Introduction
Supplementation of menaquinone (MK)-7, a subtype of
vitamin K2 (VK2), has been found to significantly improve the
age-related decline in bone health and the bone mineral density
(BMD) at the lumber spine and femoral neck (1). Among the VK2 molecules, MK-4 (VK2 with
4 isoprene units) is known to contribute to high γ-carboxylation
activity and exhibit the most potent biological activity of the
VK2s (2). Previous meta-analyses
have demonstrated the anti-fracture effect of VK2 in patients with
a high risk of fracture (3,4). In a clinical study, VK2 administration
was not shown to result in an increase in BMD, but the frequency of
bone fracture was found to be significantly reduced (1). Ovariectomized rats show significant
bone loss, which VK2 prevents by inhibiting bone resorption and
osteoclast (Oc) formation, as determined by bone histomorphometry
(5). VK2 has been shown to exhibit
the novel function of binding to the pregnane X receptor (PXR), and
PXR-dependent biological functions have been demonstrated in bone
(6); however the biological effect
of VK2 remains controversial, since Fu et al (7) reported that VK2 failed to prevent bone
loss in ovariectomized rats.
Water-immersion restraint stress (WRS), which
involves both psychological and physical stress, is widely used to
induce reproducible stress ulcers in experimental animals (8–12). WRS
consists of immobilization and cold stress, in which the exposure
temperature is usually 24°C. Rats subjected to 6 h of WRS exhibit
disruption of the non-enzymatic antioxidant defense systems in the
brain and marked increases in the serum concentrations of
adrenocorticotropic hormone and corticosterone (13); however, the effect of stress on bone
mass and bone turnover is still debated.
Senescence-accelerated mouse (SAM) strains are
developed by means of repeated inbreeding, using shortness of
lifespan and aging speed as indices (14). All SAM prone (SAMP) strains are
short-lived and exhibit accelerated senescence. SAMP strains are
categorized into 11 groups according to the senile diseases that
they develop naturally, such as senile amyloidosis (SAMP1, 2, 7 and
11), temporomandibular disorders (SAMP3), senile osteoporosis
(SAMP6), learning and memory impairments (SAMP8 and 10) and
cataract (SAMP9) (15–17). The SAMP6 strain presents decreasing
BMD from the age of 16 weeks, and has a lifespan of ~32 weeks
(18). In order to assess the
effects of various drugs affecting BMD, additional approaches to
reduce BMD must be taken in the SAMP6 strain.
In the present study, the effect of WRS on BMD was
estimated and the histomorphometrical phenotype of bone resorption
and formation in the proximal femur metaphysis of SAMP6-strain mice
was characterized. Furthermore, the effect of MK-4 on WRS-induced
bone loss was investigated using bone histomorphometry, bone
densitometry and biomarkers of bone turnover.
Materials and methods
Animal experiments
Five-week-old SAMP6 males were purchased from Japan
SLC, Inc. (Hamamatsu, Japan). The animals were given a normal
rodent chow and tap water and were acclimated to the conditions for
1 week. The mice were then divided into the following three groups
(n=6 per group): Control, WRS and WRS + MK-4. WRS was performed for
6 h per day, 5 times a week, for 4 weeks. Following WRS, MK-4 (30
mg/kg; Eisai Co., Ltd., Tokyo, Japan), a VK2 molecule, was injected
subcutaneously 3 times/week, while the control and WRS mice were
injected with saline solution as a vehicle. The WRS method has been
previously described in detail (19). Briefly, the mice were restrained in a
punctured 50-ml conical centrifuge tube and immersed vertically to
the level of the xiphoid process in a 24±1°C water bath. To assess
bone formation, the mice were double-labeled with subcutaneous
injections of 20 mg/kg tetracycline hydrochloride and 10 mg/kg
calcein (Sigma-Aldrich Corp., St. Louis, MO, USA), 5 and 2 days
before sacrifice, respectively. The body weight of each animal was
measured every other day until the last day of MK-4 administration.
Mice were placed in metabolic cages and urine samples were
collected 16 h after the final administration. Urine samples were
stored at −80°C until analysis. One day after the final MK-4
administration, the mice were anesthetized using isoflurane, and
blood samples were collected from the abdominal aorta, the mice
died due to excessive loss of blood. The blood samples were
centrifuged at 3,200 × g for 10 min at room temperature to separate
the serum, which was then stored at −80°C. Following the collection
of the blood samples, the bilateral femurs were removed, cleaned
from soft tissues and fixed in 70% ethanol. The experimental
protocol was approved by the Animal Research Committee of the
Kawasaki Medical School (Kurashiki, Japan; 11-031).
Trabecular (Tb) BMD
The fixed right femoral bones were analyzed using an
X-ray Computed Tomography system for small experimental animals
(LaTheta LCT-200; Aloka Co., Ltd, Osaka, Japan). Each bone was
placed horizontally inside a tube and scanned using a 96-µm voxel.
The scan line was adjusted using the scout view. The Tb BMD in the
secondary spongiosa was measured from the growth plate to 3 mm
proximal at the femoral distal end. The data were quantified using
the automated image analysis software supplied with the device
(LCT-200F1, v.3.22; Aloka Co., Ltd.).
Analysis of bone turnover biochemical
markers
Urinary Ca2+ concentration and serum
alkaline phosphatase (ALP) activity were determined using an
autoanalyzer (Hitachi 7180; Hitachi, Ltd., Tokyo, Japan). The total
serum protein concentration was measured using a refractometer
(Atago Co., Ltd., Tokyo, Japan). Serum levels of Gla-osteocalcin
(OCN) and tartrate-resistant acid phosphatase (TRACP) 5b were
determined using a mouse Gla-OCN Competitive Enzyme Immunoassay
(EIA) kit (Takara, Inc., Kyoto, Japan) and a mouse TRACP assay kit
(Immunodiagnostic Systems, Inc., Fountain Hills, AZ, USA),
respectively, according to the manufacturers' instructions. The
concentration of C-terminal telopeptides of type I collagen (CTX)
in the urine was determined using a commercial EIA kit
(Immunodiagnostic Systems, Inc.), according to the manufacturer's
instructions, and the levels were corrected against the creatinine
(Cr) concentration. The Cr level in the collected samples was
measured by SRL Inc. (Tokyo, Japan).
