Introduction
Breast cancer is one of the most common malignancies
among women worldwide and one of the most common causes of
cancer-related mortality among women in both developed and
developing countries (1).
According to the GLOBOCAN 2020 data, >2 million cases and
~685,000 deaths were registered in 2020 globally (2). In Asia, the incidence of new breast
cancer cases was 1,026,171 and the associated mortality rate was
346,009 cases. In India, there were 178,361 new breast cancer cases
(26.3%), and the mortality rate was 90,408 cases (21.9%). Owing to
the increasing incidence of female breast cancer (11.7%), it has
surpassed lung cancer as the most common type of cancer worldwide
(11.4%), followed by lung (11.4%) and colorectal (10.0%) cancer.
The age-standardized incidence rate of invasive breast cancer in
women in Asia was 36.8 per 100,000, whereas in Western populations,
such as North America and Europe, it was 89.4 and 74.3 per 100,000
women, respectively, which is ~50% of that in the Western
population (2).
The incidence of breast cancer is higher in
developing countries than in Western countries; however, globally,
there has been a change in the prevalence of breast cancer among
women in South America, Africa and Asia (3). GLOBOCAN has estimated that the
incidence of breast cancer will double by the year 2050(4).
Clinical and pathological examinations play crucial
roles in the diagnosis and understanding of complex diseases, such
as breast cancer. However, the published literature reveals that
there is a paucity of epidemiological data on breast cancer in
India (5). Variations are noted in
the distribution of breast cancer subtypes between the Indian
(6-10)
and Western populations (11-15).
The prognosis of patients with breast cancer is
dependent on various factors, such as the tumor histological type,
grade, lymph node involvement, hormonal receptor [estrogen receptor
(ER), progesterone receptor (PR)] and human epidermal growth factor
receptor 2 (HER2) status and the proliferative index (6,11,16).
Over the past two decades, microarray-based gene
profiling and The Cancer Genome Atlas network have established
refined subtypes of breast cancer via the extensive profiling of
various protein levels, microRNAs and DNA (17).
Breast cancer exhibits diverse clinical and
molecular features. Therefore, on the basis of its molecular
characteristics, patients can be categorized into four groups as
follows: i) Luminal A (ER+, PR+ and
HER2- with a low Ki67 expression); ii) luminal B
(ER+, PR+,
HER2+/- and a high Ki67
expression); iii) HER2-enriched (ER-, PR- and
HER2+); iv) and triple-negative breast cancer (TNBC;
ER-, PR- and HER2-) (12,18).
Each molecular subtype of breast cancer has been
associated with a different prognosis, preferential metastatic
organs and response to therapy, as well as different recurrence or
disease-free survival rates (19,20).
One of the most distinct features of cancer is the
uncontrolled proliferation of cells, and all proliferating cells
exhibit the Ki67 antigen, which indicates that it is a key
biomarker for the estimation of cell proliferation. Hence, the rate
of cell proliferation can be estimated by assessing the Ki67
antigen using immunohistochemical techniques (21). Moreover, Ki67 plays a crucial role
in determining the relative prognosis of the disease, resistance to
chemotherapy or endocrine therapy, and the residual risk assessment
of patients receiving standard therapy. In addition, assessing
treatment efficacy, specifically that of endocrine therapy, is
pertinent for patients receiving neoadjuvant therapy (22).
Of the various molecular subtypes of breast cancer,
TNBC and HER2-enriched breast cancer are highly aggressive and are
associated with short survival periods. Although they respond well
to chemotherapy (23), patients
with TNBC with tumor-infiltrating lymphocytes have a better
prognosis and survival rate than those without
lymphocyte-infiltrating tumors (24). Thus, breast cancer lesions of the
same histological type may respond differently to therapy and
exhibit varying prognoses. Therefore, molecular characterization
has become a key factor in deciding the targeted therapy for
patients (12). However, the
prevalence of molecular subtypes of breast cancer has not been
studied extensively in developing countries. Furthermore, the fact
that tumor volume plays a critical prognostic role in breast cancer
should be considered. Hence, the present study was performed in an
aim to determine the distribution of molecular phenotypes of ductal
carcinoma of the breast in patients who presented to a tertiary
care hospital of Northern Delhi, India. In addition to the
molecular subtypes, clinicopathological factors, such as age and
tumor volume, were compared among the patients with the various
molecular phenotypes.
