Introduction
In neuroblastoma patients with minimal residual
cancer following intensive multimodal therapy (combining surgical
resection, radiation and chemotherapy), immunotherapy is considered
a promising approach. Passive immunotherapy using antibodies has
been performed in high-risk neuroblastoma patients and has led to
improvements of the outcome (1,2), indicating
that immunological mechanisms can influence the eradication of
tumor cells in these patients. Induction of an immune response to
residual malignant cells is the key to development of
next-generation immunotherapy regimens.
Chemoimmunotherapy focuses on the immunological
benefits of chemotherapy agents that influence antitumor immunity
via modulation of the host immune system or inducing immunogenicity
of tumor cells (3). Combination
therapy with multiple antitumor agents induces tumor cell death.
Apoptotic tumor cells are usually engulfed by phagocytes, such as
macrophages or dendritic cells (DC), and this can subsequently
induce an immune response (4). Whether
the engulfed apoptotic cells induce immune tolerance or an
immunogenic reaction depends on the phagocytes involved (5,6). In
addition, a recent study revealed that the mechanism of cell death
induced by chemotherapy agents also influences the immunogenicity
of dying tumor cells. Our previous study demonstrated that ex
vivo treatment with doxorubicin can induce immunogenic tumor
cell death in a mouse neuroblastoma model (7). Such findings have provided specific
insight into the immunological benefits and drawbacks of
conventional antitumor agents.
The present study was performed to investigate the
targeting of innate cellular immunity against neuroblastoma. The
aim was to induce immunoactive phagocytic cells by co-culture of
bone marrow cells with neuroblastoma cells that had been killed by
exposure to doxorubicin, and analyze the characteristics of bone
marrow-derived cells that induced an immune response to
neuroblastoma cells, in order to establish a novel immunotherapy
method for high-risk neuroblastoma patients.
Materials and methods
Murine tumor cell line
A mouse neuroblastoma cell line that was developed
in A/J mice, neuro-2a (H2-Ka, CCL-131), was purchased
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). The cells were maintained in minimal essential medium (MEM)
with 10% fetal bovine serum (ATCC) and 1% penicillin-streptomycin
(10,000 U/ml) (Gibco, Thermo Fisher Scientific, Carlsbad, CA,
USA).
Animals
Female A/J mice (H2-Ka) aged 8–12 weeks
were purchased from SLC (Hamamatsu, Shizuoka, Japan) and maintained
under standard conditions. The Animal Care and Use Committee
(Medical Center, Saitama Medical University, Kawagoe, Saitama,
Japan) approved the animal procedures.
Induction of tumor cell death
Induction of cell death by doxorubicin
(Sigma-Aldrich, St. Louis, MO, USA) or cisplatin (Maruko® cisplatin
for I.V. infusion; Yakult, Tokyo, Japan) was performed as reported
previously (7). Briefly, neuro-2a
cells were plated in 10-cm culture dishes (Corning, One Riverfront
Plaza Corning, NY, USA) and cultured in RPMI-1640 medium
supplemented with 10% fetal calf serum (FCS), 50 µM
2-mercaptoethanol (2-ME) (Sigma-Aldrich), 1% MEM non-essential
amino acid solution, and 1% antibiotics/antimycotic solution
(Gibco, Thermo Fisher Scientific), containing 5 µM doxorubicin or
0.025 mg/ml cisplatin for 24 (doxorubicin) or 72 h (cisplatin).
Generation of bone marrow-derived DCs
by co-culture with killed neuro-2a cells and adjuvants
Cluster of differentiation (CD) 11c+
major histocompatibility complex (MHC II) II+ cells were
harvested as reported previously (7).
Briefly, bone marrow cells were harvested from A/J mice and
erythrocytes were lysed using erythrocyte lysis solution.
Subsequently, the surviving cells were washed and re-suspended in
RPMI-1620 medium supplemented with 10% FCS, 50 µM 2-ME
(Sigma-Aldrich), 1% MEM with non-essential amino acid and 1%
antibiotic/antimycotic solution (Gibco, Thermo Fisher Scientific).
Following the addition of 20 ng/ml recombinant mouse
granulocyte-macrophage colony stimulating factor (GM-CSF) (R&D
Systems, Inc., Minneapolis, MN, USA) to the medium, cells were
plated in 10-cm culture dishes and incubated at 37°C under 5%
CO2. Fresh medium containing 20 ng/ml GM-CSF was added
after 3 days.
On day 7 of culture, doxorubicin-treated neuro-2a
cells (2×105/well), with/without interleukin-4 at a
final concentration of 1,000 U/ml (Sigma-Aldrich), were added to
the dish for stimulation of bone marrow cells and incubation was
continued. At 12 h before the cells were harvested,
lipopolysaccharide (LPS) was added to the culture at a final
concentration of 100 ng/ml. As doxorubicin, but not cisplatin, was
previously reported to induce immunogenic death of mouse
neuroblastoma cells (7), cisplatin was
used as a negative control. The adherent cells were harvested by
trypsinization 8 days after starting bone marrow cell culture.
