Introduction
The discovery of the phenomenon that viral sequences
are removed from a pre-mRNA and the remaining sequences are joined
together led to a fundamental principle governing biology, known as
RNA splicing. The identification stimulated theories for protein
diversity, such as alternative splicing, which over time have been
realized repeatedly through experiments. Gilbert (1) first proposed the concept of
alternative splicing in 1978, which is currently the mechanism that
accounts for the discrepancy between the number of protein-coding
genes (~25,000) in humans and the >90,000 different proteins
that are actually generated (2,
3). The notion of ‘one gene-one
RNA-one protein’ is no longer relevant. More than 95% of human
genes have been found to undergo splicing in a developmental,
tissue-specific or signal transduction-dependent manner (4).
Constitutive splicing is the process of intron
removal and exon ligation of the majority of the exons in the order
in which they appear in a gene. Alternative splicing is a deviation
from this preferred sequence where certain exons are skipped
resulting in various forms of mature mRNA. Weaker splicing signals
at alternative splice sites, shorter exon length or higher sequence
conservation surrounding orthologous alternative exons influence
the exons that are ultimately included in the mature mRNA (5). This process is mediated by a dynamic
and flexible macromolecular machine, the spliceosome, which works
in a synergistic and antistatic manner (as explained below)
(6, 7). Three possible mechanisms, exon
shuffling, exonization of transposable elements and constitutively
spliced exons, have been proposed for the origin of alternative
splicing (8).
Numerous studies have reiterated the critical and
fundamental role of alternative splicing across biological systems
(9). The species of higher
eukaryotes have been discovered to exhibit a higher proportion of
alternatively spliced genes, which is an underlying indication of a
prominent role for the mechanism in evolution. Alternative splicing
mediates diverse biological processes over the entire life span of
organisms, from before birth to death (10, 11).
Conserved splicing to species-specific splice variants play a
significant functional role in species differentiation and genome
evolution (12, 13), as well as in the development of
functionally simple to complex tissues with diverse cell types,
such as the brain, testis and the immune system. Alternative
splicing even participates in RNA processing itself, from pre- to
post-transcriptional events.
Thus, alternative splicing has a role in almost
every aspect of protein function, including binding between
proteins and ligands, nucleic acids or membranes, localization and
enzymatic properties. Taken together, alternative splicing is a
central element in gene expression (14).
Molecular mechanisms of alternative
spicing
Systematic analyses of ESTs and microarray data have
so far revealed seven main types of alternative splicing (12) (Fig.
1). The most prevalent pattern (~30%) is the cassette-type
alternative exon (exon skipping) in vertebrates and invertebrates
(Fig. 1C), while in lower
metazoans, it is intron retention (Fig.
1F) (15). Intron retention in
human transcripts is positioned primarily in the untranslated
regions (UTRs) (16) and has been
associated with weaker splice sites, short intron length and the
regulation of cis-regulatory elements (17).
Alternative selection of 5′ or 3′ splice sites
within exon sequences (~25%) may lead to subtle changes in the
coding sequence (Fig. 1D and E),
and an additional layer of complexity arises with mutually
exclusive alternative exons (Fig.
1B). One example of a transcript that undergoes alternative
splicing, which generates variation in the protein, is
FGFR2. Differences in the splicing machinery in different
cell types and unique cis-acting elements in the FGF-R2
pre-mRNA lead to altered tissue specific choices that create either
FGF-R2IIIb or FGF-R2IIIc mature transcripts (18).
The protein expression is further regulated by
alternative polyadenylation of mRNA, which influences the coding
potential or the 3′UTR length by modifying the binding availability
of microRNA or RNA (19). Of note,
it has been demonstrated that each type of alternative splicing can
operate in a stochastic manner, and different splice-site
identification and processing mechanisms do not necessarily occur
at the same frequencies among all biological kingdoms (20).
The mechanisms outlined above are just one
indication of the complexity, as numerous molecules are involved in
alternative splicing in a coordinated manner. Even the basic
nucleotide components and the essential molecules that recognize
them can introduce diversity in the synthesis of mature
transcripts.
