Introduction
Chronic neuropathic pain has previously been
characterized by hyperalgesia and allodynia has become a notable
public health problem that affects a broader population worldwide
(1,2). Generally, chronic neuropathic pain
is a consequence of a disease or lesion that causes damage to the
somatosensory system (3). The
increasing amount of evidence has indicated that neuropathic pain
contributes to the pain experience for a subset of the
osteoarthritis population (4). In
addition, neuropathic pain has been suggested to be an
underestimated problem in patients following total knee replacement
(5). However, all current
therapies for neuropathic pain are far from effective and only
treat the symptoms (6). The main
obstacle hampering the development of effective therapeutics is
that the precise molecular mechanism underlying neuropathic pain
remains to be elucidated.
MicroRNAs (miRNAs or miRs) are a subset of small
non-coding RNAs with a length of ~21 nucleotides that regulate the
expression of numerous genes via targeting the 3′-untranslated
region (UTR) of messenger RNA (mRNA), resulting in mRNA
destabilization and degradation, and thus protein translational
inhibition (7,8). miRNAs have been found to be involved
in regulating numerous cellular processes that could participate in
the pathogenesis of various diseases (9,10).
In recent years, the role of miRNAs in neuropathic pain has been
highlighted (11). It has been
reported that the expression of >63 miRNAs was significantly
altered in a rat model of neuropathic pain (12). miR-182, miR-18 and
miR-96 have been revealed to be highly expressed in the
dorsal root ganglion (DRG) in a rat model of neuropathic pain
(13). Favereaux et al
(14) demonstrated that
miR-103 was decreased in neuropathic animals and intrathecal
injection of miR-103 successfully attenuated neuropathic
chronic pain. More recently, Tan et al (15) reported that inhibition of
miR-155 relieved neuroinflammation and neuropathic pain
development by upregulating suppressor of cytokine signaling 1
expression. All these findings suggest that miRNAs may be served as
a potential and effective molecular target for developing novel
therapies for treatment of neuropathic pain.
High-mobility group box 1 (HMGB1) has been
considered as an abundant and ubiquitous non-histone DNA-binding
protein that is expressed in numerous cell types, including neurons
and glial cells (16). Emerging
evidence has reported that HMGB1 is extensively involved in
regulating proinflammatory diseases, such as rheumatoid arthritis
(17) and sepsis (18). Therefore, HMGB1 has been suggested
as an alarmin to orchestrate inflammatory responses that regulate
cell migration, phagocytosis and activation of immune cells
(19). With consideration of the
important role of HMGB1 in inflammation, HMGB1 is expected to be
involved in regulating neuropathic pain, which is characterized as
an excessive proinflammation in the nervous system (20–22). Initial reports revealed that
exogenous HMGB injection induced neuropathic pain-like behavior in
rodents (23). Shibasaki et
al (24) demonstrated that
induction of HMGB1 in DRG contributes to neuropathic pain
following peripheral nerve injury. In a rat model of tibial nerve
injury, HMGB was redistributed from the nucleus to the cytoplasm in
neurons that contributed to tactile hyperalgesia (25). Administration of the HMGB1
neutralization antibody effectively reduced neuroinflammation and
improved the pain-related behavior (26). Therefore, HMGB1 may be a promising
therapeutic target for neuropathic pain.
The present study identified HMGB1 as a
predicated target gene of miR-141 by bioinformatics analysis
(http://www.targetscan.org), implying
that miR-141 may regulate HMGB1 expression and thus
participate in neuropathic pain. Therefore, the study was designed
to identify and validate whether miR-141 directly regulated
HMGB1 expression and participated in the development of
neuropathic pain, and aimed to provide an effective and potential
molecular target for the treatment of neuropathic pain.
Materials and methods
Animals
Adult male Sprague-Dawley rats, weighing 220–250 g,
were provided by the Laboratory Animal Center of Tianjin Medical
University (Tianjin, China). The animals were raised at room
temperature of 24.0±1°C with a 12/12-h light/dark-cycle and free
access to food and water. All the animal experiments were conducted
in accordance with the guidelines of the Institutional Animal Care
and Use Committee of Tianjin Hospital (Tianjin, China).
DRG culture
The primary DRG neurons were isolated and cultured
according to a previously reported method (27). Briefly, the bilateral DRG from
rats were quickly dissected under the microscope and digested for
15 min at 37°C in Dulbecco's modified Eagle's medium (DMEM; Life
Technologies, Carlsbad, CA, USA) containing trypsin (1 mg/ml) and
collagenase (2 mg/ml) (Sigma, St. Louis, MO, USA). The DRG neurons
were obtained by a pasteur pipette (Shanghai Lianshuo Biological
Technology Co., Ltd., Shanghai, China), and were plated in 6-well
plates at 1×106 cells/well in DMEM containing 5
µg/ml cytarabine (Hisunpharm, Taizhou, China) for 24 h to
suppress the growth of non-neuronal cells. Subsequently, cells were
collected and cultured in DMEM/F-12 (Gibco, Grand Island, NY, USA)
containing 10% fetal bovine serum (Gibco), 0.1 mg/ml L-glutamine,
and 10 ng/ml nerve growth factor (Life Technologies) supplemented
with 1% penicillin/streptomycin (Sigma).
Chronic constriction injury (CCI)
model
A rat model of neuropathic pain was established by
CCI according to a previously described method (28). Briefly, rats were anesthetized by
intraperitoneal (i.p.) injection of sodium pentobarbital (40 mg/kg;
Merck, Darmstadt, Germany). The left sciatic nerve was exposed and
ligated with 4–0 catgut thread (Johnson & Johnson, New
Brunswick, NJ, USA) at 4 sites with an interval of 1 mm.
Sham-operated rats were performed with left sciatic nerve exposure,
without ligation.
Intrathecal catheter implantation
Intrathecal catheter implantation was performed
according to a method reported previously (29). Briefly, rats were anaesthetised
with 40 mg/kg sodium pentobarbital (i.p.). The occipital muscles
were separated to expose the cisternal membrane. The polyethylene
catheter (PE-10; American Health & Medical Supply International
Corp., New York, NY, USA) was inserted in the cisterna magna
through an incision and advanced 7.0–7.5 cm caudally to the lumbar
enlargement. The intrathecal implantation was verified by paralysis
of the bilateral hind limbs with injection of 2% lidocaine (Sigma).