Measurement of in vitro interferon-γ
production by co-cultured CD8α+ T cells
Our previous study reported that the interferon-γ
concentration in the culture supernatant provides an index of
CD8α+ lymphocyte proliferation when CD8α+
lymphocytes are co-cultured with antigen-presenting cells and
killed neuro-2a cells (7). The ability
of bone marrow-derived adherent cells to induce proliferation of
CD8α+ lymphocytes was evaluated. Briefly,
CD8α+ cells were harvested from the inguinal and
mesenteric lymph nodes and spleens of A/J mice using CD8α magnetic
beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), followed
by positive selection with an auto MACS Pro (Miltenyi Biotec GmbH).
Subsequently, the CD8α+ lymphocytes, bone marrow-derived
adherent cells (0.5×105) and neuro-2a cells
(2.0×105) were treated overnight with ultraviolet (UV)
light and were plated in 24-well flat-bottomed plates coated with
hamster anti-mouse CD3/CD28 antibody (BD Biosciences, San Diego,
CA, USA) in 1 ml of RPMI-1640 medium supplemented with 10% FCS, 50
µM 2-ME, 1% MEM with non-essential amino acids and 1%
antibiotic/antimycotic solution. Subsequently, the concentration of
interferon-γ in the culture supernatant was measured with a mouse
interferon-γ enzyme-linked immunosorbent assay (ELISA) kit (BD
Biosciences) according to the manufacturer's instructions.
Surface antigen analysis of bone
marrow-derived DC co-cultured with killed neuro-2a cells
Surface antigens expressed by bone marrow-derived
adherent cells were analyzed by flow cytometry (FACS), with the
expression of CD11c, MHC class II antigens, CD8α and DEC-205
detected by the phycoerythrin-conjugated anti-mouse CD11c antibody
(MCD11C04; Invitrogen), fluorescein isothiocyanate-conjugated
anti-MHC class II antibody (130–102–168; Miltenyi Biotec GmbH), and
APC-conjugated CD8α and DEC-205 antibodies (110712 and 138206,
respectively; Biolegend, San Diego, CA, USA), respectively.
Following culture of bone marrow cells for 7 days, and overnight
incubation with killed neuro-2a cells with/without LPS and/or
interleukin-4, adherent cells were harvested by trypsinization,
re-suspended in MACS buffer, and incubated with antibodies for 30
