Asparaginase (ASNase) is a component of highly
effective chemotherapeutic regimens used to treat pediatric acute
lymphoblastic leukemia (ALL) (4,5)
and some lymphomas (6–8). ASNase treatment has been estimated
to have contributed to the sparing of the lives of upwards of
60,000 children in the US in the decades following its discovery
(9) and rapid introduction to the
clinic (10). However, ASNase
treatment is not without hazard; it can produce a myriad of
side-effects that include hyperglycemia, dislipidemia,
pancreatitis, vascular accidents and adverse neurological outcomes.
The physiological mode of action of ASNase is unclear. The enzyme
deaminates Asn and Gln with production of altered amino acid ratios
and ammonia (11–15). ASNase inhibits synthesis of
proteins in vitro (16)
and in vivo (17,18) by a mechanism consistent with
reduced ribosomal translocation at Asn codons. In humans, ASNase
treatment protocols cause depletion of plasma Asn and modest
reductions of plasma Gln levels accompanied by mild transient
hyperglycemia and occasional ketoacidosis (11,19,20). In mice, administration of ASNase
causes Asn depletion in plasma and some tissues, e.g., skeletal
muscle (21,22), indicating, importantly, that
intracellular Asn can also be depleted. Moreover, in mice, impaired
glucose tolerance following ASNase treatment can be improved by
amino acid supplements which serve to moderate amino acid ratio
imbalances (23) and Asn
administered directly to mice reverses adverse events initiated by
ASNase (24). In rabbits, ASNase
induces dose-dependent glycemic dysregulation extending from
transient mild glycosuria to hyperglycemia and diabetes (25,26). Prednisolone has been shown to
potentiate the action of ASNase: both drugs can cause hyperglycemia
when used alone; but predisolone synergizes with ASNase to cause
significant hyperglycemia (500–700 mg/dl) when both drugs are
administered in combination at doses that are insufficient to
produce an effect above baseline (~100 mg/dl) when either drug is
administered alone (27).
Complementing these clinical and experimental
observations, metabolomic data from the Framingham Heart study and
from diabetic patients in a Shanghai study have shown that plasma
Asn concentration is negatively correlated with fasting insulin
concentration (28), and that the
degree of negative correlation is the highest for Asn by comparison
with the 20 amino acids that are commonly incorporated into
proteins by ribosomal synthesis. By contrast, γ-amino butyric acid
(GABA) levels are 10-fold more negatively correlated with fasting
insulin levels. In the Framingham data, the maximal negative
correlation observed between Asn concentration and fasting insulin
also extends to additional diabetes metrics such as body mass index
(BMI), waist circumference (WC), homeostatic model assessment
(HOMA), and triglyceride levels. In a third study, of a different
cohort, Asn was the amino acid most negatively correlated with
adiponectin, HOMA and leptin levels (29). Because therapeutic Asn depletion
induces glycemic dysregulation, low Asn levels may not merely be
correlatively associated with poor glycemic control, but may be
causative or provocative. This raises the question of the potential
mechanisms by which Asn depletion in plasma or tissues could
adversely impact glucose homeostasis.
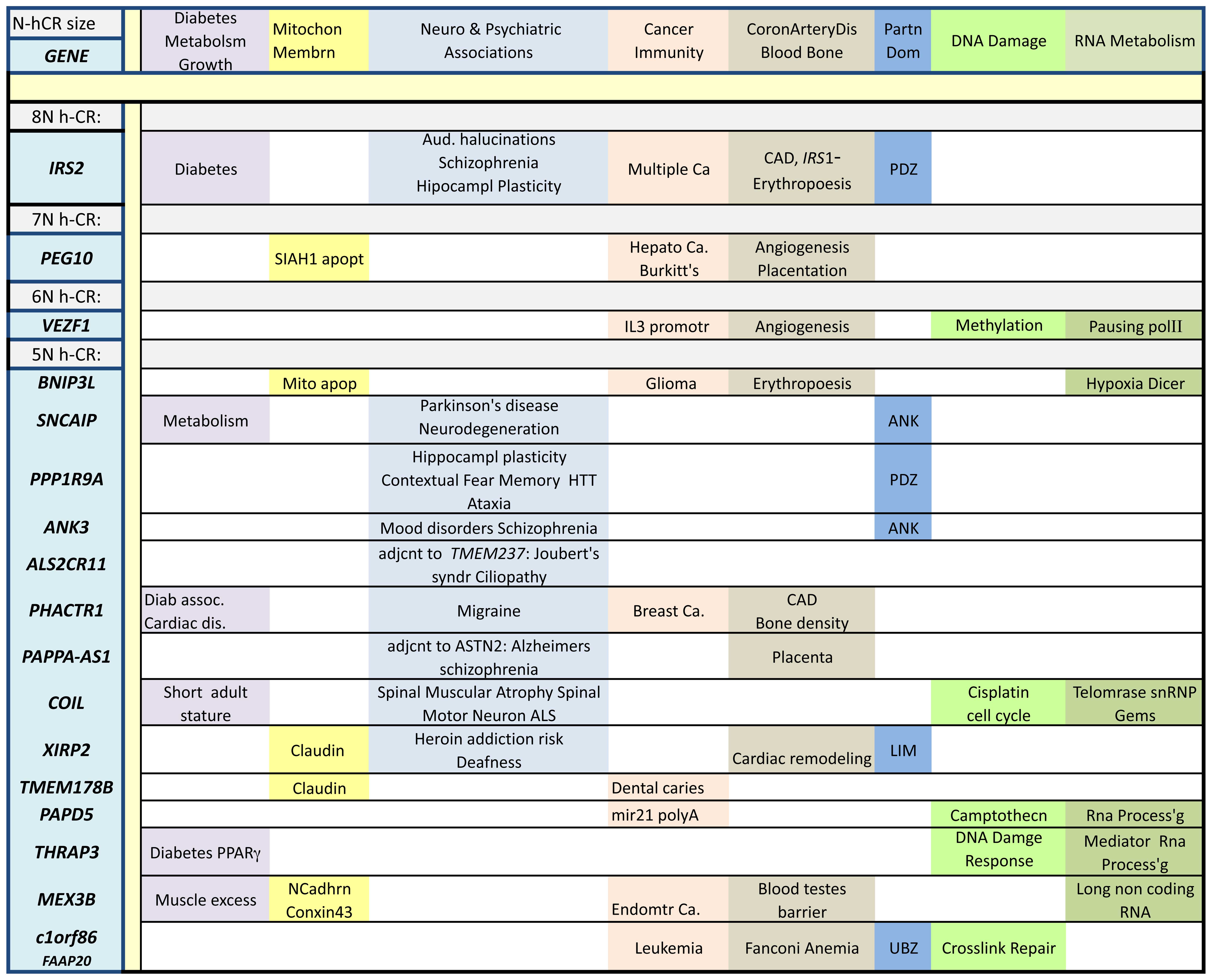
The possibility that N-hCR can be implicated in the
etiologies of some diabetic syndromes is supported by the
enrichment of genes governing metabolic balance among the list of
those containing N-hCR. Approximately one-fifth of the genes
bearing N-hCR in Table I are
associated with metabolic disorders, obesity, diabetes, urea cycle
or pancreatic islet β-cell regulation. Among these, IRS2 is
of particular note. IRS2 encodes insulin receptor
substrate-2, a labile (30,31) intracellular signal transducer that
is a substrate for a number of membrane spanning receptor tyrosine
kinases specific for extracellular cytokines that include insulin,
insulin-like-growth-factor-1, erythropoietin, thrombopoetin, growth
hormone, leukemia inhibitory factor, interleukin-4 (IL-4) and
interferon-γ (32–37). Sequence polymorphisms in the human
IRS2 locus have been associated with obesity (38), type 2-diabetes-mellitus (T2DM)
(39,40) or its complications (41,42), aspects of schizophrenia (43) and IgE immune responses (44). In transgenic mice, IRS2
deletion causes compromised maintenance of β-cell mass and produces
a diabetic state similar to T2DM (45,46). Reduced levels of IRS2 in humans
have been proposed to lead to desensitized insulin/cytokine
signalling and thus to hyperglycemia/muted immune responses, with
prolonged IRS2 deficits exacerbating islet cell mass reduction
leading to T2DM (47–50). Alterations in IRS2 expression have
been associated with altered lipid metabolism in obese subjects
(51) and have been correlated
with development of insulin nonresponsiveness in obese boys
(52). IRS2 has eight consecutive
Asn-codons located 19 codons after the initiator AUG codon.
Depletion of the levels of the cognate Asn aminoacyl-tRNA may
result in compromised elongation in the homopolymeric Asn coding
region that may be especially deleterious to the synthesis of IRS2
due to the location of the N-hCR.
Codon usage and ribosome translocation rates affect
protein expression in bacterial (53–57), viral (58,59) and human genes (60,61). Ribosomal footprinting studies have
suggested that the stability of translation initiation complexes
increases when nascent chains emerge from the exit tunnel or
folding vestibule to engage chaperones (62). Ribosomal stalling may potentially
lead to translation termination when the elongation rate is
diminished in the 'translation-initiation-ramp' or instability
region (63–65). The concept of the ramp, which may
not apply to all mammalian genes, remains controversial (66) and though potentially contributory,
it is not essential to the overall thesis proposed here. In
general, a severely diminished elongation rate may lead to
premature termination; for example in prokaryotes, ribosomal
stalling induces a translational termination mechanism through
tmRNA (67, Cf. 68). In the abstract, reduced rates of
translation anywhere along an mRNA would result directly in a
reduced overall rate of target protein synthesis and, depending on
protein halflife, result indirectly in decreased steady state
levels of such proteins. High rates of translation may even
increase the halflife of an mRNA (69).
Of the genes that have been identified with N-hCR of
length 3 or greater, approximately one third can be associated with
cancer and immune response, one quarter with neurode-generation
(20% with metabolic disorders, above), and eight percent with
vasculature and hematopoesis. Of the remaing ~14%, many can be
classified as involved with chromatin modification, DNA
maintainance and repair, RNA transcription and processing or
protein synthesis and turnover, some have Leucine rich repeats that
can serve as pattern recognition elements. Some genes fall into
multiple categories, e.g. IRS2 is associated not only with
diabetes and receptor mediated signal transduction for specific
extracellular cytokines, but also with epilepsy (70), aspects of schizophrenia (43), Alzheimer's disease (71–73), retinal degeneration (74), hippocampal synaptic plasticity
(75), long term potentiation of
hippocampal synaptic transmission (76), ataxia (77), cardiac failure (78), kidney development (79), renal disease (80), breast cancer (81,82), rhabdomyosarcoma (83) and, in conjunction with
JAK23N-hCR, hematopoesis (84,85). A limited study of an N-hCR length
polymorphism in IRS2 shows no association with diabetes
(86).
For the purpose of establishing the consequences of
N-hCR for translational sensitivity to Asn concentration, other
genes with N-hCR could be tested, including conserved genes with
nonhuman N-hCR lengths that also differ from humans in some other
parameter (such as inflammatory response profiles) (87). For example an exceptional
mammalian gene, with an N-hCR longer than the 8N-hCR of IRS2, is a
bat paralog of the IL8-receptor, CXCR2, (EPQ18419), which
has a 60N-hCR. Other genes of interest from mouse, that differ from
human in N-hCR length, include MDR1 and CFTR (a
Salmonella receptor), and TNFRSF16/BEX3A/NGFRAP1
(implicated in diabetes) (88) as
well as the redox regulators: GCLC (89) and TXNIP (90) (the former encodes the first, rate
limiting, enzyme in the glutathione synthesis pathway and has been
associated with cardiovascular events) (91); the latter encodes a conserved
thioredoxin binding protein that has an 8N-hCR in mice, vs. a
3N-hCR in nonrodent mammals. All of these TXNIP N-hCR are
invariantly located and they begin at codon 386, end 3 codons
before the stop codon. This is discussed further, below, along with
the contribution of TXNIP to host response to P. aeruginosa
bacteremia by recruitment of neutrophils in mice (92). TXNIP also affects pancreatic
β-cell biology (93), diabetic
retinopathy (94), and glucose
metabolism (indirectly regulated by mTOR) (95). Finally, a gene with the third
longest N-hCR in the mosquito genome (XM_316513) is translationally
regulated (perhaps at its N-hCR) in insect midgut in response to
plasmodium infected blood meals (96). The gene is homologous to human
FAF1/TNFRSF6 which is associated with diabetes (97) and Parkinson's disease (PD)
(98).
Genetic studies suggest an environmental component
for the etiology of diabetes (129) and the gut microbiome has been
proposed to regulate human physiology, e.g. bone mass (130). An individual's microbiome may
also produce enzymes that alter host Asn levels. Persistent
salmonellosis in mice causes pancreatitis (131,132) which is a side-effect of
therapeutic ASNase treatment (133,134). In addition, Salmonella
mediates its own virulence (135) via a cytostatic ASNase (16) and inhibits mouse T cell responses
in a manner reversible by administration of Asn (24,136); this Salmonella mediated
immune inhibition may reflect the immunosuppression noted in
ASNase-treated rabbits (137)
and rodents (138,139).
A similar location of N-hCR, between MSDs and NBDs,
is found in two genes that encode important ATP-regulated magnesium
channels: TRPM64NhCR and
TRPM73N-hCR,4NhCR. Allelic variation of the
former has been associated with elevated risk of diabetes,
osteoporosis, asthma, and heart and vascular diseases (161), whereas allelic variation of the
latter has been associated with sudden cardiac death, QT interval
prolongation and atrial fibrillation in individuals with African
ancestry (162), and ALS and PD
in Guam (163). TRPM6 can form
heterodi-mers with, and regulate function of, TRPM7; the latter is
a channel regulated enzyme that can be cleaved to modify histones
(164,165). TRPM7 affects vascularization
(166), and has been implicated
in ovarian, breast, pancreatic and prostate cancer as well as in
the metastasis of nasopharyngeal carcinoma (167). The NBDs of these ion channels,
as well as the STAS domain of SLC26A93N-hCR
(151) (which is thought to
assemble and interact with the Regulatory domain in the NBD of
CFTR), all have poly Asn regions separating them from portions of
their hydrophobic MSDs, suggesting that translocation rate at the
N-hCR, perhaps due to variation in Asn levels, may serve to
modulate the chronology of the synthesis and assembly of the
hydrophobic intracellular domains of these molecules.
ASNase treatment produces side-effects that include
vascular dysfunction. Factor V and fibrinogen are two of several
coagulation and complement factors encoded by N-hCR-bearing-genes.
Polymorphic alleles of F53N-hCR104t (encoding
coagulation Factor V) have been linked to coronary artery disease
(189), hippocampal degeneration
(190) and thrombotic events in
ASNase treated children (144,191). ASNase specifically reduces the
synthesis rate of fibrinogen (18), see below, a subunit of which is
encoded by FGB. Thus inhibition by ASNase of the synthesis
of at least two N-hCR-bearing-genes, F5 and FGB,
could potentially account for the vascular side-effects of ASNase
administration. FGB3N-hCR,
GP1BA3N-hCR, encoding the platelet membrane
receptor (for von Willebrand's factor) associated with ischemic
stroke (192), and
CD94N-hCR, a gene involved in platelet formation
(193), are candidate N-hCR
bearing genes that could be examined for their genetic association
with adverse vascular events attending ASNase treatment (as has
been reported for F5, above). Coagulation proteins have long been
considered potential risk factors of ASNase therapy (194). The steady state half-life of
autologous iodinated fibrinogen is not affected by ASNase treatment
and hence the observed reduction in steady state plasma fibrinogen
concentration that produces the hypofibrinogenemia (195) observed after ASNase treatment is
likely due to inhibition of fibrinogen synthesis (18). There are concordant studies in
rabbits (196) and humans
(197) regarding the rate of
catabolism and synthesis of fibrinogen in response to ASNase, as
well as studies on the proteomics of FGB and C3 in diabetics
(198,199). N-hCR-bearing-genes encoding
complement proteins may also contribute to other disorders such as
retinal degeneration through effects on C33N-hCR
(200) to multiple sclerosis
through effects on C73N-hCR (201) and to uptake of pathogens such as
glycosylated viruses or bacteria by any of multiple members of the
lectin and alternate complement pathway on Table I such as CLEC6A (202), CLEC10A (203) CLEC13B/LY75, MASP1
and C1QB.
Mitigating the effects of low plasma Asn, by
altering the composition of intestinal microbiota (204) or by using amino acid supplements
(23), may slow disease onset or
progression in those at risk of diabetes or its complications.
