Introduction
The ubiquitin/proteasome system is an
extra-lysosomal, ATP-dependent protein degradation system. It
participates in the control of many cellular signals that concern
proliferation, growth, differentiation and death, namely apoptosis,
of the cells (1–6). It is the 26S proteasome that serves
as the center of this degradation mechanism and is within the huge
enzyme complex, which acts as the center of the functional control
of the cells (1–7). The suppression of proteasome activity
that participates in many life phenomena leads to death, i.e.
apoptosis of the cell. The use of such inhibitors for this
multifunctional enzyme complex, ‘proteasome’ as anticancer drug has
been attempted in recent years (8–20).
Proteasome inhibitor is a drug with highly anticipated efficacy as
an anticancer drug for clinical use. PS-341, an inhibitor, is
already applied for a multiple myeloma (9,10,12,17,21,22).
However, there are scarce data available on the clinical use of a
proteasome inhibitor as an anticancer drug. In addition to noting
any systemic side effect, evaluation whether cancer cells acquiring
resistance to a proteasome inhibitor reappear after inadequate or
incomplete cancer therapy is necessary in this type of agents. To
evaluate the possible generation of cells resistant to the
inhibitor and their specific properties it is necessary to work out
a strategy for the second line chemotherapy. Therefore, a squamous
cell carcinoma cell line A431 resistant to epoxomicin (EXM)
(23), which is a
proteasome-specific inhibitor, was established and some features
were examined to overcome the resistance to treatment (24).
Normally, epithelial cells are tightly
interconnected through several junctional structures, including
tight junctions and adherens-type junctions, which are intimately
associated with the actin and intermediate cytoskeleton. The
activation of epithelial-mesenchymal transition (EMT) allows cells
to dissociate from the epithelial type to mesenchymal type. EMT is
a process vital for morphogenesis during embryonic development and
has also been implicated in the transition of early-stage tumors
into invasive malignancies. This type of conversion results in the
loss of cell-cell contacts and a dramatic remodeling of
cytoskeleton in the epithelial cell layers (25,26).
E-cadherin is a cell-cell adhesion molecule and the loss of its
expression is a hallmark of EMT. Reduction of the E-cadherin
increased cell mobility and promoted tumor cell invasion (27–29).
The loss of E-cadherin has been shown to be an
important event for the invasion of epithelial tumor cells. Several
mechanisms have revealed that the loss of E-cadherin expression can
occur either genetically or epigenetically during tumor progression
(30,31). Hyper-methylation and chromatin
remodeling of the E-cadherin gene have emerged as the main
mechanisms for the downregulation of E-cadherin in most carcinomas.
The dysfunction in the regulation of E-cadherin expression plays an
essential role in pathological processes such as tumor
progression.
We reported previously that stabilization of cell
surface E-cadherin during EMT induced by TGF-β or during scattering
by hepatocyte growth factor can be blocked by inhibiting proteasome
with MG132 and lactacystin, and that this inhibition results in
transcriptional suppression of E-cadherin mRNA (24). We concluded that the proteasome
plays a crucial role in E-cadherin trafficking during EMT (24).
The Snail family transcription factors have been
shown to play major roles in E-cadherin repression and these
factors have been proposed to act as inducers in the invasion
process, including the zinc-finger factors snail (32–34).
The two-handed zinc factor family, ZEB1 and ZEB2 (SIP-1), and the
basic helix-loop-helix family factors, E12/E47 and Twist, also
demonstrated their downregulated effects to repress E-cadherin gene
expression (35–37).
MicroRNAs (miRNAs) are small non-coding RNAs,
usually 21–23 nucleotides long, which regulate gene expression,
primarily at the posttranscriptional level. So far, more that 400
miRNAs have been identified in mammalian cells, with each miRNA
having several target genes. The broad spectrum of genes that can
be targeted by a single miRNA is attributed to the high level of
conservation of the target motifs, known as seed sequences, within
the 3′-untranslated region (UTR)2 of the target genes,
thus making them the most powerful regulators of gene expression in
complex cellular processes, including cancer cell invasion and
metastasis (38–45). miRNAs are initially synthesized by
polymerase II as long primary transcripts, which are subsequently
processed into ~70-nt stem-loop pre-miRNAs by Drosha RNase III
endonuclease (46) and are
transported out of the nucleus by exportin 5 (47). Pre-miRNAs are further processed in
the cytoplasm by Dicer to yield the final 21–23-nt mature miRNAs
(48). Binding of miRNA to target
mRNAs with perfect or near perfect complementarity induces mRNA
degradation, whereas imperfect complementarity often induces
translational repression. It is believed that 7–8 nt in the 5′ end
of miRNAs, referred to as the seed sequence, are critical for
efficient targeting.
miRNAs have been implicated in regulating complex
physiological processes such as embryogenesis (49), organ development (50), and oncogenesis (40,51).