min at 4°C. Subsequent to washing, the cells were re-suspended in
MACS running buffer (Miltenyi Biotec GmbH) and surface antigen
expression was analyzed using a FACSVerse (BD, Franklin Lakes, NJ,
USA).
Results
Induction of an immune response by
bone marrow-derived adherent cells co-cultured with
doxorubicin-treated neuro-2a cells
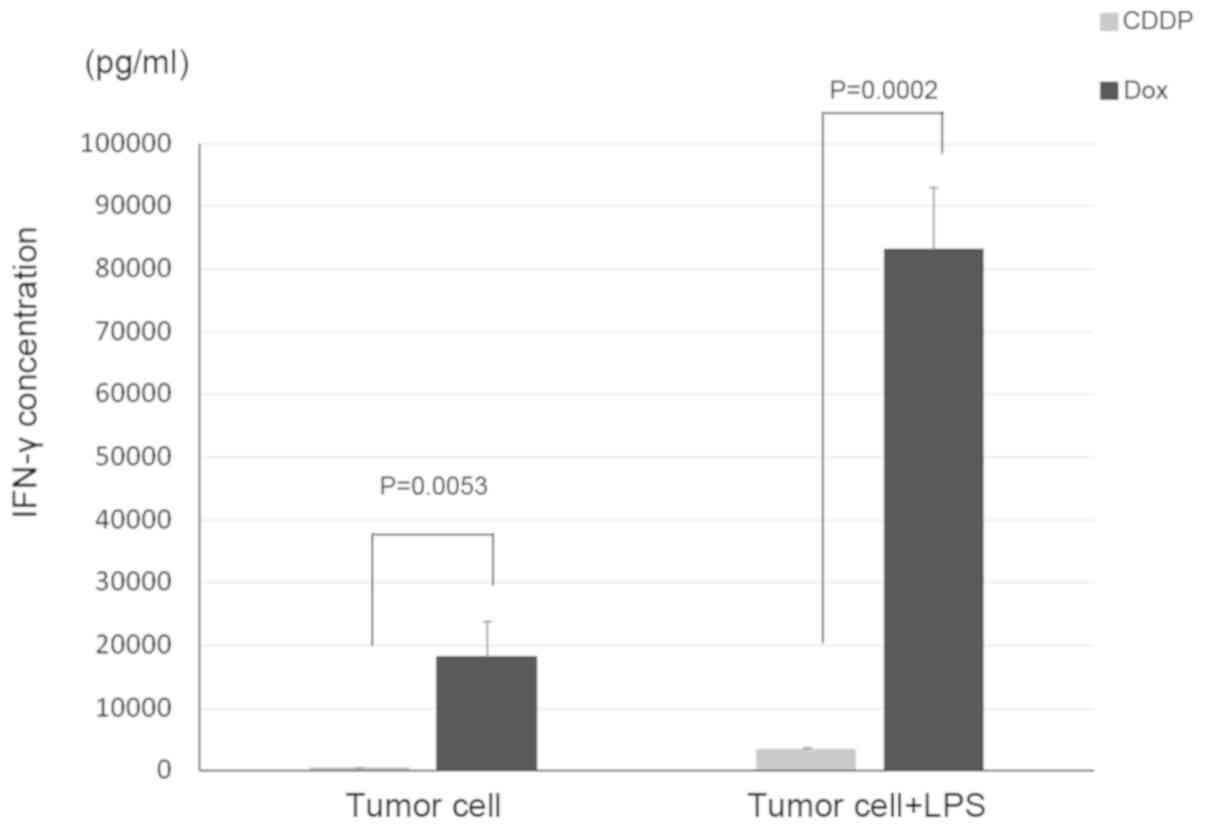
To evaluate the induction of CD8α+
lymphocyte proliferation by co-culture with bone-marrow derived
adherent cells, interferon-γ production by CD8α+
lymphocytes, UV-treated neuro-2a cells, and bone marrow-derived
adherent cells co-cultured with doxorubicin-treated or
cisplatin-treated neuro-2a cells was measured. When
CD8α+ lymphocytes were incubated with bone
marrow-derived adherent cells that had been co-cultured with
doxorubicin-treated neuro-2a cells, interferon-γ production was
higher when compared with the lymphocytes that were incubated with
bone marrow-derived adherent cells, which had been co-cultured with
cisplatin-treated neuro-2a cells. Interferon-γ production was
significantly enhanced when LPS was added to CD8α+
lymphocytes co-cultured with neuro-2a cells or to bone
marrow-derived adherent cells co-cultured with doxorubicin-treated
neuro-2a cells (Fig. 1).
Stimulation of bone marrow-derived
adherent cells by LPS and interleukin-4 during co-culture promotes
acquisition of the ability to induce antitumor immunity
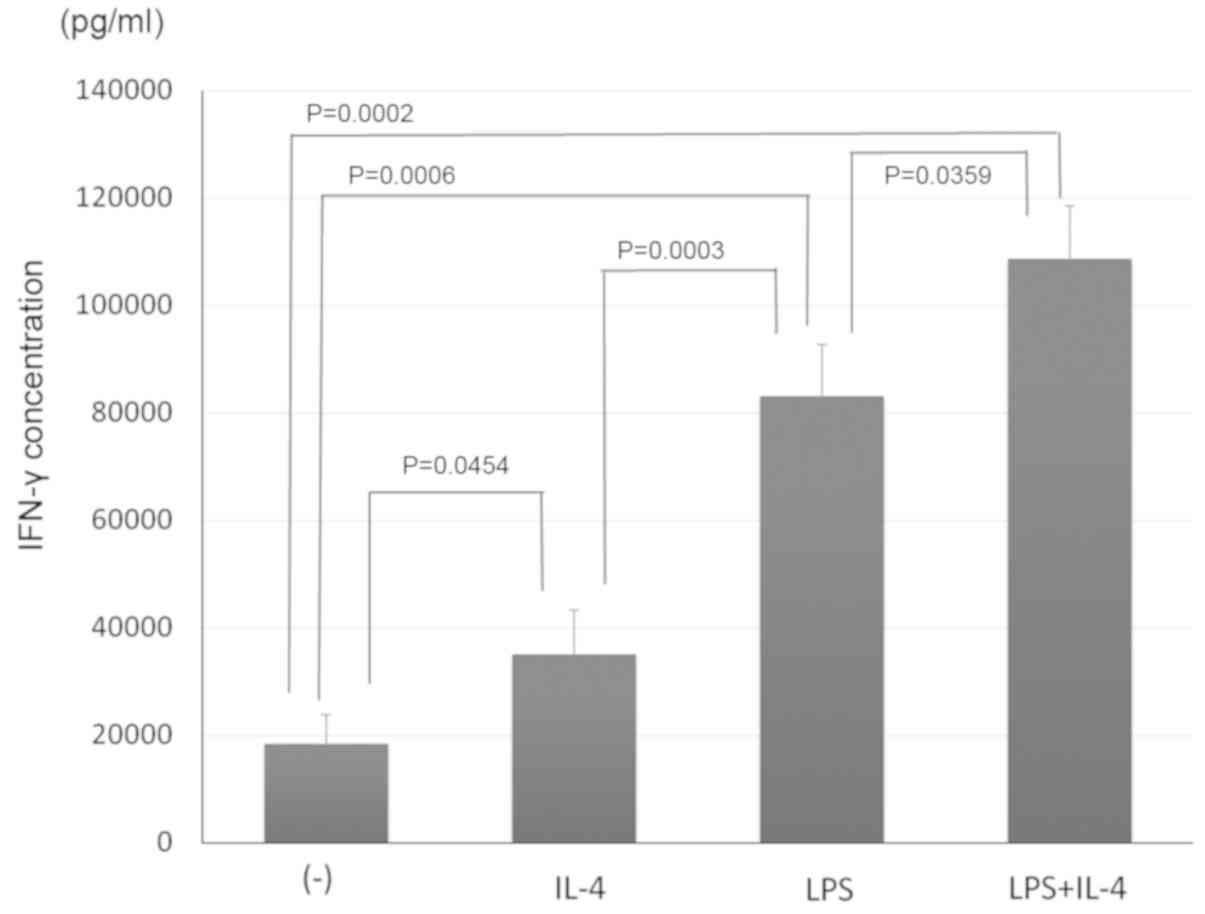
Bone marrow-derived adherent cells were co-cultured
with doxorubicin-treated neuro-2a cells plus LPS and/or
interleukin-4 to compare their adjuvant effect on the induction of
an antitumor immune response by the bone-marrow derived cells. In
addition, CD11c+ cells were co-cultured with
CD8α+ lymphocytes (as responder cells) and UV-treated
neuro-2a cells (as stimulator cells), and the interferon-γ
concentration in the supernatant was measured by ELISA as an index
of the lymphocyte response. As shown in Fig. 2, interleukin-4 or LPS stimulation of
bone marrow-derived adherent cells co-cultured with
doxorubicin-treated neuro-2a cells enhanced the lymphocyte
response, as revealed by an increased production of interferon-γ.