Dietary Asn supplementation may particularly benefit CFTR-null
homozygotes or compound heterozygotes, who frequently present with
diabetes at later stages of their disease (205). One of the N-hCR-bearing-genes in
Fig. 1,
PHACTR15N-hCR has been linked to coronary artery
disease (CAD) in diabetics (206). Diabetes and CAD are frequent
comorbidities, as are diabetes and Alzheimer's disease (72) perhaps due to a shared etiology
originating in low plasma Asn concentration. There are two
N-hCR-bearing-genes from Fig. 1
that are linked to PD and mood disorders:
SNCAIP5N-hCR and ANK35N-hCR. PD
and diabetes are comorbidities, and abnormal glucose regulation has
been reported in >50% of PD patients (207) perhaps due to altered Asn
homeostasis; correspondingly, bipolar disorder treatment outcomes
differ for patients with diabetes as compared to normal controls
(208). PD and ALS often occur
with dementia (209,210); a shared etiology may be
responsible, due to altered levels of Asn, perhaps even through
complement genes such as C1QB3N-hCR (211), or the balance between
C1QL23N-hCR, C1QL33N-hCR
(212) and
BAI23N-hCR and their non N-hCR bearing paralogs:
C1QL1 and BAI3 (213).
Multiple genes encoding N-hCR have been linked to
neuropsychiatric disorders, PD, aspects of schizophrenia,
Alzheimer's disease, mood disorders [CDH9 (214), GTF2I (215) and ALDH6A1], neurological
dysfunction (CDKL5 and TMEM106B) (216,217), breast-cancer [BRCA2,
CEACAM5/CEA (218),
CYP19A1/Aromatase (219),
IRS2, CLEC10A (220), LRP6 and TBC1D5
(221)], spinal degeneration
(COIL, FBXO38, ITGAV, ASIC2,
KIAA1217 and CHAD), age of onset of amyotrophic
lateral sclerosis (ALS) (TTLL4 and LAMA3) (222), dementia in ALS (TMEM106B)
(223) retinal dystrophy
(TTLL5) (224), large
artery stoke (TTLL5 and PHACTR1) (225) decreased bone density in
tamoxifen treated women (LRP4 and NCOA1) (226), ovarian cancer (TBC1D3 and
TBC1D3F) (227) T cell
anergy (GRAIL/RNF128/isf2) (228–230), asthma, autoimmune diseases,
innate immunity (231–233) and the link between innate and
adaptive immunity (FCGR2-A, -B, -C) (234) suggesting a common etiology of
altered Asn homeostasis may need to be considered for some of these
conditions.
LRP5, LRP6 and APC are encoded by
N-hCR-bearing-genes involved in the Wnt pathway. Rotterlin, which
is reported to accelerate the turnover rate of LRP6 (235) (a Wnt signalling co-receptor)
(236), could be co-administered
with ASNase because it may potentially synergize with ASNase to
focus the effect of ASNase on LRP6 mediated Wnt signalling
(237). We hypothesize that by
preferentially lowering the steady state level of LRP6, the
combination of drugs could regulate (238) bone mass, cancer, cardiovascular
health, vision, Alzheimer's and multiple other diseases of aging.
Notch and hedgehog signalling are also affected by N-hCR
bearing-genes such as DZIP1, MAML2, BOC and
CDON, and may present attractive targets for drug discovery
via small molecules that accelerate turnover of specific proteins
encoded by N-hCR bearing-genes, synergistically magnifying the
impact of ASNase by altering the replacement rate and perhaps by
establishing lowered steady state levels of the targeted protein.
There is already a precedent for synergism of prednisolone with
ASNase, which occurs by an as yet unknown mechanism. The halflife
of WNT signalling complexes and the contribution of DSV to turnover
of WNT coreceptors FZD and LRP6 has recently been characterized
(239).
Adverse neurological outcomes have also been
associated with N-hCR-bearing-genes ANK3, IRS2,
SNCAIP, XIRP2, PPP1R9A and CACNA1-C.
Low plasma Asn, via the 17 N-hCR-bearing-genes listed in Fig. 1, can thus also plausibly be linked
to onset of age associated(251)
and SNCAIP; dental caries and peridontal disease as a
diabetes comorbidity through TMEM178B or ANKRD17 in
children (252,253); (cf. LRP1B and
periodontitis in adults) (254).
Also affected by LRP1B are age at menarche (255), APOE and fibrinogen binding
(256), protection from
cognitive decline in aging (257) as well as BMI, insulin
resistance, optic disc size/area (cf. glaucoma), conditional
erectile dysfunction in African American men, heart rate and
multiple cancers. Deafness (258,259) is affected by XIRP2
(cf. Xeplin, PTPRQ), heroin addiction
vulnerability in African Americans (260) and heart disease by XIRP2
(261,262); heart disease by PHACTR1
(263) (cf. LRP6)
and PPP1R9A (cf. CHRM-2, -3) (264); bone density by PHACTR1
(cf. LRP4, LRP5); erythropoesis and quality control
of mitochondria by BNIP3L; nucleic acid processing by
COIL, PAPD5, THRAP3, MEX3B and
C1orf86/FAAP20; and diabetes by THRAP3 (cf.
CHRM3), PTPRD and IRS2.
The discussion above has focused on adverse events
elicited by ASNase therapy, not the induction of tumor remission.
Two N-hCR-bearing-genes, PEG10 and BNIP3L, have
transcripts with long N-hCR that are encompassed within their
initial two dozen codons. Both BNIP3L and PEG10 are
apoptosis-related genes that are candidates for mediation of the
cell death that has been observed to follow depletion of Asn either
in cell culture (265) or in
pediatric ALL. Multiple other N-hCR-bearing-genes are also
potential targets, e.g., APC, (ARID5B, IL9R
and RYR2) (266),
JAK2, KCNA3 (145), UBE2Q2 (267), COIL (268) or SMG12x3N-hCR
(269) (a Ser-Thr kinase with
homology to mTOR). Temperature sensitive mutants of Asn tRNA
synthetase undergo cell cycle arrest in early S phase at the
nonpermissive temperature, a phenomenon that has been posited to be
consistent with the existence a protein required for cell cycle
progression that is highly sensitive to the level of charged
Asn-tRNA (270), such as one
encoded by an N-hCR-bearing-gene that is eliminated and must be
resynthesized once per cell cycle (cf. COIL
above).
We have seen full length translation of templates
devoid of Asn codons under conditions of exogenously added ASNase,
but in templates containing Asn codons, translated under identical
conditions, we observe translation that extends to the N-hCR. Thus
we suggest that depletion of Asn-ylated tRNA is likely to be the
underlying cause of inhibition of synthesis seen previously by use
of random, mixed templates for characterizing the inhibition, by
Salmonella ASNase, of in vitro translation reactions
(16). There were also
unanticipated findings suggesting that frameshifting efficiency may
depend on the number of Asn codons in an artificial N-hCR that was
inserted a dozen codons upstream of the PEG10 frameshifting
site. We have not characterised the behavior of deamidated
Asn-tRNAAsn which could incorporate Asp residues at Asn
codons were it not edited and removed by a proofreading
complex.
There are differences in response to ASNase between
children and adults. They are most obvious in the ALL tumor
remission response, as well as in the type of glycemic
dysregulation: periphiral vs. central loss of responsiveness. In
the pediatric patients, the hyperglycemia is insulin reversible,
insulin is absent from circulation following an ASNase therapeutic
regimen that includes steroid hormones similar to prednisolone, and
it is likely that central control over insulin synthesis or release
may be deficient. In the metabolomic studies of diabetic adults,
Fasting Insulin levels are high, and IRS2 mediated peripheral
signalling may be deficient. In addition, the unacceptable
neurovascular complications (fugue state, cerebrovascular
accidents) in adults compared to children underscores the
difference between the physiology of children and adults.
Most of the poly Asn codon runs reported here
consist of the two isoaccepter codons AAT and AAC used in about
equal frequency with a slight bias towards homopolymeric runs of
AAC. In the gene IRS28N-hCR, from human,
zebrafish, elephant-shark, frog, python and falcon, AAC is used
exclusively in N-hCR runs of varying length and distance from the
initiator methionine, suggesting that if regulation is not
restricted only to the AAC isoacceptor species, perhaps there is a
further, structural, component to this phenomenon [CAG
homocopolymers encoding poly Q repeats can form triple stranded
structures (287), RNA sequences
enriched in AAT motifs can be labile (288)]. Interestingly,
PEG107N-hCR and BNIP3L5N-hCR
employ AAC codons exclusively in human and mouse (PEG10), or
in human, mouse, rat, lizard, ~frog and chicken (BNIP3L),
indicating that the two isoacceptor tRNAs may indeed be
differentially regulated.
N-hCR-bearing-genes encode proteins that engage in
networks whose equilibria may be affected by elongation rate, e.g.
PPP1R9A5Nx2-hCR, unique among the 17 genes of
Fig. 1 because of two separate
N-hCR, encodes neurabin, the intrinsically disordered regions
(289) of which become
conformationally restricted in regulatory complexes with PP1
(290), and which is implicated
in neurite formation (291),
neuroprotection against seizures (292), mood disorders (293), hippocampal plasticity (294), long term depression (295), dopamine mediated plasticity
(296), contextual fear memory
(297), hepatosplenic lymphoma
(298) and regulation of G
protein coupled receptor (GPCR) signalling (250). A key unstructured UBZ domain of
Fanconi's anemia gene FAAP20 can form a highly structured α
helix upon ubiquitin binding; this domain is interrupted by a
5N-hCR in certain variant isoforms. The 2N-hCR of TP53 is similarly
located: adjacent to a pair of transactivation domains (TADs) that
gain structure upon ligand binding (299,300). The N-hCR of TRPM-6 and
-7 interrupt their α kinase domain. Modulating translation
rate by varying Asn concentration, while synthesising these
proteins, could allow modulation of the protein assemblies in which
these proteins participate.
In this survey of other potential roles for the
conserved poly Asn regions in proteins, we note that they also act
as sites of post-translational modification to regulate protein
activity by glycosylation or deamination or [cleavage, by
Asparaginyl endopeptidases (301) (cf. Taspase1, an ASNase
gene family member) (302)]. The
4N-hCR of CFTR, differing in length between human, mouse and pig,
encodes a conformationally dynamic regulatory insertion (303) that may gate access to the ATP
binding site (304). A similarly
unstructured loop in Bcl-xL undergoes deamination (305,306), as does an Asn residue pair
between the TADs of TP53 (307),
a region unstructured until bound to MDM2 (308,309). The 2N-hCR of TP53 differs in
length between rats, mice and humans. N-hCR length variation in
N-hCR-bearing-genes can correlate with disease severity in animal
models of human inflamation. For example the pig model of CF more
closely reflects the physiology of the human disorder, in
comparison to the mouse model (310) perhaps because, as with TP53, the
length of the poly Asn region in pig more closely resembles that of
human rather than mouse. Also, in P. aeruginosa-induced
bacteremic shock, TXNIP exacerbates septic shock associated with
bacteremia in a mouse model (92). TXNIP of mouse has an identically
situated, but longer poly Asn region (8N-hCR) than human and most
other nonrodent mammals (3N-hCR), perhaps enabling greater redox
level changes in response to Asn level variation. These examples
may reflect divergent evolutionary choices in inflammatory and
pathogen response strategies that may partially explain the
reported differences between human and rodent models of
inflammation (311,312) and IRS2 genetic associations
(72). Altered electrophoretic
mobility, a hallmark of some deamination events, indicates that
post-translational modification may even occur at the poly Asn
region of IRS (281). Deletion
analysis of the N-terminal poly Asn containing region of
BNP3L/B5/NIX suggests that it masks apoptosis inducing
function (313,314). Regarding self association and
aggregation at poly Asn regions, Perutz stated that it is unlikely
that poly Asn repeats can form polar zippers of the kind formed by
poly Gln repeats (315), but see
(316). hCR may be tolerated at
intrinsically disordered regions of proteins (317) where proteins could accommodate
hCR expansion in their genes (318). An alternative explanation for
the action of ASNase: NH3 generated by ASNase may act as a gaseous
reactive signalling molecule, akin to NO, CO or SH2, to modify
protein structure and function (319).
At least five different human tRNA synthetases can
serve as autoantigens in inflammatory responses (320). Human tRNA synthetases AsnRS and
HisRS both serve as chemoattractants (321), ligands for cell surface proteins
CCR5 and CCR3 respectively (322). AsnRS protein levels are
upregulated by almost three orders of magnitude in a model of
preosteoblast cell proliferation driven by FGF2 (323). Filarial AsnRS, in contrast to
human AsnRS, serves as a ligand for CXCR1 and CXCR2 and is
chemotactic for neutrophils and eosinophils, with a terminal
subdomain that serves as a ligand for human IL8 receptor (324). The link between inflammatory
responses and Asn tRNA synthetases remains an open question.
Leu contributes to formation of mTOR1C, a
biochemical complex that regulates cell cycle (325) in conjunction with other amino
acids (326,327) including Arg (328,329) and Gln (105,330–332). In a related experimental
paradigm, apoptosis induced by Gln withdrawal, Asn, instead of Gln
may actually be the effector molecule whose withdrawal is sensed
(267). A biochemical mechanism
for sensing Asn levels, required either to trigger apoptosis, or to
advance through S phase of the cell cycle, perhaps mediated by
AsnRS, and not involving ribosomes may yet be discovered, but even
if such a mechanism were to exist, translational inhibition at
N-hCR would still remain a most parsimonious explanation for the
myriad clinical side-effects of ASNase treatment. Poly Asn
(2) and poly Leu (100) codon repeats (N-hCR and L-hCR)
appear in a biased manner in mammalian genomes; this bias may be
related to metabolomic differences in the levels of Asn (23,28) and Leu (333) between normal and diabetic
patients as we have discussed for the case of Asn in this study,
and as may be the case for Leu (cf. L-hCR length
polymorphisms and diabetic nephropathy in CNDP1 (107,108). mTORC1 activation is the orthodox
pathway for understanding how altered amino acid levels exert
metabolic control. This study has examined an alternative
hypothesis, of the potential for amino acid fluctuations to control
translation rate, to thereby effect a different measure of
metabolic control by reshaping the composition of the proteome.
A GWAS of ALL shows that it is affected by at least
two other N-hCR-bearing-genes, in addition to RYR2 (noted
above): IL9R (361) and
ARID5B (cf. KCNA3) (145). IL9R shares a common γ
subunit with other interleukin receptors) (362) IL9R has a 4N-hCR that is
absent from all mammals except Pan [cf. APOL1
which lacks 3N-hCR in all mammals except Gorilla (2N in
Pongo)]. ARID5B encodes part of a histone lysine
demethylase complex (363) and
is not only genetically associated with ALL (266,364–369) but is also associated with
corneal changes (370), low
birth weight (371), diastolic
blood pressure (372) rheumatoid
arthritis (373), response to
haloperidol (374) (an
anti-psychotic medication), systemic lupus erythematosus (SLE)
(375), lipid balance (376) and triglyceride metabolism in
mouse adipocytes (377), as well
as, in humans, T2DM (378). The
contribution of ASNase to these conditions, especially to ALL,
potentially by altered translation at the N-hCR of ARID5B
warrants further investigation (379).
We propose that the impaired translation which has
been described above be termed the 'translational N-hamper effect'
because there is nothing intrinsically impaired about a protein
polymerization reaction in which one of the required components,
activated Asn tRNA, is ratelimiting for the translocation reaction
on the template mRNA. The verb of choice for slowed translocation
could just as well have been cumbered movement instead of hampered
movement. If the argument was first made for Gln, the Q-cumber
effect could have encompassed this hypothetical phenomenon.
The 'translational N-hamper effect' is a mechanism
whereby protein expression is modulated by coupling fluctuations in
appropriate aminoacylated-tRNA availability to ribosome
translocation rates at corresponding hCR. Thus, ribosome movement
could pause at hCR which would serve as punctuation marks to allow
relative intracellular amino acid pool sizes to influence mRNA
decoding and protein synthesis. Amino acid level fluctuation could
potentially affect: mRNA halflife and accessibility to regulatory
complexes, ribosome frameshifting efficiency, initiation rate and
formation of stable translation complexes, and elongation rate and
vestibule residence time to affect steady state levels of these
proteins and of higher order structures in which they
participate.
Our model holds that Asn level reductions, such as
those accompanying the administration of ASNase, cause impaired
translation of N-hCR-bearing-genes to precipitate metabolic,
vascular, immunological and neurological disorders and contends
that this could result in insulin desensitization, impaired insulin
release and, ultimately, diabetes. Thus the microbiome, by
endogenously generating ASNase, could cotranslationally regulate a
constellation of N-hCR-bearing-genes to initiate complex disease
pathologies.
I thank B. Seed (MGH) for support; F. Baas (AMC,
NL), R. Movva (Basle, CH), W. Summers (Yale), T. Enoch (Berkeley,
ZC), J. Broome (New Lebanon, NY), E. Fritch (DFCI) and G.
Enikolopov (CSH) for encouragement and discussions; G.E. and B.S.
for critical editorial advice. P. Mason (MGH) for help with
database searches and Lin Sun and members of the Seed lab for help
with in vitro translation experiments.
|
1
|
Karlin S, Brocchieri L, Bergman A, Mrazek
J and Gentles AJ: Amino acid runs in eukaryotic proteomes and
disease associations. Proc Natl Acad Sci U S A. 99:333–338. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kreil DP and Kreil G: Asparagine repeats
are rare in mammalian proteins. Trends Biochem Sci. 25:270–271.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karlin S and Burge C: Trinucleotide
repeats and long homopeptides in genes and proteins associated with
nervous system disease and development. Proc Natl Acad Sci USA.