Patients and methods
The present study is an observational
cross-sectional study performed at the Department of Pathology of a
tertiary care medical college hospital in Delhi. The study was
conducted after obtaining approval from the Institutional Ethics
Committee, vide letter no. IEC/NDMC/2022/109 (dated June 17, 2022).
The present study conformed to the tenets of the Declaration of
Helsinki, and good clinical practice guidelines were followed.
Moreover, written informed consent was obtained from the patients
for their participation.
Patients
A total of 165 patients with breast cancer were
included in the present study. All women with infiltrating ductal
carcinoma-not otherwise specified (IDC-NOS) who presented from
June, 2022 to December, 2023 to the hospital were included in the
study. All males, patients with IDC-NOS for whom complete tumors
were not available, such as via needle biopsy, and those who
underwent surgery after neoadjuvant therapy were excluded from the
study.
Immunohistochemistry
The immunohistochemistry (IHC) staining procedure
was performed on formalin-fixed paraffin-embedded (FFPE) tissue
blocks utilizing an optimized IHC protocol. Sections of FFPE tissue
were sliced at a 4 µm thickness and subsequently dewaxed, followed
by rehydration in water. Heat-induced epitope retrieval was
performed using a domestic pressure cooker with retrieval buffer
(Tris-EDTA buffer, pH 9.0, Merck KGaA) for ER, PR, HER2, and Ki67.
The slides underwent a 10-min incubation at room temperature with
peroxidase-blocking solution (1% hydrogen peroxide), followed by a
wash with phosphate-buffered saline (PBS, pH 7.2-7.4).
The slides were then incubated for 1 h at room
temperature with specific ready-to-use mouse monoclonal primary
antibodies ER, PR, HER2 and Ki67 (cat. no. AN710-5ME, AN711-5ME,
AN471-5ME and AM297-5M, respectively; BioGenex), followed by
overnight incubation at 4-6˚C in a refrigerator. Subsequently, the
slides were washed with PBS solution, followed by a 30-min
incubation at room temperature with a secondary antibody (polymer
HRP; cat. no. HK595-50KN, BioGenex). Following another wash with
PBS solution, the DAB chromogen with substrate (cat. no.
HK124-025KN, BioGenex) was applied for 5 min. The slides were
counterstained with hematoxylin (Merck KGaA) for 30 sec at room
temperature and then mounted with DPX and a cover slip. Assessment
of the IHC stained sections was conducted using a compound light
microscope (Olympus Corporation) by an experienced pathologist.
All the studied patients were classified as luminal
A [ER+, PR+ and HER2 with a low (<14%)
Ki67 expression]; luminal B [ER+, PR+ and
HER2+/-, with a high (>14%)
Ki67 expression]; HER2-enriched (ER-, PR- and
HER2+); and TNBC (ER-, PR- and
HER2-). The tumor volume in cm3 was estimated
from the histological specimens of all patients included in the
study.
Statistical analysis
Statistical analyses were performed using the Python
language package V3.0 (https://www.python.org) and Jupyter V5.0 (https://jupyter.org) as the IDE for the Python
language. To assess the significance of differences in age and
tumor volume among the different molecular subtypes of breast
cancer, the Kruskal-Wallis (non-parametric) test was used. In the
case that the Kruskal-Wallis test result was significant, Dunn's
post hoc test was then performed. A P-value of <0.05 was
considered to indicate a statistically significant difference.
Results
All the cases were infiltrating ductal carcinoma,
NOS type. The age range of the patients was 25-75 years, with a
mean age of 47.1 years (SD ±11.5 years), and the median age was 45
years. The majority of the patients with breast cancer were in the
age group of 31-40 years (32.1%), followed by 41-50 years (26.1%),
51-60 years (23%), 61-70 years (10.9%), <30 years (6.1%) and
>70 years (1.8%) (Table I).
 | Table IAge distribution of the patients in
the present study. |
Table I
Age distribution of the patients in
the present study.