Bone histomorphometry
Bone histomorphometry was performed on the secondary
spongiosa of the left femoral distal end of samples at the Ito Bone
Histomorphometry Institute (Niigata, Japan). The coronal view of
the femoral distal end was observed using Villanueva Bone Staining.
Bone histomorphometrical examination was used to evaluate the
tissue volume (TV, µm2), bone volume (BV,
µm2), bone surface (BS, µm), single-labeled surface,
double-labeled surface (dLS, mm), Tb thickness (Tb.Th, µm) and the
number of osteoclasts and osteoblasts (N.Ocs and N.Obs,
respectively). Obs were further classified into type II–IV
according to morphological classification criteria (20): Type II, classical cuboidal or
columnar with adjacent nuclear clear zone, type III, intermediate
without adjacent nuclear clear zone and type IV,
cytoplasm-extremely thin (most mature population) (21). Type I Obs cannot be detected by
microscopy. The following parameters were then estimated from the
primary parameters: BV/TV (%), osteoid surface (OS)/BS (%), eroded
surface (ES)/BS (%), N.Ocs/BS (N/mm), surface area of Ocs
(Ocs.S)/BS (%) mineral apposition rate (MAR, µm/day), Obs.S/BS (%)
and N.Obs/BS (N/mm). Standard bone histomorphometrical
nomenclature, symbols and units were used as described in the
report by the American Society for Bone and Mineral Research
Histomorphometry Nomenclature Committee (22).
Statistical analysis
Data are expressed as the mean ± standard deviation.
In order to compare the differences between the control, WRS and
WRS + MK-4 groups, a one-way analysis of variance (ANOVA), followed
by the Tukey-Kramer post hoc test, was employed using JMP 10
software (SAS Institute Inc., Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Growth of SAMP6 mice and effect of WRS
on BMD
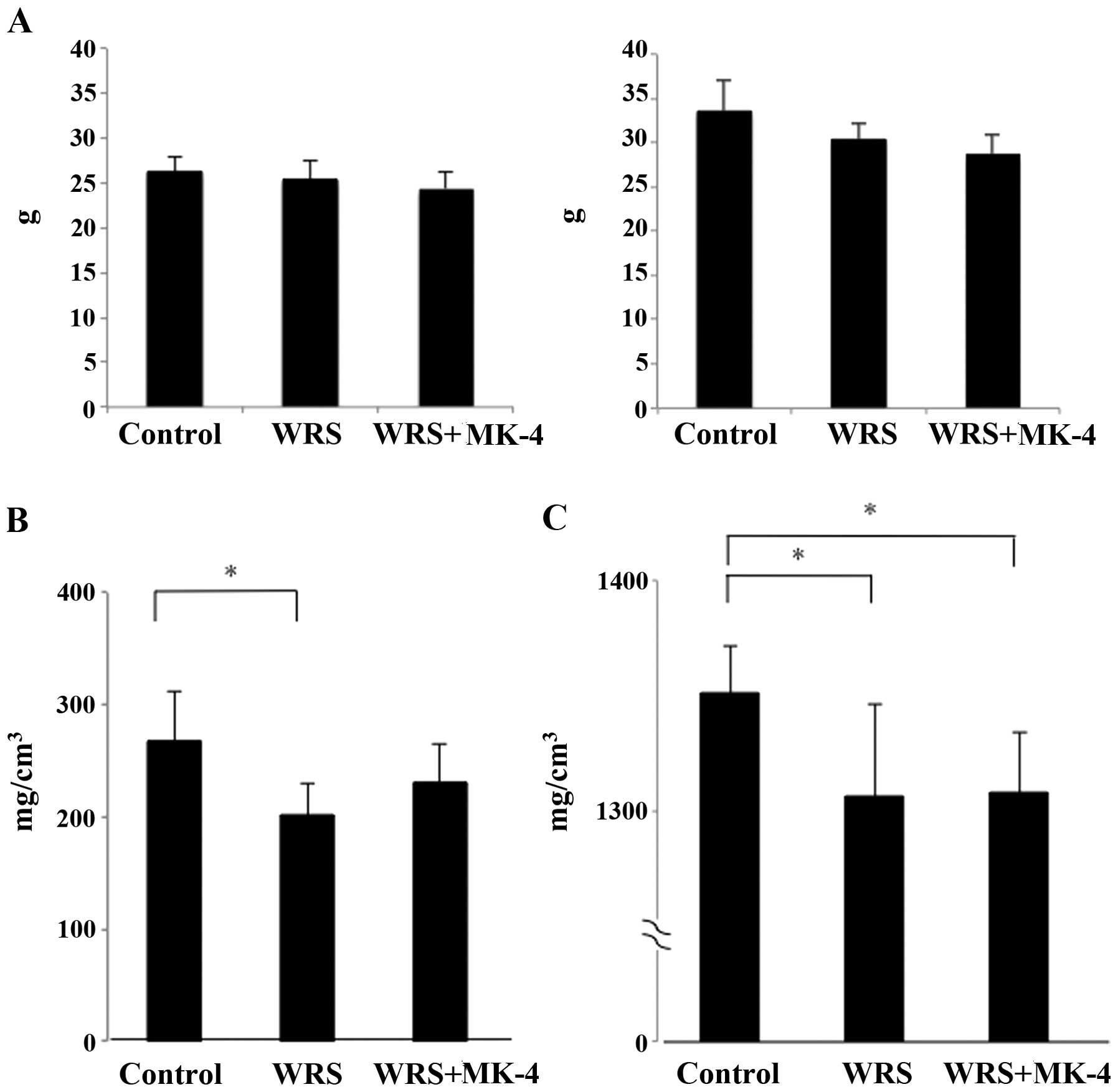
No significant differences were observed in either
the initial or final body weights among the groups, despite the
fact that the final body weights of the WRS groups had a tendency
to be lower than those of the control group (Fig. 1A). In addition, the femoral lengths
were 15 mm in all groups, and no statistically significant
differences were observed. Both the Tb and the cortical BMDs were
significantly decreased in the WRS mice compared with those in the
control mice (Fig. 1B and C).