93:1560–1565. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawedia JD and Rytting ME: Asparaginase in
acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk.
14(Suppl): S14–S17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Müller HJ and Boos J: Use of
L-Asparaginase in childhood ALL. Crit Rev Oncol Hematol. 28:97–113.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki R: Pathogenesis and treatment of
extranodal natural killer/T-cell lymphoma. Semin Hematol. 51:42–51.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fréling E, Granel-Brocard F, Serrier C,
Ortonne N, Barbaud A and Schmutz J: Extranodal NK/T-cell lymphoma,
nasal-type, revealed by cutaneous breast involvement. Ann Dermatol
Venereol. 142:104–111. 2015.In French. View Article : Google Scholar
|
|
8
|
Kidd JG: Regression of transplanted
lymphomas induced in vivo by means of normal guinea pig serum. I.
Course of transplanted cancers of various kinds in mice and rats
given guinea pig serum, horse serum, or rabbit serum. J Exp Med.
98:565–582. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Broome JD: Evidence that the
L-asparaginase of guinea pig serum is responsible for its
antilymphoma effects. I. Properties of the L-asparaginase of guinea
pig serum in relation to those of the antilymphoma substance. J Exp
Med. 118:99–120. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Essig S, Li Q, Chen Y, Hitzler J,
Leisenring W, Greenberg M, Sklar C, Hudson MM, Armstrong GT, Krull
KR, et al: Risk of late effects of treatment in children newly
diagnosed with standard-risk acute lymphoblastic leukaemia: A
report from the Childhood Cancer Survivor Study cohort. Lancet
Oncol. 15:841–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong WH, Pieters R, Hop WC,
Lanvers-Kaminsky C, Boos J and van der Sluis IM: No evidence of
increased asparagine levels in the bone marrow of patients with
acute lymphoblastic leukemia during asparaginase therapy. Pediatr
Blood Cancer. 60:258–261. 2013. View Article : Google Scholar
|
|
12
|
Fine BM, Kaspers GJ, Ho M, Loonen AH and
Boxer LM: A genome-wide view of the in vitro response to
l-asparaginase in acute lymphoblastic leukemia. Cancer Res.
65:291–299. 2005.PubMed/NCBI
|
|
13
|
Kelo E, Noronkoski T, Stoineva IB, Petkov
DD and Mononen I: Beta-aspartylpeptides as substrates of
L-asparaginases from Escherichia coli and Erwinia chrysanthemi.
FEBS Lett. 528:130–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan WK, Lorenzi PL, Anishkin A, Purwaha
P, Rogers DM, Sukharev S, Rempe SB and Weinstein JN: The
glutaminase activity of L-asparaginase is not required for
anticancer activity against ASNS-negative cells. Blood.
123:3596–3606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang L, Liu Y, Sun Y, Yan Q and Jiang Z:
Biochemical characterization of a novel L-Asparaginase with low
glutaminase activity from Rhizomucor miehei and its application in
food safety and leukemia treatment. Appl Environ Microbiol.
80:1561–1569. 2014. View Article : Google Scholar :
|
|
16
|
Iwamaru Y, Miyake M, Arii J, Tanabe Y and
Noda M: An inhibitory factor for cell-free protein synthesis from
Salmonella enteritidis exhibits cytopathic activity against Chinese
hamster ovary cells. Microb Pathog. 31:283–293. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Capizzi RL, Bertino JR, Skeel RT, Creasey
WA, Zanes R, Olayon C, Peterson RG and Handschumacher RE:
L-asparaginase: Clinical, biochemical, pharmacological, and
immunological studies. Ann Intern Med. 74:893–901. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bettigole RE, Himelstein ES, Oettgen HF
and Clifford GO: Hypofibrinogenemia due to L-asparaginase: Studies
of fibrinogen survival using autologous 131-I-fibrinogen. Blood.
35:195–200. 1970.PubMed/NCBI
|
|
19
|
Avramis VI: Is glutamine depletion needed
in ALL disease? Blood. 123:3532–3533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quintanilla-Flores DL, Flores-Caballero
MÁ, Rodríguez-Gutiérrez R, Tamez-Pérez HE and González-González JG:
Acute pancreatitis and diabetic ketoacidosis following
L-asparaginase/prednisonetherapy in acute lymphoblastic leukemia.
Case Rep Oncol Med. 2014:1391692014.
|
|
21
|
Frankel DL, Wells H and Fillios LC:
Concentrations of asparagine in tissues of prepubertal rats after
enzymic or dietary depletion of asparagine. Biochem J. 132:645–648.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holcenberg JS, Tang E and Dolowy WC:
Effect of Acinetobacter glutaminase-asparaginase treatment on free
amino acids in mouse tissues. Cancer Res. 35:1320–1325.
1975.PubMed/NCBI
|
|
23
|
Cheng S, Rhee EP, Larson MG, Lewis GD,
McCabe EL, Shen D, Palma MJ, Roberts LD, Dejam A, Souza AL, et al:
Metabolite profiling identifies pathways associated with metabolic
risk in humans. Circulation. 125:2222–2231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kullas AL, McClelland M, Yang HJ, Tam JW,
Torres A, Porwollik S, Mena P, McPhee JB, Bogomolnaya L,
Andrews-Polymenis H and van der Velden AW: L-asparaginase II
produced by Salmonella typhimurium inhibits T cell responses and
mediates virulence. Cell Host Microbe. 12:791–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lavine RL and DiCinto DM: L-asparaginase
diabetes mellitus in rabbits: Differing effects of two different
schedules of L-asparaginase administration. Horm Metab Res.
16(Suppl): 92–96. 1984.PubMed/NCBI
|
|
26
|
Khan A, Adachi M and Hill JM: Diabetogenic
effect of L-asparaginase. J Clin Endocrinol Metab. 29:1373–1376.
1969. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khan A, Adachi M and Hill JM: Potentiation
of diabetogenic effect of L-asparaginase by prednisolone. Horm
Metab Res. 2:275–276. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou Y, Qiu L, Xiao Q, Wang Y, Meng X, Xu
R, Wang S and Na R: Obesity and diabetes related plasma amino acid
alterations. Clin Biochem. 46:1447–1452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakamura H, Jinzu H, Nagao K, Noguchi Y,
Shimba N, Miyano H, Watanabe T and Iseki K: Plasma amino acid
profiles are associated with insulin, C-peptide and adiponectin
levels in type 2 diabetic patients. Nutr Diabetes. 4:e1332014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Burén J, Liu HX, Lauritz J and Eriksson
JW: High glucose and insulin in combination cause insulin receptor
substrate-1 and -2 depletion and protein kinase B desensitisation
in primary cultured rat adipocytes: possible implications for
insulin resistance in type 2 diabetes. Eur J Endocrinol.
148:157–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsunekawa S, Demozay D, Briaud I, McCuaig
J, Accili D, Stein R and Rhodes CJ: FoxO feedback control of basal
IRS-2 expression in pancreatic β-cells is distinct from that in
hepatocytes. Diabetes. 60:2883–2891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Argetsinger LS, Norstedt G, Billestrup N,
White MF and Carter-Su C: Growth hormone, interferon-gamma, and
leukemia inhibitory factor utilize insulin receptor substrate-2 in
intracellular signaling. J Biol Chem. 271:29415–29421. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Uddin S, Fish EN, Sher D, Gardziola C,
Colamonici OR, Kellum M, Pitha PM, White MF and Platanias LC: The
IRS-pathway operates distinctively from the Stat-pathway in
hematopoietic cells and transduces common and distinct signals
during engagement of the insulin or interferon-alpha receptors.
Blood. 90:2574–2582. 1997.PubMed/NCBI
|
|
34
|
O'Connor JC, Sherry CL, Guest CB and
Freund GG: Type 2 diabetes impairs insulin receptor
substrate-2-mediated phosphatidylinositol 3-kinase activity in
primary macrophages to induce a state of cytokine resistance to
IL-4 in association with overexpression of suppressor of cytokine
signaling-3. J Immunol. 178:6886–6893. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carey GB, Semenova E, Qi X and Keegan AD:
IL-4 protects the B-cell lymphoma cell line CH31 from
anti-IgM-induced growth arrest and apoptosis: Contribution of the
PI-3 kinase/AKT pathway. Cell Res. 17:942–955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Blaeser F, Bryce PJ, Ho N, Raman V,
Dedeoglu F, Donaldson DD, Geha RS, Oettgen HC and Chatila TA:
Targeted inactivation of the IL-4 receptor alpha chain I4R motif
promotes allergic airway inflammation. J Exp Med. 198:1189–1200.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wurster AL, Withers DJ, Uchida T, White MF
and Grusby MJ: Stat6 and IRS-2 cooperate in interleukin 4
(IL-4)-induced proliferation and differentiation but are
dispensable for IL-4-dependent rescue from apoptosis. Mol Cell
Biol. 22:117–126. 2002. View Article : Google Scholar
|
|
38
|
Butte NF, Voruganti VS, Cole SA, Haack K,
Comuzzie AG, Muzny DM, Wheeler DA, Chang K, Hawes A and Gibbs RA:
Resequencing of IRS2 reveals rare variants for obesity but not
fasting glucose homeostasis in Hispanic children. Physiol Genomics.
43:1029–1037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haghani K and Bakhtiyari S: The study on
the relationship between IRS-1 Gly972Arg and IRS-2 Gly1057Asp
polymorphisms and type 2 diabetes in the Kurdish ethnic group in
West Iran. Genet Test Mol Biomarkers. 16:1270–1276. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ayaz L, Karakaş Çelik S and Cayan F: The
G1057D polymorphism of insulin receptor substrate-2 associated with
gestational diabetes mellitus. Gynecol Endocrinol. 30:165–168.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pezzolesi MG, Poznik GD, Skupien J, Smiles
AM, Mychaleckyj JC, Rich SS, Warram JH and Krolewski AS: An
intergenic region on chromosome 13q33.3 is associated with the
susceptibility to kidney disease in type 1 and 2 diabetes. Kidney
Int. 80:105–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Craig DW, Millis MP and DiStefano JK:
Genome-wide SNP genotyping study using pooled DNA to identify
candidate markers mediating susceptibility to end-stage renal
disease attributed to Type 1 diabetes. Diabet Med. 26:1090–1098.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim SK, Yu GI, Park HJ, Kim YJ, Kim JW,
Baik HH and Chung JH: A polymorphism (rs4773092, Cys816Cys) of IRS2
affects auditory hallucinations in schizophrenia patients.
Psychiatry Res. 209:124–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Acevedo N, Mercado D, Vergara C, Sánchez
J, Kennedy MW, Jiménez S, Fernández AM, Gutiérrez M, Puerta L and
Caraballo L: Association between total immunoglobulin E and
antibody responses to naturally acquired Ascaris lumbricoides
infection and polymorphisms of immune system-related LIG4, TNFSF13B
and IRS2 genes. Clin Exp Immunol. 157:282–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Alvarez-Perez JC, Rosa TC, Casinelli GP,
Valle SR, Lakshmipathi J, Rosselot C, Rausell-Palamos F, Vasavada
RC and García-Ocaña A: Hepatocyte growth factor ameliorates
hyperglycemia and corrects β-cell mass in IRS2-deficient mice. Mol
Endocrinol. 28:2038–2048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Withers DJ, Gutierrez JS, Towery H, Burks
DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, et al:
Disruption of IRS-2 causes type 2 diabetes in mice. Nature.
391:900–904. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Niessen M: On the role of IRS2 in the
regulation of functional beta-cell mass. Arch Physiol Biochem.
112:65–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Park S, Hong SM, Lee JE, Sung SR and Kim
SH: Chlorpromazine attenuates pancreatic beta-cell function and
mass through IRS2 degradation, while exercise partially reverses
the attenuation. J Psychopharmacol. 22:522–531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gunasekaran U, Hudgens CW, Wright BT,
Maulis MF and Gannon M: Differential regulation of embryonic and
adult β cell replication. Cell Cycle. 11:2431–2442. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oliveira JM, Rebuffat SA, Gasa R and Gomis
R: Targeting type 2 diabetes: Lessons from a knockout model of
insulin receptor substrate 2. Can J Physiol Pharmacol. 92:613–620.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rametta R, Mozzi E, Dongiovanni P, Motta
BM, Milano M, Roviaro G, Fargion S and Valenti L: Increased insulin
receptor substrate 2 expression is associated with steatohepatitis
and altered lipid metabolism in obese subjects. Int J Obes (Lond).
37:986–992. 2013. View Article : Google Scholar
|
|
52
|
Minchenko DO, Davydov VV, Budreiko OA,
Moliavko OS, Kulieshova DK, Tiazhka OV and Minchenko OH: The
expression of CCN2, IQSEC, RSPO1, DNAJC15, RIPK2, IL13RA2, IRS1,
and IRS2 genes in blood of obese boys with insulin resistance.
Fiziol Zh. 61:10–18. 2015.PubMed/NCBI
|
|
53
|
Chen GT and Inouye M: Role of the AGA/AGG
codons, the rarest codons in global gene expression in Escherichia
coli. Genes Dev. 8:2641–2652. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mitarai N, Sneppen K and Pedersen S:
Ribosome collisions and translation efficiency: Optimization by
codon usage and mRNA destabilization. J Mol Biol. 382:236–245.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang S, Goldman E and Zubay G: Clustering
of low usage codons and ribosome movement. J Theor Biol.
170:339–354. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen GF and Inouye M: Suppression of the
negative effect of minor arginine codons on gene expression;
preferential usage of minor codons within the first 25 codons of
the Escherichia coli genes. Nucleic Acids Res. 18:1465–1473. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ivanov IG, Saraffova AA and Abouhaidar MG:
Unusual effect of clusters of rare arginine (AGG) codons on the
expression of human interferon alpha 1 gene in Escherichia coli.
Int J Biochem Cell Biol. 29:659–666. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Coleman JR, Papamichail D, Skiena S,
Futcher B, Wimmer E and Mueller S: Virus attenuation by
genome-scale changes in codon pair bias. Science. 320:1784–1787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
de Fabritus L, Nougairède A, Aubry F,
Gould EA and de Lamballerie X: Attenuation of tick-borne
encephalitis virus using large-scale random codon re-encoding. PLoS
Pathog. 11:e10047382015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sauna ZE and Kimchi-Sarfaty C:
Understanding the contribution of synonymous mutations to human
disease. Nat Rev Genet. 12:683–691. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gartner JJ, Parker SC, Prickett TD,
Dutton-Regester K, Stitzel ML, Lin JC, Davis S, Simhadri VL, Jha S,
Katagiri N, et al: NISC Comparative Sequencing Program:
Whole-genome sequencing identifies a recurrent functional
synonymous mutation in melanoma. Proc Natl Acad Sci USA.
110:13481–13486. 2013. View Article : Google Scholar
|
|
62
|
Ingolia NT: Ribosome profiling: New views
of translation, from single codons to genome scale. Nat Rev Genet.
15:205–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dana A and Tuller T: The effect of tRNA
levels on decoding times of mRNA codons. Nucleic Acids Res.
42:9171–9181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fredrick K and Ibba M: How the sequence of
a gene can tune its translation. Cell. 141:227–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li Q and Qu HQ: Human coding synonymous
single nucleotide polymorphisms at ramp regions of mRNA
translation. PLoS One. 8:e597062013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Charneski CA and Hurst LD: Positively
charged residues are the major determinants of ribosomal velocity.
PLoS Biol. 11:e10015082013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Himeno H, Nameki N, Kurita D, Muto A and
Abo T: Ribosome rescue systems in bacteria. Biochimie. 114:102–112.
2015. View Article : Google Scholar
|
|
68
|
Edenberg ER, Downey M and Toczyski D:
Polymerase stalling during replication, transcription and
translation. Curr Biol. 24:R445–R452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Faucillion ML and Larsson J: Increased
expression of X-linked genes in mammals is associated with a higher
stability of transcripts and an increased ribosome density. Genome
Biol Evol. 7:1039–1052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Che F, Fu Q, Li X, Gao N, Qi F, Sun Z, Du
Y and Li M: Association of insulin receptor H1085H C>T, insulin
receptor substrate 1 G972R and insulin receptor substrate 2 1057G/A
polymorphisms with refractory temporal lobe epilepsy in Han
Chinese. Seizure. 25:178–180. 2015. View Article : Google Scholar
|
|
71
|
de la Monte SM and Tong M: Brain metabolic
dysfunction at the core of Alzheimer's disease. Biochem Pharmacol.
88:548–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
White MF: IRS2 integrates insulin/IGF1
signalling with metabolism, neurodegeneration and longevity.
Diabetes Obes Metab. 16(Suppl 1): 4–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
de la Monte SM: Contributions of brain
insulin resistance and deficiency in amyloid-related
neurodegeneration in Alzheimer's disease. Drugs. 72:49–66. 2012.