Furthermore, stimulation with interleukin-4 and LPS induced a
stronger lymphocyte response from the cultured bone marrow-derived
adherent cells.
Stimulation of bone marrow-derived
adherent cells with LPS and interleukin-4 promotes induction of
CD11c+ MHC class II+ double-positive
cells
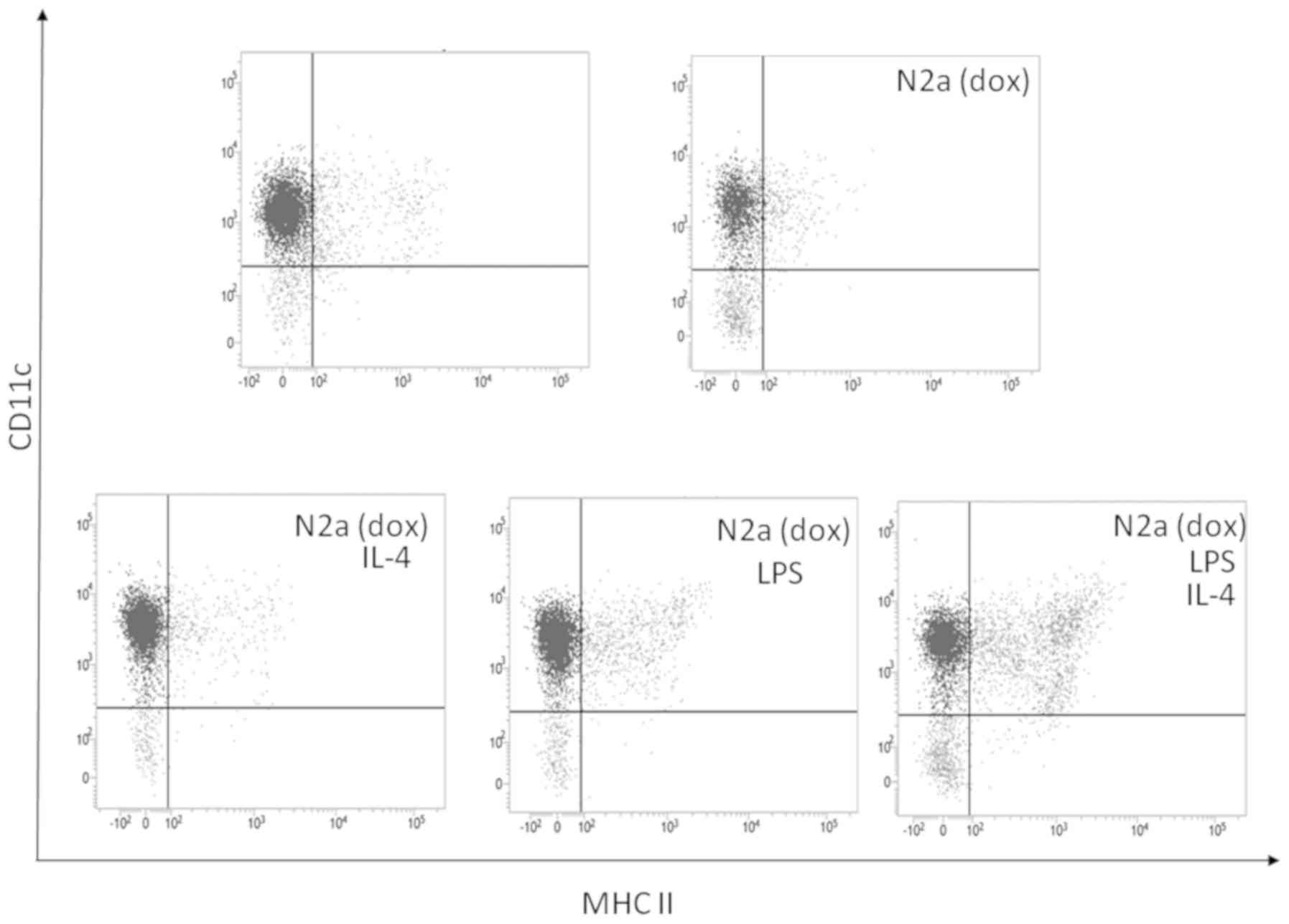
To confirm the effect of stimulation with
interleukin-4 and LPS, FACS analysis of the surface antigen and MHC
class II antigen expression by CD11c+ cells was
performed. As shown in Fig. 3, LPS
stimulation of bone marrow-derived adherent cells co-cultured with
doxorubicin-treated neuro-2a cells induced MHC class II expression
on the surface of CD11c+ cells, while stimulation with
interleukin-4 and LPS induced CD11c+ MHC II+
double-positive cells. Thus, co-culture of bone marrow-derived
adherent cells with immunogenically killed neuro-2a cells combined
with stimulation by interleukin-4 and LPS induced the maturation of
CD11c+ cells expressing MHC class II molecules.