However, the functional roles of a vast majority of miRNAs remain
unknown. Previously, several groups have used a variety of model
systems to identify different miRNAs as promoters or suppressors of
metastasis (52–56). Although these studies clearly
implicate these miRNAs in metastasis, it is unclear which step(s)
in the multistep metastatic progression these miRNAs regulate.
Several miRNAs have been found to function as tumor
suppressors, such as miR15a, miR16-1, and let-7 (40,57–61),
whereas others were found to possess oncogenic properties,
including miR155, miR17-5p and miR21 (40,62,63).
Previously, the miR200 family has been found to play a central role
in the regulation of EMT process during cancer progression and
metastasis (39,41–45,64–68).
EMT, while being a critical process during embryonic development
and wound healing (69), also
plays a fundamental role in cancer metastasis, where cancer cells
acquire their invasive phenotype by undergoing a change from the
differentiated to a more dedifferentiated state (38,66,69–72).
Applying a classical model system of inducing EMT in
several cells, some studies showed that members of the miR-200
family, existing as two clusters in the genome, are significantly
repressed during EMT, suggesting a role as suppressors of EMT.
Since the proteasome inhibitor EXM-resistant
endometrial carcinoma Ishikawa cells (Ish/EXM cells) prepared by
us, suppressed E-cadherin and induced EMT, we studied here the
mechanism of E-cadherin suppression in Ish/EXM cells for an
association between transcriptional suppression factor and
miRNA.
Materials and methods
Cell lines
Human endometrial carcinoma cell line, Ishikawa, and
EXM-resistant Ishikawa cells (Ish/EXM cells) were cultured with
RPMI-1640 (Wako Chemicals, Japan) containing 10% heat inactivated
fetal bovine serum (growth medium) under conventional
conditions.
Cytotoxicity of proteasome inhibitor and
DXR
To assess the growth inhibitory effect of proteasome
inhibitors (EXM, MG-132, PSI and PS-341, Peptide Instrument, Japan)
and DXR (Kyowa Hakko Kogyo, Japan), viable Ishikawa and Ish/EXM
cells (2×104) were cultured continuously for 96 h in a
48-well culture plate (Greiner Japan) with 0.5 ml of EXM, MG-132,
PSI, PS-341 or DXR containing growth medium at graded equivalent
concentrations of each drug. After incubation, viable cells were
determined with a colorimetric assay using MTS
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (CellTiter 96® Aqueous, Promega) as
previously described (34), and
the results were expressed by the following equation: viable cells
(%) = 100 × (absorbance at 490 nm of the treated cells)/(absorbance
at 490 nm of the untreated cells) (35–42).
Assay of proteasome activity
Proteasome activity was measured in 100 μM of
Suc-Leu-Leu-Val-Tyr-amino-methyl-coumarin (Suc-LLVY-AMC, for
chymotrypsin-like activity) or
Boc-Leu-Arg-Arg-amino-methyl-coumarin (Boc-LRR-AMC, for
trypsin-like activity), respectively, monitored for AMC liberation
at 37°C for 15 min in a spectrofluorometer at an
excitation/emission wavelength of 380/460 nm, and expressed as nmol
AMC per min per mg protein (35).
Reaction mixture contained 0.05 M HEPES-NaOH (pH 7.5), 2% glycerol,
2 mM dithiothreitol and 100 μM substrate. Triton X-100 extracts
(1%) from cells were used as enzymatic source.