| Age group
(years) | Luminal A (n) | Luminal B (n) | HER2-enriched
(n) | TNBC (n) | Total (n) | Percentage |
|---|
| Up to 30 | 1 | 3 | 3 | 3 | 10 | 6.06 |
| 31-40 | 7 | 13 | 11 | 22 | 53 | 32.1 |
| 41-50 | 7 | 12 | 7 | 17 | 43 | 26.1 |
| 51-60 | 5 | 8 | 9 | 16 | 38 | 23.0 |
| 61-70 | 4 | 4 | 4 | 6 | 18 | 10.9 |
| >70 | 2 | 0 | 0 | 1 | 3 | 1.82 |
| Total | 26 | 40 | 34 | 65 | 165 | 100 |
The most common molecular phenotype was TNBC (65
cases, 39.4%) with a median age of 45 years (range, 25-71 years),
followed by luminal B (40 cases, 24.2%) with a median age of 45
years (range, 28-70 years), HER2-enriched (34 cases, 20.6%) with a
median age of 46 years (range, 25-70 years), and luminal A (26
cases, 15.8%) with a median age of 48.5 years (range, 30-75 years)
(Figs. 1 and 2). The difference in age among the
patients with different molecular subtypes of breast cancer was not
statistically significant (P=0.686, Kruskal-Wallis test; Table I).
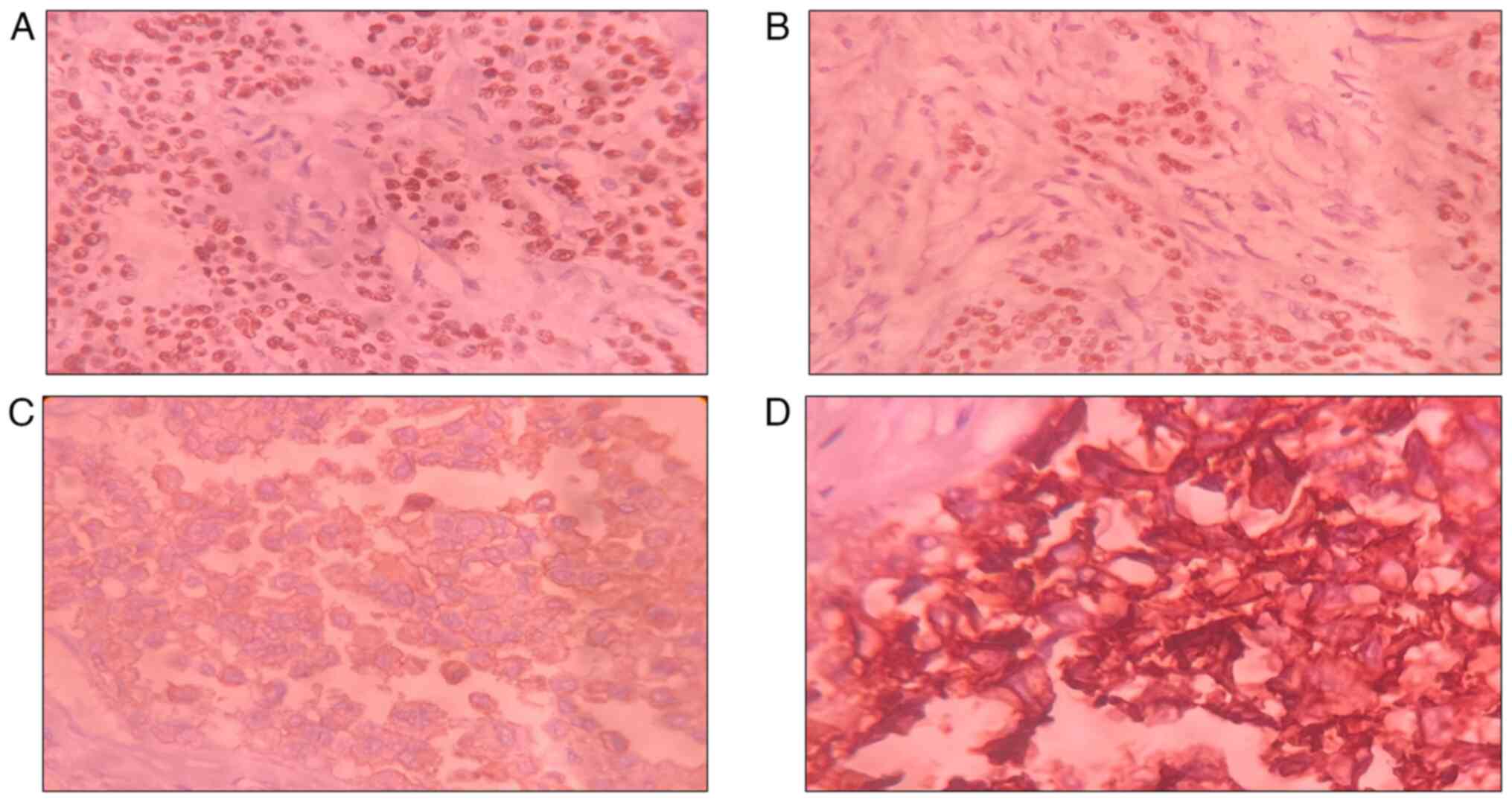 | Figure 2Photomicrographs from IHC illustrating
(A) estrogen receptor positivity (IHC for ER, DAB; magnification,
x40), (B) progesterone receptor positivity (IHC for PR, DAB;
magnification, x40), (C) HER2 positivity (2+) (IHC for HER2, DAB;
magnification, x40), and (D) HER2 positivity (3+) (IHC for HER,
DAB; magnification, x40). IHC, immunohistochemistry; HER2, human
epidermal growth factor receptor 2. |
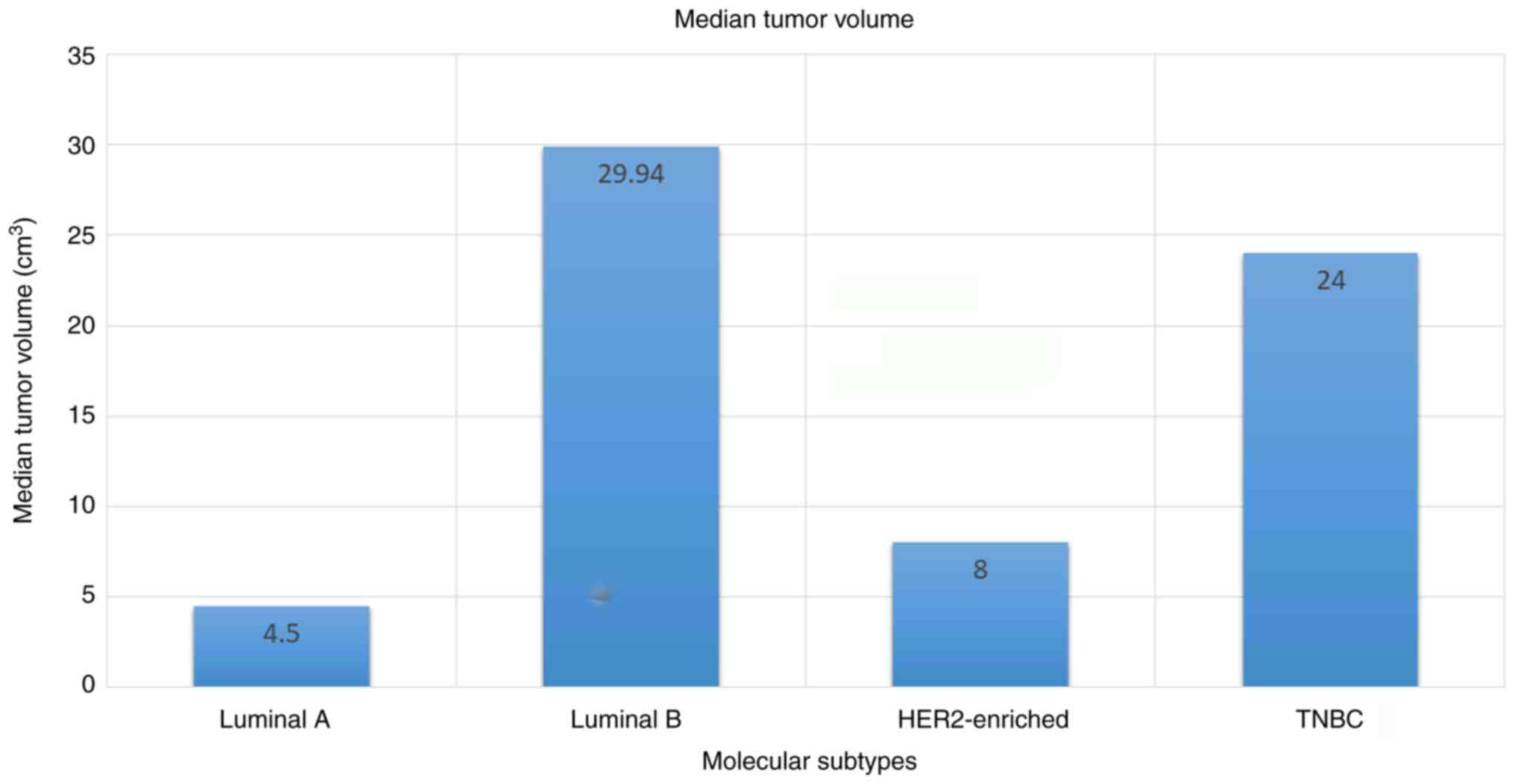
The average tumor volume was 44.16 cm3