Subsequently, the catheter was fixed and the incision was sealed.
Intrathecal lentiviral (LV)-miR-141 (GenePharma, Shanghai,
China) administration was performed using a microinjection syringe
linked with the intrathecal catheter. A total of 10 µl of
recombinant lentivirus was administrated once daily for 3 days
after CCI. For biological analysis, the rats were euthanized
quickly and the L4-L5 lumbar spinal cords were removed and the DRG
were dissected.
Examination of pain threshold
Mechanical allodynia indicated by the paw withdrawal
threshold was detected according to a method previously described
(30). Briefly, rats were plated
on a metal mesh floor in a transparent plastic box. The pressure
was created using the electronic von Frey filament (IITC, Woodland
Hills, CA, USA) to the plantar surface of each hind. The time of
paw withdrawal of each rat in response to the force was recorded.
The paw withdrawal latency in response to radiant heat was measured
according to the Hargreaves method (31). The rats were placed in a perspex
box on an elevated glass, and a radiant heat source was focused on
mid-plantar area underneath the glass. The duration between the
start of stimuli and paw withdrawal was read and recorded by a
digital timer. A cut-off time was set at 20 sec of irradiation to
avoid tissue damage according to a standard procedure (32).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNAs and miRNAs were extracted using mirVana™
miRNA isolation kit (Life Technologies) according to the
manufacturer's instructions. The cDNA was generated by a TaqMan
miRNA reverse transcription kit (Life Technologies) according to
the manufacturer's instructions. RT-qPCR was performed using SYBR
Premix Ex Taq (Takara, Dalian, China). The relative quantification
of gene expression level was compared with the internal reference
GAPDH (for mRNA) or U6 snRNA (for miRNAs) using the
2−∆∆Ct method.
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of interleukin (IL)-1β, IL-6 and
tumor necrosis factor (TNF)-α in the lumbar spinal cords were
measured by corresponding ELISA kits (R&D Systems, Minneapolis,
MN, USA) as described by the manufacturer's instructions. The
absorbances at 450 nm were read using an ELISA reader (Bio-Tek,
Winooski, VT, USA).
Western blot analysis
The protein in each sample was extracted using a
protein extraction kit (Applygen Technologies, Beijing, China).
Protein concentration was measured using the Bradford method. For
protein separation, a total of 25 µg of protein was loaded
on 12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis
followed by transfer to a nitrocellulose membrane (Bio-Rad,
Hercules, CA, USA), which was subsequently blocked with 2.5%
non-fat milk for 1 h at 37°C. Following this, primary antibodies
were added and incubated at 4°C overnight. Horseradish
peroxidase-conjugated secondary antibodies (1:2,000; bs-0295G-HRP;
Bioss, Beijing, China) were added and the sample was incubated for
1 h at room temperature. Following three washes with Tris-buffered
saline Tween-20, the immune-reactive protein bands on the membrane
were visualized using an enhanced chemiluminescence detection
system (Amersham, Little Chalfont, UK). The primary antibodies used
in the experiment were as follows: anti-p65 (sc-372; Santa Cruz
Biotechnology, Dallas, TX, USA), anti-p-p65 (#3031; Cell Signaling
Technology, Danvers, MA, USA), anti-HMGB1 (ab79823; Abcam,
Cambridge, UK) and anti-GAPDH (bs-13282R; Bioss). Relative protein
expression was quantified using Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Dual-luciferase reporter assay
The cDNA fragments of HMGB1 3′-UTR containing
the putative binding site of miR-141 were amplified and
subcloned into the pGL3 luciferase promoter vector (Promega,
Madison, WI, USA). The pGL3-HMGB1 was co-transfected with
LV-miR-141 into the human embryonic kidney 293 cells for 48
h. The human embryonic kidney HEK293 cells were obtained from Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China) and maintained in DMEM (Life Technologies) supplemented with
10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin
(Sigma). Subsequently, cells were harvested and lysed in which the
luciferase activity was quantified using the dual-luciferase
reporter system (Promega) according to the manufacturer's
instructions.
Data analysis
Data are expressed as mean ± standard deviation and
processed using SPSS version 11.5 (SPSS Inc., Chicago, IL, USA).
Statistical differences between two groups were analyzed by
Student's t-test. Statistical differences among multiple groups
were analyzed by one-way analysis of variance followed by
Bonferroni post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-141 is downregulated in
the DRG in CCI rats
To examine whether miR-141 has a potential
role in regulating neuropathic pain, the expression profiles of
miR-141 in DRG in CCI rats were examined using RT-qPCR. The
results showed that miR-141 expression was downregulated in
the DRG of CCI rats as compared with that from sham-operated rats
at postoperative days 1, 3, 7 and 14 (Fig. 1). The data suggest that
miR-141 may have an important role in regulating neuropathic
pain.
Overexpression of miR-141 attenuates
mechanical allodynia and thermal hyperalgesia in CCI rats
To investigate whether targeting miR-141
expression has a beneficial effect on neuropathic pain, the CCI
rats were subjected to intrathecal injection of lentivirus-mediated
transfer of miR-141 (LV-miR-141) or non-specific
control miRNAs (LV-miR-Ctrl). The expression level of
miR-141 was significantly increased in the LV-miR-141
group in comparison with the LV-miR-Ctrl group at postoperative day
7 and 14 (Fig. 2A). The
neuropathic pain development indicated by mechanical allodynia
(Fig. 2B) and thermal
hyperalgesia (Fig. 2C) was
markedly inhibited by miR-141 overexpression. The data
suggest that overexpression of miR-141 is capable of
attenuating neuropathic pain.