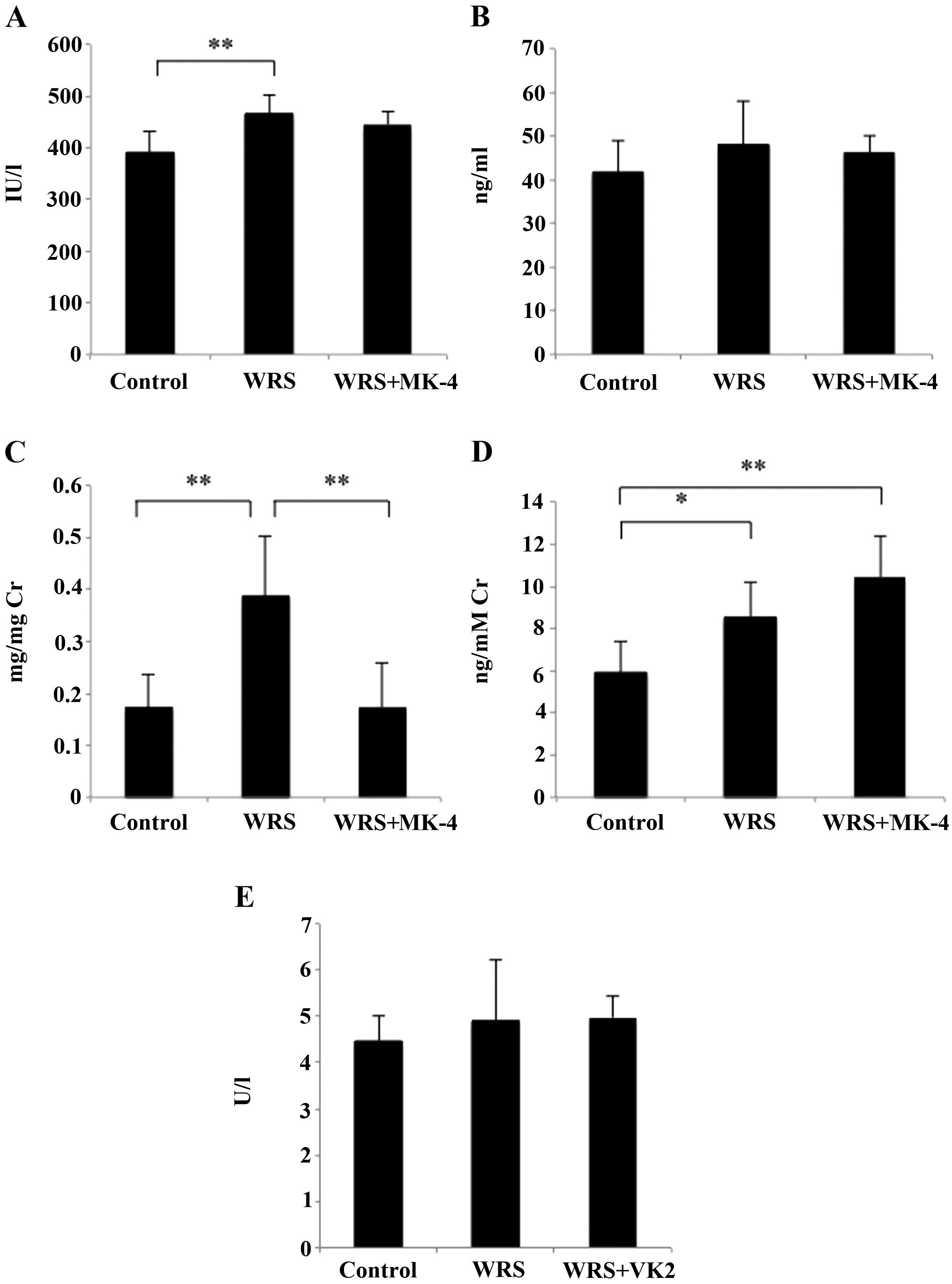
Effect of MK-4 on biochemical markers
of bone turnover following WRS
In the WRS group, levels of serum ALP, a bone
formation marker, were significantly higher than those in the
control; however, MK-4 had no effect on serum ALP in the WRS mice
(Fig. 2A). No significant
differences were identified in the Gla-OCN levels among the groups
(Fig. 2B); however, Ca2+
excretion in the urine was significantly higher in the WRS group
than that in the control group, and this excretion was suppressed
following the administration of MK-4 (Fig. 2C). The levels of urinary CTX, a
degradation product of collagen, were significantly higher in the
WRS and WRS + MK-4 groups than those in the control (Fig. 2D); however, no significant
differences were identified in TRACP 5b, a bone resorption marker
(Fig. 2E).