(SD ±71.96 cm3) and ranged from 0.13 to 440
cm3. The mean tumor volume was 14.6 cm3 (SD
±27.56 cm3) in the luminal A subgroup with a median of
4.5 cm3 (range, 1.13-110.0 cm3), 69.4
cm3 (SD ±78.23 cm3) in the luminal B subgroup
with a median of 29.94 cm3 (range, 4.0-224.0
cm3), 36.15 cm3 (SD ±47.66 cm3) in
the HER2-enriched cohort with a median of 8.0 cm3
(range, 1.8-162.5 cm3) and 51.31 cm3 (SD
±91.1 cm3) in TNBC with a median of 24.0 cm3
(range, 1.15-440.0 cm3) (Fig. 3).
The tumor volume differed significantly among the
molecular subtypes of breast cancer (P=0.001, Kruskal-Wallis test).
The difference in tumor volume between the luminal A and luminal B
subtype, as well as between the luminal A and TNBC groups was
statistically significant (P=0.001 and P=0.001, respectively,
Dunn's test). Similarly, the difference in tumor volume between the
luminal B and HER2-enriched, as well as between the luminal B and
TNBC subtype was statistically significant (P=0.001 and P=0.001,
respectively, Dunn's test). Furthermore, the difference in tumor
volume between the HER2-enriched and TNBC subtype was also
statistically significant (P=0.001, Dunn's test) However, the
difference in tumor volume between the luminal A and HER2-enriched
subtype was not statistically significant (P=0.158, Dunn's test)
(Table II).
 | Table IIDifferences in tumor volume among the
different molecular subtypes of breast cancer. |
Table II
Differences in tumor volume among the