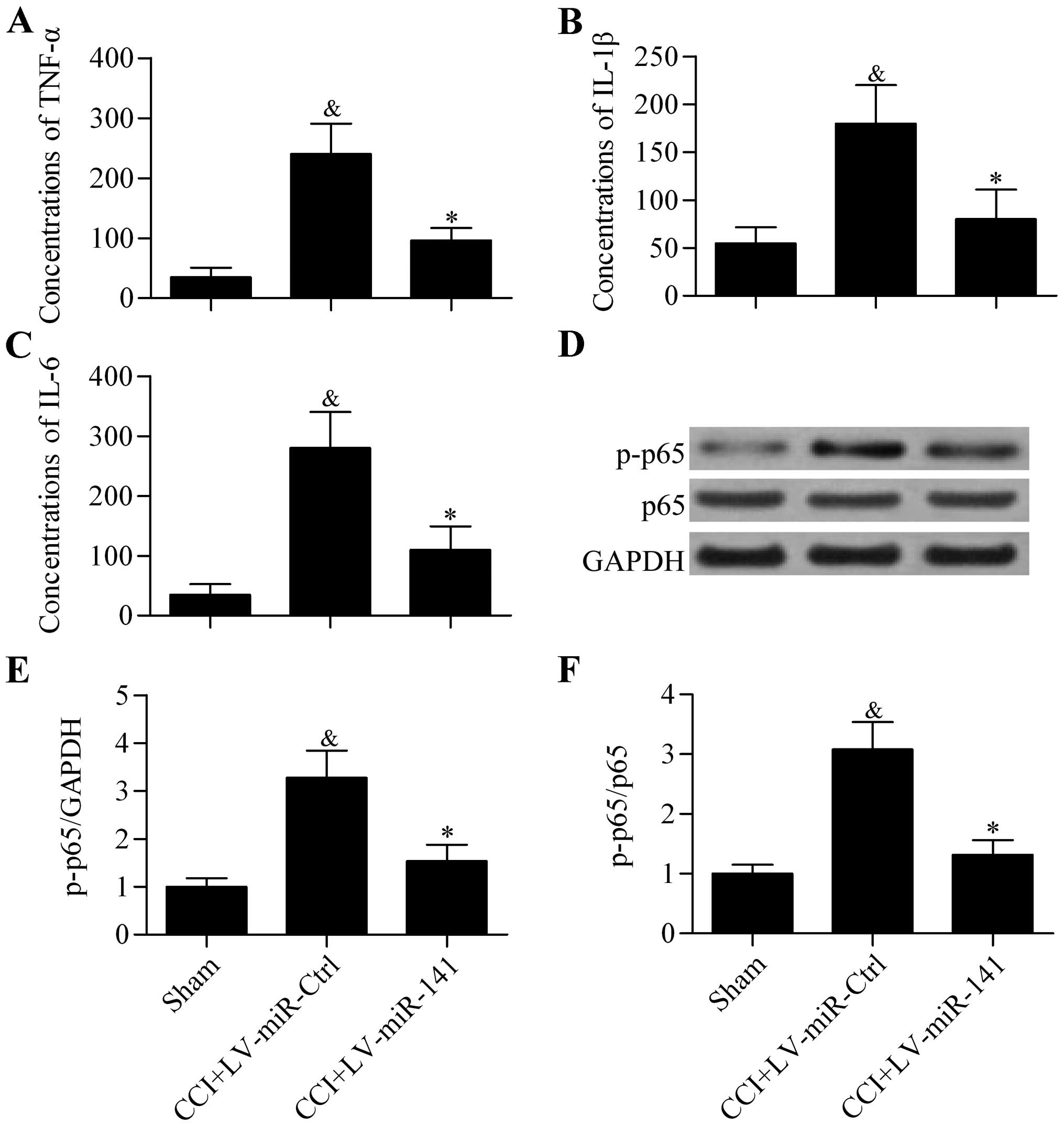
Overexpression of miR-141 inhibits
proinflammatory cytokine expression in CCI rats
To further explore the effect of miR-141
overexpression on neuropathic pain development, the expression of
proinflammatory cytokines in the spinal cord of CCI rats was
analyzed. The results showed that the protein concentrations of
TNF-α (Fig. 3A), IL-1β (Fig. 3B) and IL-6 (Fig. 3C) in rat spinal cord that were
significantly elevated in CCI rats were significantly decreased by
miR-141 overexpression, as detected by ELISA. Furthermore,
the activity of proinflammatory transcription factor NF-κB p65
indicated by phosphorylation of p65 was also significantly
decreased by miR-141 overexpression (Fig. 3D–F). These results indicate that
overexpression of miR-141 suppresses neuroinflammation in
CCI rats.