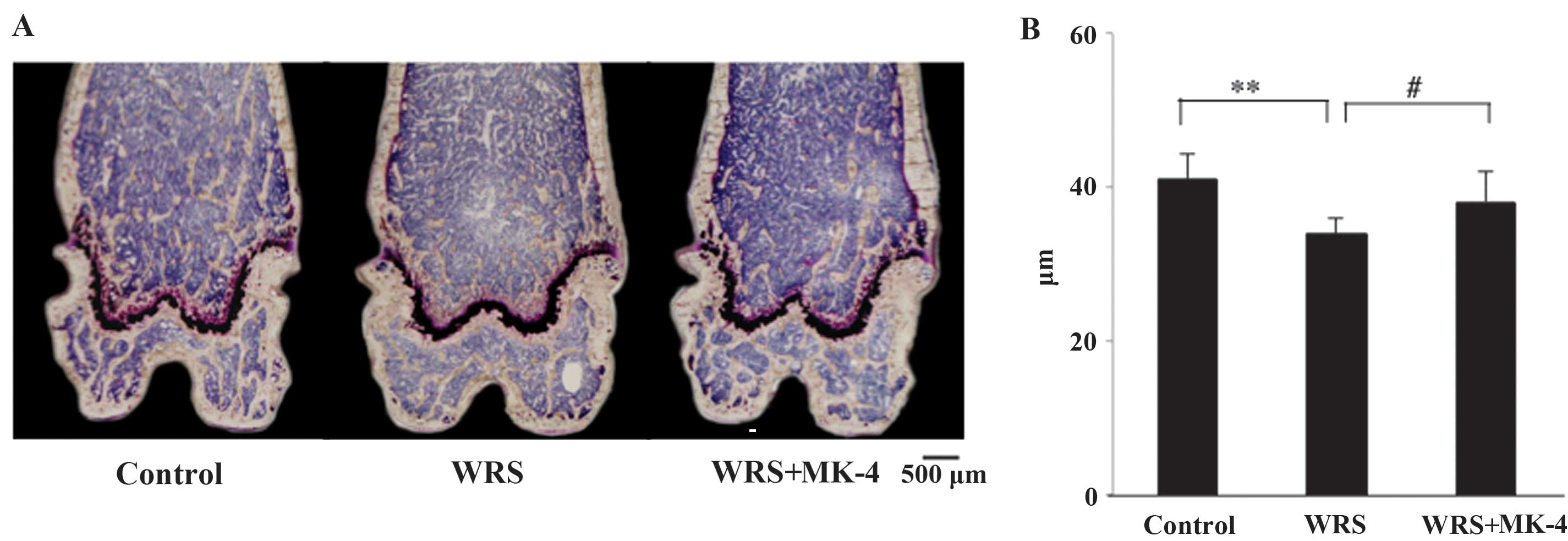
Microscopic observation of the distal
femurs of SAMP6 mice
The sagittal view of the left distal femurs was
observed using Villanueva Bone Staining (Fig. 3A). Microscopic observation showed
that the growth plate and primary spongiosa were thin in all
groups. These findings suggested that SAMP6 mice were senescent at
10 weeks of age. When the SAMP6 mice were exposed to WRS, the
quantity of Tb bone in the secondary spongiosa of the distal femur
decreased. The Tb bone mass was recovered when MK-4 was
administered. The Tb.Th was significantly lower in the WRS group
than that in the control group, and this reduction was attenuated
in the WRS + MK-4 group (Fig.
3B).
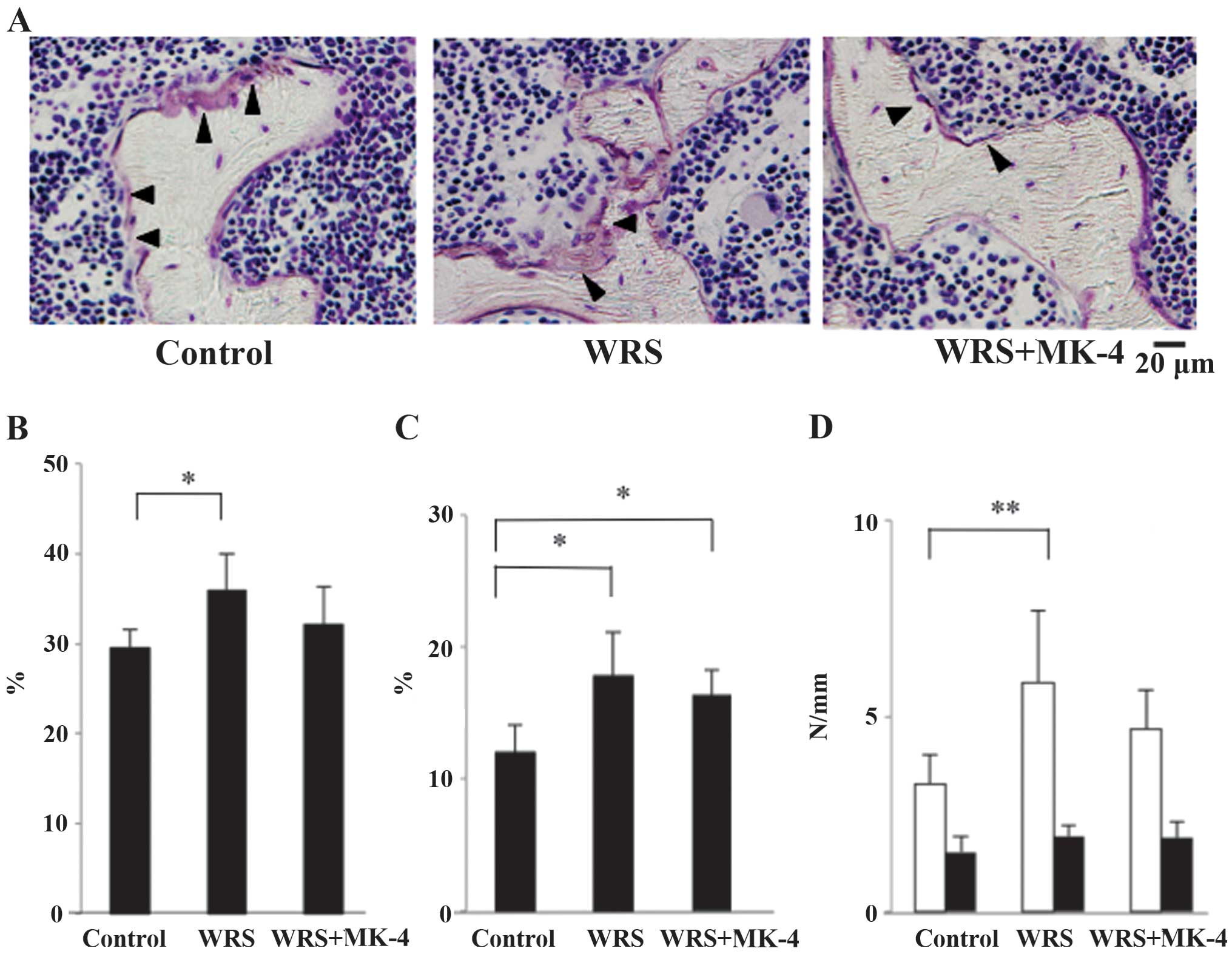
Bone resorption in the Tb bone of
SAMP6 mice
The bone resorption area was first evaluated using
bone histomorphometry (Fig. 4A). The
ES/BS percent age was significantly higher in the WRS group than
that in the control group (Fig. 4A and
B) and was effectively reduced by MK-4.