Two major steps constitute the basic process of
splicing: Assembly of the spliceosome followed by the actual
splicing of pre-mRNA. The spliceosome is mainly composed of U1, U2
small nuclear ribonucleic proteins (snRNPs) and the U4/U6.U5
tri-snRNP, and configure in identify a core set of splicing
signals: The 5′ splice site, the branch point sequence and the 3′
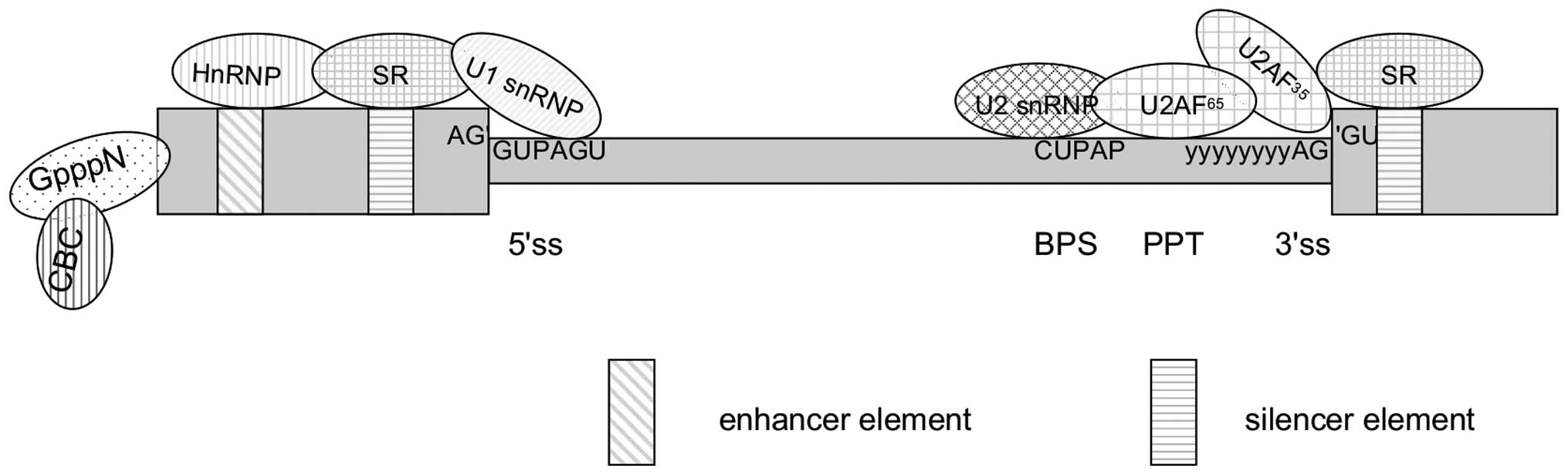
splice site (Fig. 2). Specific
spliceosomal complexes (E, A, B and others) and eight
evolutionarily conserved DExD/H-type RNA-dependent
ATPases/helicases assemble in a proposed stepwise manner and
execute multiple splicing steps that result in exon ligation and
intron excision. Numerous steps in the pathway are reversible
(21).
The exons that end up in the mature mRNA during the
process of alternative splicing is entirely defined by the
interaction between cis-acting elements and trans-acting factors.
Cis-acting elements include exonic splicing enhancers (ESEs) and
intronic splicing enhancers (ISE) that are bound by positive
trans-acting factors, such as SR proteins (serine/arginine-rich
family of nuclear phosphoproteins), whereas exonic splicing
silencers (ESSs) and intronic splicing silencers are bound by
negative acting factors, such as heterogeneous nuclear
ribonucleoproteins (hnRNPs). The collaboration between these
elements results in the promotion or inhibition of splicesome
assembly of the weak splice sites, respectively (Fig. 2) (22, 23).
In general, the cis-acting elements function additively. The
enhancing elements tend to play dominant roles in constitutive
splicing, while the silencers are relatively more important in the
control of alternative splicing (22). Enhancer activity has been shown to
be abolished by a stable stem-loop structure as short as 7 base
pairs in an RNA transcript owing to the mechanisms of physical
competition, long-range RNA pairing, a structure splice code and
co-transcription splicing (24,
25). Furthermore, the specificity
of cis-acting enhancer elements for introns or exons has been
investigated. In these experiments, an ESE was found to act as an
ISE depending on its location in an exon or intron (26).
HnRNPs are highly conserved from nematodes to
mammals and have several critical roles in pre-mRNA maturation.