View Article : Google Scholar
|
|
74
|
Albert-Fort M, Hombrebueno JR,
Pons-Vazquez S, Sanz-Gonzalez S, Diaz-Llopis M and Pinazo-Durán MD:
Retinal neurodegenerative changes in the adult insulin receptor
substrate-2 deficient mouse. Exp Eye Res. 124:1–10. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Costello DA, Claret M, Al-Qassab H,
Plattner F, Irvine EE, Choudhury AI, Giese KP, Withers DJ and
Pedarzani P: Brain deletion of insulin receptor substrate 2
disrupts hippocampal synaptic plasticity and metaplasticity. PLoS
One. 7:e311242012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Martín ED, Sánchez-Perez A, Trejo JL,
Martin-Aldana JA, Cano Jaimez M, Pons S, Acosta Umanzor C, Menes L,
White MF and Burks DJ: IRS-2 deficiency impairs NMDA
receptor-dependent long-term potentiation. Cereb Cortex.
22:1717–1727. 2012. View Article : Google Scholar :
|
|
77
|
Sadagurski M, Cheng Z, Rozzo A, Palazzolo
I, Kelley GR, Dong X, Krainc D and White MF: IRS2 increases
mitochondrial dysfunction and oxidative stress in a mouse model of
Huntington disease. J Clin Invest. 121:4070–4081. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Qi Y, Xu Z, Zhu Q, Thomas C, Kumar R, Feng
H, Dostal DE, White MF, Baker KM and Guo S: Myocardial loss of IRS1
and IRS2 causes heart failure and is controlled by p38α MAPK during
insulin resistance. Diabetes. 62:3887–3900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Carew RM, Sadagurski M, Goldschmeding R,
Martin F, White MF and Brazil DP: Deletion of Irs2 causes reduced
kidney size in mice: Role for inhibition of GSK3beta? BMC Dev Biol.
10:732010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hookham MB, O'Donovan HC, Church RH,
Mercier-Zuber A, Luzi L, Curran SP, Carew RM, Droguett A, Mezzano
S, Schubert M, et al: Insulin receptor substrate-2 is expressed in
kidney epithelium and up-regulated in diabetic nephropathy. FEBS J.
280:3232–3243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Landis J and Shaw LM: Insulin receptor
substrate 2-mediated phosphatidylinositol 3-kinase signaling
selectively inhibits glycogen synthase kinase 3β to regulate
aerobic glycolysis. J Biol Chem. 289:18603–18613. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Porter HA, Perry A, Kingsley C, Tran NL
and Keegan AD: IRS1 is highly expressed in localized breast tumors
and regulates the sensitivity of breast cancer cells to
chemotherapy, while IRS2 is highly expressed in invasive breast
tumors. Cancer Lett. 338:239–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Nishimura R, Takita J, Sato-Otsubo A, Kato
M, Koh K, Hanada R, Tanaka Y, Kato K, Maeda D, Fukayama M, et al:
Characterization of genetic lesions in rhabdomyosarcoma using a
high-density single nucleotide polymorphism array. Cancer Sci.
104:856–864. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Verma R, Su S, McCrann DJ, Green JM, Leu
K, Young PR, Schatz PJ, Silva JC, Stokes MP and Wojchowski DM:
RHEX, a novel regulator of human erythroid progenitor cell
expansion and erythroblast development. J Exp Med. 211:1715–1722.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bunn HF: Erythropoietin. Cold Spring Harb
Perspect Med. 3:a0116192013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang H, Rissanen J, Miettinen R,
Kärkkäinen P, Kekäläinen P, Kuusisto J, Mykkänen L, Karhapää P and
Laakso M: New amino acid substitutions in the IRS-2 gene in Finnish
and Chinese subjects with late-onset type 2 diabetes. Diabetes.
50:1949–1951. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Seok J, Warren HS, Cuenca AG, Mindrinos
MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy
L, et al: Inflammation and Host Response to Injury, Large Scale
Collaborative Research Program: Genomic responses in mouse models
poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA.
110:3507–3512. 2013. View Article : Google Scholar
|
|
88
|
Taborsky GJ Jr, Mei Q, Hackney DJ and
Mundinger TO: The search for the mechanism of early sympathetic
islet neuropathy in autoimmune diabetes. Diabetes Obes Metab.
16(Suppl 1): 96–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Nichenametla SN, Lazarus P and Richie JP
Jr: A GAG trinucleotide-repeat polymorphism in the gene for
glutathione biosynthetic enzyme, GCLC, affects gene expression
through translation. FASEB J. 25:2180–2187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Feuer SK, Liu X, Donjacour A, Lin W,
Simbulan RK, Giritharan G, Piane LD, Kolahi K, Ameri K, Maltepe E,
et al: Use of a mouse in vitro fertilization model to understand
the developmental origins of health and disease hypothesis.
Endocrinology. 155:1956–1969. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Campolo J, Penco S, Bianchi E, Colombo L,
Parolini M, Caruso R, Sedda V, Patrosso MC, Cighetti G, Marocchi A,
et al: Glutamate-cysteine ligase polymorphism, hypertension, and
male sex are associated with cardiovascular events. Biochemical and
genetic characterization of Italian subpopulation. Am Heart J.
154:1123–1129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Piao ZH, Kim MS, Jeong M, Yun S, Lee SH,
Sun HN, Song HY, Suh HW, Jung H, Yoon SR, et al: VDUP1 exacerbates
bacteremic shock in mice infected with Pseudomonas aeruginosa. Cell
Immunol. 280:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Shalev A: Minireview:
Thioredoxin-interacting protein: regulation and function in the
pancreatic β-cell. Mol Endocrinol. 28:1211–1220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Coucha M, Elshaer SL, Eldahshan WS, Mysona
BA and El-Remessy AB: Molecular mechanisms of diabetic retinopathy:
Potential therapeutic targets. Middle East Afr J Ophthalmol.
22:135–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kaadige MR, Yang J, Wilde BR and Ayer DE:
MondoA-Mlx transcriptional activity is limited by mTOR-MondoA
interaction. Mol Cell Biol. 35:101–110. 2015. View Article : Google Scholar :
|
|
96
|
Mead EA, Li M, Tu Z and Zhu J:
Translational regulation of Anopheles gambiae mRNAs in the midgut
during Plasmodium falciparuminfection. BMC Genomics. 13:3662012.
View Article : Google Scholar
|
|
97
|
Mahajan A, Go MJ, Zhang W, Below JE,
Gaulton KJ, Ferreira T, Horikoshi M, Johnson AD, Ng MC, Prokopenko
I, et al: DIAbetes Genetics Replication And Meta-analysis (DIAGRAM)
Consortium; Asian Genetic Epidemiology Network Type 2 Diabetes
(AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D)
Consortium; Mexican American Type 2 Diabetes (MAT2D) Consortium;
Type 2 Diabetes Genetic Exploration by Nex-generation sequencing in
muylti-Ethnic Samples (T2D-GENES) Consortium: Genome-wide
trans-ancestry meta-analysis provides insight into the genetic
architecture of type 2 diabetes susceptibility. Nat Genet.
46:234–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Betarbet R, Anderson LR, Gearing M, Hodges
TR, Fritz JJ, Lah JJ and Levey AI: Fas-associated factor 1 and
Parkinson's disease. Neurobiol Dis. 31:309–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Amelio I, Cutruzzolá F, Antonov A,
Agostini M and Melino G: Serine and glycine metabolism in cancer.
Trends Biochem Sci. 39:191–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Labaj PP, Leparc GG, Bardet AF, Kreil G
and Kreil DP: Single amino acid repeats in signal peptides. FEBS J.
277:3147–3157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Depledge DP and Dalby AR: COPASAAR - a
database for proteomic analysis of single amino acid repeats. BMC
Bioinformatics. 6:1962005. View Article : Google Scholar
|
|
102
|
Khan A, Hill JM and Adachi M: Inhibition
of anti-tumour effect of L-asparaginase by methionine and choline.
Lancet. 2:10821970. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rudman D, Vogler WR, Howard CH and Gerron
GG: Observations on the plasma amino acids of patients with acute
leukemia. Cancer Res. 31:1159–1165. 1971.PubMed/NCBI
|
|
104
|
Jewell JL, Kim YC, Russell RC, Yu FX, Park
HW, Plouffe SW, Tagliabracci VS and Guan KL: Metabolism.
Differential regulation of mTORC1 by leucine and glutamine.
Science. 347:194–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Jewell JL, Russell RC and Guan KL: Amino
acid signalling upstream of mTOR. Nat Rev Mol Cell Biol.
14:133–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yang J, Chi Y, Burkhardt BR, Guan Y and
Wolf BA: Leucine metabolism in regulation of insulin secretion from
pancreatic beta cells. Nutr Rev. 68:270–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Riedl E, Koeppel H, Brinkkoetter P,
Sternik P, Steinbeisser H, Sauerhoefer S, Janssen B, van der Woude
FJ and Yard BA: A CTG polymorphism in the CNDP1 gene determines the
secretion of serum carnosinase in Cos-7 transfected cells.
Diabetes. 56:2410–2413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Freedman BI, Hicks PJ, Sale MM, Pierson
ED, Langefeld CD, Rich SS, Xu J, McDonough C, Janssen B, Yard BA,
et al: A leucine repeat in the carnosinase gene CNDP1 is associated
with diabetic end-stage renal disease in European Americans.
Nephrol Dial Transplant. 22:1131–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zachariah RM, Olson CO, Ezeonwuka C and
Rastegar M: Novel MeCP2 isoform-specific antibody reveals the
endogenous MeCP2E1 expression in murine brain, primary neurons and
astrocytes. PLoS One. 7:e497632012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
No authors listed. The Huntington's
Disease Collaborative Research Group: A novel gene containing a
trinucleotide repeat that is expanded and unstable on Huntington's
disease chromosomes. Cell. 72:971–983. 1993. View Article : Google Scholar
|
|
111
|
Klesert TR, Otten AD, Bird TD and Tapscott
SJ: Trinucleotide repeat expansion at the myotonic dystrophy locus
reduces expression of DMAHP. Nat Genet. 16:402–406. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Korade-Mirnics Z, Babitzke P and Hoffman
E: Myotonic dystrophy: Molecular windows on a complex etiology.
Nucleic Acids Res. 26:1363–1368. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lozano R, Rosero CA and Hagerman RJ:
Fragile X spectrum disorders. Intractable Rare Dis Res. 3:134–146.
2014. View Article : Google Scholar
|
|
114
|
Laaksovirta H, Peuralinna T, Schymick JC,
Scholz SW, Lai SL, Myllykangas L, Sulkava R, Jansson L, Hernandez
DG, Gibbs JR, et al: Chromosome 9p21 in amyotrophic lateral
sclerosis in Finland: A genome-wide association study. Lancet
Neurol. 9:978–985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gijselinck I, Van Langenhove T, van der
Zee J, Sleegers K, Philtjens S, Kleinberger G, Janssens J, Bettens
K, Van Cauwenberghe C, Pereson S, et al: A C9orf72 promoter repeat
expansion in a Flanders-Belgian cohort with disorders of the
frontotemporal lobar degeneration-amyotrophic lateral sclerosis
spectrum: A gene identification study. Lancet Neurol. 11:54–65.
2012. View Article : Google Scholar
|
|
116
|
Rohrer JD, Isaacs AM, Mizielinska S, Mead
S, Lashley T, Wray S, Sidle K, Fratta P, Orrell RW, Hardy J, et al:
C9orf72 expansions in frontotemporal dementia and amyotrophic
lateral sclerosis. Lancet Neurol. 14:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Walsh MJ, Cooper-Knock J, Dodd JE,
Stopford MJ, Mihaylov SR, Kirby J, Shaw PJ and Hautbergue GM:
Invited review: decoding the pathophysiological mechanisms that
underlie RNA dysregulation in neurodegenerative disorders: a review
of the current state of the art. Neuropathol Appl Neurobiol.
41:109–134. 2015. View Article : Google Scholar :
|
|
118
|
Cleary JD and Ranum LP: Repeat associated
non-ATG (RAN) translation: New starts in microsatellite expansion
disorders. Curr Opin Genet Dev. 26:6–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yan S, Wen JD, Bustamante C and Tinoco I
Jr: Ribosome excursions during mRNA translocation mediate broad
branching of frameshift pathways. Cell. 160:870–881. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Scoles DR, Ho MH, Dansithong W, Pflieger
LT, Petersen LW, Thai KK and Pulst SM: Repeat Associated Non-AUG
Translation (RAN Translation) Dependent on Sequence Downstream of
the ATXN2 CAG Repeat. PLoS One. 10:e01287692015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Muerdter F and Stark A: Genomics: Hiding
in plain sight. Nature. 512:374–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
La Spada AR, Paulson HL and Fischbeck KH:
Trinucleotide repeat expansion in neurological disease. Ann Neurol.
36:814–822. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kayatekin C, Matlack KE, Hesse WR, Guan Y,
Chakrabortee S, Russ J, Wanker EE, Shah JV and Lindquist S:
Prion-like proteins sequester and suppress the toxicity of
huntingtin exon 1. Proc Natl Acad Sci USA. 111:12085–12090. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ripaud L, Chumakova V, Antonin M, Hastie
AR, Pinkert S, Körner R, Ruff KM, Pappu RV, Hornburg D, Mann M, et
al: Overexpression of Q-rich prion-like proteins suppresses polyQ
cytotoxicity and alters the polyQ interactome. Proc Natl Acad Sci
USA. 111:18219–18224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chambers JW, Maguire TG and Alwine JC:
Glutamine metabolism is essential for human cytomegalovirus
infection. J Virol. 84:1867–1873. 2010. View Article : Google Scholar :
|
|
126
|
Mangiarini L, Sathasivam K, Seller M,
Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach
H, Davies SW, et al: Exon 1 of the HD gene with an expanded CAG
repeat is sufficient to cause a progressive neurological phenotype
in transgenic mice. Cell. 87:493–506. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Rosas HD, Reuter M, Doros G, Lee SY,
Triggs T, Malarick K, Fischl B, Salat DH and Hersch SM: A tale of
two factors: what determines the rate of progression in
Huntington's disease? A longitudinal MRI study. Mov Disord.
26:1691–1697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lee JM, Ramos EM, Lee JH, Gillis T, Mysore
JS, Hayden MR, Warby SC, Morrison P, Nance M, Ross CA, et al:
PREDICT-HD study of the Huntington Study Group (HSG); REGISTRY
study of the European Huntington's Disease Network; HD-MAPS Study
Group; COHORT study of the HSG: CAG repeat expansion in Huntington
disease determines age at onset in a fully dominant fashion.
Neurology. 78:690–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F,
Liang S, Zhang W, Guan Y, Shen D, et al: A metagenome-wide
association study of gut microbiota in type 2 diabetes. Nature.
490:55–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ohlsson C and Sjögren K: Effects of the
gut microbiota on bone mass. Trends Endocrinol Metab. 26:69–74.
2015. View Article : Google Scholar
|
|
131
|
DelGiorno KE, Tam JW, Hall JC, Thotakura
G, Crawford HC and van der Velden AW: Persistent salmonellosis
causes pancreatitis in a murine model of infection. PLoS One.
9:e928072014. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Whitcomb DC: Genetic aspects of
pancreatitis. Annu Rev Med. 61:413–424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Wu F, Qu L, Tan Y, Zhang Y and Hu C:
L-asparaginase-induced severe acute pancreatitis in an adult with
extranodal natural killer/T-cell lymphoma, nasal type: A case
report and review of the literature. Oncol Lett. 7:1305–1307.
2014.PubMed/NCBI
|
|
134
|
Kaya I, Citil M, Sozmen M, Karapehlivan M
and Cigsar G: Investigation of protective effect of L-carnitine on
L-asparaginase-induced acute pancreatic injury in male Balb/c mice.
Dig Dis Sci. 2014.PubMed/NCBI
|
|
135
|
Bueno SM, Riquelme S, Riedel CA and
Kalergis AM: Mechanisms used by virulent Salmonella to impair
dendritic cell function and evade adaptive immunity. Immunology.
