1. Introduction
The characterization of ovarian masses and
distinguishing between benign and malignant pathology is important
both to decrease unnecessary anxiety and enable decisions regarding
optimal treatment. Benign pathology may be best treated
conservatively or in a general gynecology unit using a minimal
access approach. Conversely, suspected malignant masses should be
referred to specialized units for further management. Thus prior
knowledge of the nature of ovarian masses is essential not only for
the patient but in order to organize clinical services in terms of
planning, costs and overall management (1).
Transvaginal ultrasonography (TVS) is the most
commonly employed imaging modality for the assessment of adnexal
masses, and a number of prediction models have been created to
maximize its predictive capability. In many countries the risk of
malignancy index (RMI) (2) which
combines ultrasound features, serum CA125 levels and the menopausal
status of the patient is still used to characterize ovarian
pathology. However, more recently logistic regression models and
simple rules created by the International Ovarian Tumor Analysis
(IOTA) group have been shown to perform better than the RMI
(3–7). The most recent systematic review and
meta-analysis has concluded that based on currently available
evidence, these IOTA rules and models should now be used in
clinical practice (3).
Notwithstanding these advances, the optimal approach to
characterizing ovarian masses remains the subjective interpretation
of the ultrasound features of a mass by an expert operator
(8–10).
For the purposes of this review, the term ‘pattern
recognition’ refers to the subjective evaluation of adnexal masses
using grey-scale and power/color Doppler ultrasonography (11,12).
In the hands of experienced examiners pattern recognition has a
high sensitivity (77–86%) and specificity (94–100%) to diagnose
teratomas/dermoid cysts, endometriomas, hydrosalpinges and
peritoneal pseudocysts (13). It
has however, not been found to be as useful for the diagnosis of
fibromas, paraovarian cysts and rare benign tumors, and may have
difficulty in differentiating between physiological and other
‘simple’ cysts on the basis of a single scan (sensitivity 8–17%)
(13).
These findings suggest that with adequate training
and knowledge of the common features associated with particular
pathologies, ultrasound examiners should be able to reliably
diagnose and differentiate between certain specific types of
adnexal pathology. It is important to remember that when evaluating
women with an adnexal mass, ultrasound characteristics need to be
correlated with the clinical history, as well as signs and symptoms
before arriving at a diagnosis. This review describes only the
features that may be found using ultrasound that may be used to
predict common specific types of adnexal pathology.
2. Physiological, peritoneal and tubal
cystic pathology
Follicular cysts
They are usually unilocular and thin walled with
anechoic contents (12). They
rarely exceed 8–10 cm in diameter and typically spontaneously
resolve within 6 weeks (14).
Posterior wall hyperechoic enhancement is a feature due to
reflection of the ultrasound beam off the posterior wall having
travelled through the anechoic window formed by the clear cyst
contents (14) (Fig. 1).
Corpus luteum cysts
These are formed following the rupture of a mature
Graafian follicle. They are thick walled hyperechoic cysts that
typically demonstrate peripheral circumferential blood flow,
sometimes known as the ‘ring of fire’ (12). Some cysts may show areas of
internal hemorrhage. The cyst contents typically have a
spider-web-like appearance (Fig.
2) due to a small amount of internal hemorrhage, but can
frequently show different features including blood clots within the
cyst resembling solid components. Doppler examination may be useful
in these circumstances as the blood clot will have no blood flow,
although perhaps more useful is the a typical jelly-like ‘wobbling’
movement that can be elicited from the blood clot within the cyst
if the vaginal probe is used to gently prod the ovary during the
examination (15). In most cases,
hemorrhagic cysts resolve within 6–12 weeks without intervention
(15).
Peritoneal pseudocysts
Peritoneal pseudocysts, are collections of
peritoneal fluid trapped in adhesions usually caused by previous
pelvic surgery, pelvic inflammatory disease or endometriosis. They
usually occur in premenopausal women, because of the presence of
functional ovaries that release small amounts of fluid into the
peritoneal cavity (15–18). They grow gradually and may reach
several centimeters in size. They can cause abdominal pain or
distension, but in the majority of cases are asymptomatic (15–18).