different molecular subtypes of breast cancer.
| | Molecular subtype,
median tumor volume (range), cm3 |
|---|
| Molecular
subtype | Luminal A 4.5
(1.13-110.0) | Luminal B 29.94
(4.0-224.0) | HER2-enriched 8
(1.8-162.5) | TNBC 24.0
(1.15-440.0) |
|---|
| Luminal A | 0.999 | 0.001 | 0.158 | 0.001 |
| Luminal B | 0.001 | 0.999 | 0.001 | 0.001 |
| HER2-enriched | 0.158 | 0.001 | 0.999 | 0.001 |
| TNBC | 0.001 | 0.001 | 0.001 | 0.999 |
Discussion
Breast cancer remains a leading cause of
cancer-related mortality among women worldwide. Breast cancer is a
highly heterogeneous and complex disease and can be attributed to
various clinical, pathological and biological factors that vary
from one population to another. Identifying these factors is
crucial as many of these factors have prognostic significance and
play a key role in the successful treatment of the disease. Hence,
the molecular classification of breast cancer has emerged as a
vital tool for the optimal management of patients.
In the present study, the mean age of the patients
was 47.1 years (SD ±11.5 years). The age of the patients ranged
from 25 to 75 years, which was very close to that reported in the
study by Sharma et al (7),
in which the mean age was 48.14 years and the median age was 47
years. This finding is unlike that of other studies, as for
instance in the studies of Jain et al (8), Kumar et al (18) and Pereira et al (9), in which the mean age of the patients
was slightly higher.