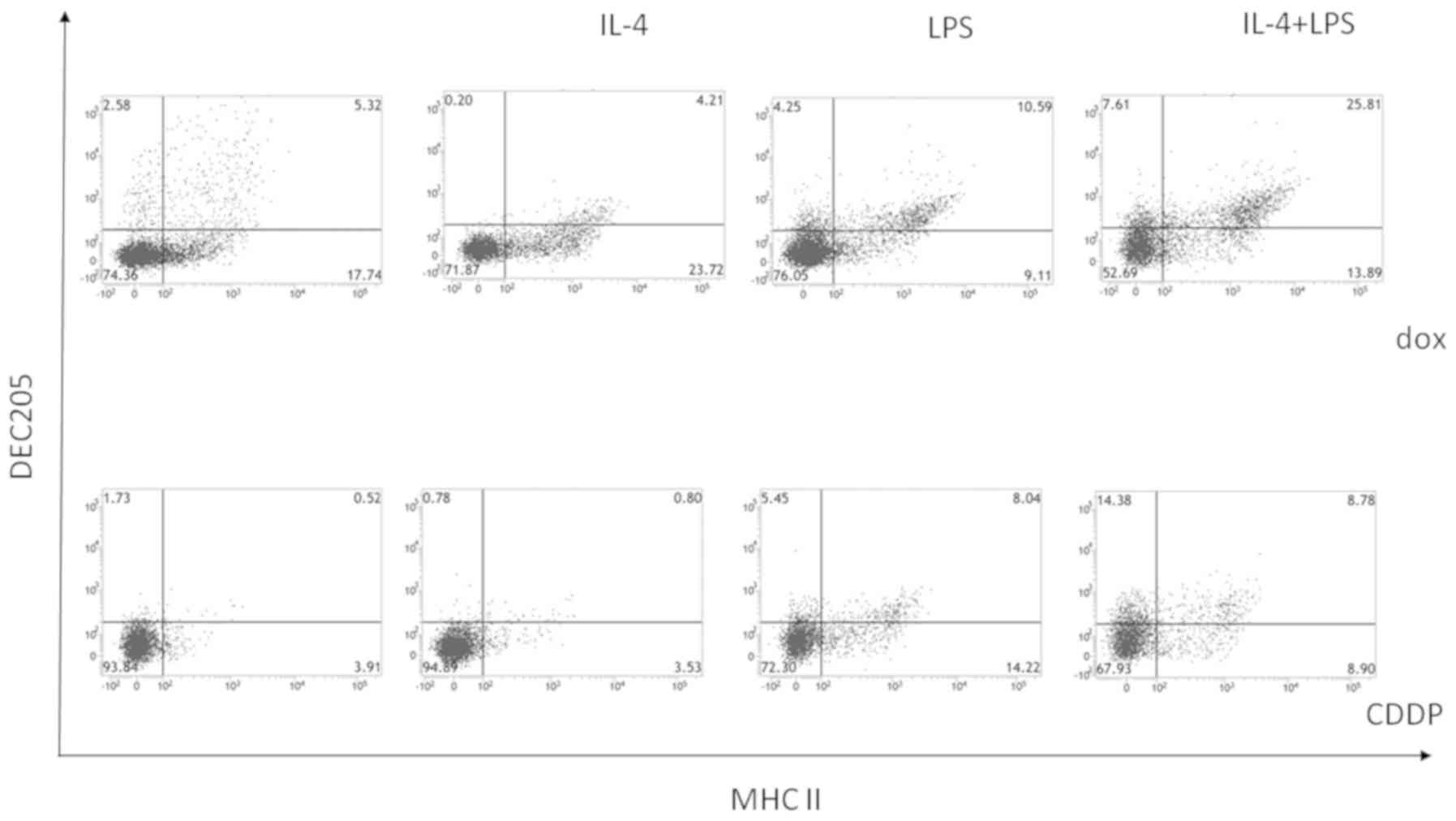
Stimulation by LPS and interleukin-4
during co-culture of bone marrow-derived cells with
doxorubicin-treated neuro-2a cells induces DEC-205+
CD11c+ MHC class II+ cells, but not
CD8α+ cells
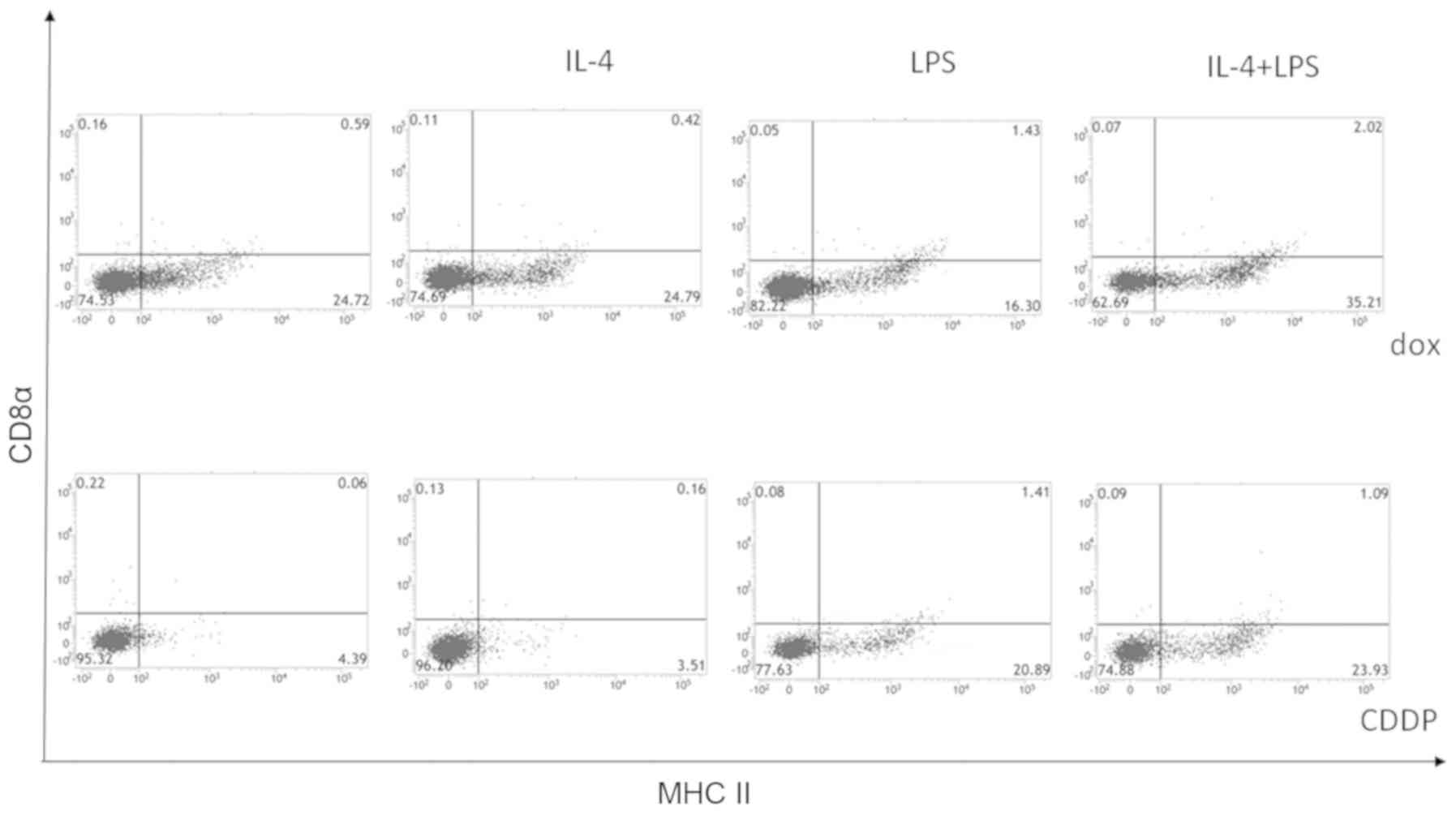
To analyze the characteristics of the cells inducing
an immune response to neuro-2a cells, the surface expression of
CD8α and DEC-205 by CD11c+ MHC class II+
double-positive cells co-cultured with doxorubicin-treated neuro-2a
cells and stimulated by interleukin-4 and LPS was investigated.
Cisplatin-treated neuro-2a cells were used as a negative control.
As shown in Fig. 4, the percentage of
CD8α− CD11c+ MHC II+ cells was
higher when adherent cells were co-cultured with
doxorubicin-treated neuro-2a cells compared to with
cisplatin-treated neuro-2a cells, and this effect was enhanced when
co-cultures were stimulated by interleukin-4 and LPS. As shown in
Fig. 5, DEC-205+
CD11c+ MHC II+ cells increased when bone
marrow-derived CD11c+ cells were co-cultured with
doxorubicin-treated neuro-2a cells and stimulated by interleukin-4
and LPS. Accordingly, stimulation by interleukin-4 and LPS during
co-culture of bone marrow-derived CD11c+ cells with
neuro-2a cells (which had been immunogenically killed by
doxorubicin) induced an immune response to neuroblastoma cells by
promoting maturation of CD11c+ MHC II+
DEC-205+ cells from bone marrow cells, although
CD11c+ MHC II+ CD8α+ cells were
not increased.