137:28–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kafkewitz D and Bendich A: Enzyme-induced
asparagine and glutamine depletion and immune system function. Am J
Clin Nutr. 37:1025–1030. 1983.PubMed/NCBI
|
|
137
|
Etheredge EE, Shons A, Harris N and
Najarian JS: Prolongation of skin xenograft survival by
L-asparaginase. Transplantation. 11:353–354. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Khan A and Levine S: Further studies on
the inhibition of allergic encephalomyelitis by L-asparaginase. J
Immunol. 113:367–370. 1974.PubMed/NCBI
|
|
139
|
Friedman H: L-asparaginase induced
immunosuppression: Inhibition of bone marrow derived antibody
precursor cells. Science. 174:139–141. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Xu J, Wang P, Li Y, Li G, Kaczmarek LK, Wu
Y, Koni PA, Flavell RA and Desir GV: The voltage-gated potassium
channel Kv1.3 regulates peripheral insulin sensitivity. Proc Natl
Acad Sci USA. 101:3112–3117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wang T, Lee MH, Choi E, Pardo-Villamizar
CA, Lee SB, Yang IH, Calabresi PA and Nath A: Granzyme B-induced
neurotoxicity is mediated via activation of PAR-1 receptor and
Kv1.3 channel. PLoS One. 7:e439502012. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
LaRusch J and Whitcomb DC: Genetics of
pancreatitis. Curr Opin Gastroenterol. 27:467–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Blackman SM, Commander CW, Watson C,
Arcara KM, Strug LJ, Stonebraker JR, Wright FA, Rommens JM, Sun L,
Pace RG, et al: Genetic modifiers of cystic fibrosis-related
diabetes. Diabetes. 62:3627–3635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Santoro N, Colombini A, Silvestri D,
Grassi M, Giordano P, Parasole R, Barisone E, Caruso R, Conter V,
Valsecchi MG, et al: Screening for coagulopathy and identification
of children with acute lymphoblastic leukemia at a higher risk of
symptomatic venous thrombosis: An AIEOP experience. J Pediatr
Hematol Oncol. 35:348–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Ellinghaus E, Stanulla M, Richter G,
Ellinghaus D, te Kronnie G, Cario G, Cazzaniga G, Horstmann M,
Panzer Grümayer R, Cavé H, et al: Identification of germline
susceptibility loci in ETV6-RUNX1-rearranged childhood acute
lymphoblastic leukemia. Leukemia. 26:902–909. 2012. View Article : Google Scholar :
|
|
146
|
Xu J, Koni PA, Wang P, Li G, Kaczmarek L,
Wu Y, Li Y, Flavell RA and Desir GV: The voltage-gated potassium
channel Kv1.3 regulates energy homeostasis and body weight. Hum Mol
Genet. 12:551–559. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Tu L, Khanna P and Deutsch C:
Transmembrane segments form tertiary hairpins in the folding
vestibule of the ribosome. J Mol Biol. 426:185–198. 2014.
View Article : Google Scholar
|
|
148
|
Kosolapov A and Deutsch C: Tertiary
interactions within the ribosomal exit tunnel. Nat Struct Mol Biol.
16:405–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Delaney E, Khanna P, Tu L, Robinson JM and
Deutsch C: Determinants of pore folding in potassium channel
biogenesis. Proc Natl Acad Sci USA. 111:4620–4625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH,
Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, et al: Gating of
CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol.
6:343–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Gray MA: Bicarbonate secretion: It takes
two to tango. Nat Cell Biol. 6:292–294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Chang MH, Plata C, Sindic A, Ranatunga WK,
Chen AP, Zandi-Nejad K, Chan KW, Thompson J, Mount DB and Romero
MF: Slc26a9 is inhibited by the R-region of the cystic fibrosis
transmembrane conductance regulator via the STAS domain. J Biol
Chem. 284:28306–28318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ishiguro H, Yamamoto A, Nakakuki M, Yi L,
Ishiguro M, Yamaguchi M, Kondo S and Mochimaru Y: Physiology and
pathophysiology of bicarbonate secretion by pancreatic duct
epithelium. Nagoya J Med Sci. 74:1–18. 2012.PubMed/NCBI
|
|
154
|
Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE,
Calcagno AM, Ambudkar SV and Gottesman MM: A 'silent' polymorphism
in the MDR1 gene changes substrate specificity. Science.
315:525–528. 2007. View Article : Google Scholar
|
|
155
|
Chong PA, Kota P, Dokholyan NV and
Forman-Kay JD: Dynamics intrinsic to cystic fibrosis transmembrane
conductance regulator function and stability. Cold Spring Harb
Perspect Med. 3:a0095222013. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
LaRusch J, Jung J, General IJ, Lewis MD,
Park HW, Brand RE, Gelrud A, Anderson MA, Banks PA, Conwell D, et
al North American Pancreatitis Study Group: Mechanisms of CFTR
functional variants that impair regulated bicarbonate permeation
and increase risk for pancreatitis but not for cystic fibrosis.
PLoS Genet. 10:e10043762014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
El Khouri E and Touré A: Functional
interaction of the cystic fibrosis transmembrane conductance
regulator with members of the SLC26 family of anion transporters
(SLC26A8 and SLC26A9): Physiological and pathophysiological
relevance. Int J Biochem Cell Biol. 52:58–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Bozoky Z, Krzeminski M, Muhandiram R,
Birtley JR, Al-Zahrani A, Thomas PJ, Frizzell RA, Ford RC and
Forman-Kay JD: Regulatory R region of the CFTR chloride channel is
a dynamic integrator of phospho-dependent intra-and intermolecular
interactions. Proc Natl Acad Sci USA. 110:E4427–E4436. 2013.
View Article : Google Scholar
|
|
159
|
Pier GB, Grout M, Zaidi T, Meluleni G,
Mueschenborn SS, Banting G, Ratcliff R, Evans MJ and Colledge WH:
Salmonella typhi uses CFTR to enter intestinal epithelial cells.
Nature. 393:79–82. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
160
|
Lazrak A, Fu L, Bali V, Bartoszewski R,
Rab A, Havasi V, Keiles S, Kappes J, Kumar R, Lefkowitz E, et al:
The silent codon change I507-ATC->ATT contributes to the
severity of the DeltaF508 CFTR channel dysfunction. FASEB J.
27:4630–4645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
van der Wijst J, Bindels RJ and Hoenderop
JG: Mg2+ homeostasis: The balancing act of TRPM6. Curr
Opin Nephrol Hypertens. 23:361–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Smith JG, Avery CL, Evans DS, Nalls MA,
Meng YA, Smith EN, Palmer C, Tanaka T, Mehra R, Butler AM, et al:
CARe and COGENT consortia: Impact of ancestry and common genetic
variants on QT interval in African Americans. Circ Cardiovasc
Genet. 5:647–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Hermosura MC and Garruto RM: TRPM7 and
TRPM2-Candidate susceptibility genes for Western Pacific ALS and
PD? Biochim Biophys Acta. 1772:822–835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Krapivinsky G, Krapivinsky L, Manasian Y
and Clapham DE: The TRPM7 chanzyme is cleaved to release a
chromatin-modifying kinase. Cell. 157:1061–1072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Wrighton KH: Epigenetics: The TRPM7 ion
channel modifies histones. Nat Rev Mol Cell Biol. 15:4272014.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zeng Z, Inoue K, Sun H, Leng T, Feng X,
Zhu L and Xiong ZG: TRPM7 regulates vascular endothelial cell
adhesion and tube formation. Am J Physiol Cell Physiol.
308:C308–C318. 2015. View Article : Google Scholar
|
|
167
|
Chen JP, Wang J, Luan Y, Wang CX, Li WH,
Zhang JB, Sha D, Shen R, Cui YG, Zhang Z, et al: TRPM7 promotes the
metastatic process in human nasopharyngeal carcinoma. Cancer Lett.
356:483–490. 2015. View Article : Google Scholar
|
|
168
|
Hunt RC, Simhadri VL, Iandoli M, Sauna ZE
and Kimchi-Sarfaty C: Exposing synonymous mutations. Trends Genet.
30:308–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Wu DF, Yin RX, Cao XL, Chen WX, Aung LH,
Wang W, Huang KK, Huang P, Zeng XN and Wu J: Scavenger receptor
class B type 1 gene rs5888 single nucleotide polymorphism and the
risk of coronary artery disease and ischemic stroke: A case-control
study. Int J Med Sci. 10:1771–1777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Constantineau J, Greason E, West M, Filbin
M, Kieft JS, Carletti MZ, Christenson LK and Rodriguez A: A
synonymous variant in scavenger receptor, class B, type I gene is
associated with lower SR-BI protein expression and function.
Atherosclerosis. 210:177–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Willer CJ, Schmidt EM, Sengupta S, Peloso
GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S,
et al: Global Lipids Genetics Consortium: Discovery and refinement
of loci associated with lipid levels. Nat Genet. 45:1274–1283.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Meyer JM, Graf GA and van der Westhuyzen
DR: New developments in selective cholesteryl ester uptake. Curr
Opin Lipidol. 24:386–392. 2013.PubMed/NCBI
|
|
173
|
Nofer JR: Signal transduction by HDL:
Agonists, receptors, and signaling cascades. Handb Exp Pharmacol.
224:229–256. 2015. View Article : Google Scholar
|
|
174
|
Tong WH, Pieters R, de Groot-Kruseman HA,
Hop WC, Boos J, Tissing WJ and van der Sluis IM: The toxicity of
very prolonged courses of PEGasparaginase or Erwinia asparaginase
in relation to asparaginase activity, with a special focus on
dyslipidemia. Haematologica. 99:1716–1721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Stanislovaitiene D, Lesauskaite V,
Zaliuniene D, Smalinskiene A, Gustiene O, Zaliaduonyte-Peksiene D,
Tamosiunas A, Luksiene D, Petkeviciene J and Zaliunas R: SCARB1
single nucleotide polymorphism (rs5888) is associated with serum
lipid profile and myocardial infarction in an age- and
gender-dependent manner. Lipids Health Dis. 12:242013. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Purdue MP, Johansson M, Zelenika D, Toro
JR, Scelo G, Moore LE, Prokhortchouk E, Wu X, Kiemeney LA,
Gaborieau V, et al: Genome-wide association study of renal cell
carcinoma identifies two susceptibility loci on 2p21 and 11q13.3.
Nat Genet. 43:60–65. 2011. View
Article : Google Scholar :
|
|
177
|
Pośpiech E, Ligęza J, Wilk W, Gołas A,
Jaszczyński J, Stelmach A, Ryś J, Blecharczyk A, Wojas-Pelc A, Jura
J, et al: Variants of SCARB1 and VDR involved in complex genetic
interactions may be implicated in the genetic susceptibility to
clear cell renal cell carcinoma. Biomed Res Int. 2015:8604052015.
View Article : Google Scholar
|
|
178
|
Suchindran S, Rivedal D, Guyton JR,
Milledge T, Gao X, Benjamin A, Rowell J, Ginsburg GS and McCarthy
JJ: Genome-wide association study of Lp-PLA(2) activity and mass in
the Framingham Heart Study. PLoS Genet. 6:e10009282010. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Song GJ, Kim SM, Park KH, Kim J, Choi I
and Cho KH: SR-BI mediates high density lipoprotein (HDL)-induced
anti-inflammatory effect in macrophages. Biochem Biophys Res
Commun. 457:112–118. 2015. View Article : Google Scholar
|
|
180
|
Gao M, Zhao D, Schouteden S, Sorci-Thomas
MG, Van Veldhoven PP, Eggermont K, Liu G, Verfaillie CM and Feng Y:
Regulation of high-density lipoprotein on hematopoietic
stem/progenitor cells in atherosclerosis requires scavenger
receptor type BI expression. Arterioscler Thromb Vasc Biol.
34:1900–1909. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Sticozzi C, Belmonte G, Cervellati F,
Muresan XM, Pessina F, Lim Y, Forman HJ and Valacchi G: Resveratrol
protects SR-B1 levels in keratinocytes exposed to cigarette smoke.
Free Radic Biol Med. 69:50–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Christianson MS and Yates M: Scavenger
receptor class B type 1 gene polymorphisms and female fertility.
Curr Opin Endocrinol Diabetes Obes. 19:115–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Meyers KJ, Mares JA, Igo RP Jr, Truitt B,
Liu Z, Millen AE, Klein M, Johnson EJ, Engelman CD, Karki CK, et
al: Genetic evidence for role of carotenoids in age-related macular
degeneration in the carotenoids in age-related eye disease study
(CAREDS). Invest Ophthalmol Vis Sci. 55:587–599. 2014. View Article : Google Scholar :
|
|
184
|
Reboul E, Goncalves A, Comera C, Bott R,
Nowicki M, Landrier JF, Jourdheuil-Rahmani D, Dufour C, Collet X
and Borel P: Vitamin D intestinal absorption is not a simple
passive diffusion: Evidences for involvement of cholesterol
transporters. Mol Nutr Food Res. 55:691–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Goncalves A, Margier M, Roi S, Collet X,
Niot I, Goupy P, Caris-Veyrat C and Reboul E: Intestinal scavenger
receptors are involved in vitamin K1 absorption. J Biol Chem.
289:30743–30752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Major JM, Yu K, Wheeler W, Zhang H,
Cornelis MC, Wright ME, Yeager M, Snyder K, Weinstein SJ, Mondul A,
et al: Genome-wide association study identifies common variants
associated with circulating vitamin E levels. Hum Mol Genet.
20:3876–3883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Schulman S and Furie B: How I treat
poisoning with vitamin K antagonists. Blood. 125:438–442. 2015.
View Article : Google Scholar
|
|
188
|
Ibarrola-Jurado N, Salas-Salvadó J,
Martínez-González MA and Bulló M: Dietary phylloquinone intake and
risk of type 2 diabetes in elderly subjects at high risk of
cardiovascular disease. Am J Clin Nutr. 96:1113–1118. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Tang W, Schwienbacher C, Lopez LM,
Ben-Shlomo Y, Oudot-Mellakh T, Johnson AD, Samani NJ, Basu S,
Gögele M, Davies G, et al: Genetic associations for activated
partial thromboplastin time and prothrombin time, their gene
expression profiles, and risk of coronary artery disease. Am J Hum
Genet. 91:152–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Melville SA, Buros J, Parrado AR,
Vardarajan B, Logue MW, Shen L, Risacher SL, Kim S, Jun G, DeCarli
C, et al: Alzheimer's Disease Neuroimaging Initiative: Multiple
loci influencing hippocampal degeneration identified by genome
scan. Ann Neurol. 72:65–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Nowak-Göttl U, Wermes C, Junker R, Koch
HG, Schobess R, Fleischhack G, Schwabe D and Ehrenforth S:
Prospective evaluation of the thrombotic risk in children with
acute lymphoblastic leukemia carrying the MTHFR TT 677 genotype,
the prothrombin G20210A variant, and further prothrombotic risk
factors. Blood. 93:1595–1599. 1999.PubMed/NCBI
|
|
192
|
Schmalbach B, Stepanow O, Jochens A,
Riedel C, Deuschl G and Kuhlenbäumer G: Determinants of
platelet-leukocyte aggregation and platelet activation in stroke.
Cerebrovasc Dis. 39:176–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Gieger C, Radhakrishnan A, Cvejic A, Tang
W, Porcu E, Pistis G, Serbanovic-Canic J, Elling U, Goodall AH,
Labrune Y, et al: New gene functions in megakaryopoiesis and
platelet formation. Nature. 480:201–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Hunault-Berger M, Chevallier P, Delain M,
Bulabois CE, Bologna S, Bernard M, Lafon I, Cornillon J, Maakaroun
A, Tizon A, et al GOELAMS (Groupe Ouest-Est des Leucémies Aiguës et
Maladies du Sang): Changes in antithrombin and fibrinogen levels
during induction chemotherapy with L-asparaginase in adult patients
with acute lymphoblastic leukemia or lymphoblastic lymphoma. Use of
supportive coagulation therapy and clinical outcome: The CAPELAL
study Haematologica. 93:1488–1494. 2008.
|
|
195
|
López Herce Cid J, Martínez A, González M
and García S: Diabetic ketoacidosis and hypofibrinogenemia as a
complication of the treatment with L-asparaginase of acute
lymphoblastic leukemia. Sangre (Barc). 31:195–199. 1986.In
Spanish.
|
|
196
|
Alving BM, Barr CF and Tang DB:
L-asparaginase: Acute effects on protein synthesis in rabbits with
normal and increased fibrinogen production. Blood. 63:823–827.
1984.PubMed/NCBI
|
|
197
|
Brodsky I, Kahn SB, Vash G, Ross EM and
Petkov G: Fibrinogen survival with [75Se]Selenomethionine during
L-asparaginase therapy. Br J Haematol. 20:477–487. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Sleddering MA, Markvoort AJ, Dharuri HK,
Jeyakar S, Snel M, Juhasz P, Lynch M, Hines W, Li X, Jazet IM, et
al: Proteomic analysis in type 2 diabetes patients before and after
a very low calorie diet reveals potential disease state and
intervention specific biomarkers. PLoS One. 9:e1128352014.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Barazzoni R, Kiwanuka E, Zanetti M,
Cristini M, Vettore M and Tessari P: Insulin acutely increases
fibrinogen production in individuals with type 2 diabetes but not
in individuals without diabetes. Diabetes. 52:1851–1856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Luo C, Zhao J, Madden A, Chen M and Xu H:
Complement expression in retinal pigment epithelial cells is
modulated by activated macrophages. Exp Eye Res. 112:93–101. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Kallio SP, Jakkula E, Purcell S, Suvela M,
Koivisto K, Tienari PJ, Elovaara I, Pirttilä T, Reunanen M,
Bronnikov D, et al: Use of a genetic isolate to identify rare
disease variants: C7 on 5p associated with MS. Hum Mol Genet.
18:1670–1683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Brudner M, Karpel M, Lear C, Chen L,
Yantosca LM, Scully C, Sarraju A, Sokolovska A, Zariffard MR, Eisen
DP, et al: Lectin-dependent enhancement of Ebola virus infection
via soluble and transmembrane C-type lectin receptors. PLoS One.