Semi-quantitative PCR analysis
Total RNA of Ishikawa and Ish/EXM cells were
purified using the RNeasy Plus mini kit (Promega). The cDNA was
prepared by reverse transcription (Prime Script reverse
transcription kit, Takara) using total RNA, and the expression
level of CDH1 (E-cadherin), ZEB1, ZEB2, Snail, Slug, and Twist were
measured by PCR (GoTaq Green Master Mix, Promega) using the
obtained cDNA as a template; the amplification number for each gene
was 26, 28, 35, 45, 35 and 33 cycles, respectively. Each factor was
compared with β-actin as an internal standard. The primers used
(Fasmac Co. Ltd., Greiner Japan) for each factor were: β-actin (254
bp): AACACCCCAGCCATGTAC (sense), ATGTCACGCA CGATTTCC (antisense),
CDH1 (567 bp): GGTTCAAGC TGCTGACCTTC (sense), AGCCAGTTGGCAGTGTCTCT
(antisense), COL1A2 (486 bp): TGCTCAGCTTTGTGGAT ACG (sense),
CCTGTGGTCCAACAACTCCT (antisense), CNX26 (421 bp):
CTACTTCCCCATCTCCCACA (sense), GACATTCAGCAGGATGCAAA (antisense),
CTNNB1 (394 bp): CCCACTAATGTCCAGCGTTT (sense), AATCC
ACTGGTGAACCAAGC (antisense), VIN (170 bp): GAG AACTTTGCCGTTGAAGC
(sense), TCCAGCAGCTTCC TGTAGGT (antisense), CDH2 (527 bp):
GGACAGTTCCTG AGGGATCA (sense), TGGTTTGACCACGGTGACTA (antisense),
FN1 (196 bp): TGTTCGTGCAGCTGTTTACC (sense), GCCACCGTAAGTCTGGGTTA
(antisense), ZEB1 (537 bp): GCACCTGAAGAGGACCAGAG (sense), TGGTGATGC
TGAAAGAGACG (antisense), ZEB2 (393 bp): TTCCTGGG CTACGACCATAC
(sense), TTTACCTTCCAGCAGCCCTA (antisense), Snail (415 bp):
TTTACCTTCCAGCAGCCCTA (sense), CCAGGCTGAGGTATTCCTTG (antisense),
Slug (415 bp): CTTTTTCTTGCCCTCACTGC (sense), ACAGCA GCCAGATTCCTCAT
(antisense), Twist (468 bp): CTGAGC AACAGCGAGGAAG (sense),
CATCTTGGAGTCCAG CTCGT (antisense), and E47/E12 (567 bp): GCACTGGCCT
CGATCTACTC (sense), GGCCTTCAGCTCCTTCTTCT (antisense).
Transwell invasion assay
For the invasion assay, 1×105 cells were
plated in the top chamber onto a Matrigelcoated membrane (24-well
insert; pore size, 8 μm, Greiner Japan). Each well was coated
freshly with Matrigel (60 mg) before the invasion assay. Cells were
plated in medium without serum or growth factors, and medium
supplemented with serum was used in the lower chamber. The cells
were incubated for 24 h and cells that did not invade through the
pores were removed by a cotton swab. Cells on the lower surface of
the membrane were fixed with methanol and stained with crystal
violet. The number of cells invading through the membrane was
counted under a light microscope (three random fields per
well).
Promoter methylation assay
Genome DNA from both Ishikawa and Ish/EXM cells was
purified using the QIAamp DNA Mini kit (Qiagen). Methylation of the
promoter CpG island in the CDH1 gene was detected using the
MethylEasy Xceed: Rapid DNA Bisulphite Modification kit (Human
Genetic Signatures). Treatment with bisulphate converts cytosines
to uracils whereas 5-methylcytosines remain unreactive. Methylation
of the E-cadherin promoter was determined by PCR using
bisulphate-treated DNA as a template. Primers used for
unmethylation and methylation of the promoter CpG island in the
CDH1 gene were as follows: unmethylation, TAATTTTAGGTTAGAGGGTTATTGT
(sense) and AACTC ACAAATCTTTACAATTCCAACA (antisense); methylation,
TTAGGTTAGAGGGTTATCGCGT (sense) and CTCACAA ATACTTTACAATTCCGACG
(antisense).
Detection of miRNA
miRNA was analyzed using the QuantiMir kit (System
Bioscience: SBI, USA). Briefly, total RNA purified by the above
described method including the small RNA fraction, was used as
starting material. miRNA was tailed with polyA, annealed with
oligo-dT adaptor, and then first strand cDNA was created by reverse
transcription. The expression level of miRNA was measured by PCR
using the obtained cDNA as a template, and the primers used were as
follows: forward, miRNA-specific sequence; reverse, universal
reverse primer into the oligo-dT adaptor sequence.
Knockdown of transcriptional suppression
factor in Ish/EXM cells
SiRNA for human ZEB1, ZEB2, Snail, Slug or Twist
(150 pmol in 10-cm dish) was transfected into Ish/EXM cells using
the siPORT NeoFX transfection reagent (Life Technologies).
Regulation of miR-200 family expression
in Ishikawa and Ish/EXM cells
Pre- and anti-miRNA for the miR-200 family
(chemically modified double-stranded RNAs that mimic the endogenous
miR-200 family, Life Technologies) (150 pmol in a 10-cm dish) were
transiently transfected into Ish/EXM and Ishikawa cells,
respectively, using Lipofectamine RNAiMax. Pre-miR Negative Control
(a random sequence miRNA mimic molecule that has been extensively
tested in human cell lines and tissues and validated to not produce
identifiable effects on known miRNA function) was transfected using
Lipofectamine RNAiMax. Following transfection, cells were starved
overnight and then the total RNA was extracted as described
previously. Using RT-PCR, expression of the miR-200 family,
E-cadherin and ZEB1 was measured as described above.