Discussion
Adoption of intensive multimodal therapy
significantly improved the initial survival rate of pediatric
cancer patients between 1975 and 1995 (8,9). However, it
was recently reported that the overall survival of patients with
advanced or high-risk neuroblastoma only showed a slight
improvement due to the heterogeneity of this tumor and/or
acquisition of resistance to conventional therapy (8,10). In a
previous clinical trial, the anti-GD2 antibody showed promise for
the treatment of high-risk or advanced neuroblastoma (1,2,11–13). GD2 is
an antigen that is normally expressed on the surface of neuronal
cells, peripheral nerve fibers and skin melanocytes, as well as
being highly expressed by neuroblastoma (12,13).
Anti-GD2 antibody therapy for neuroblastoma aims to induce passive
antibody-dependent cellular cytotoxicity following apoptosis of
tumor cells, and also aims to induce complement-dependent
cytotoxicity (12). Generating an
immune response to tumor cells is considered to be the ‘key’ to
effective immunotherapy for high-risk neuroblastoma.
Previous studies have revealed that specific
conventional antitumor agents modify the immune response to certain
tumor cells by affecting the host immune system or by modulating
tumor cell immunogenicity (3,4). In patients with advanced neuroblastoma,
initial tumor regression is usually induced by intensive multimodal
treatment that includes chemotherapy (12); however, residual tumor cells are not
eradicated by the chemotherapy agents. Combining chemoimmunotherapy
with passive induction of an antitumor immune response is
considered a promising approach to the treatment of high-risk
neuroblastoma. Our previous study reported that doxorubicin, a
major chemotherapy agent for neuroblastoma, induces immunogenic
death of neuroblastoma cells in a murine model (7). In that model, CD11c+ cells
derived from the bone marrow promoted lymphocyte proliferation more
effectively compared to CD11b+ spleen cells (7). The present study was performed to
investigate the lymphocyte response subsequent to the
CD11c+ cells from the bone marrow phagocytosed
neuroblastoma cells that had been immunogenically killed by
doxorubicin. Cisplatin was used as the negative control as it was
revealed that doxorubicin induces immunogenic death of mouse
neuroblastoma cells, while cisplatin does not (7). As shown in Figs.
1 and 2, interferon-γ production
in co-cultures of CD8α+ lymphocytes (responder cells)
with UV-treated neuro-2a cells (stimulator cells) and
CD11c+ cells (antigen-presenting effector cells) was
promoted when CD11c+ cells and doxorubicin-treated
neuro-2a cells were co-cultured with interleukin-4 or LPS prior to
harvesting for subsequent co-culture with CD8α+
lymphocytes. Therefore, CD11c+ cells derived from the
bone marrow can be ‘educated’ by co-culture with tumor cells that
have been immunogenically killed by doxorubicin. In addition,
interleukin-4 and/or LPS act as adjuvants to promote the
‘educational’ effect of immunogenically killed neuro-2a cells.
Interleukin-4 and LPS showed a stronger adjuvant effect when the
two agents were added to cultures simultaneously. During
conventional chemotherapy for malignancy, Apetoh et al
(14) found that Toll-like receptor 4
stimulation is essential for DC to cross-present antigens from
dying tumor cells, leading to induction of antitumor immunity. In
addition, interleukin-4 has been reported to expand and activate DC
and stimulate antitumor immunity in vivo (15,16). In
addition, interleukin-4 was reported to prevent blockade of DC
maturation by renal cell carcinoma (17). These immunomodulatory mechanisms may
have been involved in the induction of antitumor immunity to
neuroblastoma cells in the present study.
To establish effective immunotherapy for advanced
neuroblastoma, promoting the presentation of tumor antigens by
specific cells involved in innate cellular immune responses is
vital for initiation of antitumor immunity.
Tumor cells exhibit poor immunogenicity (18). Our previous study demonstrated that the
immunogenicity of neuroblastoma cells could be increased by
applying the concepts of chemoimmunotherapy (7). In the present study, the initiation of DC
maturation and the promotion of tumor antigen presentation were
assessed in order to establish a new immunotherapy method. DEC-205
is a cell surface antigen expressed by DC, B cells and T cells
(19), including CD8+ DC.
CD8+ DEC-205+ DC are considered to specialize
in the uptake of dying cells and presentation of MHC class I
antigens. By contrast, CD11c+ CD8−
33D1+ cells were reported to specialize in presenting
MHC class II antigens and the initiation of CD4+ T cell
responses (20). Neubert et al
(21) demonstrated that
CD11c+ CD8−33D1+ DC and
CD11c+ CD8+ DEC-205+ DC could
present tumor antigens and reduce tumor growth, thus prolonging the
survival of melanoma-bearing mice. In the present study,
CD11c+ MHC II+ DEC-205+ DC
increased, while CD8α+ cells did not, when
GM-CSF-dependent bone marrow cells were co-cultured with
immunogenically killed neuroblastoma cells along with stimulation
by LPS and interleukin-4, and these CD11c+ MHC
II+ DEC-205+ DC promoted interferon-γ
production when co-cultured with CD8α+ lymphocytes.
Lahoud et al (22) reported
that DEC-205 expressed on DC is a key receptor for binding CpG
oligodendronucleotides and has an important role in the production
of various cytokines. Thus, not only direct presentation of tumor
antigens but also other indirect mechanisms could be involved in
promoting the response of CD8α+ lymphocytes when
CD11c+ cells were co-cultured with immunogenically
killed tumor cells and stimulated by LPS and interleukin-4.