8:e608382013. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
van Vliet SJ, Steeghs L, Bruijns SC,
Vaezirad MM, Snijders Blok C, Arenas Busto JA, Deken M, van Putten
JP and van Kooyk Y: Variation of Neisseria gonorrhoeae
lipooligosaccharide directs dendritic cell-induced T helper
responses. PLoS Pathog. 5:e10006252009. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Chen P, Zhang Q, Dang H, Liu X, Tian F,
Zhao J, Chen Y, Zhang H and Chen W: Antidiabetic effect of
Lactobacillus casei CCFM0412 on mice with type 2 diabetes induced
by a high-fat diet and streptozotocin. Nutrition. 30:1061–1068.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Meyre D and Pare G: Genetic dissection of
diabetes: Facing the giant. Diabetes. 62:3338–3340. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Qi L, Parast L, Cai T, Powers C, Gervino
EV, Hauser TH, Hu FB and Doria A: Genetic susceptibility to
coronary heart disease in type 2 diabetes: 3 independent studies. J
Am Coll Cardiol. 58:2675–2682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Sandyk R: The relationship between
diabetes mellitus and Parkinson's disease. Int J Neurosci.
69:125–130. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Calkin CV, Ruzickova M, Uher R, Hajek T,
Slaney CM, Garnham JS, O'Donovan MC and Alda M: Insulin resistance
and outcome in bipolar disorder. Br J Psychiatry. 206:52–57. 2015.
View Article : Google Scholar
|
|
209
|
Cosgrove J, Alty JE and Jamieson S:
Cognitive impairment in Parkinson's disease. Postgrad Med J.
91:212–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Talbot K: Amyotrophic lateral sclerosis:
cell vulnerability or system vulnerability? J Anat. 224:45–51.
2014. View Article : Google Scholar
|
|
211
|
Carbutt S, Duff J, Yarnall A, Burn DJ and
Hudson G: Variation in complement protein C1q is not a major
contributor to cognitive impairment in Parkinson's disease.
Neurosci Lett. 594:66–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Ressl S, Vu BK, Vivona S, Martinelli DC,
Südhof TC and Brunger AT: Structures of C1q-like proteins reveal
unique features among the C1q/TNF superfamily. Structure.
23:688–699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Sigoillot SM, Iyer K, Binda F,
González-Calvo I, Talleur M, Vodjdani G, Isope P and Selimi F: The
secreted protein C1QL1 and its receptor BAI3 control the synaptic
connectivity of excitatory inputs converging on cerebellar purkinje
cells. Cell Rep. 10:820–832. 2015. View Article : Google Scholar
|
|
214
|
Wang K, Zhang H, Ma D, Bucan M, Glessner
JT, Abrahams BS, Salyakina D, Imielinski M, Bradfield JP, Sleiman
PM, et al: Common genetic variants on 5p14.1 associate with autism
spectrum disorders. Nature. 459:528–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Malenfant P, Liu X, Hudson ML, Qiao Y,
Hrynchak M, Riendeau N, Hildebrand MJ, Cohen IL, Chudley AE,
Forster-Gibson C, et al: Association of GTF2i in the
Williams-Beuren syndrome critical region with autism spectrum
disorders. J Autism Dev Disord. 42:1459–1469. 2012. View Article : Google Scholar
|
|
216
|
Lu RC, Wang H, Tan MS, Yu JT and Tan L:
TMEM106B and APOE polymorphisms interact to confer risk for
late-onset Alzheimer's disease in Han Chinese. J Neural Transm.
121:283–287. 2014. View Article : Google Scholar
|
|
217
|
Stagi M, Klein ZA, Gould TJ, Bewersdorf J
and Strittmatter SM: Lysosome size, motility and stress response
regulated by fronto-temporal dementia modifier TMEM106B. Mol Cell
Neurosci. 61:226–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Paoletti C and Hayes DF: Molecular testing
in breast cancer. Annu Rev Med. 65:95–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Ma CX, Reinert T, Chmielewska I and Ellis
MJ: Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer.
15:261–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Nollau P, Wolters-Eisfeld G, Mortezai N,
Kurze AK, Klampe B, Debus A, Bockhorn M, Niendorf A and Wagener C:
Protein domain histochemistry (PDH): binding of the carbohydrate
recognition domain (CRD) of recombinant human glycoreceptor CLEC10A
(CD301) to formalin-fixed, paraffin-embedded breast cancer tissues.
J Histochem Cytochem. 61:199–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Chen W, Salto-Tellez M, Palanisamy N,
Ganesan K, Hou Q, Tan LK, Sii LH, Ito K, Tan B, Wu J, et al:
Targets of genome copy number reduction in primary breast cancers
identified by integrative genomics. Genes Chromosomes Cancer.
46:288–301. 2007. View Article : Google Scholar
|
|
222
|
Ahmeti KB, Ajroud-Driss S, Al-Chalabi A,
Andersen PM, Armstrong J, Birve A, Blauw HM, Brown RH, Bruijn L,
Chen W, et al: Age of onset of amyotrophic lateral sclerosis is
modulated by a locus on 1p34.1. Neurobiol Aging. 34(357): e7–e19.
2013.
|
|
223
|
Brady OA, Zheng Y, Murphy K, Huang M and
Hu F: The frontotemporal lobar degeneration risk factor, TMEM106B,
regulates lysosomal morphology and function. Hum Mol Genet.
22:685–695. 2013. View Article : Google Scholar :
|
|
224
|
Sergouniotis PI, Chakarova C, Murphy C,
Becker M, Lenassi E, Arno G, Lek M, MacArthur DG, Bhattacharya SS,
Moore AT, et al: UCL-Exomes Consortium: Biallelic variants in
TTLL5, encoding a tubulin glutamylase, cause retinal dystrophy. Am
J Hum Genet. 94:760–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Dichgans M, Malik R, König IR, Rosand J,
Clarke R, Gretarsdottir S, Thorleifsson G, Mitchell BD, Assimes TL,
Levi C, et al: METASTROKE Consortium; CARDIoGRAM Consortium; C4D
Consortium; International Stroke Genetics Consortium: Shared
genetic susceptibility to ischemic stroke and coronary artery
disease: A genome-wide analysis of common variants. Stroke.
45:24–36. 2014. View Article : Google Scholar :
|
|
226
|
Hartmaier RJ, Richter AS, Gillihan RM,
Sallit JZ, McGuire SE, Wang J, Lee AV, Osborne CK, O'Malley BW,
Brown PH, et al: A SNP in steroid receptor coactivator-1 disrupts a
GSK3β phosphorylation site and is associated with altered tamoxifen
response in bone. Mol Endocrinol. 26:220–227. 2012. View Article : Google Scholar :
|
|
227
|
Pharoah PD, Tsai YY, Ramus SJ, Phelan CM,
Goode EL, Lawrenson K, Buckley M, Fridley BL, Tyrer JP, Shen H, et
al: GWAS meta-analysis and replication identifies three new
susceptibility loci for ovarian cancer. Nat Genet. 45:362–370.
370e1–2. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Kriegel MA, Rathinam C and Flavell RA: E3
ubiquitin ligase GRAIL controls primary T cell activation and oral
tolerance. Proc Natl Acad Sci USA. 106:16770–16775. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
229
|
MacKenzie DA, Schartner J, Lin J, Timmel
A, Jennens-Clough M, Fathman CG and Seroogy CM: GRAIL is
up-regulated in CD4+ CD25+ T regulatory cells
and is sufficient for conversion of T cells to a regulatory
phenotype. J Biol Chem. 282:9696–9702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Seroogy CM1, Soares L, Ranheim EA, Su L,
Holness C, Bloom D and Fathman CG: The gene related to anergy in
lymphocytes, an E3 ubiquitin ligase, is necessary for anergy
induction in CD4 T cells. J Immunol. 173:79–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
No authors listed. A death attributed to
antitoxin. Boston Med Surg J. 132:337–341. 1895.
|
|
232
|
Hunt EL: Death from allergic shock. N Engl
J Med. 228:502–507. 1943. View Article : Google Scholar
|
|
233
|
Kortright JL: Practical experiences with
antitoxin. Brooklyn MJ (Medical Society of the County of Kings).
10:87–101. 1896.
|
|
234
|
Gillis C, Gouel-Chéron A, Jönsson F and
Bruhns P: Contribution of human FcγRs to disease with evidence from
human polymorphisms and transgenic animal studies. Front Immunol.
5:2542014. View Article : Google Scholar
|
|
235
|
Lu W, Lin C and Li Y: Rottlerin induces
Wnt co-receptor LRP6 degradation and suppresses both Wnt/β-catenin
and mTORC1 signaling in prostate and breast cancer cells. Cell
Signal. 26:1303–1309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Malinauskas T and Jones EY: Extracellular
modulators of Wnt signalling. Curr Opin Struct Biol. 29:77–84.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Joiner DM, Ke J, Zhong Z, Xu HE and
Williams BO: LRP5 and LRP6 in development and disease. Trends
Endocrinol Metab. 24:31–39. 2013. View Article : Google Scholar :
|
|
238
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Jiang X, Charlat O, Zamponi R, Yang Y and
Cong F: Dishevelled Promotes Wnt Receptor Degradation through
Recruitment of ZNRF3/RNF43 E3 Ubiquitin Ligases. Mol Cell.
58:522–533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Holland J, Fasanello S and Onuma T:
Psychiatric symptoms associated with L-asparaginase administration.
J Psychiatr Res. 10:105–113. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Feinberg WM and Swenson MR:
Cerebrovascular complications of L-asparaginase therapy. Neurology.
38:127–133. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Rodrigo R, Cauli O, Boix J, ElMlili N,
Agusti A and Felipo V: Role of NMDA receptors in acute liver
failure and ammonia toxicity: Therapeutical implications. Neurochem
Int. 55:113–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Davidovic L, Jaglin XH, Lepagnol-Bestel
AM, Tremblay S, Simonneau M, Bardoni B and Khandjian EW: The
fragile X mental retardation protein is a molecular adaptor between
the neurospecific KIF3C kinesin and dendritic RNA granules. Hum Mol
Genet. 16:3047–3058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Darnell JC and Klann E: The translation of
translational control by FMRP: Therapeutic targets for FXS. Nat
Neurosci. 16:1530–1536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Poliakov E, Koonin EV and Rogozin IB:
Impairment of translation in neurons as a putative causative factor
for autism. Biol Direct. 9:162014. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Cauchi RJ: Gem depletion: Amyotrophic
lateral sclerosis and spinal muscular atrophy crossover. CNS
Neurosci Ther. 20:574–581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Häggmark A, Mikus M, Mohsenchian A, Hong
MG, Forsström B, Gajewska B, Barańczyk-Kuźma A, Uhlén M, Schwenk
JM, Kuźma-Kozakiewicz M, et al: Plasma profiling reveals three
proteins associated to amyotrophic lateral sclerosis. Ann Clin
Transl Neurol. 1:544–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Ingre C, Roos PM, Piehl F, Kamel F and
Fang F: Risk factors for amyotrophic lateral sclerosis. Clin
Epidemiol. 7:181–193. 2015.PubMed/NCBI
|
|
249
|
Smith WW, Liu Z, Liang Y, Masuda N, Swing
DA, Jenkins NA, Copeland NG, Troncoso JC, Pletnikov M, Dawson TM,
et al: Synphilin-1 attenuates neuronal degeneration in the A53T
alpha-synuclein transgenic mouse model. Hum Mol Genet.
19:2087–2098. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Wang X, Zeng W, Kim MS, Allen PB,
Greengard P and Muallem S: Spinophilin/neurabin reciprocally
regulate signaling intensity by G protein-coupled receptors. EMBO
J. 26:2768–2776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Latourelle JC, Pankratz N, Dumitriu A,
Wilk JB, Goldwurm S, Pezzoli G, Mariani CB, DeStefano AL, Halter C,
Gusella JF, et al: PROGENI Investigators, Coordinators and
Molecular Genetic Laboratories; GenePD Investigators, Coordinators
and Molecular Genetic Laboratories: Genomewide association study
for onset age in Parkinson disease. BMC Med Genet. 10:982009.
View Article : Google Scholar
|
|
252
|
Lalla E and Papapanou PN: Diabetes
mellitus and periodontitis: A tale of two common interrelated
diseases. Nat Rev Endocrinol. 7:738–748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Zeng Z, Feingold E, Wang X, Weeks DE, Lee
M, Cuenco DT, Broffitt B, Weyant RJ, Crout R, McNeil DW, et al:
Genome-wide association study of primary dentition pit-and-fissure
and smooth surface caries. Caries Res. 48:330–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Teumer A, Holtfreter B, Völker U,
Petersmann A, Nauck M, Biffar R, Völzke H, Kroemer HK, Meisel P,
Homuth G, et al: Genome-wide association study of chronic
periodontitis in a general German population. J Clin Periodontol.
40:977–985. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Elks CE, Perry JR, Sulem P, Chasman DI,
Franceschini N, He C, Lunetta KL, Visser JA, Byrne EM, Cousminer
DL, et al: GIANT Consortium: Thirty new loci for age at menarche
identified by a meta-analysis of genome-wide association studies.
Nat Genet. 42:1077–1085. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
256
|
Haas J, Beer AG, Widschwendter P,
Oberdanner J, Salzmann K, Sarg B, Lindner H, Herz J, Patsch JR and
Marschang P: LRP1b shows restricted expression in human tissues and
binds to several extracellular ligands, including fibrinogen and
apoE-carrying lipoproteins. Atherosclerosis. 216:342–347. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Poduslo SE, Huang R and Spiro A III: A
genome screen of successful aging without cognitive decline
identifies LRP1B by haplotype analysis. Am J Med Genet B
Neuropsychiatr Genet. 153B:114–119. 2010.
|
|
258
|
Scheffer DI, Zhang DS, Shen J,
Indzhykulian A, Karavitaki KD, Xu YJ, Wang Q, Lin JJ, Chen ZY and
Corey DP: XIRP2, an Actin-Binding Protein Essential for Inner Ear
Hair-Cell Stereocilia. Cell Rep. 10:1811–1818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Francis SP, Krey JF, Krystofiak ES, Cui R,
Nanda S, Xu W, Kachar B, Barr-Gillespie PG and Shin JB: A short
splice form of Xin-actin binding repeat containing 2 (XIRP2)
lacking the Xin repeats is required for maintenance of stereocilia
morphology and hearing function. J Neurosci. 35:1999–2014. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Nielsen DA, Ji F, Yuferov V, Ho A, He C,
Ott J and Kreek MJ: Genome-wide association study identifies genes
that may contribute to risk for developing heroin addiction.
Psychiatr Genet. 20:207–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
McCalmon SA, Desjardins DM, Ahmad S,
Davidoff KS, Snyder CM, Sato K, Ohashi K, Kielbasa OM, Mathew M,
Ewen EP, et al: Modulation of angiotensin II-mediated cardiac
remodeling by the MEF2A target gene Xirp2. Circ Res. 106:952–960.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Wang Q, Lin JL, Erives AJ, Lin CI and Lin
JJ: New insights into the roles of Xin repeat-containing proteins
in cardiac development, function, and disease. Int Rev Cell Mol
Biol. 310:89–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Matsuoka R, Abe S, Tokoro F, Arai M, Noda
T, Watanabe S, Horibe H, Fujimaki T, Oguri M, Kato K, et al:
Association of six genetic variants with myocardial infarction. Int
J Mol Med. 35:1451–1459. 2015.PubMed/NCBI
|
|
264
|
Roy A, Guatimosim S, Prado VF, Gros R and
Prado MA: Cholinergic activity as a new target in diseases of the
heart. Mol Med. 20:527–537. 2014.PubMed/NCBI
|
|
265
|
Zhang J, Fan J, Venneti S, Cross JR,
Takagi T, Bhinder B, Djaballah H, Kanai M, Cheng EH, Judkins AR, et
al: Asparagine plays a critical role in regulating cellular
adaptation to glutamine depletion. Mol Cell. 56:205–218. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Treviño LR, Yang W, French D, Hunger SP,
Carroll WL, Devidas M, Willman C, Neale G, Downing J, Raimondi SC,
et al: Germline genomic variants associated with childhood acute
lymphoblastic leukemia. Nat Genet. 41:1001–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Seghatoleslam A, Monabati A,
Bozorg-Ghalati F, Nikseresht M, Bordbar MR, Rahvar M and Owji AA:
Expression of UBE2Q2, a putative member of the
ubiquitin-conjugating enzyme family in pediatric acute
lymphoblastic leukemia. Arch Iran Med. 15:352–355. 2012.PubMed/NCBI
|
|
268
|
Velma V, Broome HJ and Hebert MD:
Regulated specific proteolysis of the Cajal body marker protein
coilin. Chromosoma. 121:629–642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Gubanova E, Brown B, Ivanov SV, Helleday
T, Mills GB, Yarbrough WG and Issaeva N: Downregulation of SMG-1 in
HPV-positive head and neck squamous cell carcinoma due to promoter
hypermethylation correlates with improved survival. Clin Cancer
Res. 18:1257–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Diamond G, Cedar H and Marcus M: A
temperature-sensitive mutation in asparaginyl-tRNA synthetase
causes cell-cycle arrest in early S phase. Exp Cell Res. 184:53–60.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Reitzer LJ and Magasanik B: Asparagine
synthetases of Klebsiella aerogenes: Properties and regulation of
synthesis. J Bacteriol. 151:1299–1313. 1982.PubMed/NCBI
|
|
272
|
Srikhanta YN, Atack JM, Beacham IR and
Jennings MP: Distinct physiological roles for the two
L-asparaginase isozymes of Escherichia coli. Biochem Biophys Res
Commun. 436:362–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Brigotti M, Rambelli F, Nanetti A, Zamboni
M, Sperti S and Montanaro L: Isolation of an inhibitor of cell-free
protein synthesis from Salmonella enteritidis. Microbiologica.