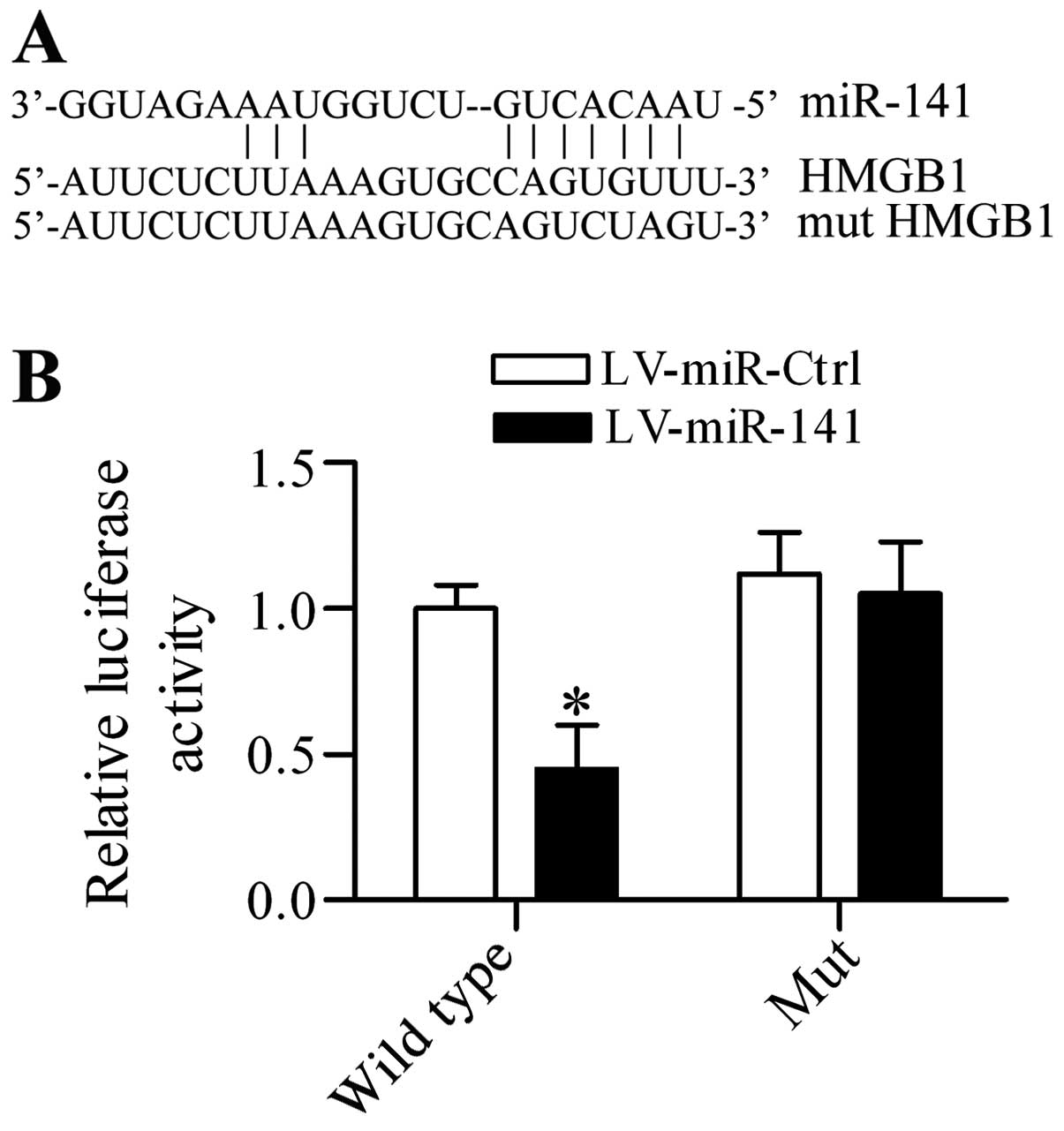
miR-141 targets the 3′-UTR of HMGB1 and
regulates HMGB1 expression DRG neurons in vitro
To investigate the potential underlying mechanism of
miR-141 in regulating neuropathic pain, the putative target
gene of miR-141 was screened and HGMB1, which has been
suggested to be an important proinflammatory mediator in regulating
neuropathic pain (33), contained
the predicted targeting sequences in the 3′-UTR (Fig. 4A). To verify that this association
was authentic, a dual-luciferase reporter assay was performed. The
results demonstrated that overexpression of miR-141
significantly inhibited the luciferase activity in
pGL3-HMGB1 3′-UTR transfected cells, whereas it had no
apparent effect on pGL3-mut HMGB1 3′-UTR transfected cells
(Fig. 4B). Additionally, the
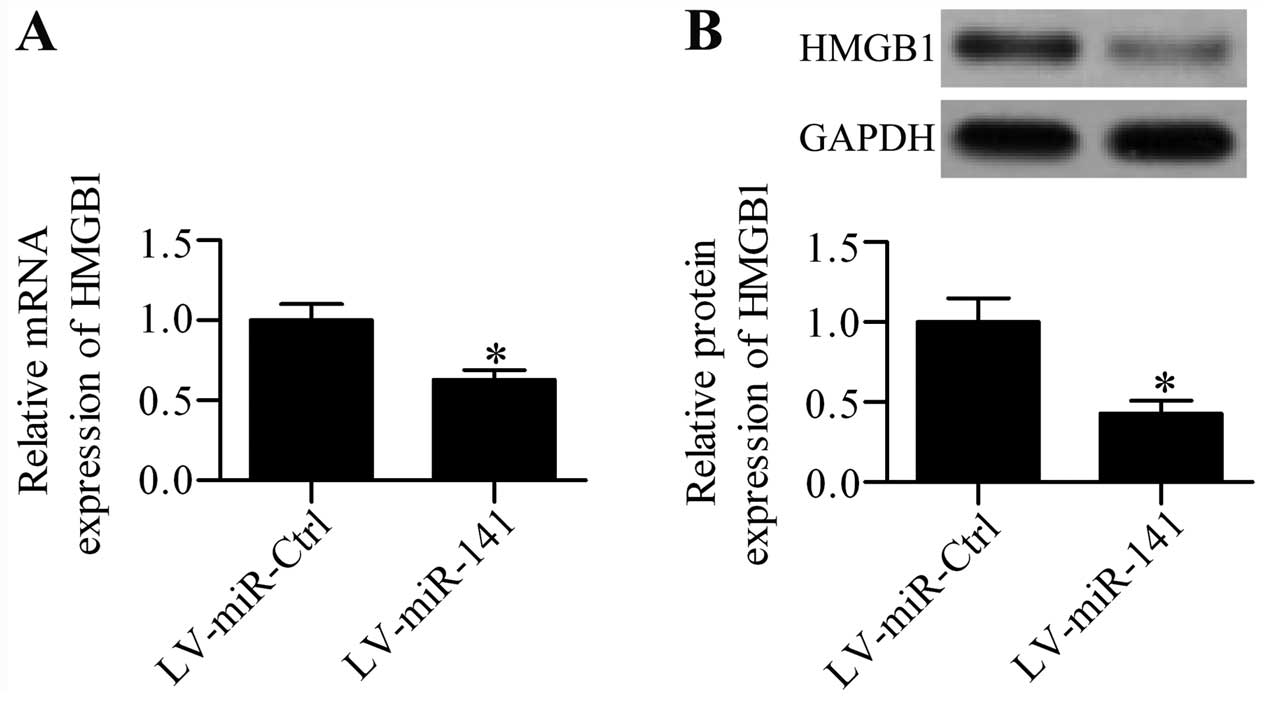
regulatory effect of miR-141 on HMGB1 expression in
cultured DRG neurons was further detected. RT-qPCR analysis showed
that the mRNA expression of HMGB1 was significantly
inhibited in LV-miR-141 infected cells (Fig. 5A), as compared with LV-miR-Ctrl
infected cells. The effect of miR-141 over expression on
HMGB1 protein expression was also validated by western blot
analysis, which demonstrated that the HMGB1 protein expression
level was markedly decreased by miR-141 overexpression
(Fig. 5B).