Pseudocysts appear mainly as multilocular cysts,
with a high number of septa that are adherent to the ovarian
surface. Septa are most frequently complete and thin (15–18)
(Fig. 3). In contrast to septae
within true ovarian cysts the septae in pseudocysts generally move
and ‘flap’ when the cystic area is prodded by the transvaginal
ultrasound probe. This has been described as the ‘flapping sail
sign’ (18). They have an
irregular shape, that follows the contours of the pouch of Douglas
or pelvic sidewall and surrounding pelvic organs, giving a ‘lumpy’,
‘star-like’ or ‘tubular’ appearance (15–18).
The ipsilateral ovary is visible in almost all cases
(Fig. 4). It can be external to
the lesion or entrapped within the cyst (17,18).
The cyst contents are generally anechoic, but may show low-level
echogenicity (16,18).
Paraovarian cysts
Paraovarian cysts arise in the broad ligament
between the ovary and the fallopian tube. They account for 5–20% of
adnexal masses (19,20). The incidence of borderline and
malignant paraovarian tumors is low but cases have been reported
(20,21). They appear as thin walled
unilocular anechoic masses close to but separate from the ovary
(Fig. 5). However they can show
papillary projections in ~30% of cases (20).
Their mean diameter is usually <5 cm with no
evidence of any follicles or significant vascularity. In almost all
cases, it is possible to visualize the ipsilateral normal ovary,
and to detect movement of the cyst in the opposite direction to the
ovary when the area is pushed with the vaginal probe - the ‘split
sign’. This may help to differentiate between a paraovarian and
ovarian cyst when the ipsilateral ovary is not clearly visible
(20).
Tubal pathology
A normal Fallopian tube is rarely visible during an
ultrasound examination. Hydrosalpinges have typical diagnostic
features on ultrasound with anechoic contents and incomplete septae
(15) (Fig. 6). In the case of an acute or
chronic inflammatory process the tube may become detectable and
some specific characteristics have been described.