Their function is to bind to the ESS to the exclusion of SR
proteins. A looping out pre-mRNA leads to exonic sequestration from
the rest of pre-mRNA transcript (27). HnRNPs A/B are a family of
RNA-binding proteins, its diversification roles in the modulation
of alternative splicing have evolved based on differing affinities
for their cognate nucleic acids (28). HnRNP H and F serve to alter the
proteolipid protein (PLP/DM20) ratio via the variation in the
recruitment of U1 snRNP (29).
Similarly, the antagonistic role of hnRNP M to the splicing factor
Nova-1 generates alternatively spliced dopamine receptor pre-mRNAs,
which create isoforms associated with diverse key physical
functions, such as control, reward, learning and memory (30). In addition, hnRNP L and
phosphorylation of ser513 have been recently shown to be involved
in the regulation of alternative splicing through dynamic membrane
depolarization and Ca2+/calmodulin-dependent protein
kinase IV activation (31, 32).
In addition to the coupling of SR proteins to
enhancer elements, SR proteins interact with U1 snRNP and the 35
kDa subunit of the heterodimeric factor, U2AF. The second subunit
of U2AF, U2AF65, binds SF1 and the pyrimidine tract
simultaneously, on the basis of the arginine/serine (RS)-rich
domain, which results in recognition and stability of the branch
point, as well as polypyrimidine tract sequences. Approximately
10–12 serines in the N-terminal region of the RS domain are rapidly
phosphorylated by the binding of SR-specific protein kinase to
serine/arginine-rich splicing factor 1 with an unusually high
affinity. This continuous phosphorylation/dephosphorylation cycle
of SR proteins facilitates the shuttling of SR proteins between the
nucleus and the cytoplasm, and is critically required for the
regulation of alternative splicing by growth signals transduced to
the nucleus (33, 34). SR proteins have also been proposed
to participate in post-splicing activities, such as mRNA nuclear
export, nonsense-mediated decay (NMD) and mRNA translation
(35).
In general, positive or negative splice-site
recognition is regulated through various mechanisms, such as the
local concentration or activity of splicing regulatory factors,
under diverse physiological or pathological conditions. How these
elements function together to precisely select a regulated splice
site is, however, only partially explained by these results
(36).
Coupling of alternative splicing to
transcription
Since the first significant observation of
co-transcriptional spliceosome assembly from electron micrographs
of Drosophila melanogaster embryonic transcription units
(37), increasing evidence supports
the idea that transcription and splicing are physically and
functionally coupled, and has also uncovered the intricate
association between mRNA splicing, RNA polymerase II (Pol II) and
chromatin structure (38, 39).
A large number of components associated with the
physical interaction between splicing and transcription have been
purified, with particular attention on the carboxyl terminal domain
(CTD) of the large subunit of RNAPII (40). The CTD consists of 52 tandem repeats
of the heptapeptide YSPTSPS in mammals (26 tandem repeats in yeast)
(41), which act as a special
platform to recruit different factors to the nascent transcripts
via dynamic phosphorylation of serine residues. Kinases that
phosphorylate specific CTD serine residues have been identified and
are components of the protein apparatus driving the specific
function. For example, ser5 phosphorylation is associated with
transcription initiation through cyclin-dependent kinase 7 (CDK7)
of the general transcription factor IIH (TFIIH), whereas ser2
phosphorylation is preferentially linked with CTD activity at the
3′-end of genes through CDK9 of the positive transcription
elongation factor b (42). In
addition, phosphorylation of ser7 has been found to facilitate
elongation and splicing (43).
Thus, phosphorylation is a mechanism that clearly demonstrates that
functional coupling exists between transcription and alternative
splicing.
CTD participates in gene expression-related
functions ranging from 5′ capping, splicing, poly-adenylation and
chromatin remodeling (44). Of
note, mutation and deletion analysis of CTD has revealed multiple
defects in mRNA processing (45),
therefore, CTD and additional components of the two machineries
have emerged as a central element in governing the interactions
between transcription and splicing. Taken together, functional
coupling appears to maintain an important role in alternative
splicing in driving determinative physiological changes, and
fine-tune gene expression in mathematical modeling approaches
(46).
Two models have been suggested to explain the
co-transcription process of how transcription coupled repair
influences alternative splicing. The mechanism of the recruitment
model may mainly depend on specific features of CTD (as mentioned
above), whereas the kinetic model is based on the different
elongation rates of Pol II, which in turn determine the timing of
the presentation of splices sites (47, 48).
