:180–190. 2018. View Article : Google Scholar
|
|
56
|
Lin R, Zhang Z, Chen L, Zhou Y, Zou P,
Feng C, Wang L and Liang G: Dihydroartemisinin (DHA) induces
ferroptosis and causes cell cycle arrest in head and neck carcinoma
cells. Cancer Lett. 381:165–175. 2016. View Article : Google Scholar
|
|
57
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View Article : Google Scholar
|
|
60
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS
and Stockwell BR: Pharmacological inhibition of cystine-glutamate
exchange induces endoplasmic reticulum stress and ferroptosis.
Elife. 3:e025232014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sato H, Tamba M, Ishii T and Bannai S:
Cloning and expression of a plasma membrane cystine/glutamate
exchange transporter composed of two distinct proteins. J Biol
Chem. 274:11455–11458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar
|
|
63
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar
|
|
64
|
Shi JF, Liu Y, Wang Y, Gao R, Wang Y and
Liu J: Targeting ferroptosis, a novel programmed cell death, for
the potential of alcohol-related liver disease therapy. Front
Pharmacol. 14:11943432023. View Article : Google Scholar
|
|
65
|
Seibt TM, Proneth B and Conrad M: Role of
GPX4 in ferroptosis and its pharmacological implication. Free Radic
Biol Med. 133:144–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Maiorino M, Conrad M and Ursini F: GPx4,
Lipid peroxidation, and cell death: Discoveries, rediscoveries, and
open issues. Antioxid Redox Signal. 29:61–74. 2018. View Article : Google Scholar
|
|
67
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wu M, Xu LG, Li X, Zhai Z and Shu HB:
AMID, an apoptosis-inducing factor-homologous
mitochondrion-associated protein, induces caspase-independent
apoptosis. J Biol Chem. 277:25617–25623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Marshall KR, Gong M, Wodke L, Lamb JH,
Jones DJ, Farmer PB, Scrutton NS and Munro AW: The human
apoptosis-inducing protein AMID is an oxidoreductase with a
modified flavin cofactor and DNA binding activity. J Biol Chem.
280:30735–30740. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Elguindy MM and Nakamaru-Ogiso E:
Apoptosis-inducing factor (AIF) and its family member protein,
AMID, are rotenone-sensitive NADH:Ubiquinone oxidoreductases
(NDH-2). J Biol Chem. 290:20815–20826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shimada K, Skouta R, Kaplan A, Yang WS,
Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ and
Stockwell BR: Global survey of cell death mechanisms reveals
metabolic regulation of ferroptosis. Nat Chem Biol. 12:497–503.
2016. View Article : Google Scholar
|
|
73
|
Xu J, Wu Y, Song P, Zhang M, Wang S and
Zou MH: Proteasome-dependent degradation of guanosine
5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin
deficiency in diabetes mellitus. Circulation. 116:944–953. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Soula M, Weber RA, Zilka O, Alwaseem H, La
K, Yen F, Molina H, Garcia-Bermudez J, Pratt DA and Birsoy K:
Metabolic determinants of cancer cell sensitivity to canonical
ferroptosis inducers. Nat Chem Biol. 16:1351–1360. 2020. View Article : Google Scholar
|
|
75
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP Cyclohydrolase
1/Tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar
|
|
76
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ward JF: DNA damage produced by ionizing
radiation in mammalian cells: Identities, mechanisms of formation,
and reparability. Prog Nucleic Acid Res Mol Biol. 35:95–125. 1988.
View Article : Google Scholar
|
|
78
|
Santivasi WL and Xia F: Ionizing
radiation-induced DNA damage, response, and repair. Antioxid Redox
Signal. 21:251–259. 2014. View Article : Google Scholar
|
|
79
|
Azzam EI, Jay-Gerin JP and Pain D:
Ionizing radiation-induced metabolic oxidative stress and prolonged
cell injury. Cancer Lett. 327:48–60. 2012. View Article : Google Scholar
|
|
80
|
Reisz JA, Bansal N, Qian J, Zhao W and
Furdui CM: Effects of ionizing radiation on biological
molecules-mechanisms of damage and emerging methods of detection.
Antioxid Redox Signal. 21:260–292. 2014. View Article : Google Scholar
|
|
81
|
Adjemian S, Oltean T, Martens S, Wiernicki
B, Goossens V, Van den Berghe T, Cappe B, Ladik M, Riquet FB,
Heyndrickx L, et al: Ionizing radiation results in a mixture of
cellular outcomes including mitotic catastrophe, senescence,
methuosis, and iron-dependent cell death. Cell Death Dis.