The Ocs.S/BS percentage was significantly higher in
the WRS groups than that in the control group (Fig. 4C). Furthermore, the number of
multinucleated Ocs was significantly higher in the WRS group than
that in the control group (Fig. 4D),
an increase that was not observed in the WRS + MK-4 group. No
significant differences were observed in the number of
mononucleated Ocs among these groups.
Bone formation in the Tb bone of SAMP6
mice
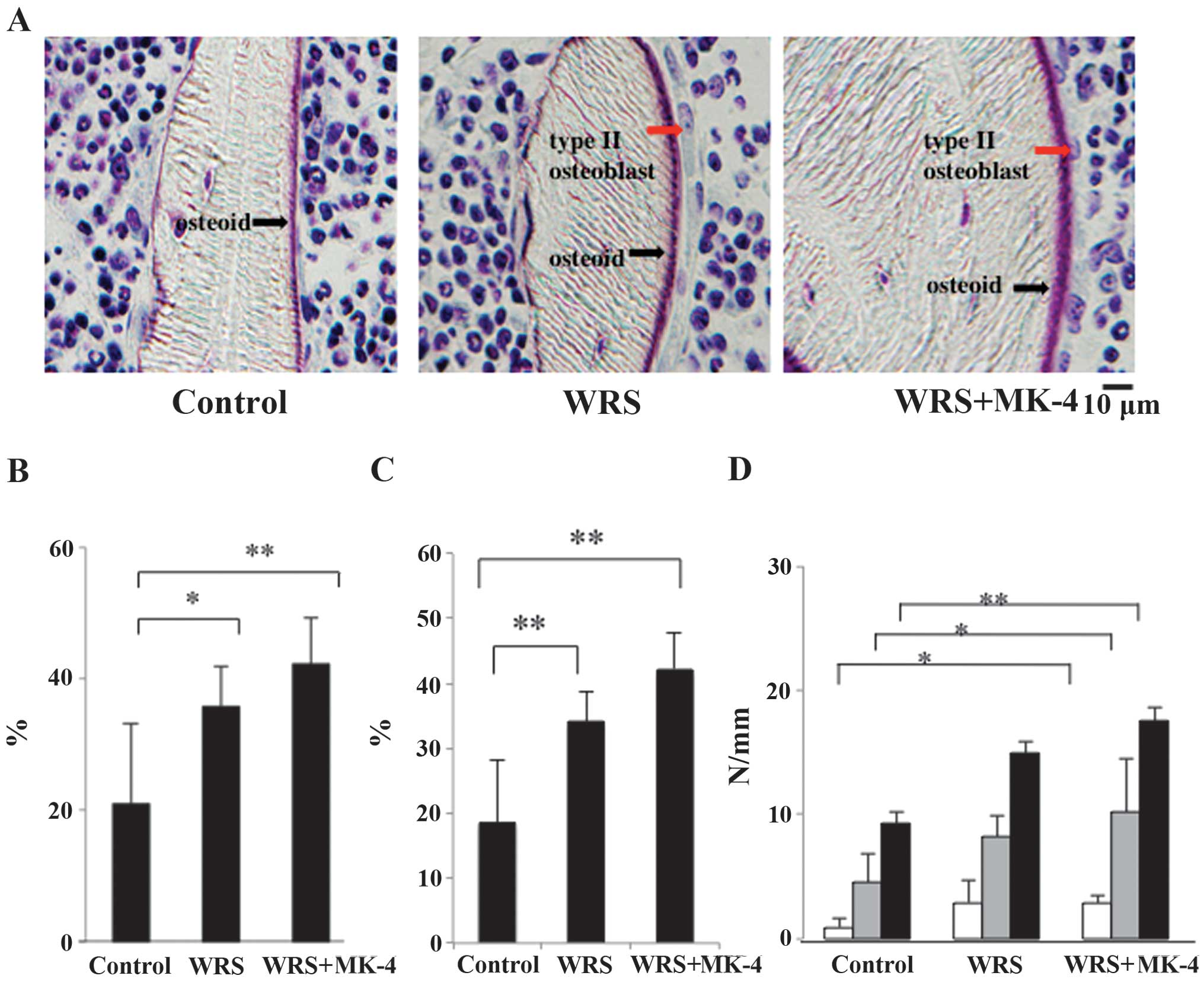
Microscopic observation showed the osteoid width to
be thicker in the WRS + MK-4 group than that in the control group
(Fig. 5A). The OS/BS percentages in
the WRS groups were significantly higher than those in the control
group (Fig. 5B), indicating that WRS
induced bone formation. Furthermore, the Obs.S/BS percentages in
the WRS groups were significantly higher than those in the control
group (Fig. 5C). Although no
difference was observed between the WRS and WRS + MK-4 groups, all
the numbers of all Ob types were significantly increased in the WRS
+ MK-4 group compared with the control group (Fig. 5D). These results indicated that MK-4
increased the N.Obs. In combination with its effect on Ocs, WRS may
cause both bone resorption and formation, resulting in increased
bone turnover. In addition, MK-4 may stimulate recovery from
WRS-induced bone loss.