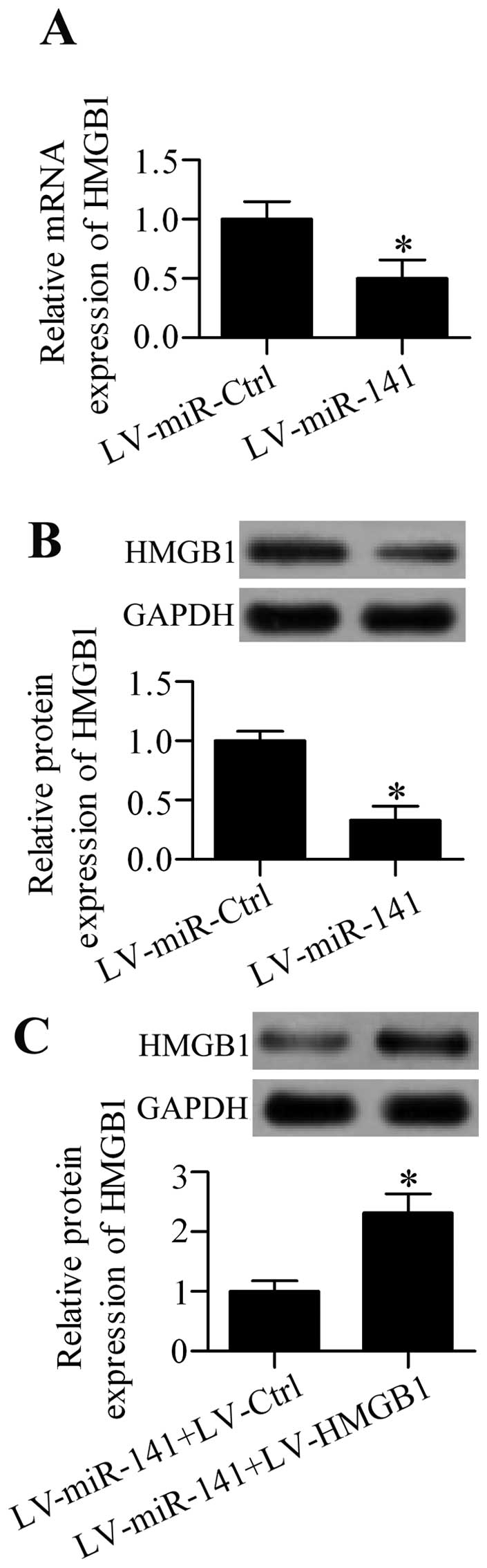
Overexpression of miR-141 regulates HMGB1
expression in the DGR of neuropathic pain in rats in vivo
To ascertain the possible role of miR-141 in
regulating HMGB1 expression, the effect of miR-141
overexpression was further analyzed on HMGB1 expression in
CCI rats in vivo. The results showed that intrathecal
injection of LV-miR-141 significantly decreased the mRNA
(Fig. 6A) and protein (Fig. 6B) expression of HMGB1 in the DRG
from CCI rats. To further clarify that miR-141 had an
important role in regulating neuropathic pain via regulating
HMGB1, a rescue experiment was performed by co-infection of
the rats with LV-miR-141 and LV-HMGB1 containing no
specific targeting sites of miR-141 in 3′-UTR. The results
showed that HMGB1 protein expression was overexpressed in
LV-HMGB1-infected rats (Fig.
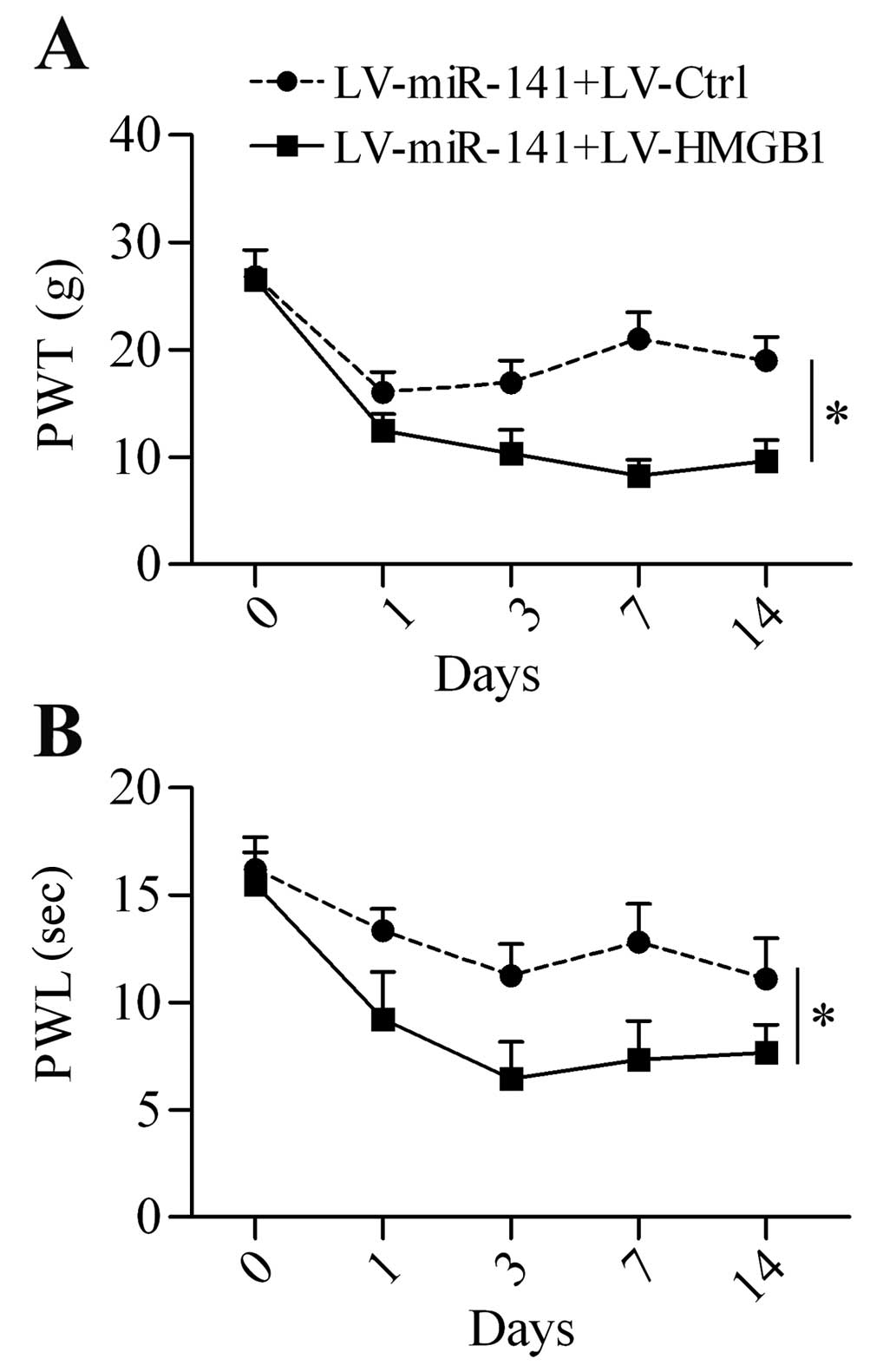
6C). Additionally, HMGB1 overexpression restored the
neuropathic pain development reduced by miR-141
overexpression (Fig. 7A and
B).
Discussion
The present study demonstrated that overexpression
of miR-141 significantly attenuated neuropathic pain via
targeting and inhibiting the expression of HMGB1. The
downregulated expression of miR-141 was responsible for the
highly increased expression of HMGB1, whereas overexpression
of miR-141 significantly inhibited the upregulated
expression of HMGB1 and suppressed the mechanical allodynia
and thermal hyperalgesia, as well as the proinflammatory cytokines
release in neuropathic pain rats. In addition, overexpression of
HMGB1 abolished the protective effect of miR-141
overexpression on neuropathic pain that further confirmed the
direct interaction between miR-141 and HMGB1 in
neuropathic pain. The present data implied that blocking
HMGB1 by miR-141 could be a useful therapeutic
strategy for neuropathic pain.
Previously, the role of miRNAs in regulating
neuropathic pain has been widely studied (34,35). Im et al (36,37) reported that ectopic miR-23b
expression ameliorates neuropathic pain by inhibiting nicotinamide
adenine dinucleotide phosphate oxidase 4 in the spinal cord.
Intrathecal miR-124 treatment prevented persistent
inflammatory and persistent hyperalgesia in chronic hyperalgesia
mice (38). Upregulated
miR-195 was identified in the spinal microglia of rats with
spinal nerve ligation and the miR-195 inhibitor prevented
neuronal inflammation and neuropathic pain through increasing
autophagy (39). Intrathecal
injection of miR-96 has been reported to inhibit neuropathic