11:10032020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Srinivas US, Tan BWQ, Vellayappan BA and
Jeyasekharan AD: ROS and the DNA damage response in cancer. Redox
Biol. 25:1010842019. View Article : Google Scholar
|
|
83
|
Yang WS, Kim KJ, Gaschler MM, Patel M,
Shchepinov MS and Stockwell BR: Peroidation of polyunsaturated
fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci
USA. 113:E4966–E4975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lei G, Mao C, Yan Y, Zhuang L and Gan B:
Ferroptosis, radiotherapy, and combination therapeutic strategies.
Protein Cell. 12:836–857. 2021. View Article : Google Scholar
|
|
85
|
Herrera FG, Irving M, Kandalaft LE and
Coukos G: Rational combinations of immunotherapy with radiotherapy
in ovarian cancer. Lancet Oncol. 20:e417–e433. 2019. View Article : Google Scholar
|
|
86
|
Lang X, Green MD, Wang W, Yu J, Choi JE,
Jiang L, Liao P, Zhou J, Zhang Q, Dow A, et al: Radiotherapy and
immunotherapy promote tumoral lipid oxidation and ferroptosis via
synergistic repression of SLC7A11. Cancer Discov. 9:1673–1685.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar
|
|
88
|
Wang H, An P, Xie E, Wu Q, Fang X, Gao H,
Zhang Z, Li Y, Wang X, Zhang J, et al: Characterization of
ferroptosis in murine models of hemochromatosis. Hepatology.
66:449–465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dong H, Qiang Z, Chai D, Peng J, Xia Y, Hu
R and Jiang H: Nrf2 inhibits ferroptosis and protects against acute
lung injury due to intestinal ischemia reperfusion via regulating
SLC7A11 and HO-1. Aging (Albany NY). 12:12943–12959. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wei R, Zhao Y, Wang J, Yang X, Li S, Wang
Y, Yang X, Fei J, Hao X, Zhao Y, et al: Tagitinin C induces
ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal
cancer cells. Int J Biol Sci. 17:2703–2717. 2021. View Article : Google Scholar
|
|
91
|
Bump EA and Brown JM: Role of glutathione
in the radiation response of mammalian cells in vitro and in vivo.
Pharmacol Ther. 47:117–136. 1990. View Article : Google Scholar
|
|
92
|
Zou Y and Schreiber SL: Progress in
understanding ferroptosis and challenges in its targeting for
therapeutic benefit. Cell Chem Biol. 27:463–471. 2020. View Article : Google Scholar
|
|
93
|
Feng L, Zhao K, Sun L, Yin X, Zhang J, Liu
C and Li B: SLC7A11 regulated by NRF2 modulates esophageal squamous
cell carcinoma radiosensitivity by inhibiting ferroptosis. J Transl
Med. 19:3672021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Steinbichler TB, Dudás J, Skvortsov S,
Ganswindt U, Riechelmann H and Skvortsova II: Therapy resistance
mediated by exosomes. Mol Cancer. 18:582019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang W, Sun Y, Bai L, Zhi L, Yang Y, Zhao
Q, Chen C, Qi Y, Gao W, He W, et al: RBMS1 regulates lung cancer
ferroptosis through translational control of SLC7A11. J Clin
Invest. 131:e1520672021. View Article : Google Scholar
|
|
96
|
Yang M, Wu X, Hu J, Wang Y, Wang Y, Zhang
L, Huang W, Wang X, Li N, Liao L, et al: COMMD10 inhibits HIF1α/CP
loop to enhance ferroptosis and radiosensitivity by disrupting
Cu-Fe balance in hepatocellular carcinoma. J Hepatol. 76:1138–1150.
2022. View Article : Google Scholar
|
|
97
|
Zheng Z, Su J, Bao X, Wang H, Bian C, Zhao
Q and Jiang X: Mechanisms and applications of radiation-induced
oxidative stress in regulating cancer immunotherapy. Front Immunol.
14:12472682023. View Article : Google Scholar
|
|
98
|
Koppula P, Lei G, Zhang Y, Yan Y, Mao C,
Kondiparthi L, Shi J, Liu X, Horbath A, Das M, et al: A targetable
CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1
inactive lung cancers. Nat Commun. 13:22062022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Olcina MM, Grand RJ and Hammond EM: ATM
activation in hypoxia-causes and consequences. Mol Cell Oncol.