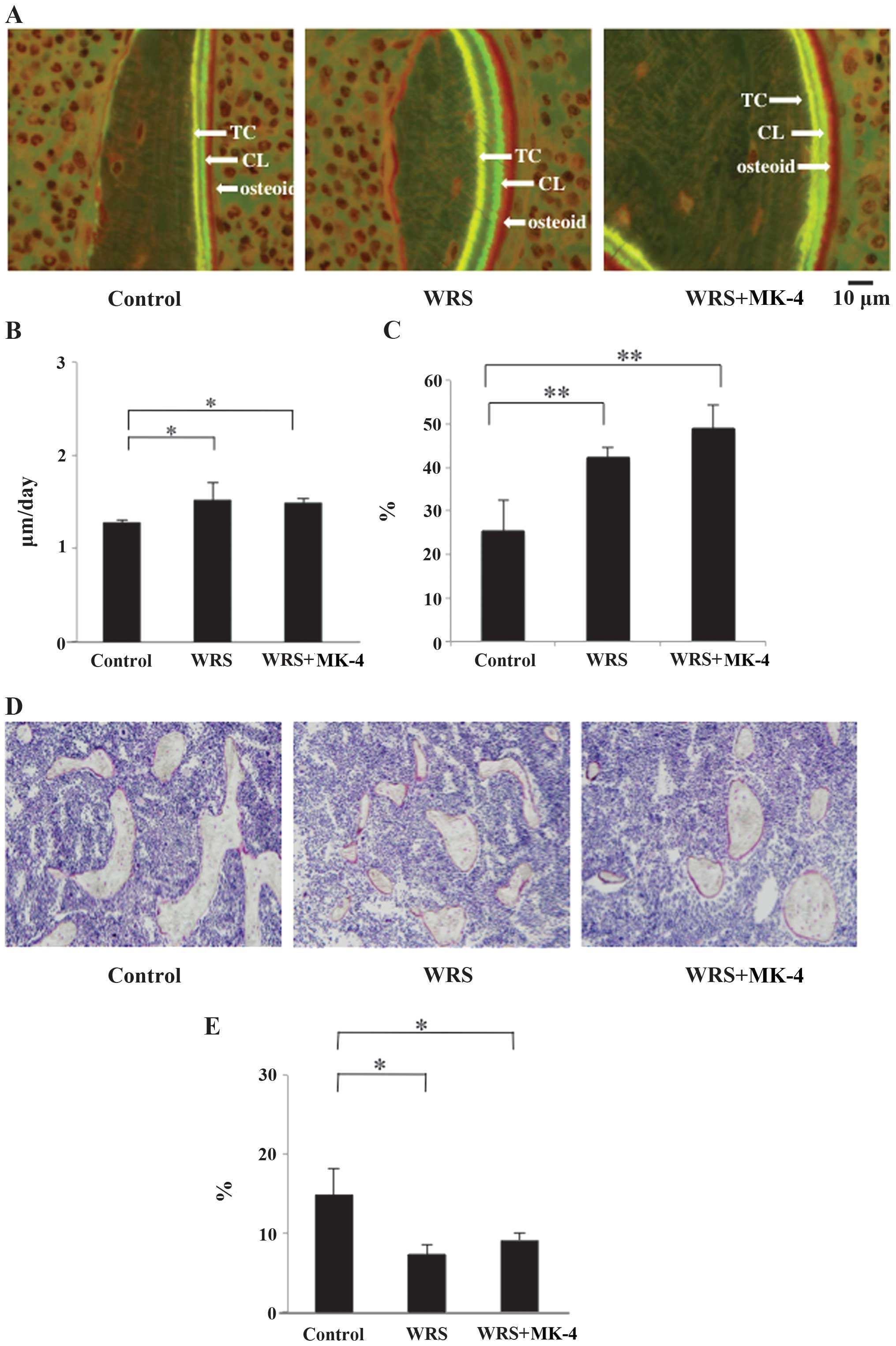
MAR and BV
Since tetracycline and calcein were used for double
staining, the time interval between the injections of the two
labels enabled the determination of the MAR, with a wide physical
distance between the labeled layers indicating rapid
mineralization. The WRS and WRS + MK-4 groups exhibited a
significantly higher MAR than the control group (Fig. 6A and B), and the dLS/BS was
significantly higher in the WRS and WRS + MK-4 groups compared with
that in the control group (Fig. 6C).
These results indicated that WRS promoted bone formation and MK-4
also appeared to promote bone mineralization.
Microscopic observation of the bones showed that the
Tb bones of all groups were small, rounded and isolated (Fig. 6D). This phenomenon was considered
characteristic of the SAMP6 strain. In addition, the Tb bone shape
in the WRS group was small and scattered compared with the control
and WRS + MK-4 groups, and the BV/TV percentage was significantly
lower in the WRS and WRS + MK-4 groups than that in the control
group (Fig. 6E).
Discussion
The SAMP6 strain shows the characteristic Tb bone
shape in bone histomorphometry, and WRS successfully reduces BV/TV.
The SAMP6 strain starts losing bone at 16 weeks and exhibits BV and
BMD loss at 28 weeks (18); however,
ovariectomy of female SAMP6 mice does not lead to an increase in
osteoclastic activity or a further decrease in bone mass, since the
SAMP6 strain is a hormone-independent model of osteoporosis
(23). Despite attempts in the
present study to induce bone loss by ovariectomy of female SAMP6
mice, the females showed no bone loss at 12 weeks (data not shown).
Thus, SAMP6 males were used, since they are hormonally stable in
adolescence compared with SAMP6 females.
WRS is often used as a stress treatment to induce
stress response syndromes in animals (8–13,24). In
the present study, WRS successfully reduced the BMD via a high bone
turnover rate; WRS induced an increase in bone resorption and
formation. There are several mouse models for the reduction of BMD,
such as ovariectomy of female mice (25), administration of soluble receptor
activator of nuclear factor κB ligand (RANKL) (26) and tail suspension (27). Ovariectomy leads to BMD loss for 8
weeks, but requires specific surgical skills, while the
intraperitoneal injection of soluble RANKL reduces BMD for only 50
h; however, soluble RANKL is expensive and difficult to obtain.
Tail suspension leads to a reduction of BMD for 14 days, caused by
immobilization. The type of bone loss caused by WRS is different
from immobilization-induced bone loss, since immobilization leads
to incremental bone resorption but to a decrease in bone formation
(27). In the present study, WRS did
not trigger growth retardation, and bone reduction was observed
after a 4-week treatment. Thus, WRS could be a model of stress
response and osteopenia in the SAMP6 strain.