Fundamentally, the aforementioned mechanism
influences patterns of alternative splicing via the variations in
Pol II elongation and recruitment of splicing factors by specific
histone marks (49). Thus,
alternative splicing is highly influenced not only by
transcription, but also by the chromatin structure, which
underscores chromatin as another layer in the regulation of
alternative splicing. The resultant mature mRNA is thus a
reflection of numerous DNA modifications, such as patterns of
histone methylation at exons, modulation of histone modifications
and increased DNA methylation at exons (50, 51).
Conversely, a previous study indicated that splicing may mediate
chromatin remodeling via deposition of histone marks on DNA or
numerous associations between splicing factors and elongation
proteins (38).
Adding additional complexity to the regulation
network is alternative transcription initiation (ATI) and
alternative transcription termination (ATT) sites. ATI and ATT
significantly contribute to the diversity of the human and mouse
transcriptomes to a degree that may exceed alternative splicing,
when considering the number of possibilities available through
alternative nucleotides, isoforms and introns (52, 53).
In contrast to the prevalence of alternative splicing that occurs
within coding sequences (CDSs), the dominant class of alternative
events, which includes ATI and ATT, occur in UTRs. This discovery
reflects the preferential regulation of large distinct groups of
genes with different mechanisms, such as strong coupling with
alternative splicing in 5′ and 3′UTRs (54).
Despite the strong correlation between alternative
splicing and transcription, alternative transcription mainly
results in variations of the transcript number or the 5′/3′
terminal protein variants due to differential transcriptional start
or terminal sites. By contrast, alternative splicing associated
alterations mostly lie within the protein sequence, potentially
affecting almost all areas of protein function (14, 55).
Alternative splicing and nonsense-mediated
decay
NMD is an extensive and complicated mechanism,
ranging from yeast to human, exploited to achieve another level of
robustness in post-transcriptional gene expression control. Studies
have revealed that up to one-third of human alternative splicing
events contain premature termination codons (PTC), which are
recognized and lead to the degradation of transcripts containing
NMD cis-elements in their 3′ UTRs (56, 57).
In vertebrates, it has been proposed that the coupling of the exon
junction complex (EJC) to mRNA transcripts, followed by binding of
3′UTRs to EJCs, triggers vertebrate specific NMD (58). The sensitivity of mRNA transcripts
to NMD is modulated by alternative splicing events in the 5′ or
3′UTRs and aids with the wide range of protein biosynthesis
(59). Furthermore, analysis of
quantitative alternative splicing microarray profiling has
demonstrated that individual knockdown of NMD factors
[Up-Frameshift (UPF)] strongly affects PTC-introducing alternative
splicing events, indicating a role for different UPF factor
requirements in alternative splicing regulation (60). In a second example, regulation of
intron retention by alternative splicing-NMD in a specific
differentiation event has been recently observed (61).
Trans-splicing
Trans-splicing is a common phenomenon in
trypanosomes, nematodes, Drosophila and even humans, and
refers to the novel and unusual splicing of exons from independent
pre-mRNAs (62, 63). The phenomenon has been explored as a
therapeutic option for a variety of genetic diseases, particularly
in the treatment of cancer (64).
The carcinoembryonic antigen (CEA), for example, is associated with
a variety of neoplastic processes and was exploited as a target for
trans-splicing. A CEA RNA-targeting trans-splicing ribozyme was
designed to perform RNA replacement through a trans-splicing
reaction specifically in CEA expressing cells (65). The activity of the ribozyme
simultaneously reduced CEA expression and introduced the thymidine
kinase gene, which rendered the cells sensitive to ganciclovir
treatment. RNA trans-splicing has also been utilized for the
potential treatment of neurodegenerative diseases through a novel
technology, spliceosome mediated trans-splicing (SMaRT). SMaRT was
successfully used in vivo to re-engineer tau mRNA
transcripts to include E10, and therefore, offers the opportunity
potential to correct tau mis-splicing and treat the underlying
disease (66).
Alternative splicing and non-coding RNA
Non-coding RNAs (ncRNAs), including microRNA and
small interfering RNA, have recently emerged as novel regulators in
alternative splicing, generally through the modulation of the
expression of key splicing factors during development and
differentiation (67).