Western blot analysis
E-cadherin and ZEB1 in the cell extract with 1%
Triton X-100 were separated by SDS-PAGE (5–20% gradient acrylamide)
and analyzed by western blotting using anti-E-cadherin antibody
(Cosmo Bio Co.) and anti-ZEB1 antibody (Sigma-Aldrich Japan) as the
primary antibody and alkaline phosphatase-labeled anti-rabbit IgG
as the secondary antibody (Sigma-Aldrich, Japan).
Protein determination
Protein concentration was assayed by a Bio-Rad
protein assay kit using bovine serum albumin as the standard.
Results
Establishment of EXM-resistant
variants
EXM-resistant variants of Ishikawa cells were
obtained by exposure to EXM. Initial induction of resistance was
achieved by continuous exposure of Ishikawa cells to EXM (6.25 nM)
over 2 months. Growing resistant cells were further treated with
gradually increasing concentrations of EXM (increasing every 4
weeks) until the concentration finally reached 60 nM of EXM. The
resistant Ishikawa cells that survived exposure to 60 nM EXM were
designated as Ish/EXM cells. Ish/EXM cells were cloned by the
limiting dilution method in a 96-well culture plate. Acquirement of
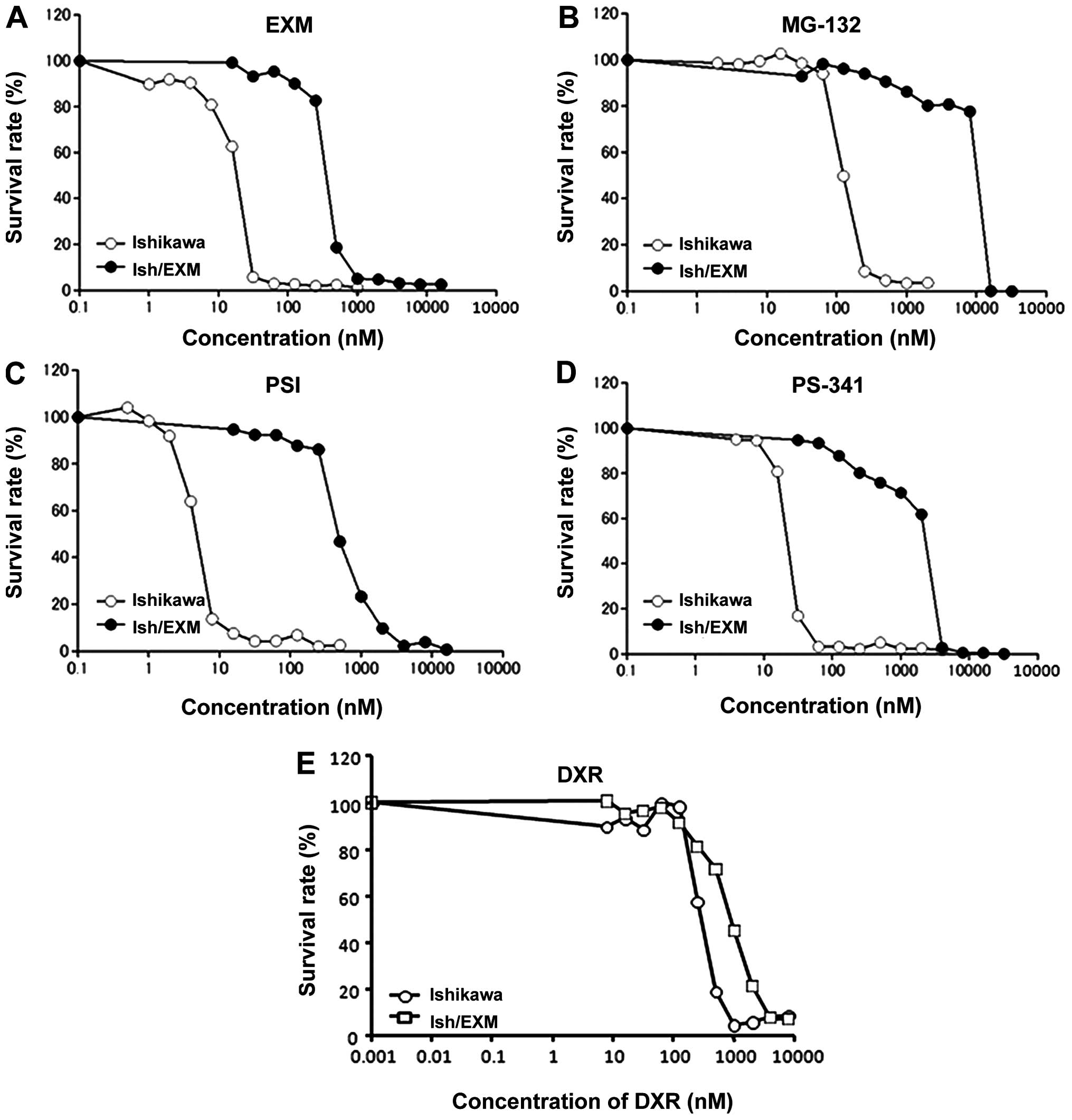
EXM-resistance in Ishikawa cells is shown in Fig. 1A.