Since VK2 consists of 14 isoprene units and MK-4 is
the most potent form of VK2, MK-4 was administered subcutaneously
to SAMP6 mice as VK2 treatment. The biological function of VK2 is
associated with anticoagulation and γ-carboxylation in various
proteins (28). Most VK-dependent
enzymes are involved in the hemostatic process and are associated
with bone metabolism. OCN and matrix Gla protein are two major Gla
proteins in the bone (29), but
their beneficial effects on the bone remain unclear. Studies have
shown that VK2 attenuates Tb bone loss in glucocorticoid-treated
rats (30) and prevents bone loss by
inhibiting bone resorption in ovariectomized rats (5). In vitro studies revealed that
VK2 influences Ob differentiation (31), binds to the steroid xenobiotic
receptor (6) and accelerates bone
mineralization (32). In addition, a
clinical study demonstrated that VK2 administration effectively
reduced the osteoporotic fracture incidence (1). Furthermore, VK2 induces Oc apoptosis
and reduces the bone resorption area (33). Based on these reports, VK2 may
maintain bone strength through the activation of osteoblastic
function and the suppression of osteoclastic function. The bone
histomorphometrical examination in the present study showed that
MK-4 attenuated WRS-induced Tb bone loss by inhibiting Oc activity
and increasing Ob activity. Furthermore, the Tb bone shape in the
WRS mice was quite different from that in the WRS + MK-4 mice.
Despite the fact that bone strength was not measured, the
aforementioned results indicate that MK-4 may improve BMD.
The present study had several limitations. First,
although MK-4 was administered subcutaneously to mice, the
absorption of MK-4 was not considered and the serum concentration
of MK-4 was not measured. Secondly, the SAMP6 strain was used for
all experiments; the results obtained in this study could therefore
be strain-specific phenomena.
In conclusion, WRS effectively reduced BMD in a
short period of time in the SAMP6 mice. Furthermore, MK-4 treatment
was efficient in recovering the WRS-induced bone mineral loss,
indicating its effect on the suppression of Oc function and the
increase in Ob function. Further studies are required in order to
clarify the association between MK-4 administration and bone
quality using a different mouse strain.
Acknowledgements
This study was supported by Grants-in-Aid for
Scientific Research (KAKENHI; grant no. 22500684). The authors
would like to acknowledge Editage for their English editing
service.
Glossary
Abbreviations
Abbreviations:
|
MK-4
|
menaquinone-4
|
|
BMD
|
bone mineral density
|
|
SAMP6
|
senescence-accelerated mouse prone
6
|
|
VK2
|
vitamin K2
|
|
WRS
|
water-immersion restraint stress
|
|
ALP
|
alkaline phosphatase
|
|
OCN
|
osteocalcin
|
|
TRACP
|
tartrate-resistant acid
phosphatase
|
|
CTX
|
C-terminal telopeptides of type I
collagen
|
|
Oc
|
osteoclast
|
|
Ob
|
osteoblast
|
References
|
1
|
Knapen MH, Drummen NE, Smit E, Vermeer C
and Theuwissen E: Three-year low-dose menaquinone-7 supplementation
helps decrease bone loss in healthy postmenopausal women.
Osteoporos Int. 24:2499–2507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koshihara Y, Hoshi K, Ishibashi H and
Shiraki M: Vitamin K2 promotes 1alpha,25(OH)2 vitamin D3-induced
mineralization in human periosteal osteoblasts. Calcif Tissue Int.
59:466–473. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwamoto J, Matsumoto H and Takeda T:
Efficacy of menatetrenone (vitamin K2) against non-vertebral and
hip fractures in patients with neurological diseases: Meta-analysis
of three randomized, controlled trials. Clin Drug Investig.
29:471–479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cockayne S, Adamson J, Lanham-New S,
Shearer MJ, Gilbody S and Torgerson DJ: Vitamin K and the
prevention of fractures: Systematic review and meta-analysis of
randomized controlled trials. Arch Intern Med. 166:1256–1261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akiyama Y, Hara K, Kobayashi M, Tomiuga T
and Nakamura T: Inhibitory effect of vitamin K2 (menatetrenone) on
bone resorption in ovariectomized rats: A histomorphometric and
dual energy X-ray absorptiometric study. Jpn J Pharmacol. 80:67–74.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Azuma K, Casey SC, Ito M, Urano T, Horie
K, Ouchi Y, Kirchner S, Blumberg B and Inoue S: Pregnane X receptor
knockout mice display osteopenia with reduced bone formation and
enhanced bone resorption. J Endocrinol. 207:257–263. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu X, Moreines J and Booth SL: Vitamin K
supplementation does not prevent bone loss in ovariectomized Norway
rats. Nutr Metab (Lond). 9:122012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Landeira-Fernandez J: Analysis of the
cold-water restraint procedure in gastric ulceration and body
temperature. Physiol Behav. 82:827–833. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang P, Zhou ZR, Zheng MQ and Shi FX:
Effect of the IGF-1/PTEN/Akt/FoxO signaling pathway in the duodenal
mucosa of rats subjected to water immersion and restraint stress.
Genet Mol Res 11 (AOP). 4775–4788. 2012.
|
|
10
|
Szlachcic A, Sliwowski Z, Krzysiek-Maczka
G, Majka J, Surmiak M, Pajdo R, Drozdowicz D, Konturek SJ and
Brzozowski T: New satiety hormone nesfatin-1 protects gastric
mucosa against stress-induced injury: Mechanistic roles of
prostaglandins, nitric oxide, sensory nerves and vanilloid
receptors. Peptides. 49:9–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nur Azlina MF, Kamisah Y, Chua KH and
Qodriyah HM: Tocotrienol attenuates stress-induced gastric lesions
via activation of prostaglandin and upregulation of COX-1 mRNA.