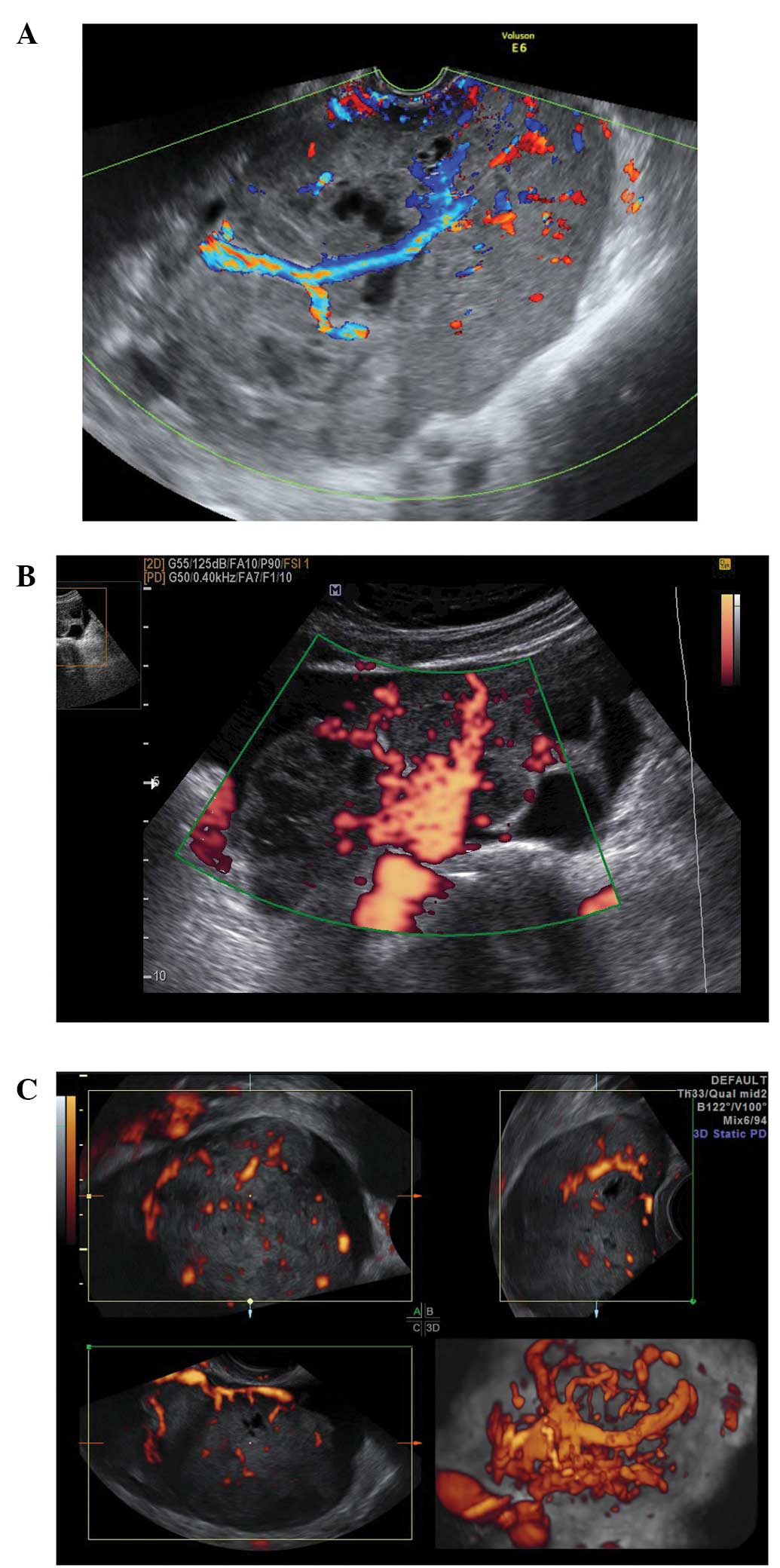
Acute salpingitis typically appears like a
pear-shaped unilocular mass with anechoic or low-level content,
characterized by thickening of the wall (>5 mm) and the presence
of incomplete septae (Fig. 7). In
transverse section it often shows the well described ‘cogwheel
sign’ appearance (15,22) (Fig.
8). Color or power Doppler examination generally shows
significant vascularity in cases of an acute inflammatory process
as well as the presence of fluid in the pouch of Douglas (23).
In chronic salpingitis the tube appears as an
elongated fluid-filled mass, with incomplete septae, but the
thickening of the wall is no longer visible. It is characterized by
the typical sonographic ‘beads on a string’ sign, due to 2–3 mm
sized hyperechoic structures on the tubal wall, seen on transverse
section (15,22–24).
A tubo-ovarian complex represents the involvement of
ovarian tissue in the inflammatory process. Normal ovarian
parenchyma is visible, but it is usually seen separate from tubal
structures (15,22–24)
(Fig. 9).
In a tubo-ovarian abscess, ovarian tissue is no
longer visible; the lesion may be unilocular, solid or
multilocular-solid with mixed or ground-glass echogenicity. On the
basis of the ultrasound features, these have to be differentiated
from endometriomas or hemorrhagic cysts (15,22–24).
In practice the clinical features associated with an abscess make
the diagnosis relatively straightforward.
3. Ovarian pathology
Serous cystadenomas
These appear as smooth, thin walled, anechoic,
fluid-filled structures. They are bilateral in 15% of cases and
their mean size is 5–8 cm (25).
Some contain fine septations whilst others have areas of
haemorrhage appearing as small echogenic areas (25) (Fig.
10).
Mucinous cystadenomas
Mucinous cysts are classically thin walled, large
and unilateral. They consist of internal thin-walled locules
containing mucin which appears as fluid with low level echogenicity
(25) (Fig. 11). In general neither serous nor
mucinous cystadenomas are associated with significant vascularity
(25).