Alternative splicing and disease
Stringent regulation of alternative splicing is
necessary for the functional requirements of complex tissues under
normal conditions, whereas aberrant splicing appears to an
underlying cause for an extremely high fraction of dysfunction and
disease (68). Aberrant splicing
has been suggested to root in alterations of the cellular
concentration, composition, localization and activity of regulatory
splicing factors, as well as mutations in components of core
splicing machinery (69). A changed
efficiency of splice site recognition is the immediate consequence,
while irregularities in protein isoforms in different systems
ultimately establish the disease state. Any of these alterations
affecting alternative splicing can facilitate the appearance of
characteristics in cancer cells, including the inappropriate
proliferation, migration, methylation changes and resistance to
apoptosis and chemotherapy (70).
Alternative splicing has been implicated in nearly all aspects of
cancer development, and therefore, is a main participant in the
disease.
Understanding the basic mechanisms and patterns of
splicing in tumor progress will shed light on the biology of cancer
and lay the foundation for diagnostic, prognostic and therapeutic
tools with minimum treatment toxicity in cancer (71). Extensive research efforts have
already committed to developing drugs that target specific cancer
protein isoforms. Several examples are genes associated with
apoptosis [BCL2L1 (BCL-X), FAS, BIRC5 (survivin) and
MDM2], immortality (human telomerase reverse transcriptase),
and angiogenesis (vascular endothelial growth factor-A) (72, 73).
However, limited success has been achieved by simply
activating or inhibiting cancer-associated genes, possibly due to
the expression of target genes in normal and cancers cells, such as
angiogenic and anti-angiogenic isoforms (74). The lack of specificity of numerous
molecular targets for cancer cells favors the development of
isoform-specific diagnostic markers as therapeutic targets
(75). Therefore, the key task for
cancer treatment in the future should be to detect and target the
expression of a gene at the gene level.
Conclusion
The combination of an alternative splicing database,
tandem mass spectrometry, and even the latest synthetic alternative
splicing database may aid with the identification, analysis and
characterization of potential alternative splicing isoforms. Over
two-thirds of human genes and 40% of Drosophila genes
contain one or more alternative exons, and >90% of the
protein-coding genes associated with alternative splicing events
according to the >60,000 studies since the discovery of splicing
(76). Alternative splicing appears
to be prevalent in almost all multi-exon genes. However, what
limits our insight into a more complete and accurate usage of
alternative splicing are factors such as biased coverage of ESTs
toward the 5′- and 3′-ends of transcripts, insufficient widespread
analyses, subtle alternative splicing associated changes and
advanced alternative splicing networks involved in various
mechanisms and numbers of regulatory proteins. All these
deficiencies lead to an incomplete understanding of the alternative
splicing mechanism and may prevent the correct prediction of splice
events in other species, such as the chimpanzee or plant (77, 78).
Distinguishing alternative splicing from other regulatory
mechanisms in the gene regulation is also difficult. Alternative
splicing, alternative trans-splicing, NMD, transcriptional
efficiency, exon duplication and RNA editing (79) all contribute to an extensive
mechanism for generating protein diversity. In addition, the
difference between artificial experimental systems and real-life
scenarios makes it challenging to transfer functional studies from
cells to whole organisms. Numerous questions remain regarding the
global impact of alternative splicing on cellular and organismal
homeostasis, as well as its underlying molecular mechanisms.
Finally, with regards to cancer-associated alternative splicing,
whether a particular splice site selection causes the observed
effect or is merely the result of the cancerous transformation is
hard to distinguish. The data collected regarding alternative
splicing is likely to represent only the tip of the iceberg, with
further information yet to be revealed in future studies.
Acknowledgements
The present study was supported by grants from the
National Science Foundation of China (No. 81271912) and the
Education Department of Jiangxi province, China (No. GJJ13041).
References
|
1
|
Gilbert W: Why genes in pieces? Nature.
271:5011978. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
C. elegans Sequencing Consortium, . Genome
sequence of the nematode C. elegans: a platform for investigating
biology. Science. 282:2012–2018. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Human Genome Sequencing Consortium I
International Human Genome Sequencing C, . Finishing the
euchromatic sequence of the human genome. Nature. 431:931–945.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nilsen TW and Graveley BR: Expansion of
the eukaryotic proteome by alternative splicing. Nature.