pain of CCI rats via inhibiting Nav1.3 expression (27). More recently, miR-155 was
found to be highly expressed in the spinal cord of CCI rats and
administration of miR-155 inhibitor attenuated neuropathic
pain and proinflammatory cytokine expression through regulating the
suppressor of cytokine signalling 1, which was an inhibitor of
proinflammation (15). In the
present study, miR-141 expression was altered in CCI rats
and intrathecal administration of lentivirus expressing
miR-141 reversed the neuropathic pain and neuronal
inflammation in CCI rats. The role of miR-141 in diseases
has been widely studied, such as cancer (40,41). Certain studies also indicated an
important role of miR-141 in regulating
inflammation-associated diseases (42–44). The present study revealed that
HMGB1 was a direct target gene of miR-141, both of
which were involved in neuropathic pain.
As the critical role of HMGB1 has been reported in
neuropathic pain (23), numerous
strategies targeting HMGB1 have been carried out to treat
neuropathic pain. A neutralizing antibody against HMGB1
(anti-HMGB1) has been shown to successfully alleviate the
mechanical allodynia in rats with spinal nerve ligation (24). Treatment of anti-HMGB1
neutralization antibody significantly inhibited proinflammatory
cytokine expression in the DRG and improved the pain-related
behavior (26). Ren et al
(45) reported that intrathecal
injection of anti-HMGB1 repressed mechanical allodynia induced by
diabetes. Nakamura et al (46) provided evidence that intravenous
injection of anti-HMGB1 relieved neuropathic pain in rats followed
by partial sciatic nerve ligation. Furthermore, anti-HMGB1 also
showed an effective anti-allodynia effect in a rat model of bone
cancer pain (47). In addition,
it has been reported that Tanshinone IIA reversed thermal
hyperalgesia and mechanical allodynia induced by spinal nerve
ligation via modulating HMGB1 and its receptor, Toll-like receptor
4 (48). Intrathecal injection of
lentivirus-mediated transfer of IL-10 inhibited neuropathic pain
via regulating spinal HMGB1 expression in CCI rats (49). All the aforementioned studies
indicate that HMGB1-based therapeutic strategies could be an
effective method for treatment of neuropathic pain. In the present
study, miR-141 could regulate HMGB1 expression through
directly targeting the 3′-UTR of HMGB1. In addition,
intrathecal injection of lentivirus-mediated transfer of
miR-141 significantly attenuated neuropathic pain and
proinflammatory cytokine release, including TNF-α, IL-1β and IL-6,
in the spinal cord of CCI rats.