Evid Based Complement Alternat Med. 2013:8047962013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Magierowski M, Jasnos K, Pawlik M,
Krzysiek-Maczka G, Ptak-Belowska A, Olszanecki R, Kwiecien S,
Korbut R and Brzozowski T: Role of angiotensin-(1–7) in
gastroprotection against stress-induced ulcerogenesis. The
involvement of mas receptor, nitric oxide, prostaglandins and
sensory neuropeptides. J Pharmacol Exp Ther. 347:717–726. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohta Y, Yashiro K, Ohashi K and Imai Y:
Disruption of non-enzymatic antioxidant defense systems in the
brain of rats with water-immersion restraint stress. J Clin Biochem
Nutr. 51:136–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takeda T, Hosokawa M, Takeshita S, Irino
M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H and
Shimizu K: A new murine model of accelerated senescence. Mech
Ageing Dev. 17:183–194. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsushita M, Tsuboyama T, Kasai R,
Okumura H, Yamamuro T, Higuchi K, Higuchi K, Kohno A, Yonezu T,
Utani A, et al: Age-related changes in bone mass in the
senescence-accelerated mouse (SAM). SAM-R/3 and SAM-P/6 as new
murine models for senile osteoporosis. Am J Pathol. 125:276–283.
1986.PubMed/NCBI
|
|
16
|
Ohnishi K, Tomimoto H, Akiguchi I, Seriu
N, Kawamata T, Nakamura S, Kimura J, Nishio T, Higuchi K and
Hosokawa M: Age-related decrease of nerve growth factor-like
immunoreactivity in the basal forebrain of senescence-accelerated
mice. Acta Neuropathol. 90:11–16. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeda T, Hosokawa M and Higuchi K:
Senescence-accelerated mouse (SAM): A novel murine model of
senescence. Exp Gerontol. 32:105–109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Washimi Y, Chen H, Ito A, Takao R, Uzawa
T, Yamamoto Y, Yamada H and Shoumura S: Effect of intermittent
treatment with human parathyroid hormone 1–34 in SAMP6
senescence-accelerated mice. J Encocrinol Invest. 33:395–400. 2010.
View Article : Google Scholar
|
|
19
|
Takagi K and Okabe S: The effects of drugs
on the production and recovery processes of the stress ulcer. Jpn J
Pharmacol. 18:9–18. 1968. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parfitt AM: The cellular basis of bone
remodeling: The quantum concept reexamined in light of recent
advances in the cell biology of bone. Calcif Tissue Int 36. (Suppl
1):37–45. 1984. View Article : Google Scholar
|
|
21
|
Kawamori Y, Katayama Y, Asada N, Minagawa
K, Sato M, Okamura A, Shimoyama M, Nakagawa K, Okano T, Tanimoto M,
et al: Role for vitamin D receptor in the neuronal control of the
hematopoietic stem cell niche. Blood. 116:5528–5535. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dempster DW, Compston JE, Drezner MK,
Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR
and Parfitt AM: Standardized nomenclature, symbols and units for
bone histomorphometry: A 2012 update of the report of the ASBMR
Histomorphometry Nomenclature Committee. J Bone Miner Res. 28:2–17.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duque G, Macoritto M, Dion N, Ste-Marie LG
and Kremer R: 1,25(OH)2D3 acts as a bone-forming agent in the
hormone-independent senescence-accelerated mouse (SAM-P/6). Am J
Physiol Endocrinol Metab. 288:E723–E730. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomita M, Katsuyama H, Okuyama T, Watanabe
Y, Hidaka K, Otsuki T and Nata M: The effect of CAG repeat
polymorphism in the glucocorticoid receptor on stress responses of
mice exposed to water-immersion restraint stress. Int J Mol Med.
25:415–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sasaki H, Miyakoshi N, Kasukawa Y, Maekawa
S, Noguchi H, Kamo K and Shimada Y: Effects of combination
treatment with alendronate and vitamin K2 on bone mineral density
and strength in ovariectomized mice. J Bone Miner Metab.
28:403–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomimori Y, Mori K, Koide M, Nakamichi Y,
Ninomiya T, Udagawa N and Yasuda H: Evaluation of pharmaceuticals
with a novel 50-hour animal model of bone loss. J Bone Miner Res.
24:1194–1205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakata T, Sakai A, Tsurukami H, Okimoto N,
Okazaki Y, Ikeda S, Norimura T and Nakamura T: Trabecular bone
turnover and bone marrow cell development in tail-suspended mice. J
Bone Miner Res. 14:1596–1604. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohsaki Y, Shirakawa H, Miura A, Giriwono
PE, Sato S, Ohashi A, Iribe M, Goto T and Komai M: Vitamin K
suppresses the lipopolysaccharide-induced expression of
inflammatory cytokines in cultured macrophage-like cells via the
inhibition of the activation of nuclear factor κB through the
repression of IKKα/β phosphorylation. J Nutr Biochem. 21:1120–1126.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ichikawa T, Horie-Inoue K, Ikeda K,
Blumberg B and Inoue S: Vitamin K2 induces phosphorylation of
protein kinase A and expression of novel target genes in
osteoblastic cells. J Mol Endocrinol. 39:239–247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Iwamoto J, Matsumoto H, Tadeda T, Sato Y
and Yeh JK: Comparison of the effect of vitamin K(2) and
residronate on trabecular bone in glucocorticoid-treated rats: A
bone histomorphometry study. Yonsei Med J. 50:189–194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Katsuyama H, Saijoh K, Otsuki T, Tomita M,
Fukunaga M and Sunami S: Menaquinone-7 regulates gene expression in
osteoblastic MC3T3E1 cells. Int J Mol Med. 19:279–284.
2007.PubMed/NCBI
|
|
32
|
Atkins GJ, Welldon KJ, Wijenayaka AR,
Bonewald LF and Findlay DM: Vitamin K promotes mineralization,
osteoblast-to-osteocyte transition and an anticatabolic phenotype
by {gamma}-carboxylation-dependent and -independent mechanisms. Am
J Physiol Cell Physiol. 297:C1358–C1367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koshihara Y, Hoshi K, Okawara R, Ishibashi
H and Yamamoto S: Vitamin K stimulates osteoblastogenesis and
inhibits osteoclastogenesis in human bone marrow cell culture. J
Endocrinol. 176:339–348. 2003. View Article : Google Scholar : PubMed/NCBI
|