1:e299032014. View Article : Google Scholar
|
|
100
|
Fallone F, Britton S, Nieto L, Salles B
and Muller C: ATR controls cellular adaptation to hypoxia through
positive regulation of hypoxia-inducible factor 1 (HIF-1)
expression. Oncogene. 32:4387–4396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Olcina M, Lecane PS and Hammond EM:
Targeting hypoxic cells through the DNA damage response. Clin
Cancer Res. 16:5624–5629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hammond EM, Asselin MC, Forster D,
O'Connor JP, Senra JM and Williams KJ: The meaning, measurement and
modification of hypoxia in the laboratory and the clinic. Clin
Oncol (R Coll Radiol). 26:277–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Xie L, Song X, Yu J, Guo W, Wei L, Liu Y
and Wang X: Solute carrier protein family may involve in
radiation-induced radioresistance of non-small cell lung cancer. J
Cancer Res Clin Oncol. 137:1739–1747. 2011. View Article : Google Scholar
|
|
104
|
Pan X, Lin Z, Jiang D, Yu Y, Yang D, Zhou
H, Zhan D, Liu S, Peng G, Chen Z and Yu Z: Erastin decreases
radioresistance of NSCLC cells partially by inducing GPX4-mediated
ferroptosis. Oncol Lett. 17:3001–3008. 2019.
|
|
105
|
Chen Q, Zheng W, Guan J, Liu H, Dan Y, Zhu
L, Song Y, Zhou Y, Zhao X, Zhang Y, et al: SOCS2-enhanced
ubiquitination of SLC7A11 promotes ferroptosis and
radiosensitization in hepatocellular carcinoma. Cell Death Differ.
30:137–151. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ivanov SD, Semenov AL, Kovan'ko EG and
Yamshanov VA: Effects of iron ions and iron chelation on the
efficiency of experimental radiotherapy of animals with gliomas.
Bull Exp Biol Med. 158:800–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang Z, Lu M, Chen C, Tong X, Li Y, Yang
K, Lv H, Xu J and Qin L: Holo-lactoferrin: The link between
ferroptosis and radiotherapy in triple-negative breast cancer.
Theranostics. 11:3167–3182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shibata Y, Yasui H, Higashikawa K,
Miyamoto N and Kuge Y: Erastin, a ferroptosis-inducing agent,
sensitized cancer cells to X-ray irradiation via glutathione
starvation in vitro and in vivo. PLoS One. 14:e02259312019.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ye LF, Chaudhary KR, Zandkarimi F, Harken
AD, Kinslow CJ, Upadhyayula PS, Dovas A, Higgins DM, Tan H, Zhang
Y, et al: Radiation-induced lipid peroxidation triggers ferroptosis
and synergizes with ferroptosis inducers. ACS Chem Biol.
15L:469–484. 2020. View Article : Google Scholar
|
|
110
|
Lei G, Zhang Y, Koppula P, Liu X, Zhang J,
Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H and Gan B: The role of
ferroptosis in ionizing radiation-induced cell death and tumor
suppression. Cell Res. 30:146–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Jhunjhunwala S, Hammer C and Delamarre L:
Antigen presentation in cancer: Insights into tumour immunogenicity
and immune evasion. Nat Rev Cancer. 21:298–312. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wang W, Green M, Choi JE, Gijón M, Kennedy
PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al: CD8+T
cells regulate tumour ferroptosis during cancer immunotherapy.
Nature. 569:270–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Xu L and Fan W: Efficacy of sorafenib
combined with radiofrequency ablation in renal cancer and its
effects on immunity and inflammation in patients. J BUON.
25:514–519. 2020.PubMed/NCBI
|
|
114
|
Li S, Liu Y, Li J, Zhao X and Yu D:
Mechanisms of Ferroptosis and application to head and neck squamous
cell carcinoma treatments. DNA Cell Biol. 40:720–732. 2021.
View Article : Google Scholar
|
|
115
|
Ma P, Xiao H, Yu C, Liu J, Cheng Z, Song
H, Zhang X, Li C, Wang J, Gu Z and Lin J: Enhanced cisplatin
chemotherapy by iron oxide nanocarrier-mediated generation of
highly toxic reactive oxygen species. Nano Lett. 17:928–937. 2017.
View Article : Google Scholar
|
|
116
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar
|
|
117
|
Gout PW, Buckley AR, Simms CR and
Bruchovsky N: Sulfasalazine, a potent suppressor of lymphoma growth
by inhibition of the x(c)-cystine transporter: A new action for an
old drug. Leukemia. 15:1633–1640. 2001. View Article : Google Scholar
|
|
118
|
Liang F, Wang R, Du Q and Zhu S: An
Epithelial-mesenchymal transition hallmark gene-based risk score
system in head and neck squamous-cell carcinoma. Int J Gen Med.
14:4219–4227. 2021. View Article : Google Scholar : PubMed/NCBI
|