However, in a study by Pandit et al (10), the median age of the patients was
50.02 years, ranging from 22 to 100 years, and the majority of the
patients were in the age group of 41-50 years (31.3%), followed by
51-60 years (27.6%). In the present study, the majority of the
patients were in the age group of 31-40 years (32.1%), followed by
41-50 years (26.1%). The findings of the present study are similar
to those of the study by Gupta et al (6), in which a total of 60 patients were
included. The most prevalent age group was 31-40 years (41.7%),
followed by 41-50 years (26.7%) (6). In the study by Sharma et al
(7), the majority of the patients
were >40 years (73.4%) and 26.6% were <40 years. Jain et
al (8) also reported that
41-70 years was the predominant age group, accounting for 76.1% of
breast cancer cases; 13.9 and 10% of the patients were <41 years
and >70, respectively.
In the present study, TNBC was the most common
subtype of breast cancer, followed by luminal B, HER2-enriched and
luminal A. This finding agrees to a certain extent with the
findings presented in the study by Pereira et al (9) in Mangalore (South India), in which
TNBC was the most prevalent subtype, followed by the luminal B,
luminal A and HER2-enriched subtypes.
By contrast, in the study by Pandit et al
(10) performed in Maharashtra
(West India), the most common molecular subtype of breast carcinoma
was luminal A, followed by TNBC (26%), HER2-enriched (11%) and
luminal B (8%). In that study, the remaining 18% of the total 2,062
patients were unclassified owing to an equivocal HER2 status
(10). Similarly, another study
from North India was performed by Gupta et al (6), in which luminal A was the most common
subtype, followed by the TNBC, HER2-enriched and luminal B
subtypes. However, in the studies by Jain et al (8) and Sharma et al (7) performed in Ludhiana (North India) and
Guwahati (North-East India), respectively, luminal B was the most
prevalent subtype, followed by the TNBC, luminal A and
HER2-enriched subtypes. According to the findings of various Indian
studies, there is heterogeneity in the distribution of molecular
subtypes of breast cancer in the Indian population (7,8).
However, studies from other countries have revealed a predominance
of the luminal A subtype of breast cancer. The studies performed in
Thailand (Asia) and Morocco (North Africa) by Tubtimhin et
al (13) and Elidrissi et
al (14), respectively,
reported similar findings, with luminal A being the most common
subtype, followed by the luminal B, TNBC and HER2-enriched
subtypes.
In the study performed in Saudi Arabia (the Middle
East) by Alnegheimish et al (12), the most common subtype was luminal
A, followed by the TNBC, luminal B and HER2-enriched subtypes. The
study conducted by Lin et al (15) in Taiwan (Asia) also documented that
luminal A was the most prevalent subtype, followed by the TNBC,
HER2-enriched and luminal B subtypes. Furthermore, yet another
study from Bahrain (Asia) performed by AlZaman et al
(11) observed that luminal A was
the most common subtype, followed by the HER2-enriched, luminal B
and TNBC subtypes (Table
III).
 | Table IIIPrevalence of molecular subtypes of
breast carcinoma among the different populations. |
Table III
Prevalence of molecular subtypes of
breast carcinoma among the different populations.
| Authors
(country) | Luminal A (%) | Luminal B (%) | HER2- enriched
(%) | TNBC (%) | Total no. of
cases | (Refs.) |
|---|
| Pandit et al
(India) | 37 | 08 | 11 | 26 | 2,062 | (10) |
| Jain et al
(India) | 19.7 | 47.2 | 11.4 | 21.7 | 360 | (8) |
| Pereira et
al (India) | 17 | 33.4 | 15.3 | 34.3 | 300 | (9) |
| Sharma et al
(India) | 18.7 | 43 | 15.5 | 22.8 | 568 | (7) |
| Gupta et al
(India) | 60.6 | 3.3 | 10 | 26.7 | 60 | (6) |
| Tubtimhin et
al (Thailand) | 31.6 | 15.6 | 9.9 | 11.3 | 523 | (13) |
| Elidrissi et
al (Morocco) | 61.1 | 16.1 | 8.6 | 14.2 | 2,260 | (14) |
| Lin et al
(Taiwan) | 62 | 9 | 12 | 13 | 978 | (15) |
| Alnegheimish et
al (Saudi Arabia) | 58.5 | 14.5 | 12.3 | 14.8 | 359 | (12) |
| AlZaman et
al (Bahrain) | 41.3 | 22 | 23 | 13.7 | 109 | (11) |
| Present study
(India) | 15.8 | 24.2 | 20.6 | 39.4 | 165 | |
All the surveyed studies considered only the largest
dimension of the tumor in terms of tumor size; however, in the
present study, the tumor volume (cm3) was instead
considered, which is considered more appropriate for assessing the
clinicopathological features of patients. The findings presented
herein indicated that the average tumor volume was 44.16
cm3 (SD ±71.96 cm3), ranging from 0.13 to 440
cm3. Furthermore, luminal A tumors were found to have
the smallest median tumor volume (4.5 cm3), followed by
HER2-enriched tumors (8 cm3), TNBC tumors (24
cm3) and luminal B tumors (29.94 cm3). The
differences in the median tumor volume between the luminal A and
luminal B groups, and between the luminal A and TNBC groups were
statistically significant (P=0.001 and P=0.001, respectively).