463:457–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng CL, Fu XD and Gribskov M:
Characteristics and regulatory elements defining constitutive
splicing and different modes of alternative splicing in human and
mouse. RNA. 11:1777–1787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Effenberger KA, Perriman RJ, Bray WM, et
al: A high-throughput splicing assay identifies new classes of
inhibitors of human and yeast spliceosomes. J Biomol Screen.
18:1110–1120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wahl MC, Will CL and Luhrmann R: The
spliceosome: design principles of a dynamic RNP machine. Cell.
136:701–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim E, Goren A and Ast G: Alternative
splicing: current perspectives. Bioessays. 30:38–47. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Irimia M, Penny D and Roy SW: Coevolution
of genomic intron number and splice sites. Trends Genet.
23:321–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu G, Condon KC, Epton MJ, et al:
Female-specific insect lethality engineered using alternative
splicing. Nat Biotechnol. 25:353–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hurt KJ, Sezen SF, Champion HC, et al:
Alternatively spliced neuronal nitric oxide synthase mediates
penile erection. Proc Natl Acad Sci USA. 103:3440–3443. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blencowe BJ: Alternative splicing: new
insights from global analyses. Cell. 126:37–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nygard AB, Cirera S, Gilchrist MJ, et al:
A study of alternative splicing in the pig. BMC Res Notes.
3:1232010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kelemen O, Convertini P, Zhang Z, et al:
Function of alternative splicing. Gene. 514:1–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim E, Magen A and Ast G: Different levels
of alternative splicing among eukaryotes. Nucleic Acids Res.
35:125–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galante PA, Sakabe NJ, Kirschbaum-Slager N
and de Souza SJ: Detection and evaluation of intron retention
events in the human transcriptome. RNA. 10:757–765. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakabe NJ and de Souza SJ: Sequence
features responsible for intron retention in human. BMC Genomics.
8:592007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldstrohm AC, Greenleaf AL and
Garcia-Blanco MA: Co-transcriptional splicing of pre-messenger
RNAs: considerations for the mechanism of alternative splicing.
Gene. 277:31–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Giammartino DC, Nishida K and Manley
JL: Mechanisms and consequences of alternative polyadenylation. Mol
Cell. 43:853–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mastrangelo AM, Marone D, Laido G, et al:
Alternative splicing: enhancing ability to cope with stress via
transcriptome plasticity. Plant Sci. 185–186:40–49. 2012.
View Article : Google Scholar
|
|
21
|
Hoskins AA and Moore MJ: The spliceosome:
a flexible, reversible macromolecular machine. Trends Biochem Sci.
37:179–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z and Burge CB: Splicing regulation:
from a parts list of regulatory elements to an integrated splicing
code. RNA. 14:802–813. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Xiao X, Van Nostrand E and Burge
CB: General and specific functions of exonic splicing silencers in
splicing control. Mol Cell. 23:61–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin Y, Yang Y and Zhang P: New insights
into RNA secondary structure in the alternative splicing of
pre-mRNAs. RNA Biol. 8:450–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu W, Zhou Y, Hu Z, et al: Regulation of
splicing enhancer activities by RNA secondary structures. FEBS
Lett. 584:4401–4407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McManus CJ and Graveley BR: RNA structure
and the mechanisms of alternative splicing. Curr Opin Genet Dev.
21:373–379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nasim FU, Hutchison S, Cordeau M and
Chabot B: High-affinity hnRNP A1 binding sites and duplex-forming
inverted repeats have similar effects on 5′ splice site selection
in support of a common looping out and repression mechanism. RNA.
8:1078–1089. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han SP, Kassahn KS, Skarshewski A, Ragan
MA, Rothnagel JA and Smith R: Functional implications of the
emergence of alternative splicing in hnRNP A/B transcripts. RNA.