Caspi et al described the presence of
variable echogenicity among different tumor locules as an
ultrasound feature of multilocular mucinous cystadenomas (26) (Fig.
12), however this has not been confirmed in larger studies to
date.
Cystadenofibromas
Cystadenofibromas represent a relatively rare type
of benign epithelial ovarian tumor. They are mainly serous although
mucinous subtypes do exist (27).
Descriptions of the sonographic features of cystadenofibromas are
limited but some specific appearances have been described. They may
appear as unilocular-solid, or less frequently, multilocular-solid
masses with thin cyst walls and anechoic contents (15,27).
The diagnosis may be aided by the presence of hyperechoic solid
components with acoustic shadows and low to moderate vascularity
(15,27). They are often seen as
unilocular-solid lesions with single papillary projections. The key
feature to look for then is acoustic shadowing even within these
small papillations (15,27). Differentiating between
cystadenofibromas and borderline or malignant ovarian masses can be
difficult (15,27) (Fig.
13).
Mature teratoma/dermoid cysts
Mature cystic teratomas are benign germ cell tumors.
They usually have the highest sensitivity and specificity for a
specific diagnosis with ultrasound as they generally have rather
typical features (28). They are
cystic and unilocular in the majority of cases, with mixed
echogenicity representing the different components of fat, bone and
fluid (28). Pathognomonic of
dermoid cysts is a Rokitansky nodule, a distinct hyperechoic mural
nodule representing areas of floating hair in low-density fluid
(29,30) (Fig.
14). There are often bright echoes and sharp acoustic shadows
associated with hair or even teeth in the cyst.
Endometriomas
Ultrasonography is particularly sensitive for
accurately diagnosing ‘typical’ endometriomas, most commonly seen
in premenopausal women. Typically an endometrioma is a unilocular
tumor and has low-level echogenicity representing old blood in the
cyst cavity (commonly termed ‘ground glass’). It is this ‘ground
glass’ feature that is the most typical feature (28,31–33)
(Fig. 15).
Endometriomas may also have atypical features, and
frequently debris within the cyst may give the impression that it
is a unilocular-solid lesion with solid papillary projections. In
postmenopausal women the appearances of an atypical endometrioma
should be examined very carefully as there is a significant risk of
malignancy in such lesions in this age group (29,32)
(Fig. 16).
During pregnancy endometriomas can change their
appearance secondary to decidualization. The features may become
quite alarming, with solid vascular projections into the cyst
cavity. When no pre-existing scan of the ovary is documented it is
difficult in these cases not to suspect malignancy (Fig. 17), although papillary projections
were a more frequent sonographic feature among malignant lesions
than among benign endometrioid cysts (34,35).
Ovarian fibromas and fibrothecomas
These are benign tumors of stromal origin. Fibromas
originate from spindle cells producing collagen and can be
associated with ascites or Meig’s syndrome. Fibrothecomas originate
from both spindle and theca cells and may produce a small amount of
estrogens (36,37).
Their characteristic sonographic appearance is of a
round or oval solid tumor, with regular margins. They may have
stripy acoustic shadows, but these are present in just a small
percentage of cases (15,36,37)
(Fig. 18). Fibromas and
fibrothecomas can also show cystic areas, due to hemorrhage, edema
or necrosis within the stromal tissue (Fig. 19). Doppler findings are variable,
but frequently the lesions show little peripheral vascularity
(36,37) (Fig.
18).
Ovarian stromal tumors (struma
ovarii)
Struma ovarii is a rare subtype of mature teratoma
characterized by the presence of ectopic thyroid tissue. They
account for <5% of mature teratomas (38). Although a preoperative diagnosis is
not always possible, they have been described as having a similar
appearances to mature teratomas but with increased vascularity in
the central part of the mass (39). They are difficult to classify
(40), but are of interest
morphologically because they have been associated with a
sonographic sign called the ‘struma pearl’. These are rounded
hyperechogenic structures with smooth surfaces, with increased
vascularity on Doppler examination (40) (Fig.