Inhibiting HMGB1 by miRNAs has been reported in
various studies. For instance, miR-22 repressed osteosarcoma
via targeting HMGB1 (50).
miR-181b has been reported to regulate drug sensitivity of
acute myeloid leukemia by targeting and inhibiting HMGB1 (51). However, the present data suggested
that miR-141 could target and inhibit HMGB1 expression in
DRG neurons in vitro and in vivo. In conclusion, the
present data revealed that miR-141 was significantly
decreased in the DRG of CCI rats, which directly regulated HMGB1
expression and implied that targeting miR-141-HGMB1 could be
a useful therapeutic strategy for the treatment of neuropathic
pain.
Abbreviations:
|
HMGB1
|
high-mobility group box 1
|
|
miRNAs
|
microRNAs
|
|
DRG
|
dorsal root ganglion
|
|
UTR
|
untranslated region
|
|
CCI
|
chronic constriction injury
|
References
|
1
|
Sorge RE, Trang T, Dorfman R, Smith SB,
Beggs S, Ritchie J, Austin JS, Zaykin DV, Vander Meulen H, Costigan
M, et al: Genetically determined P2X7 receptor pore formation
regulates variability in chronic pain sensitivity. Nat Med.
18:595–599. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neville A, Peleg R, Singer Y, Sherf M and
Shvartzman P: Chronic pain: A population-based study. Isr Med Assoc
J. 10:676–680. 2008.PubMed/NCBI
|
|
3
|
Baron R: Peripheral neuropathic pain: From
mechanisms to symptoms. Clin J Pain. 16(Suppl): S12–S20. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dimitroulas T, Duarte RV, Behura A, Kitas
GD and Raphael JH: Neuropathic pain in osteoarthritis: A review of
pathophysiological mechanisms and implications for treatment. Semin
Arthritis Rheum. 44:145–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Phillips JR, Hopwood B, Arthur C, Stroud R
and Toms AD: The natural history of pain and neuropathic pain after
knee replacement: A prospective cohort study of the point
prevalence of pain and neuropathic pain to a minimum three-year
follow-up. Bone Joint J. 96-B:1227–1233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haanpää M, Attal N, Backonja M, Baron R,
Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA,
Iannetti GD, et al: NeuPSIG guidelines on neuropathic pain
assessment. Pain. 152:14–27. 2011. View Article : Google Scholar
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ranganathan K and Sivasankar V: MicroRNAs
- Biology and clinical applications. J Oral Maxillofac Pathol.
18:229–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakai A and Suzuki H: Emerging roles of
microRNAs in chronic pain. Neurochem Int. 77:58–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
von Schack D, Agostino MJ, Murray BS, Li
Y, Reddy PS, Chen J, Choe SE, Strassle BW, Li C, Bates B, et al:
Dynamic changes in the microRNA expression profile reveal multiple
regulatory mechanisms in the spinal nerve ligation model of
neuropathic pain. PLoS One. 6:e176702011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aldrich BT, Frakes EP, Kasuya J, Hammond
DL and Kitamoto T: Changes in expression of sensory organ-specific
microRNAs in rat dorsal root ganglia in association with mechanical
hypersensitivity induced by spinal nerve ligation. Neuroscience.
164:711–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Favereaux A, Thoumine O, Bouali-Benazzouz
R, Roques V, Papon MA, Salam SA, Drutel G, Léger C, Calas A, Nagy
F, et al: Bidirectional integrative regulation of Cav1.2 calcium
channel by microRNA miR-103: Role in pain. EMBO J. 30:3830–3841.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan Y, Yang J, Xiang K, Tan Q and Guo Q:
Suppression of microRNA-155 attenuates neuropathic pain by
regulating SOCS1 signalling pathway. Neurochem Res. 40:550–560.
2015. View Article : Google Scholar
|
|
16
|
Andersson U, Erlandsson-Harris H, Yang H
and Tracey KJ: HMGB1 as a DNA-binding cytokine. J Leukoc Biol.
72:1084–1091. 2002.PubMed/NCBI
|
|
17
|
Taniguchi N, Kawahara K, Yone K,
Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K,
Matsunaga S, et al: High mobility group box chromosomal protein 1
plays a role in the pathogenesis of rheumatoid arthritis as a novel
cytokine. Arthritis Rheum. 48:971–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andersson U and Tracey KJ: HMGB1 in
sepsis. Scand J Infect Dis. 35:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oppenheim JJ and Yang D: Alarmins:
Chemotactic activators of immune responses. Curr Opin Immunol.
17:359–365. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Genevay S, Finckh A, Payer M, Mezin F,
Tessitore E, Gabay C and Guerne PA: Elevated levels of tumor
necrosis factor-alpha in periradicular fat tissue in patients with
radiculopathy from herniated disc. Spine. 33:2041–2046. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCarron RF, Wimpee MW, Hudkins PG and
Laros GS: The inflammatory effect of nucleus pulposus. A possible
element in the pathogenesis of low-back pain. Spine. 12:760–764.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vallejo R, Tilley DM, Vogel L and Benyamin
R: The role of glia and the immune system in the development and
maintenance of neuropathic pain. Pain Pract. 10:167–184. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chacur M, Milligan ED, Gazda LS, Armstrong
C, Wang H, Tracey KJ, Maier SF and Watkins LR: A new model of
sciatic inflammatory neuritis (SIN): Induction of unilateral and
bilateral mechanical allodynia following acute unilateral
peri-sciatic immune activation in rats. Pain. 94:231–244. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shibasaki M, Sasaki M, Miura M, Mizukoshi
K, Ueno H, Hashimoto S, Tanaka Y and Amaya F: Induction of high
mobility group box-1 in dorsal root ganglion contributes to pain
hypersensitivity after peripheral nerve injury. Pain. 149:514–521.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feldman P, Due MR, Ripsch MS, Khanna R and
White FA: The persistent release of HMGB1 contributes to tactile
hyperalgesia in a rodent model of neuropathic pain. J
Neuroinflammation. 9(180)2012. View Article : Google Scholar
|
|
26
|
Otoshi K, Kikuchi S, Kato K, Sekiguchi M
and Konno S: Anti-HMGB1 neutralization antibody improves
pain-related behavior induced by application of autologous nucleus
pulposus onto nerve roots in rats. Spine. 36:E692–E698. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen HP, Zhou W, Kang LM, Yan H, Zhang L,
Xu BH and Cai WH: Intrathecal miR-96 inhibits Nav1.3 expression and
alleviates neuropathic pain in rat following chronic construction
injury. Neurochem Res. 39:76–83. 2014. View Article : Google Scholar
|
|
28
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yaksh TL and Rudy TA: Chronic
catheterization of the spinal subarachnoid space. Physiol Behav.