Acquirement of EXM-resistance in Ishikawa
cells and proteasome activity
Cytotoxic effect of EXM on Ishikawa and Ish/EXM
cells was measured using the MTS method. The 50% growth inhibition
concentration (IC50) for EXM against Ishikawa and
Ish/EXM cells was 20±1.7 and 400±28 nM, respectively (Fig. 1A, 3 independent experiments). Cytotoxicity
of the other proteasome inhibitors, MG132, PSI and PS-341 against
these cells was assayed. Ish/EXM cells were also acquired for these
inhibitors, and the IC50 values of Ishikawa and Ish/EXM
cells were: 125±11 and 1200±105 nM for MG132, 4±0.32 and 500±35 nM
for PSI, and 20±1.2 and 3000±250 nM for PS-341, respectively
(Fig. 1B, C and D), 3 independent
experiments). However, sensitivity to DXR against Ish/EXM cells
showed 3-fold resistance compared to that against Ishikawa cells
(Fig. 1D). Proteasomal activity in
Ishikawa and Ish/EXM cells was 7.2±0.8 and 1.0±0.2 nmol/min/mg
protein for chymotrypsin-like activity, and 6.9±0.8 and 4.7±0.6
nmol/min/mg protein for trypsin-like activity, respectively (3
independent experiments).
Suppression of E-cadherin and expression
of transcriptional suppression factor in Ish/EXM cells/Induction of
EMT in Ish/EXM cells
Acquirement of EXM-resistance led to the
disappearance of both E-cadherin mRNA (CDH1) and its protein in
Ish/EXM cells (Fig. 2) and
induction of EMT in Ish/EXM cells. Moreover, suppression of the
epithelial markers COL1A2, CNX26 and CTNNB1, and overexpression of
the mesenchymal markers VIN, CDH2 and FN1 were observed in Ish/EXM
cells relative to Ishikawa cells. Since E-cadherin expression is
known to be regulated by a transcriptional suppression factor, we
used RT-PCR to measure mRNA expression of several factors related
to E-cadherin suppression, specifically Snail, Slug, ZEB1, ZEB2,
E12/E47 and Twist, in Ish/EXM cells. Among these suppressors
concerning EMT-induction, expression of ZEB1 and ZEB2 was
especially enhanced in Ish/EXM cells, and expression of the ZEB1
protein was markedly increased (Fig.
2A). Expression of Slug, Snail and Twist mRNA was also
substantially increased in the cells. However, no change in E12/E47
mRNA was observed in Ishikawa or Ish/EXM cells.