20).
Brenner tumors
Brenner tumors also arise from the ovarian stroma
but are benign in 99% of cases. Their diagnosis is often an
incidental finding in women between the fifth and the seventh
decade of life. They are usually small and often coexist with
serous or mucinous cystadenomas (Fig.
21). They are more frequently unilateral, mainly within the
left ovary (41–43). Brenner tumors are sometimes
associated with acoustic shadowing and so may be confused with an
ovarian fibroma or pedunculated fibroid from the uterus (Fig. 21) (41–43).
Primary invasive ovarian epithelial
cancer
Stage 1 primary invasive ovarian epithelial cancers
share similar ultrasound characteristics to borderline tumors, but
they differ significantly from the appearances of later stage
disease (44) (Fig. 22). They often contain papillary
projections and less commonly are purely solid (44).
Later stage primary ovarian tumors are usually
multilocular with a high proportion of solid tissue and are
frequently associated with ascites as well as metastatic disease to
the peritoneum, omentum and elsewhere in the abdomen and pelvis
(44). They are also significantly
vascular with high color scores (3–4)
(44) (Fig. 23).
Borderline tumors
The presence of papillary projections within a cyst
has been used as a discriminatory factor for serous borderline
tumors (45). However, the
potential for misdiagnosis between borderline tumors (BOT),
cystadenomas, cystadenofibromas and invasive malignant tumors is
significant (45). Doppler
assessment of tumor vascularity is not useful in distinguishing
between borderline and invasive tumors (45,46).
The size and characteristics of the surface of the papillary
projections are however thought to be helpful with the angle the
projection makes with the cyst wall being significantly different
(47) (Figs. 24–26). In this review the mean size of
papillary projections was 9.6, 15.7, and 35.3 mm in benign,
borderline, and malignant tumors, respectively. In benign masses an
acute angle was present between the cyst wall and projection in 68%
of cases and an obtuse angle in 40% of borderline and 89% when the
mass was an invasive malignancy. These observations are of
interest, but have not yet been validated in larger prospective
studies (47).
Serous and mucinous endocervical type BOTs are
usually unilocular solid tumors with a high number of vascular
papillary projections within the cyst. Mucinous intestinal type BOT
are more often very large, unilateral, multilocular tumors with a
high number of locules encased by thick, hyperechoic tissue with no
evidence of solid components (Figs.
24–26). They are associated
with the ‘honeycomb’ sign formed by tightly interrelated septae
within the cyst. Intestinal-type mucinous BOT are generally less
vascular than both serous and endocervical BOT (48,49).
Tumors that have metastasized to the
ovary
Ovarian metastasis from breast, gastric, and uterine
cancers as well as lymphomas appear as solid tumors on ultrasound
examination (Figs. 27 and
28). In contrast, ovarian
metastasis from the colon, rectum and biliary tract, tend to be
multilocular-solid or multilocular with anechoic or low-level
echogenicity (50) (Figs. 29 and 30). The latter group demonstrate, a
larger diameter and more frequently the presence of an irregular
external surface (50). The
detection of papillary projections is rare in metastatic tumors
(50) (Figs. 27–30). The presence of rich vascularity
(color score 3–4) is characteristic of all metastatic tumors
(44), but metastatic tumors from
the colon, rectum and biliary tract tend to be less vascular
compared to those from the stomach, breast, uterus or lymphomas
(50).
The vascularity of metastatic tumors is
characterized by the presence of a ‘lead vessel’ - a single large
vessel penetrating from the periphery to the central part of the
lesion (Fig. 27). Further
research is needed to determine the diagnostic performance of this
sign (51).
Conclusion
Predicting the specific histopathology of an adnexal
mass is important as it may lead to surgery being avoided or being
less invasive in some cases whilst ensuring appropriate referral to
a gynecological oncology surgeon in the case of malignancy. In
general there is an intense focus on excluding malignancy when the
characterization of ovarian pathology is considered. However the
field has moved on, both in terms of tailoring treatment to
individual patients and with what we know about the features of
different types of ovarian pathology. In this review we hope we
have illustrated some of the pathognomonic features of some of the
more commonly found adnexal masses in clinical practice. By
improving the specific classification of masses we hope that
management decisions in relation to such pathology will become more
patient specific and lead to improved outcomes.