17:1031–1036. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang H, Cang CL, Kawasaki Y, Liang LL,
Zhang YQ, Ji RR and Zhao ZQ: Neurokinin-1 receptor enhances TRPV1
activity in primary sensory neurons via PKCepsilon: A novel pathway
for heat hyperalgesia. J Neurosci. 27:12067–12077. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maeda T, Ozaki M, Kobayashi Y, Kiguchi N
and Kishioka S: HMGB1 as a potential therapeutic target for
neuropathic pain. J Pharmacol Sci. 123:301–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong Q, Lu Z, Huang Q, Ruan L, Chen J,
Liang Y, Wang H, Yue Y and Feng S: Altered microRNAs expression
profiling in mice with diabetic neuropathic pain. Biochem Biophys
Res Commun. 456:615–620. 2015. View Article : Google Scholar
|
|
35
|
Norcini M, Sideris A, Martin Hernandez LA,
Zhang J, Blanck TJ and Recio-Pinto E: An approach to identify
microRNAs involved in neuropathic pain following a peripheral nerve
injury. Front Neurosci. 8(266)2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Im YB, Jee MK, Jung JS, Choi JI, Jang JH
and Kang SK: miR23b ameliorates neuropathic pain in spinal cord by
silencing NADPH oxidase 4. Antioxid Redox Signal. 16:1046–1060.
2012. View Article : Google Scholar
|
|
37
|
Im YB, Jee MK, Choi JI, Cho HT, Kwon OH
and Kang SK: Molecular targeting of NOX4 for neuropathic pain after
traumatic injury of the spinal cord. Cell Death Dis. 3:e4262012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Willemen HL, Huo XJ, Mao-Ying QL, Zijlstra
J, Heijnen CJ and Kavelaars A: MicroRNA-124 as a novel treatment
for persistent hyperalgesia. J Neuroinflammation. 9(143)2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi G, Shi J, Liu K, Liu N, Wang Y, Fu Z,
Ding J, Jia L and Yuan W: Increased miR-195 aggravates neuropathic
pain by inhibiting autophagy following peripheral nerve injury.
Glia. 61:504–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou X, Xia Y, Su J and Zhang G:
Down-regulation of miR-141 induced by helicobacter pylori promotes
the invasion of gastric cancer by targeting STAT4. Cell Physiol
Biochem. 33:1003–1012. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zuo QF, Zhang R, Li BS, Zhao YL, Zhuang Y,
Yu T, Gong L, Li S, Xiao B and Zou QM: MicroRNA-141 inhibits tumor
growth and metastasis in gastric cancer by directly targeting
transcriptional co-activator with PDZ-binding motif, TAZ. Cell
Death Dis. 6:e16232015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sipert CR, Morandini AC, Dionísio TJ,
Machado MA, Oliveira SH, Campanelli AP, Kuo WP and Santos CF: In
vitro regulation of CCL3 and CXCL12 by bacterial by-products is
dependent on site of origin of human oral fibroblasts. J Endod.
40:95–100. 2014. View Article : Google Scholar
|
|
43
|
Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi
H, Dong L, Zhang C, Zeng K, Chen J, et al: miR-141 Regulates
colonic leukocytic trafficking by targeting CXCL12β during murine
colitis and human Crohn's disease. Gut. 63:1247–1257. 2014.
View Article : Google Scholar
|
|
44
|
Lam WY, Yeung AC, Ngai KL, Li MS, To KF,
Tsui SK and Chan PK: Effect of avian influenza A H5N1 infection on
the expression of microRNA-141 in human respiratory epithelial
cells. BMC Microbiol. 13(104)2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ren PC, Zhang Y, Zhang XD, An LJ, Lv HG,
He J, Gao CJ and Sun XD: High-mobility group box 1 contributes to
mechanical allodynia and spinal astrocytic activation in a mouse
model of type 2 diabetes. Brain Res Bull. 88:332–337. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakamura Y, Morioka N, Abe H, Zhang FF,
Hisaoka-Nakashima K, Liu K, Nishibori M and Nakata Y: Neuropathic
pain in rats with a partial sciatic nerve ligation is alleviated by
intravenous injection of monoclonal antibody to high mobility group
box-1. PLoS One. 8:e736402013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tong W, Wang W, Huang J, Ren N, Wu SX and
Li YQ: Spinal high-mobility group box 1 contributes to mechanical
allodynia in a rat model of bone cancer pain. Biochem Biophys Res
Commun. 395:572–576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma YQ, Chen YR, Leng YF and Wu ZW:
Tanshinone IIA downregulates HMGB1 and TLR4 expression in a spinal
nerve ligation model of neuropathic pain. Evid Based Complement
Alternat Med. 2014(639563)2014.
|
|
49
|
He Z, Guo Q, Xiao M, He C and Zou W:
Intrathecal lentivirus-mediated transfer of interleukin-10
attenuates chronic constriction injury-induced neuropathic pain
through modulation of spinal high-mobility group box 1 in rats.
Pain Physician. 16:E615–E625. 2013.PubMed/NCBI
|
|
50
|
Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang
Y, Wang Y, Zhao W and Wang W: miR-22 inhibits osteosarcoma cell
proliferation and migration by targeting HMGB1 and inhibiting
HMGB1-mediated autophagy. Tumour Biol. 35:7025–7034. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu F, Zhang J, Ji M, Li P, Du Y, Wang H,
Zang S, Ma D, Sun X and Ji C: miR-181b increases drug sensitivity
in acute myeloid leukemia via targeting HMGB1 and Mcl-1. Int J
Oncol. 45:383–392. 2014.PubMed/NCBI
|