In conclusion, the antigen-presenting cells that
were induced from bone marrow cells promoted interferon-γ
production in co-cultures of CD8α+ lymphocytes and
murine neuroblastoma cells. Induction of these cells was achieved
by co-culture with tumor cells (subsequent to causing immunogenic
death by exposure to doxorubicin) plus stimulation by LPS and
interleukin-4. These cells showed surface expression of CD11c, MHC
II and DEC-205. However, CD11c+ MHC II+
CD8α+ cells were not induced by this co-culture
system.
Acknowledgements
The present study was supported in part by a Saitama
Medical University Internal grant (no. 25B105) and by a
Grant-in-Aid for Scientific Research (C) (no. 15K10927).
References
|
1
|
Yu AL, Gilman AL, Ozkaynak MF, London WB,
Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay
KK, et al: Children's Oncology Group: Anti-GD2 antibody with
GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J
Med. 363:1324–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Navid F, Sondel PM, Barfield R, Shulkin
BL, Kaufman RA, Allay JA, Gan J, Hutson P, Seo S, Kim K, et al:
Phase I trial of a novel anti-GD2 monoclonal antibody,
Hu14.18K322A, designed to decrease toxicity in children with
refractory or recurrent neuroblastoma. J Clin Oncol. 32:1445–1452.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen G and Emens LA: Chemoimmunotherapy:
Reengineering tumor immunity. Cancer Immunol Immunother.
62:203–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Emens LA: Chemoimmunotherapy. Cancer J.
16:295–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ullrich E, Ménard C, Flament C, Terme M,
Mignot G, Bonmort M, Plumas J, Chaperot L, Chaput N and Zitvogel L:
Dendritic cells and innate defense against tumor cells. Cytokine
Growth Factor Rev. 19:79–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sica A: Role of tumour-associated
macrophages in cancer-related inflammation. Exp Oncol. 32:153–158.
2010.PubMed/NCBI
|
|
7
|
Inoue S, Setoyama Y and Odaka A:
Doxorubicin treatment induces tumor cell death followed by
immunomodulation in a murine neuroblastoma model. Exp Ther Med.
7:703–708. 2014.PubMed/NCBI
|
|
8
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith MA, Altekruse SF, Adamson PC, Reaman
GH and Seibel NL: Declining childhood and adolescent cancer
mortality. Cancer. 120:2497–2506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hara J: Development of treatment
strategies for advanced neuroblastoma. Int J Clin Oncol.
17:196–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parsons K, Bernhardt B and Strickland B:
Targeted immunotherapy for high-risk neuroblastoma - the role of
monoclonal antibodies. Ann Pharmacother. 47:210–218. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mackall CL, Merchant MS and Fry TJ:
Immune-based therapies for childhood cancer. Nat Rev Clin Oncol.
11:693–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roth MD, Gitlitz BJ, Kiertscher SM, Park
AN, Mendenhall M, Moldawer N and Figlin RA: Granulocyte macrophage
colony-stimulating factor and interleukin 4 enhance the number and
antigen-presenting activity of circulating CD14+ and
CD83+ cells in cancer patients. Cancer Res.
60:1934–1941. 2000.PubMed/NCBI
|
|
16
|
Kiertscher SM, Gitlitz BJ, Figlin RA and
Roth MD: Granulocyte/macrophage-colony stimulating factor and
interleukin-4 expand and activate type-1 dendritic cells (DC1) when
administered in vivo to cancer patients. Int J Cancer. 107:256–261.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Menetrier-Caux C, Thomachot MC, Alberti L,
Montmain G and Blay JY: IL-4 prevents the blockade of dendritic
cell differentiation induced by tumor cells. Cancer Res.
61:3096–3104. 2001.PubMed/NCBI
|
|
18
|
Ashman LK: The immunogenicity of tumour
cells. Immunol Cell Biol. 65:271–277. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inaba K, Swiggard WJ, Inaba M, Meltzer J,
Mirza A, Sasagawa T, Nussenzweig MC and Steinman RM: Tissue
distribution of the DEC-205 protein that is detected by the
monoclonal antibody NLDC-145. I. Expression on dendritic cells and
other subsets of mouse leukocytes. Cell Immunol. 163:148–156. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dudziak D, Kamphorst AO, Heidkamp GF,
Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW,
Park CG, et al: Differential antigen processing by dendritic cell
subsets in vivo. Science. 315:107–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neubert K, Lehmann CH, Heger L, Baranska
A, Staedtler AM, Buchholz VR, Yamazaki S, Heidkamp GF, Eissing N,
Zebroski H, et al: Antigen delivery to
CD11c+CD8− dendritic cells induces protective
immune responses against experimental melanoma in mice in vivo. J
Immunol. 192:5830–5838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lahoud MH, Ahmet F, Zhang JG, Meuter S,
Policheni AN, Kitsoulis S, Lee CN, O'Keeffe M, Sullivan LC, Brooks
AG, et al: DEC-205 is a cell surface receptor for CpG
oligonucleotides. Proc Natl Acad Sci USA. 109:16270–16275. 2012.
View Article : Google Scholar : PubMed/NCBI
|