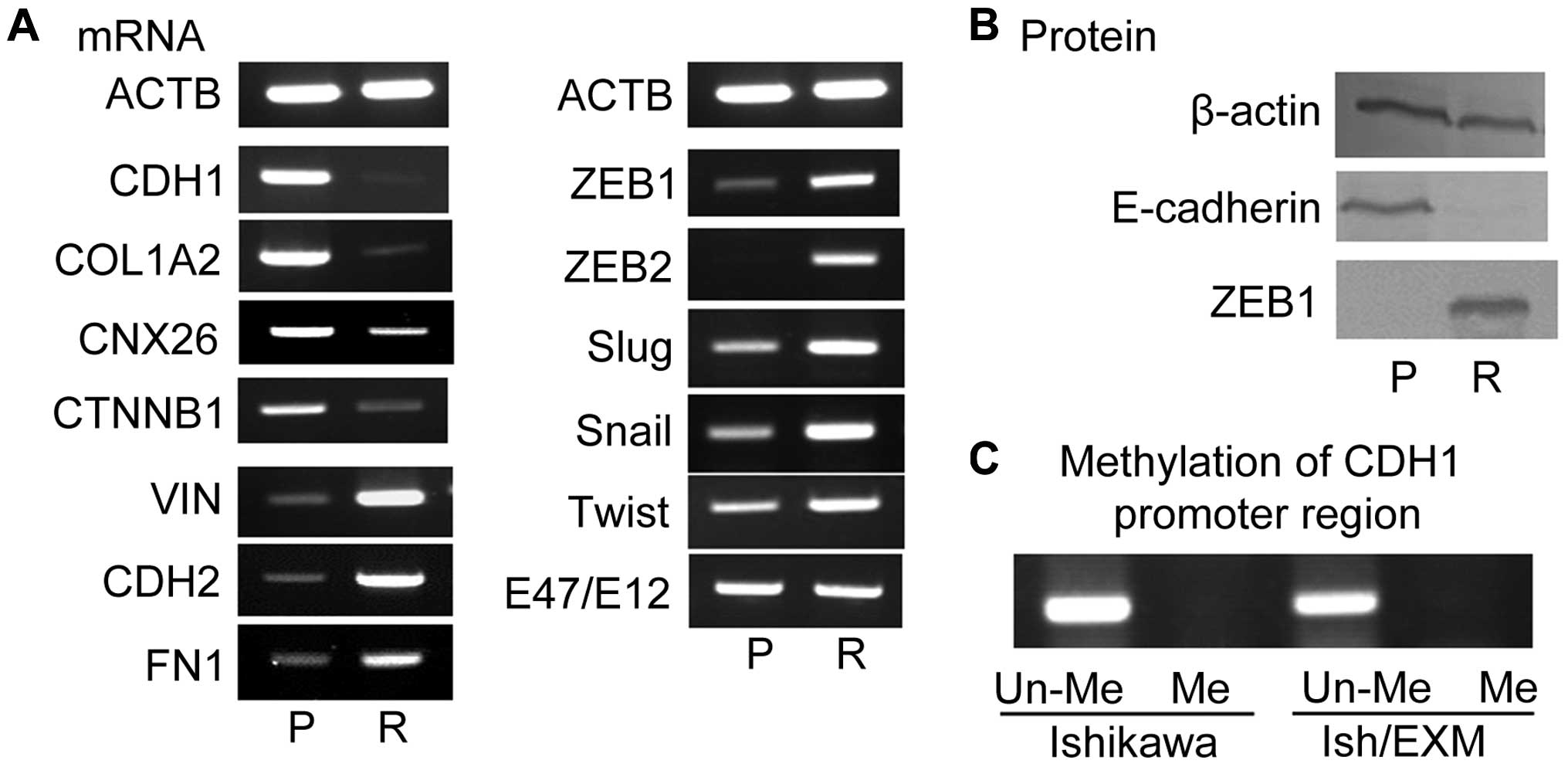 | Figure 2Expression of mRNA level of
epithelial marker (CDH1, COL1A2, CNX26, CNTB1), mesenchymal marker
(VIN, CDH2, FN1), and transcriptional repressors (ZEB1, ZEB2, Slug,
Snail, Twist, E47/E12) by acquirement of EXM-resistance (A), and of
the protein level of E-cadherin and ZEB1 (B). P, Ishikawa cells, R,
Ish/EXM cells. The methylation profile of this E-cadherin promoter
fragment in CpG islands of both Ishikawa and Ish/EXM cells (C).
Un-Me, unmethylation in CpG islands; Me, methylation in CpG
islands. |
Methylation profile of E-cadherin
promoter in Ishikawa and Ish/EXM cells
Since promoter hyper-methylation is known to result
in transcriptional downregulation of the E-cadherin gene, we
measured methylation of the E-cadherin promoter. The methylation
profile of this E-cadherin promoter fragment contained unmethylated
CpG islands in both Ishikawa and Ish/EXM cells (Fig. 2C).
Increase in invasive capacity of Ish/EXM
cells
Since E-cadherin was suppressed in Ish/EXM cells, it
was expected that EMT would be induced in these cells. Therefore,
we measured migration of both Ishikawa and Ish/EXM cells. Invasive
capacity of Ish/EXM cells increased 5.7-fold compared with that of
Ishikawa cells (Fig. 3, 3
independent experiments).
E-cadherin expression by suppression of
transcriptional repression factor in Ish/EXM cells
For identification of the primary transcriptional
suppressor for E-cadherin suppression, each factor of Snail, Slug,
ZEB1, ZEB2 and Twist, was knocked down in Ish/EXM cells. Treatment
of Ish/EXM cells with ZEB1 siRNA but not with ZEB2, Snail, Slug or
Twist siRNA restored the expression of both E-cadherin mRNA and its
protein (Fig. 4). Of note,
treatment of Ish/EXM cells with ZEB2 siRNA partially restored
expression of E-cadherin mRNA (Fig.