16:1760–1768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang E and Cambi F: Heterogeneous nuclear
ribonucleoproteins H and F regulate the proteolipid protein/DM20
ratio by recruiting U1 small nuclear ribonucleoprotein through a
complex array of G runs. J Biol Chem. 284:11194–11204. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park E, Iaccarino C, Lee J, et al:
Regulatory roles of heterogeneous nuclear ribonucleoprotein M and
Nova-1 protein in alternative splicing of dopamine D2 receptor
pre-mRNA. J Biol Chem. 286:25301–25308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu G, Razanau A, Hai Y, et al: A
conserved serine of heterogeneous nuclear ribonucleoprotein L
(hnRNP L) mediates depolarization-regulated alternative splicing of
potassium channels. J Biol Chem. 287:22709–22716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu J, Hai Y, Liu G, Fang T, Kung SK and
Xie J: The heterogeneous nuclear ribonucleoprotein L is an
essential component in the Ca2+/calmodulin-dependent protein kinase
IV-regulated alternative splicing through cytidine-adenosine
repeats. J Biol Chem. 284:1505–1513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghosh G and Adams JA: Phosphorylation
mechanism and structure of serine-arginine protein kinases. FEBS J.
278:587–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Twyffels L, Gueydan C and Kruys V:
Shuttling SR proteins: more than splicing factors. FEBS J.
278:3246–3255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Long JC and Caceres JF: The SR protein
family of splicing factors: master regulators of gene expression.
Biochem J. 417:15–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kalnina Z, Zayakin P, Silina K and Linē A:
Alterations of pre-mRNA splicing in cancer. Genes Chromosomes
Cancer. 42:342–357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beyer AL, Bouton AH and Miller OL Jr:
Correlation of hnRNP structure and nascent transcript cleavage.
Cell. 26:155–165. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kornblihtt AR, de la Mata M, Fededa JP, et
al: Multiple links between transcription and splicing. RNA.
10:1489–1498. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Montes M, Becerra S, Sanchez-Alvarez M and
Sune C: Functional coupling of transcription and splicing. Gene.
501:104–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ryman K, Fong N, Bratt E, Bentley DL and
Ohman M: The C-terminal domain of RNA Pol II helps ensure that
editing precedes splicing of the GluR-B transcript. RNA.
13:1071–1078. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bentley DL: Rules of engagement:
co-transcriptional recruitment of pre-mRNA processing factors. Curr
Opin Cell Biol. 17:251–256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bartkowiak B, Liu P, Phatnani HP, et al:
CDK12 is a transcription elongation-associated CTD kinase, the
metazoan ortholog of yeast Ctk1. Genes Dev. 24:2303–2316. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim H, Erickson B, Luo W, et al:
Gene-specific RNA polymerase II phosphorylation and the CTD code.
Nat Struct Mol Biol. 17:1279–1286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Phatnani HP and Greenleaf AL:
Phosphorylation and functions of the RNA polymerase II CTD. Genes
Dev. 20:2922–2936. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rosonina E and Blencowe BJ: Analysis of
the requirement for RNA polymerase II CTD heptapeptide repeats in
pre-mRNA splicing and 3′-end cleavage. RNA. 10:581–589. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kalsotra A and Cooper TA: Functional
consequences of developmentally regulated alternative splicing. Nat
Rev Genet. 12:715–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schor IE, Gómez Acuña LI and Kornblihtt
AR: Coupling between transcription and alternative splicing. Cancer
Treat Res. 158:1–24. 2013.PubMed/NCBI
|
|
48
|
Dujardin G, Lafaille C, Petrillo E, et al:
Transcriptional elongation and alternative splicing. Biochim
Biophys Acta. 1829:134–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gómez Acuña LI, Fiszbein A, Alló M, et al:
Connections between chromatin signatures and splicing. Wiley
Interdiscip Rev RNA. 4:77–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Iannone C and Valcárcel J: Chromatin's
thread to alternative splicing regulation. Chromosoma. 122:465–474.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shukla S and Oberdoerffer S:
Co-transcriptional regulation of alternative pre-mRNA splicing.
Biochim Biophys Acta. 1819:673–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ma X, Li-Ling J, Huang Q, et al:
Systematic analysis of alternative promoters correlated with
alternative splicing in human genes. Genomics. 93:420–425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xin D, Hu L and Kong X: Alternative
promoters influence alternative splicing at the genomic level. PLoS
One. 3:e23772008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shabalina SA, Spiridonov AN, Spiridonov NA
and Koonin EV: Connections between alternative transcription and
alternative splicing in mammals. Genome Biol Evol. 2:791–799. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Roy B, Haupt LM and Griffiths LR: Review:
Alternative splicing (AS) of genes as an approach for generating
protein complexity. Curr Genomics. 14:182–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lareau LF, Brooks AN, Soergel DA, Meng Q
and Brenner SE: The coupling of alternative splicing and
nonsense-mediated mRNA decay. Adv Exp Med Biol. 623:190–211.