13:55–60. 1990.PubMed/NCBI
|
|
274
|
Bartalena L, Martino E, Antonelli A,
Pacchiarotti A, Robbins J and Pinchera A: Effect of the
antileukemic agent L-asparaginase on thyroxine-binding globulin and
albumin synthesis in cultured human hepatoma (HEP G2) cells.
Endocrinology. 119:1185–1188. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Stahl PD and Wainszelbaum MJ:
Human-specific genes may offer a unique window into human cell
signaling. Sci Signal. 2:pe592009.PubMed/NCBI
|
|
276
|
Kong C, Lange JJ, Samovski D, Su X, Liu J,
Sundaresan S and Stahl PD: Ubiquitination and degradation of the
hominoid-specific oncoprotein TBC1D3 is regulated by protein
palmitoylation. Biochem Biophys Res Commun. 434:388–393. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Frasa MA, Koessmeier KT, Ahmadian MR and
Braga VM: Illuminating the functional and structural repertoire of
human TBC/RABGAPs. Nat Rev Mol Cell Biol. 13:67–73. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Pei L, Peng Y, Yang Y, Ling XB, Van
Eyndhoven WG, Nguyen KC, Rubin M, Hoey T, Powers S and Li J: PRC17,
a novel oncogene encoding a Rab GTPase-activating protein, is
amplified in prostate cancer. Cancer Res. 62:5420–5424.
2002.PubMed/NCBI
|
|
279
|
Seaman MN, Harbour ME, Tattersall D, Read
E and Bright N: Membrane recruitment of the cargo-selective
retromer subcomplex is catalysed by the small GTPase Rab7 and
inhibited by the Rab-GAP TBC1D5. J Cell Sci. 122:2371–2382. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Popovic D and Dikic I: TBC1D5 and the AP2
complex regulate ATG9 trafficking and initiation of autophagy. EMBO
Rep. 15:392–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Frittoli E, Palamidessi A, Pizzigoni A,
Lanzetti L, Garrè M, Troglio F, Troilo A, Fukuda M, Di Fiore PP,
Scita G, et al: The primate-specific protein TBC1D3 is required for
optimal macropinocytosis in a novel ARF6-dependent pathway. Mol
Biol Cell. 19:1304–1316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
He Z, Tian T, Guo D, Wu H, Chen Y, Zhang
Y, Wan Q, Zhao H, Wang C, Shen H, et al: Cytoplasmic retention of a
nucleocytoplasmic protein TBC1D3 by microtubule network is required
for enhanced EGFR signaling. PLoS One. 9:e941342014. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Scheufele F, Wolf B, Kruse M, Hartmann T,
Lempart J, Muehlich S, Pfeiffer AF, Field LJ, Charron MJ, Pan ZQ,
et al: Evidence for a regulatory role of Cullin-RING E3 ubiquitin
ligase 7 in insulin signaling. Cell Signal. 26:233–239. 2014.
View Article : Google Scholar :
|
|
284
|
Wainszelbaum MJ, Liu J, Kong C, Srikanth
P, Samovski D, Su X and Stahl PD: TBC1D3, a hominoid-specific gene,
delays IRS-1 degradation and promotes insulin signaling by
modulating p70 S6 kinase activity. PLoS One. 7:e312252012.
View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Copps KD and White MF: Regulation of
insulin sensitivity by serine/threonine phosphorylation of insulin
receptor substrate proteins IRS1 and IRS2. Diabetologia.
55:2565–2582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Chantranupong L, Wolfson RL and Sabatini
DM: Nutrient-sensing mechanisms across evolution. Cell. 161:67–83.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
287
|
Mirkin SM: Expandable DNA repeats and
human disease. Nature. 447:932–940. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Shaw G and Kamen R: A conserved AU
sequence from the 3′ untranslated region of GM-CSF mRNA mediates
selective mRNA degradation. Cell. 46:659–667. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Uversky VN: Functional roles of
transiently and intrinsically disordered regions within proteins.
FEBS J. 282:1182–1189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
290
|
Ragusa MJ, Dancheck B, Critton DA, Nairn
AC, Page R and Peti W: Spinophilin directs protein phosphatase 1
specificity by blocking substrate binding sites. Nat Struct Mol
Biol. 17:459–464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
291
|
Nakanishi H, Obaishi H, Satoh A, Wada M,
Mandai K, Satoh K, Nishioka H, Matsuura Y, Mizoguchi A and Takai Y:
Neurabin: A novel neural tissue-specific actin filament-binding
protein involved in neurite formation. J Cell Biol. 139:951–961.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
292
|
Chen Y, Liu Y, Cottingham C, McMahon L,
Jiao K, Greengard P and Wang Q: Neurabin scaffolding of adenosine
receptor and RGS4 regulates anti-seizure effect of endogenous
adenosine. J Neurosci. 32:2683–2695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
293
|
Kim SS, Wang H, Li XY, Chen T, Mercaldo V,
Descalzi G, Wu LJ and Zhuo M: Neurabin in the anterior cingulate
cortex regulates anxiety-like behavior in adult mice. Mol Brain.
4:62011. View Article : Google Scholar : PubMed/NCBI
|
|
294
|
Hu XD, Huang Q, Roadcap DW, Shenolikar SS
and Xia H: Actin-associated neurabin-protein phosphatase-1 complex
regulates hippocampal plasticity. J Neurochem. 98:1841–1851. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
295
|
Hu XD, Huang Q, Yang X and Xia H:
Differential regulation of AMPA receptor trafficking by
neurabin-targeted synaptic protein phosphatase-1 in synaptic
transmission and long-term depression in hippocampus. J Neurosci.
27:4674–4686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
296
|
Allen PB, Zachariou V, Svenningsson P,
Lepore AC, Centonze D, Costa C, Rossi S, Bender G, Chen G, Feng J,
et al: Distinct roles for spinophilin and neurabin in
dopamine-mediated plasticity. Neuroscience. 140:897–911. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
297
|
Wu LJ, Ren M, Wang H, Kim SS, Cao X and
Zhuo M: Neurabin contributes to hippocampal long-term potentiation
and contextual fear memory. PLoS One. 3:e14072008. View Article : Google Scholar : PubMed/NCBI
|
|
298
|
Finalet Ferreiro J, Rouhigharabaei L,
Urbankova H, van der Krogt JA, Michaux L, Shetty S, Krenacs L,
Tousseyn T, De Paepe P, Uyttebroeck A, et al: Integrative genomic
and transcriptomic analysis identified candidate genes implicated
in the pathogenesis of hepatosplenic T-cell lymphoma. PLoS One.
9:e1029772014. View Article : Google Scholar : PubMed/NCBI
|
|
299
|
Rowell JP, Simpson KL, Stott K, Watson M
and Thomas JO: HMGB1-facilitated p53 DNA binding occurs via
HMG-Box/p53 transactivation domain interaction, regulated by the
acidic tail. Structure. 20:2014–2024. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
300
|
Teufel DP, Freund SM, Bycroft M and Fersht
AR: Four domains of p300 each bind tightly to a sequence spanning
both transactivation subdomains of p53. Proc Natl Acad Sci USA.
104:7009–7014. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
301
|
Zhang Z, Song M, Liu X, Kang SS, Kwon IS,
Duong DM, Seyfried NT, Hu WT, Liu Z, Wang JZ, et al: Cleavage of
tau by asparagine endopeptidase mediates the neurofibrillary
pathology in Alzheimer's disease. Nat Med. 20:1254–1262. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
302
|
Hsieh JJ, Cheng EH and Korsmeyer SJ:
Taspase1: A threonine aspartase required for cleavage of MLL and
proper HOX gene expression. Cell. 115:293–303. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
303
|
Aleksandrov AA, Kota P, Aleksandrov LA, He
L, Jensen T, Cui L, Gentzsch M, Dokholyan NV and Riordan JR:
Regulatory insertion removal restores maturation, stability and
function of DeltaF508 CFTR. J Mol Biol. 401:194–210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
304
|
Lewis HA, Zhao X, Wang C, Sauder JM,
Rooney I, Noland BW, Lorimer D, Kearins MC, Conners K, Condon B, et
al: Impact of the deltaF508 mutation in first nucleotide-binding
domain of human cystic fibrosis transmembrane conductance regulator
on domain folding and structure. J Biol Chem. 280:1346–1353. 2005.
View Article : Google Scholar
|
|
305
|
Muchmore SW, Sattler M, Liang H, Meadows
RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong
SL, et al: X-ray and NMR structure of human Bcl-xL, an inhibitor of
programmed cell death. Nature. 381:335–341. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
306
|
Dho SH, Deverman BE, Lapid C, Manson SR,
Gan L, Riehm JJ, Aurora R, Kwon KS and Weintraub SJ: Control of
cellular Bcl-xL levels by deamidation-regulated degradation. PLoS
Biol. 11:e10015882013. View Article : Google Scholar : PubMed/NCBI
|
|
307
|
Lee JC, Kang SU, Jeon Y, Park JW, You JS,
Ha SW, Bae N, Lubec G, Kwon SH, Lee JS, et al: Protein
L-isoaspartyl methyltransferase regulates p53 activity. Nat Commun.
3:9272012. View Article : Google Scholar : PubMed/NCBI
|
|
308
|
Dawson R, Müller L, Dehner A, Klein C,
Kessler H and Buchner J: The N-terminal domain of p53 is natively
unfolded. J Mol Biol. 332:1131–1141. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
309
|
Schon O, Friedler A, Freund S and Fersht
AR: Binding of p53-derived ligands to MDM2 induces a variety of
long range conformational changes. J Mol Biol. 336:197–202. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
310
|
Rogers CS, Abraham WM, Brogden KA,
Engelhardt JF, Fisher JT, McCray PB Jr, McLennan G, Meyerholz DK,
Namati E, Ostedgaard LS, et al: The porcine lung as a potential
model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol.
295:L240–L263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
311
|
Seok J, Warren HS, Cuenca AG, Mindrinos
MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy
L, et al: Inflammation and Host Response to Injury, Large Scale
Collaborative Research Program: Genomic responses in mouse models
poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA.
110:3507–3512. 2013. View Article : Google Scholar
|
|
312
|
Patterson PH: Immune involvement in
schizophrenia and autism: etiology, pathology and animal models.
Behav Brain Res. 204:313–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
313
|
Ohi N, Tokunaga A, Tsunoda H, Nakano K,
Haraguchi K, Oda K, Motoyama N and Nakajima T: A novel adenovirus
E1B19K-binding protein B5 inhibits apoptosis induced by Nip3 by
forming a heterodimer through the C-terminal hydrophobic region.
Cell Death Differ. 6:314–325. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
314
|
Zhang J, Loyd MR, Randall MS, Waddell MB,
Kriwacki RW and Ney PA: A short linear motif in BNIP3L (NIX)
mediates mitochondrial clearance in reticulocytes. Autophagy.
8:1325–1332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
315
|
Perutz M: Polar zippers: their role in
human disease. Protein Sci. 3:1629–1637. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
316
|
Perutz MF, Pope BJ, Owen D, Wanker EE and
Scherzinger E: Aggregation of proteins with expanded glutamine and
alanine repeats of the glutamine-rich and asparagine-rich domains
of Sup35 and of the amyloid beta-peptide of amyloid plaques. Proc
Natl Acad Sci USA. 99:5596–5600. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
317
|
Simon M and Hancock JM: Tandem and cryptic
amino acid repeats accumulate in disordered regions of proteins.
Genome Biol. 10:R592009. View Article : Google Scholar : PubMed/NCBI
|
|
318
|
Tompa P: Intrinsically unstructured
proteins evolve by repeat expansion. Bioessays. 25:847–855. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
319
|
Li L and Moore PK: An overview of the
biological significance of endogenous gases: New roles for old
molecules. Biochem Soc Trans. 35:1138–1141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
320
|
Levine SM, Rosen A and Casciola-Rosen LA:
Anti-aminoacyl tRNA synthetase immune responses: Insights into the
pathogenesis of the idiopathic inflammatory myopathies. Curr Opin
Rheumatol. 15:708–713. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
321
|
Beaulande M, Tarbouriech N and Härtlein M:
Human cytosolic asparaginyl-tRNA synthetase: cDNA sequence,
functional expression in Escherichia coli and characterization as
human autoantigen. Nucleic Acids Res. 26:521–524. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
322
|
Howard OM, Dong HF, Yang D, Raben N,
Nagaraju K, Rosen A, Casciola-Rosen L, Härtlein M, Kron M, Yang D,
et al: Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase,
autoantigens in myositis, activate chemokine receptors on T
lymphocytes and immature dendritic cells. J Exp Med. 196:781–791.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
323
|
Park SJ, Kim SH, Choi HS, Rhee Y and Lim
SK: Fibroblast growth factor 2-induced cytoplasmic asparaginyl-tRNA
synthetase promotes survival of osteoblasts by regulating
anti-apoptotic PI3K/Akt signaling. Bone. 45:994–1003. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
324
|
Kron MA, Wang C, Vodanovic-Jankovic S,
Howard OM and Kuhn LA: Interleukin-8-like activity in a filarial
asparaginyl-tRNA synthetase. Mol Biochem Parasitol. 185:66–69.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
325
|
Bonfils G, Jaquenoud M, Bontron S,
Ostrowicz C, Ungermann C and De Virgilio C: Leucyl-tRNA synthetase
controls TORC1 via the EGO complex. Mol Cell. 46:105–110. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
326
|
Avruch J, Long X, Ortiz-Vega S, Rapley J,
Papageorgiou A and Dai N: Amino acid regulation of TOR complex 1.
Am J Physiol Endocrinol Metab. 296:E592–E602. 2009. View Article : Google Scholar :
|
|
327
|
Hara K, Yonezawa K, Weng QP, Kozlowski MT,
Belham C and Avruch J: Amino acid sufficiency and mTOR regulate p70
S6 kinase and eIF-4E BP1 through a common effector mechanism. J
Biol Chem. 273:14484–14494. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
328
|
Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant
GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, et
al: Metabolism. Lysosomal amino acid transporter SLC38A9 signals
arginine sufficiency to mTORC1. Science. 347:188–194. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
329
|
Rebsamen M, Pochini L, Stasyk T, de Araújo
ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya
EL, Bruckner M, et al: SLC38A9 is a component of the lysosomal
amino acid sensing machinery that controls mTORC1. Nature.
519:477–481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
330
|
Bar-Peled L and Sabatini DM: Regulation of
mTORC1 by amino acids. Trends Cell Biol. 24:400–406. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
331
|
Efeyan A, Zoncu R and Sabatini DM: Amino
acids and mTORC1: From lysosomes to disease. Trends Mol Med.
18:524–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
332
|
Abraham RT: Cell biology. Making sense of
amino acid sensing. Science. 347:128–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
333
|
Weng L, Quinlivan E, Gong Y, Beitelshees
AL, Shahin MH, Turner ST, Chapman AB, Gums JG, Johnson JA, Frye RF,
et al: Association of branched and aromatic amino acids levels with
metabolic syndrome and impaired fasting glucose in hypertensive
patients. Metab Syndr Relat Disord. 13:195–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
334
|
Björkegren JL, Kovacic JC, Dudley JT and
Schadt EE: Genome-wide significant loci: How important are they?
Systems genetics to understand heritability of coronary artery
disease and other common complex disorders. J Am Coll Cardiol.
65:830–845. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
335
|
Zhang X, Bailey SD and Lupien M: Laying a
solid foundation for Manhattan - 'setting the functional basis for
the post-GWAS era'. Trends Genet. 30:140–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
336
|
Gusev A, Bhatia G, Zaitlen N, Vilhjalmsson
BJ, Diogo D, Stahl EA, Gregersen PK, Worthington J, Klareskog L,
Raychaudhuri S, et al: Quantifying missing heritability at known
GWAS loci. PLoS Genet. 9:e10039932013. View Article : Google Scholar
|
|
337
|
Manolio TA, Collins FS, Cox NJ, Goldstein
DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR,
Chakravarti A, et al: Finding the missing heritability of complex
diseases. Nature. 461:747–753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
338
|
Cross-Disorder Group of the Psychiatric
Genomics Consortium: Identification of risk loci with shared
effects on five major psychiatric disorders: A genome-wide
analysis. Lancet. 381:1371–1379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
339
|
Nikoletopoulou V, Lickert H, Frade JM,
Rencurel C, Giallonardo P, Zhang L, Bibel M and Barde YA:
Neurotrophin receptors TrkA and TrkC cause neuronal death whereas
TrkB does not. Nature. 467:59–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
340
|
Yoon K, Jang HD and Lee SY: Direct
interaction of Smac with NADE promotes TRAIL-induced apoptosis.