Acknowledgements
T.B. was supported by the National Institute for
Health Research (NIHR) Biomedical Research Centre based at Imperial
College Healthcare NHS Trust and Imperial College London. The views
expressed are those of the author(s) and not necessarily those of
the NHS, the NIHR or the Department of Health. D.T. is Fundamental
Clinical Researcher of the FWO-Flanders.
References
|
1
|
Carley ME, Klingele CJ, Gebhart JB, Webb
MJ and Wilson TO: Laparoscopy versus laparotomy in the management
of benign unilateral adnexal masses. J Am Assoc Gynecol Laparosc.
9:321–326. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jacobs I, Oram D, Fairbanks J, Turner J,
Frost C and Grudzinskas JG: A risk of malignancy index
incorporating CA 125, ultrasound and menopausal status for the
accurate preoperative diagnosis of ovarian cancer. Br J Obstet
Gynaecol. 97:922–929. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaijser J, Sayasneh A, Van Hoorde K, et
al: Presurgical diagnosis of adnexal tumours using mathematical
models and scoring systems: a systematic review and meta-analysis.
Hum Reprod Update. 20:449–462. 2014. View Article : Google Scholar
|
|
4
|
Sayasneh A, Wynants L, Preisler J, et al:
Multicentre external validation of IOTA prediction models and RMI
by operators with varied training. Br J Cancer. 108:2448–2454.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Timmerman D, Van Calster B, Testa AC, et
al: Ovarian cancer prediction in adnexal masses using
ultrasound-based logistic regression models: a temporal and
external validation study by the IOTA group. Ultrasound Obstet
Gynecol. 36:226–234. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Timmerman D, Ameye L, Fischerova D, et al:
Simple ultrasound rules to distinguish between benign and malignant
adnexal masses before surgery: prospective validation by IOTA
group. BMJ. 341:c68392010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Testa A, Kaijser J, Wynants L, et al:
Strategies to diagnosie ovarian cancer: new evidence from phase 3
of the multicentre international IOTA study. Br J Cancer.
111:680–688. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valentin L, Ameye L, Savelli L, et al:
Adnexal masses difficult to classify as benign or malignant using
subjective assessment of gray-scale and Doppler ultrasound
findings: logistic regression models do not help. Ultrasound Obstet
Gynecol. 38:456–465. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Timmerman D, Schwarzler P, Collins WP, et
al: Subjective assessment of adnexal masses with the use of
ultrasonography: an analysis of interobserver variability and
experience. Ultrasound Obstet Gynecol. 13:11–16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Timmerman D: The use of mathematical
models to evaluate pelvic masses; can they beat an expert operator?
Best Pract Res Clin Obstet Gynaecol. 18:91–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valentin L, Hagen B, Tingulstad S and
Eik-Nes S: Comparison of ‘pattern recognition’ and logistic
regression models for discrimination between benign and malignant
pelvic masses: a prospective cross validation. Ultrasound Obstet
Gynecol. 18:357–365. 2001. View Article : Google Scholar
|
|
12
|
Valentin L: Pattern recognition of pelvic
masses by gray-scale ultrasound imaging: the contribution of
Doppler ultrasound. Ultrasound Obstet Gynecol. 14:338–347. 1999.