4).
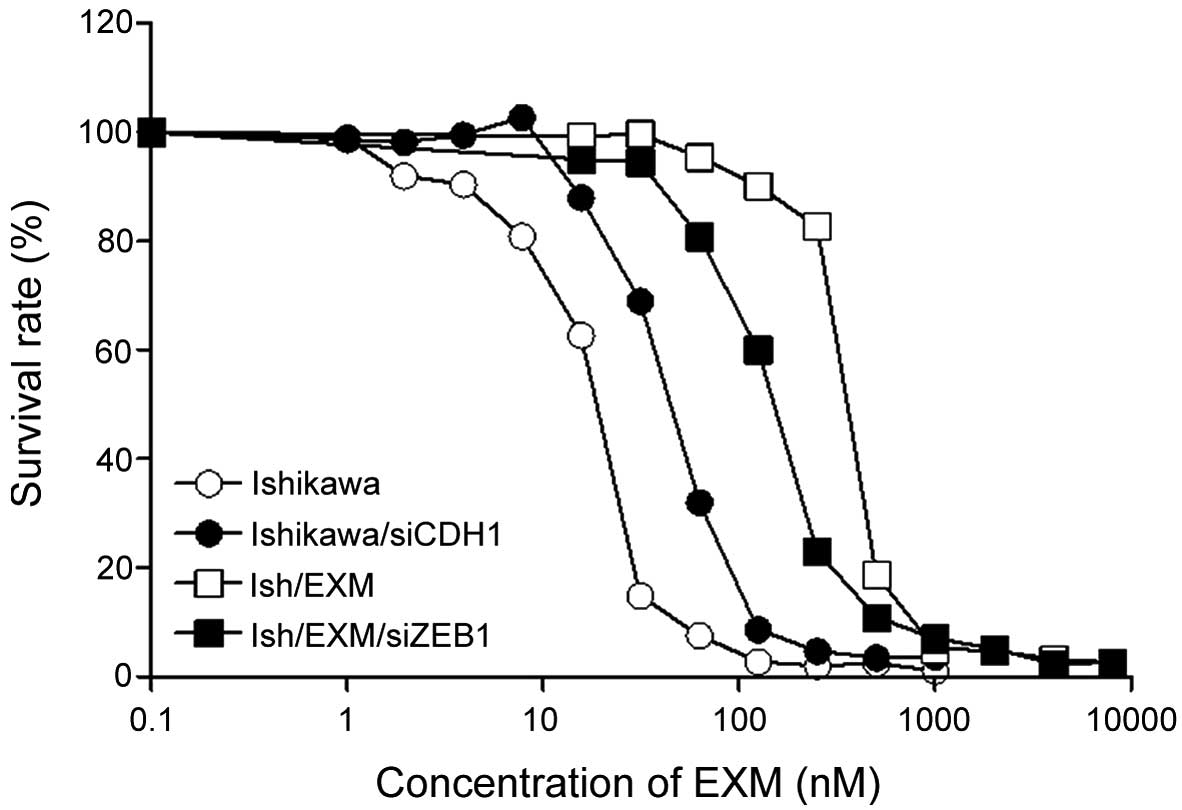
Cytotoxic effect of EXM on
CDH1-suppressed Ishikawa cells and ZEB1-repressed Ish/EXM
cells
We studied whether the sensitivity of EXM decreased
with CDH1-supressed Ishikawa cells by transfection of siRNA for
CDH1. Conversely, we studied whether the sensitivity of EXM rose
with Ish/EXM cells by transfection of siRNA for ZEB1. Acquirement
of EXM-resistance led to the disappearance of both E-cadherin mRNA
(CDH1) and its protein in Ish/EXM cells (Fig. 2) and induction of EMT in Ish/EXM
cells.
Since the IC50 value for EXM against
CDH1-supressed Ishikawa cells was 40±4.8 nM (3 independent
experiments), sensitivity to EXM against CDH1-supressed Ishikawa
cells showed 2-fold resistance compared to that against Ishikawa
cells (Fig. 5). Since the
IC50 value for EXM against the ZEB1-supressed Ish/EXM
cells was 300±45 nM (3 independent experiments), sensitivity to EXM
against ZEB1-supressed Ish/EXM cells showed 1.3-fold higher
susceptibility compared to that against Ish/EXM cells (Fig. 5).