2007.PubMed/NCBI
|
|
57
|
Lewis BP, Green RE and Brenner SE:
Evidence for the widespread coupling of alternative splicing and
nonsense-mediated mRNA decay in humans. Proc Natl Acad Sci USA.
100:189–192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nyiko T, Kerenyi F, Szabadkai L, et al:
Plant nonsense-mediated mRNA decay is controlled by different
autoregulatory circuits and can be induced by an EJC-like complex.
Nucleic Acids Res. 41:6715–6728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kalyna M, Simpson CG, Syed NH, et al:
Alternative splicing and nonsense-mediated decay modulate
expression of important regulatory genes in Arabidopsis. Nucleic
Acids Res. 40:2454–2469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Saltzman AL, Kim YK, Pan Q, et al:
Regulation of multiple core spliceosomal proteins by alternative
splicing-coupled nonsense-mediated mRNA decay. Mol Cell Biol.
28:4320–4330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ge Y and Porse BT: The functional
consequences of intron retention: alternative splicing coupled to
NMD as a regulator of gene expression. Bioessays. 36:236–243. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Herai RH and Yamagishi ME: Detection of
human interchromosomal trans-splicing in sequence databanks. Brief
Bioinform. 11:198–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Horiuchi T and Aigaki T: Alternative
trans-splicing: a novel mode of pre-mRNA processing. Biol Cell.
98:135–140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lee SW and Jeong JS: Use of
tumor-targeting trans-splicing ribozyme for cancer treatment.
Methods Mol Biol. 1103:83–95. 2014.PubMed/NCBI
|
|
65
|
Jung HS and Lee SW: Ribozyme-mediated
selective killing of cancer cells expressing carcinoembryonic
antigen RNA by targeted trans-splicing. Biochem Biophys Res Commun.
349:556–563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Avale ME, Rodriguez-Martin T and Gallo JM:
Trans-splicing correction of tau isoform imbalance in a mouse model
of tau mis-splicing. Hum Mol Genet. 22:2603–2611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Luco RF and Misteli T: More than a
splicing code: integrating the role of RNA, chromatin and
non-coding RNA in alternative splicing regulation. Curr Opin Genet
Dev. 21:366–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Singh RK and Cooper TA: Pre-mRNA splicing
in disease and therapeutics. Trends Mol Med. 18:472–482. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang J and Manley JL: Misregulation of
pre-mRNA alternative splicing in cancer. Cancer Discov.
3:1228–1237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shkreta L, Bell B, Revil T, et al:
Cancer-associated perturbations in alternative pre-messenger RNA
splicing. Cancer Treat Res. 158:41–94. 2013.PubMed/NCBI
|
|
71
|
Kim YJ and Kim HS: Alternative splicing
and its impact as a cancer diagnostic marker. Genomics Inform.
10:74–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lapuk AV, Volik SV, Wang Y and Collins CC:
The role of mRNA splicing in prostate cancer. Asian J Androl.
16:515–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pajares MJ, Ezponda T, Catena R, et al:
Alternative splicing: an emerging topic in molecular and clinical
oncology. Lancet Oncol. 8:349–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sitohy B, Nagy JA and Dvorak HF:
Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer
Res. 72:1909–1914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pal S, Gupta R and Davuluri RV:
Alternative transcription and alternative splicing in cancer.
Pharmacol Ther. 136:283–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pan Q, Shai O, Lee LJ, et al: Deep
surveying of alternative splicing complexity in the human
transcriptome by high-throughput sequencing. Nat Genet.
40:1413–1415. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
77
|
Calarco JA, Xing Y, Caceres M, et al:
Global analysis of alternative splicing differences between humans
and chimpanzees. Genes Dev. 21:2963–2975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Reddy AS, Rogers MF, Richardson DN,
Hamilton M and Ben-Hur A: Deciphering the plant splicing code:
experimental and computational approaches for predicting
alternative splicing and splicing regulatory elements. Front Plant
Sci. 3:182012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tang W, Fei Y and Page M: Biological
significance of RNA editing in cells. Mol Biotechnol. 52:91–100.
2012. View Article : Google Scholar : PubMed/NCBI
|