View Article : Google Scholar
|
|
13
|
Sokalska A, Timmerman D, Testa AC, et al:
Diagnostic accuracy of transvaginal ultrasound examination for
assigning a specific diagnosis to adnexal masses. Ultrasound Obstet
Gynecol. 34:462–470. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeong YY, Outwater EK and Kang HK: Imaging
evaluation of ovarian masses. Radiographics. 20:1445–1470. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Valentin L: Use of morphology to
characterize and manage common adnexal masses. Best Pract Res Clin
Obstet Gynaecol. 18:71–89. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kurachi H, Murakami T, Nakamura H, et al:
Imaging of peritoneal pseudocysts: value of MR imaging compared
with sonography and CT. AJR Am J Roentgenol. 161:589–591. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jain KA: Imaging of peritoneal inclusion
cysts. AJR Am J Roentgenol. 174:1559–1563. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Savelli L, de Iaco P, Ghi T, Bovicelli L,
Rosati F and Cacciatore B: Transvaginal sonographic appearance of
peritoneal pseudocysts. Ultrasound Obstet Gynecol. 23:284–288.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dorum A, Blom GP, Ekerhovd E and Granberg
S: Prevalence and histologic diagnosis of adnexal cysts in
postmenopausal women: an autopsy study. Am J Obstet Gynecol.
192:48–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Savelli L, Ghi T, De Iaco P, Ceccaroni M,
Venturoli S and Cacciatore B: Paraovarian/paratubal cysts:
comparison of transvaginal sonographic and pathological findings to
establish diagnostic criteria. Ultrasound Obstetrics Gynecol.
28:330–334. 2006. View Article : Google Scholar
|
|
21
|
Smorgick N, Herman A, Schneider D,
Halperin R and Pansky M: Paraovarian cysts of neoplastic origin are
underreported. JSLS. 13:22–26. 2009.PubMed/NCBI
|
|
22
|
Timor-Tritsch IE, Lerner JP, Monteagudo A,
Murphy KE and Heller DS: Transvaginal sonographic markers of tubal
inflammatory disease. Ultrasound Obstet Gynecol. 12:56–66. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Romosan G, Bjartling C, Skoog L and
Valentin L: Ultrasound for diagnosing acute salpingitis: a
prospective observational diagnostic study. Hum Reprod.
28:1569–1579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guerriero S, Ajossa S, Lai MP, Mais V,
Paoletti AM and Melis GB: Transvaginal ultrasonography associated
with colour Doppler energy in the diagnosis of hydrosalpinx. Hum
Reprod. 15:1568–1572. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karlan BY, Bristow RE and Li AJ:
Gynecologic Oncology: Clinical Practice & Surgical Atlas.
McGraw-Hill Medical; New York, NY: 2012
|
|
26
|
Caspi B, Hagay Z and Appelman Z: Variable
echogenicity as a sonographic sign in the preoperative diagnosis of
ovarian mucinous tumors. J Ultrasound Med. 25:1583–1585.
2006.PubMed/NCBI
|
|
27
|
Alcazar JL, Errasti T, Minguez JA, Galan
MJ, Garcia-Manero M and Ceamanos C: Sonographic features of ovarian
cystadenofibromas: spectrum of findings. J Ultrasound Med.
20:915–919. 2001.PubMed/NCBI
|
|
28
|
Ameye L, Timmerman D, Valentin L, et al:
Clinically oriented three-step strategy for assessment of adnexal
pathology. Ultrasound Obstet Gynecol. 40:582–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jermy K, Luise C and Bourne T: The
characterization of common ovarian cysts in premenopausal women.
Ultrasound Obstet Gynecol. 17:140–144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cohen L and Sabbagha R: Echo patterns of
benign cystic teratomas by transvaginal ultrasound. Ultrasound
Obstet Gynecol. 3:120–123. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guerriero S, Ajossa S, Mais V, Risalvato
A, Lai MP and Melis GB: The diagnosis of endometriomas using colour
Doppler energy imaging. Hum Reprod. 13:1691–1695. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van Holsbeke C, Van Calster B, Guerriero
S, et al: Endometriomas: their ultrasound characteristics.
Ultrasound Obstet Gynecol. 35:730–740. 2010.PubMed/NCBI
|
|
33
|
Asch E and Levine D: Variations in
appearance of endometriomas. J Ultrasound Med. 26:993–1002.