Similarly, the difference in tumor volume between the luminal B and
both HER2-enriched and TNBC subtype was also found statistically
significant (P=0.001 for both). Moreover, a statistically
significant difference in tumor volume was also found between the
HER2-enriched and TNBC subtypes (P=0.001).
In the study by Jain et al (8), the mean tumor size was 3.7 cm and
ranged from 0.8 to 10 cm, with a median of 3 cm. The mean tumor
size was the least in the luminal A subgroup (3.2 cm) and the
highest in the HER2-enriched subgroup (4 cm) (8). Their study further demonstrated that
there was a significant difference in the mean tumor size between
luminal A breast cancer and other subtypes of breast carcinoma
(luminal B, HER2-enriched and TNBC, P=0.03) (8).
In the study by Kumar et al (18), the mean tumor size was 3.4 cm and
ranged from 1.1 to 7.8 cm. According to their study, the luminal A
subtype had the maximum percentage of tumors <2 cm (smallest),
whereas the TNBC subtype had the maximum percentage of tumors >5
cm (largest). In addition, there was a significant difference in
the mean tumor size among all molecular subtypes of breast cancer
(P=0.004) (18).
In the study by Pereira et al (9), the mean tumor size was 3.4 cm (SD
±1.6 cm), with luminal A having the maximum percentage of tumors
<2 cm in size and TNBC having the maximum percentage of tumors
>5 cm in size. Moreover, there was no significant difference
between the molecular subtypes of breast cancer and tumor size
(9).
Of note, a limitation of the present study is the
small sample size of 165 patients, which resulted in a small number
of cases in all four molecular subtypes of breast carcinoma.
In conclusion, in the present study, it was found
that TNBC was the most common molecular phenotype of breast cancer
in Delhi (North India). The tumor volume was the smallest in the
luminal A, followed by the HER2-enriched subtype, than in the other
molecular subtypes of breast cancer. As tumor volume has prognostic
significance, patients with TNBC have a poor prognosis, and those
with HER2-enriched breast cancer can be treated using targeted
therapy. The findings of the present study may prove to be useful
for the management of patients with breast cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (SKS, SS and SK) contributed to the
conception and design of the study. Material preparation was
performed by SKS and SS. Data collection and analysis were
performed by SKS and SS. Analysis was performed by SKS and SS. The
first draft of the manuscript was written by SKS, and all authors
commented on previous versions of the manuscript. SS and SK confirm
the authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted after obtaining
approval from the Institutional Ethics Committee, NDMC Medical
College and Hindu Rao Hospital, Delhi (vide letter No.
IEC/NDMC/2022/109 dated June 17, 2022), and written informed
consent was obtained from the patients for participation in the
study. The present study was conducted in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Rosenberg PS, Chen WQ, Hartman M,
Lim WY, Chia KS, Wai-Kong Mang O, Chiang CJ, Kang D, Ngan RK, et
al: Female breast cancer incidence among Asian and Western
populations: More similar than expected. J Natl Cancer Inst.
107(djv107)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Youlden DR, Cramb SM, Yip CH and Baade PD:
Incidence and mortality of female breast cancer in the Asia-Pacific
region. Cancer Biol Med. 11:101–115. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rangarajan B, Shet T, Wadasadawala T, Nair
NS, Sairam RM, Hingmire SS and Bajpai J: Breast cancer: An overview
of published Indian data. South Asian J Cancer. 5:86–92.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gupta P, Rai NN, Agarwal L and Namdev S:
Comparison of molecular subtypes of carcinoma of the breast in two
different age groups: A single institution experience. Cureus.