Biochem Biophys Res Commun. 319:649–654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
341
|
Zhang CK, Stein PB, Liu J, Wang Z, Yang R,
Cho JH, Gregersen PK, Aerts JM, Zhao H, Pastores GM, et al:
Genome-wide association study of N370S homozygous Gaucher disease
reveals the candidacy of CLN8 gene as a genetic modifier
contributing to extreme phenotypic variation. Am J Hematol.
87:377–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
342
|
Bultron G, Kacena K, Pearson D, Boxer M,
Yang R, Sathe S, Pastores G and Mistry PK: The risk of Parkinson's
disease in type 1 Gaucher disease. J Inherit Metab Dis. 33:167–173.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
343
|
Urano M, Nagao T, Miyabe S, Ishibashi K,
Higuchi K and Kuroda M: Characterization of mammary analogue
secretory carcinoma of the salivary gland: Discrimination from its
mimics by the presence of the ETV6-NTRK3 translocation and novel
surrogate markers. Hum Pathol. 46:94–103. 2015. View Article : Google Scholar
|
|
344
|
Lannon CL and Sorensen PH: ETV6-NTRK3: A
chimeric protein tyrosine kinase with transformation activity in
multiple cell lineages. Semin Cancer Biol. 15:215–223. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
345
|
Genevois AL, Ichim G, Coissieux MM,
Lambert MP, Lavial F, Goldschneider D, Jarrosson-Wuilleme L,
Lepinasse F, Gouysse G, Herceg Z, et al: Dependence receptor TrkC
is a putative colon cancer tumor suppressor. Proc Natl Acad Sci
USA. 110:3017–3022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
346
|
Luo Y, Kaz AM, Kanngurn S, Welsch P,
Morris SM, Wang J, Lutterbaugh JD, Markowitz SD and Grady WM: NTRK3
is a potential tumor suppressor gene commonly inactivated by
epigenetic mechanisms in colorectal cancer. PLoS Genet.
9:e10035522013. View Article : Google Scholar : PubMed/NCBI
|
|
347
|
Ivanov SV, Panaccione A, Brown B, Guo Y,
Moskaluk CA, Wick MJ, Brown JL, Ivanova AV, Issaeva N, El-Naggar
AK, et al: TrkC signaling is activated in adenoid cystic carcinoma
and requires NT-3 to stimulate invasive behavior. Oncogene.
32:3698–3710. 2013. View Article : Google Scholar
|
|
348
|
Kim MS, Kim GM, Choi YJ, Kim HJ, Kim YJ
and Jin W: TrkC promotes survival and growth of leukemia cells
through Akt-mTOR-dependent up-regulation of PLK-1 and Twist-1. Mol
Cells. 36:177–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
349
|
Weinkauf C, Salvador R and Pereiraperrin
M: Neurotrophin receptor TrkC is an entry receptor for Trypanosoma
cruzi in neural, glial, and epithelial cells. Infect Immun.
79:4081–4087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
350
|
Capewell P, Cooper A, Clucas C, Weir W and
Macleod A: A co-evolutionary arms race: Trypanosomes shaping the
human genome, humans shaping the trypanosome genome. Parasitology.
142(Suppl 1): S108–S119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
351
|
Marx SO, Reiken S, Hisamatsu Y, Jayaraman
T, Burkhoff D, Rosemblit N and Marks AR: PKA phosphorylation
dissociates FKBP12.6 from the calcium release channel (ryanodine
receptor): Defective regulation in failing hearts. Cell.
101:365–376. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
352
|
Zhou L, He M, Mo Z, Wu C, Yang H, Yu D,
Yang X, Zhang X, Wang Y, Sun J, et al: A genome wide association
study identifies common variants associated with lipid levels in
the Chinese population. PLoS One. 8:e824202013. View Article : Google Scholar
|
|
353
|
Del-Aguila JL, Beitelshees AL,
Cooper-Dehoff RM, Chapman AB, Gums JG, Bailey K, Gong Y, Turner ST,
Johnson JA and Boerwinkle E: Genome-wide association analyses
suggest NELL1 influences adverse metabolic response to HCTZ in
African Americans. Pharmacogenomics J. 14:35–40. 2014. View Article : Google Scholar
|
|
354
|
Jeong SW, Chung M, Park SJ, Cho SB and
Hong KW: Genome-wide association study of metabolic syndrome in
koreans. Genomics Inform. 12:187–194. 2014. View Article : Google Scholar
|
|
355
|
Wellcome Trust Case Control Consortium:
Genome-wide association study of 14,000 cases of seven common
diseases and 3,000 shared controls. Nature. 447:661–678. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
356
|
Eirís N, González-Lara L, Santos-Juanes J,
Queiro R, Coto E and Coto-Segura P: Genetic variation at IL12B,
IL23R and IL23A is associated with psoriasis severity, psoriatic
arthritis and type 2 diabetes mellitus. J Dermatol Sci. 75:167–172.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
357
|
Zhang M, Cai ZR, Zhang B, Cai X, Li W, Guo
Z and Ma L: Functional polymorphisms in interleukin-23 receptor and
susceptibility to coronary artery disease. DNA Cell Biol.
33:891–897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
358
|
Mizuki N, Meguro A, Ota M, Ohno S, Shiota
T, Kawagoe T, Ito N, Kera J, Okada E, Yatsu K, et al: Genome-wide
association studies identify IL23R-IL12RB2 and IL10 as Behçet's
disease susceptibility loci. Nat Genet. 42:703–706. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
359
|
Daryabor G, Mahmoudi M, Jamshidi A,
Nourijelyani K, Amirzargar A, Ahmadzadeh N, Farhadi E and Nicknam
MH: Determination of IL-23 receptor gene polymorphism in Iranian
patients with ankylosing spondylitis. Eur Cytokine Netw. 25:24–29.
2014.PubMed/NCBI
|
|
360
|
Zhang F, Liu H, Chen S, Low H, Sun L, Cui
Y, Chu T, Li Y, Fu X, Yu Y, et al: Identification of two new loci
at IL23R and RAB32 that influence susceptibility to leprosy. Nat
Genet. 43:1247–1251. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
361
|
Hornakova T, Staerk J, Royer Y, Flex E,
Tartaglia M, Constantinescu SN, Knoops L and Renauld JC: Acute
lymphoblastic leukemia-associated JAK1 mutants activate the Janus
kinase/STAT pathway via interleukin-9 receptor alpha homodimers. J
Biol Chem. 284:6773–6781. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
362
|
Leonard WJ: The defective gene in X-linked
severe combined immunodeficiency encodes a shared interleukin
receptor subunit: Implications for cytokine pleiotropy and
redundancy. Curr Opin Immunol. 6:631–635. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
363
|
Baba A, Ohtake F, Okuno Y, Yokota K, Okada
M, Imai Y, Ni M, Meyer CA, Igarashi K, Kanno J, et al:
PKA-dependent regulation of the histone lysine demethylase complex
PHF2-ARID5B. Nat Cell Biol. 13:668–675. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
364
|
Papaemmanuil E, Hosking FJ, Vijayakrishnan
J, Price A, Olver B, Sheridan E, Kinsey SE, Lightfoot T, Roman E,
Irving JA, et al: Loci on 7p12.2, 10q21.2 and 14q11.2 are
associated with risk of childhood acute lymphoblastic leukemia. Nat
Genet. 41:1006–1010. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
365
|
Chokkalingam AP, Hsu LI, Metayer C, Hansen
HM, Month SR, Barcellos LF, Wiemels JL and Buffler PA: Genetic
variants in ARID5B and CEBPE are childhood ALL susceptibility loci
in Hispanics. Cancer Causes Control. 24:1789–1795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
366
|
Xu H, Cheng C, Devidas M, Pei D, Fan Y,
Yang W, Neale G, Scheet P, Burchard EG, Torgerson DG, et al: ARID5B
genetic polymorphisms contribute to racial disparities in the
incidence and treatment outcome of childhood acute lymphoblastic
leukemia. J Clin Oncol. 30:751–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
367
|
Gutiérrez-Camino Á, López-López E,
Martín-Guerrero I, Sánchez-Toledo J, García de Ann N, Carboné
Bañeres A, García-Miguel P, Navajas A and García-Orad Á: Intron 3
of the ARID5B gene: A hot spot for acute lymphoblastic leukemia
susceptibility. J Cancer Res Clin Oncol. 139:1879–1886. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
368
|
Guo LM, Xi JS, Ma Y, Shao L, Nie CL and
Wang GJ: ARID5B gene rs10821936 polymorphism is associated with
childhood acute lymphoblastic leukemia: a meta-analysis based on
39,116 subjects. Tumour Biol. 35:709–713. 2014. View Article : Google Scholar
|
|
369
|
Lin CY, Li MJ, Chang JG, Liu SC, Weng T,
Wu KH, Yang SF, Huang FK, Lo WY and Peng CT: High-resolution
melting analyses for genetic variants in ARID5B and IKZF1 with
childhood acute lymphoblastic leukemia susceptibility loci in
Taiwan. Blood Cells Mol Dis. 52:140–145. 2014. View Article : Google Scholar
|
|
370
|
Lu Y, Vitart V, Burdon KP, Khor CC,
Bykhovskaya Y, Mirshahi A, Hewitt AW, Koehn D, Hysi PG, Ramdas WD,
et al: NEIGHBOR Consortium: Genome-wide association analyses
identify multiple loci associated with central corneal thickness
and keratoconus. Nat Genet. 45:155–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
371
|
Engel SM, Joubert BR, Wu MC, Olshan AF,
Håberg SE, Ueland PM, Nystad W, Nilsen RM, Vollset SE, Peddada SD,
et al: Neonatal genome-wide methylation patterns in relation to
birth weight in the Norwegian Mother and Child Cohort. Am J
Epidemiol. 179:834–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
372
|
Newton-Cheh C, Johnson T, Gateva V, Tobin
MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S,
et al: Wellcome Trust Case Control Consortium: Genome-wide
association study identifies eight loci associated with blood
pressure. Nat Genet. 41:666–676. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
373
|
Okada Y, Terao C, Ikari K, Kochi Y, Ohmura
K, Suzuki A, Kawaguchi T, Stahl EA, Kurreeman FA, Nishida N, et al:
Meta-analysis identifies nine new loci associated with rheumatoid
arthritis in the Japanese population. Nat Genet. 44:511–516. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
374
|
Drago A, Giegling I, Schäfer M, Hartmann
AM, Konte B, Friedl M, Serretti A and Rujescu D: Genome-wide
association study supports the role of the immunological system and
of the neurodevelopmental processes in response to haloperidol
treatment. Pharmacogenet Genomics. 24:314–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
375
|
Yang W, Tang H, Zhang Y, Tang X, Zhang J,
Sun L, Yang J, Cui Y, Zhang L, Hirankarn N, et al: Meta-analysis
followed by replication identifies loci in or near CDKN1B, TET3,
CD80, DRAM1, and ARID5B as associated with systemic lupus
erythematosus in Asians. Am J Hum Genet. 92:41–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
376
|
Whitson RH, Tsark W, Huang TH and Itakura
K: Neonatal mortality and leanness in mice lacking the ARID
transcription factor Mrf-2. Biochem Biophys Res Commun.
312:997–1004. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
377
|
Yamakawa T, Sugimoto K, Whitson RH and
Itakura K: Modulator recognition factor-2 regulates triglyceride
metabolism in adipocytes. Biochem Biophys Res Commun. 391:277–281.
2010. View Article : Google Scholar
|
|
378
|
Wang G, Watanabe M, Imai Y, Hara K, Manabe
I, Maemura K, Horikoshi M, Ozeki A, Itoh C, Sugiyama T, et al:
Associations of variations in the MRF2/ARID5B gene with
susceptibility to type 2 diabetes in the Japanese population. J Hum
Genet. 57:727–733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
379
|
Urayama KY, Chokkalingam AP, Manabe A and
Mizutani S: Current evidence for an inherited genetic basis of
childhood acute lymphoblastic leukemia. Int J Hematol. 97:3–19.
2013. View Article : Google Scholar
|
|
380
|
Prakash T, Sharma VK, Adati N, Ozawa R,
Kumar N, Nishida Y, Fujikake T, Takeda T and Taylor TD: Expression
of conjoined genes: Another mechanism for gene regulation in
eukaryotes. PLoS One. 5:e132842010. View Article : Google Scholar : PubMed/NCBI
|
|
381
|
Geer LY, Marchler-Bauer A, Geer RC, Han L,
He J, He S, Liu C, Shi W and Bryant SH: The NCBI BioSystems
database. Nucleic Acids Res. 38:D492–D496. 2010. View Article : Google Scholar :
|
|
382
|
Parge HE, Arvai AS, Murtari DJ, Reed SI
and Tainer JA: Human CksHs2 atomic structure: A role for its
hexameric assembly in cell cycle control. Science. 262:387–395.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
383
|
Liberal V, Martinsson-Ahlzén HS, Liberal
J, Spruck CH, Widschwendter M, McGowan CH and Reed SI:
Cyclin-dependent kinase subunit (Cks) 1 or Cks2 overexpression
overrides the DNA damage response barrier triggered by activated
oncoproteins. Proc Natl Acad Sci USA. 109:2754–2759. 2012.
View Article : Google Scholar :
|
|
384
|
Agirre X, Román-Gómez J, Jiménez-Velasco
A, Garate L, Montiel-Duarte C, Navarro G, Vázquez I, Zalacain M,
Calasanz MJ, Heiniger A, et al: ASPP1, a common activator of TP53,
is inactivated by aberrant methylation of its promoter in acute
lymphoblastic leukemia. Oncogene. 25:1862–1870. 2006. View Article : Google Scholar
|
|
385
|
Khattar V and Thottassery JV: Cks1:
Structure, emerging roles and implications in multiple cancers. J
Cancer Ther. 4:1341–1354. 2013. View Article : Google Scholar
|
|
386
|
Lee SW, Lin CY, Tian YF, Sun DP, Lin LC,
Chen LT, Hsing CH, Huang CT, Hsu HP, Huang HY, et al:
Overexpression of CDC28 protein kinase regulatory subunit 1B
confers an independent prognostic factor in nasopharyngeal
carcinoma. APMIS. 122:206–214. 2014. View Article : Google Scholar
|
|
387
|
Vigneron AM and Vousden KH: An indirect
role for ASPP1 in limiting p53-dependent p21 expression and
cellular senescence. EMBO J. 31:471–480. 2012. View Article : Google Scholar :
|
|
388
|
Valaperta R, Rizzo V, Lombardi F, Verdelli
C, Piccoli M, Ghiroldi A, Creo P, Colombo A, Valisi M, Margiotta E,
et al: Adenine phosphoribosyltransferase (APRT) deficiency:
Identification of a novel nonsense mutation. BMC Nephrol.
15:1022014. View Article : Google Scholar : PubMed/NCBI
|
|
389
|
Ibrahim L, Aladle D, Mansour A, Hammad A,
Al Wakeel AA and Abd El-Hameed SA: Expression and prognostic
significance of livin/BIRC7 in childhood acute lymphoblastic
leukemia. Med Oncol. 31:9412014. View Article : Google Scholar : PubMed/NCBI
|
|
390
|
Mulcahy ME, Geoghegan JA, Monk IR,
O'Keeffe KM, Walsh EJ, Foster TJ and McLoughlin RM: Nasal
colonisation by Staphylococcus aureus depends upon clumping factor
B binding to the squamous epithelial cell envelope protein
loricrin. PLoS Pathog. 8:e10030922012. View Article : Google Scholar
|
|
391
|
Hawkes WC, Wang TT, Alkan Z, Richter BD
and Dawson K: Selenoprotein W modulates control of cell cycle
entry. Biol Trace Elem Res. 131:229–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
392
|
Pekarsky Y, Drusco A, Kumchala P, Croce CM
and Zanesi N: The long journey of TCL1 transgenic mice: Lessons
learned in the last 15 years. Gene Expr. 16:129–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
393
|
Chalouhi N, Theofanis T, Starke RM, Zanaty
M, Jabbour P, Dooley SA and Hasan D: Potential role of
granulocyte-monocyte colony-stimulating factor in the progression
of intracranial aneurysms. DNA Cell Biol. 34:78–81. 2015.
View Article : Google Scholar
|
|
394
|
Small KS, Hedman AK, Grundberg E, Nica AC,
Thorleifsson G, Kong A, Thorsteindottir U, Shin SY, Richards HB,
Soranzo N, et al: GIANT Consortium; MAGIC Investigators; DIAGRAM
Consortium; MuTHER Consortium: Identification of an imprinted
master trans regulator at the KLF14 locus related to multiple
metabolic phenotypes. Nat Genet. 43:561–564. 2011. View Article : Google Scholar : PubMed/NCBI
|