2007.PubMed/NCBI
|
|
34
|
Sayasneh A, Naji O, Abdallah Y, Stalder C
and Bourne T: Changes seen in the ultrasound features of a presumed
decidualised ovarian endometrioma mimicking malignancy. J Obstet
Gynaecol. 32:807–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Testa AC, Timmerman D, Van Holsbeke C, et
al: Ovarian cancer arising in endometrioid cysts: ultrasound
findings. Ultrasound Obstet Gynecol. 38:99–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yen P, Khong K, Lamba R, Corwin MT and
Gerscovich EO: Ovarian fibromas and fibrothecomas: sonographic
correlation with computed tomography and magnetic resonance
imaging: a 5-year single-institution experience. J Ultrasound Med.
32:13–18. 2013.
|
|
37
|
Paladini D, Testa A, Van Holsbeke C,
Mancari R, Timmerman D and Valentin L: Imaging in gynecological
disease (5): clinical and ultrasound characteristics in fibroma and
fibrothecoma of the ovary. Ultrasound Obstet Gynecol. 34:188–195.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Roth LM and Talerman A: The enigma of
struma ovarii. Pathology. 39:139–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zalel Y, Seidman DS, Oren M, et al:
Sonographic and clinical characteristics of struma ovarii. J
Ultrasound Med. 19:857–861. 2000.PubMed/NCBI
|
|
40
|
Savelli L, Testa AC, Timmerman D, Paladini
D, Ljungberg O and Valentin L: Imaging of gynecological disease
(4): clinical and ultrasound characteristics of struma ovarii.
Ultrasound Obstet Gynecol. 32:210–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Green GE, Mortele KJ, Glickman JN and
Benson CB: Brenner tumors of the ovary: sonographic and computed
tomographic imaging features. J Ultrasound Med. 25:1245–1254.
2006.PubMed/NCBI
|
|
42
|
Sherer DM, Dalloul M, Salame G, et al:
Color Doppler sonographic features of a Brenner tumor in pregnancy.
J Ultrasound Med. 28:1405–1408. 2009.PubMed/NCBI
|
|
43
|
Dierickx I, Valentin L, Van Holsbeke C, et
al: Imaging in gynecological disease (7): clinical and ultrasound
features of Brenner tumors of the ovary. Ultrasound Obstet Gynecol.
40:706–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Valentin L, Ameye L, Testa A, et al:
Ultrasound characteristics of different types of adnexal
malignancies. Gynecol Oncol. 102:41–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Exacoustos C, Romanini ME, Rinaldo D, et
al: Preoperative sonographic features of borderline ovarian tumors.
Ultrasound Obstet Gynecol. 25:50–59. 2005. View Article : Google Scholar
|
|
46
|
Pascual MA, Tresserra F, Grases PJ,
Labastida R and Dexeus S: Borderline cystic tumors of the ovary:
gray-scale and color Doppler sonographic findings. J Clin
Ultrasound. 30:76–82. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hassen K, Ghossain MA, Rousset P, et al:
Characterization of papillary projections in benign versus
borderline and malignant ovarian masses on conventional and color
Doppler ultrasound. AJR Am J Roentgenol. 196:1444–1449. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fruscella E, Testa AC, Ferrandina G, et
al: Ultrasound features of different histopathological subtypes of
borderline ovarian tumors. Ultrasound Obstet Gynecol. 26:644–650.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Darai E, Teboul J, Walker F, et al:
Epithelial ovarian carcinoma of low malignant potential. Eur J
Obstet Gynecol Reprod Biol. 66:141–145. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Testa AC, Ferrandina G, Timmerman D, et
al: Imaging in gynecological disease (1): ultrasound features of
metastases in the ovaries differ depending on the origin of the
primary tumor. Ultrasound Obstet Gynecol. 29:505–511. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Testa AC, Mancari R, Di Legge A, et al:
The ‘lead vessel’: a vascular ultrasound feature of metastasis in
the ovaries. Ultrasound Obstet Gynecol. 31:218–221. 2008.
View Article : Google Scholar : PubMed/NCBI
|