10(e2834)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sharma JD, Khanna S, Ramchandani S, Kakoti
LM, Baruah A and Mamidala V: Prevalence of molecular subtypes of
breast carcinoma and its comparison between two different age
groups: A retrospective study from a tertiary care center of
northeast India. South Asian J Cancer. 10:220–224. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jain S, Narang V, Jain K, Paul D, Singh J,
Sohi AS, Sood S, Aggarwal R, Sood N and Brar GS: Prevalence of
molecular subtypes in operated cases of breast cancer and its
clinicopathological correlation: A single institute study from a
tertiary cancer centre in North India. Indian J Surg Oncol.
12:538–544. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pereira C, Martis M, D'Souza R and Tauro
LF: Correlation of clinicopathological features of breast cancer
with molecular subtypes taking Ki-67 into Consideration: Single
institution experience over 5 years. Curr Health Sci J. 47:348–352.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pandit P, Patil R, Palwe V, Gandhe S,
Patil R and Nagarkar R: Prevalence of molecular subtypes of breast
cancer: A single institutional experience of 2062 patients. Eur J
Breast Health. 16:39–43. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
AlZaman AS, Mughal SA, AlZaman YS and
AlZaman ES: Correlation between hormone receptor status and age,
and its prognostic implications in breast cancer patients in
Bahrain. Saudi Med J. 37:37–42. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alnegheimish NA, Alshatwi RA, Alhefdhi RM,
Arafah MM, AlRikabi AC and Husain S: Molecular subtypes of breast
carcinoma in Saudi Arabia. A retrospective study. Saudi Med J.
37:506–512. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tubtimhin S, Promthet S, Suwanrungruang K
and Supaattagorn P: Molecular subtypes and prognostic factors among
premenopausal and postmenopausal thai women with invasive breast
cancer: 15 Years Follow-up Data. Asian Pac J Cancer Prev.
19:3167–3174. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Elidrissi Errahhali M, Elidrissi Errahhali
M, Ouarzane M, El Harroudi T, Afqir S and Bellaoui M: First report
on molecular breast cancer subtypes and their clinico-pathological
characteristics in Eastern Morocco: Series of 2260 cases. BMC
Womens Health. 17(3)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lin CH, Liau JY, Lu YS, Huang CS, Lee WC,
Kuo KT, Shen YC, Kuo SH, Lan C, Liu JM, et al: Molecular subtypes
of breast cancer emerging in young women in Taiwan: Evidence for
more than just westernization as a reason for the disease in Asia.
Cancer Epidemiol Biomarkers Prev. 18:1807–1814. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Engstrøm MJ, Opdahl S, Hagen AI,
Romundstad PR, Akslen LA, Haugen OA, Vatten LJ and Bofin AM:
Molecular subtypes, histopathological grade and survival in a
historic cohort of breast cancer patients. Breast Cancer Res Treat.
140:463–473. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cancer Genome Atlas Network. Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kumar N, Patni P, Agarwal A, Khan MA and
Parashar N: Prevalence of molecular subtypes of invasive breast
cancer: A retrospective study. Med J Armed Forces India.
71:254–258. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Parker JS, Mullins M, Cheang MC, Leung S,
Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, et al:
Supervised risk predictor of breast cancer based on intrinsic
subtypes. J Clin Oncol. 27:1160–1167. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dowsett M, Nielsen TO, A'Hern R, Bartlett
J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et
al: Assessment of Ki67 in breast cancer: Recommendations from the
International Ki67 in Breast Cancer working group. J Natl Cancer
Inst. 103:1656–1664. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Soliman NA and Yussif SM: Ki-67 as a
prognostic marker according to breast cancer molecular subtype.
Cancer Biol Med. 13:496–504. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rouzier R, Perou CM, Symmans WF, Ibrahim
N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P,
et al: Breast cancer molecular subtypes respond differently to
preoperative chemotherapy. Clin Cancer Res. 11:5678–5685.
2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Marra A, Viale G and Curigliano G: Recent
advances in triple negative breast cancer: The immunotherapy era.
BMC Med. 17(90)2019.PubMed/NCBI View Article : Google Scholar
|