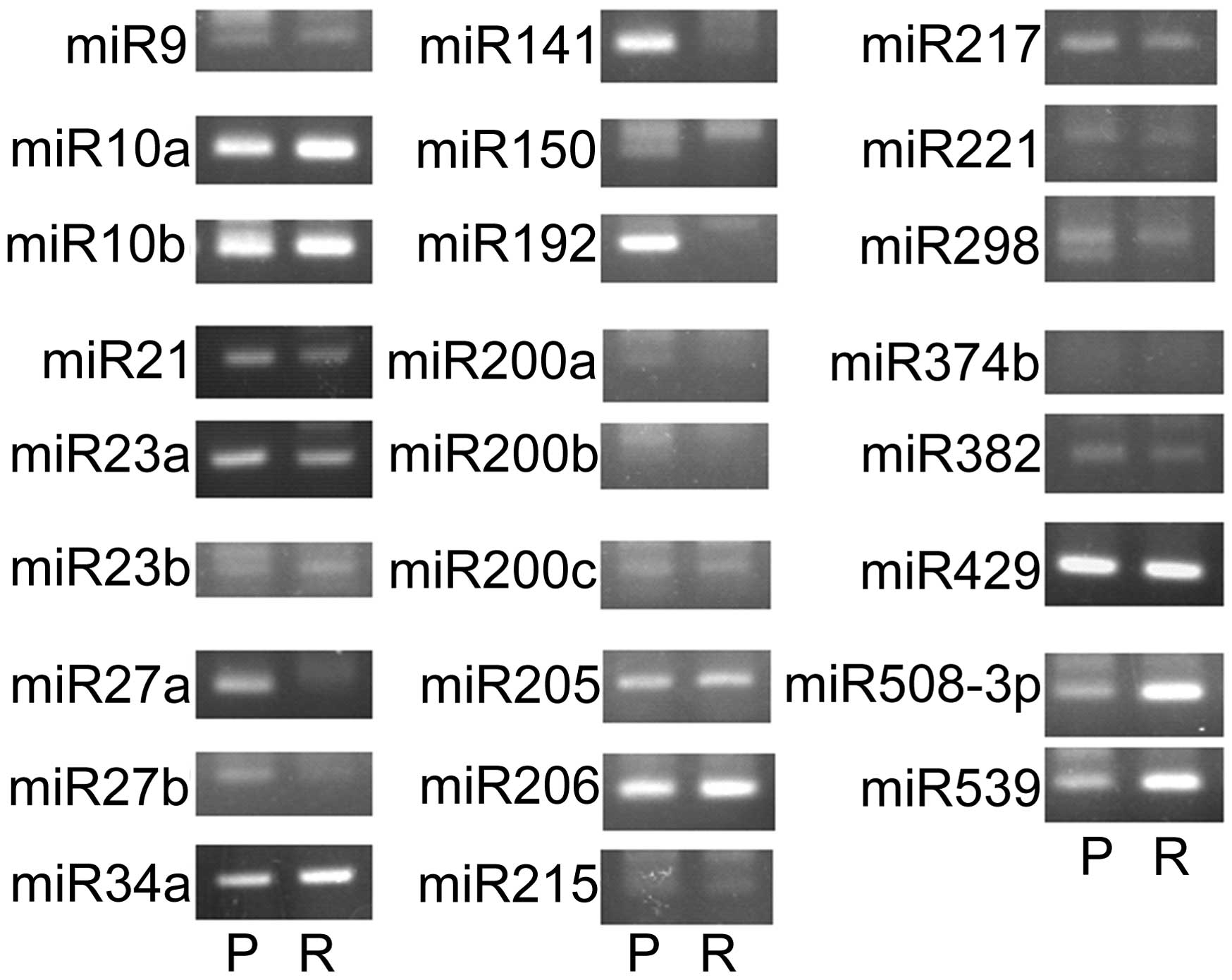
Suppression of miRNA controlling EMT in
Ish/EXM cells
Since several miRNAs have been found to regulate
EMT, such as miR-9, miR-10a, miR-10b, miR-21, miR-23a, miR-23b,
miR-27a, miR-27b, miR-34a, miR-141, miR-150, miR-192, miR-200a,
miR-200b, miR-200c, miR-205, miR-206, miR-215, miR-217, miR-221,
miR-298, miR-374b, miR-382, miR-429, miR-508-3p, and miR-539, we
measured the expression of these miRNAs in Ishikawa and Ish/EXM
cells. Shown in Fig. 6, miR-200a,
miR-200b, miR-200c and miR-141 (miR-200 family) were suppressed in
Ish/EXM cells. Therefore, the miR-200 family was regarded as a
candidate for inducing EMT in Ish/EXM cells.
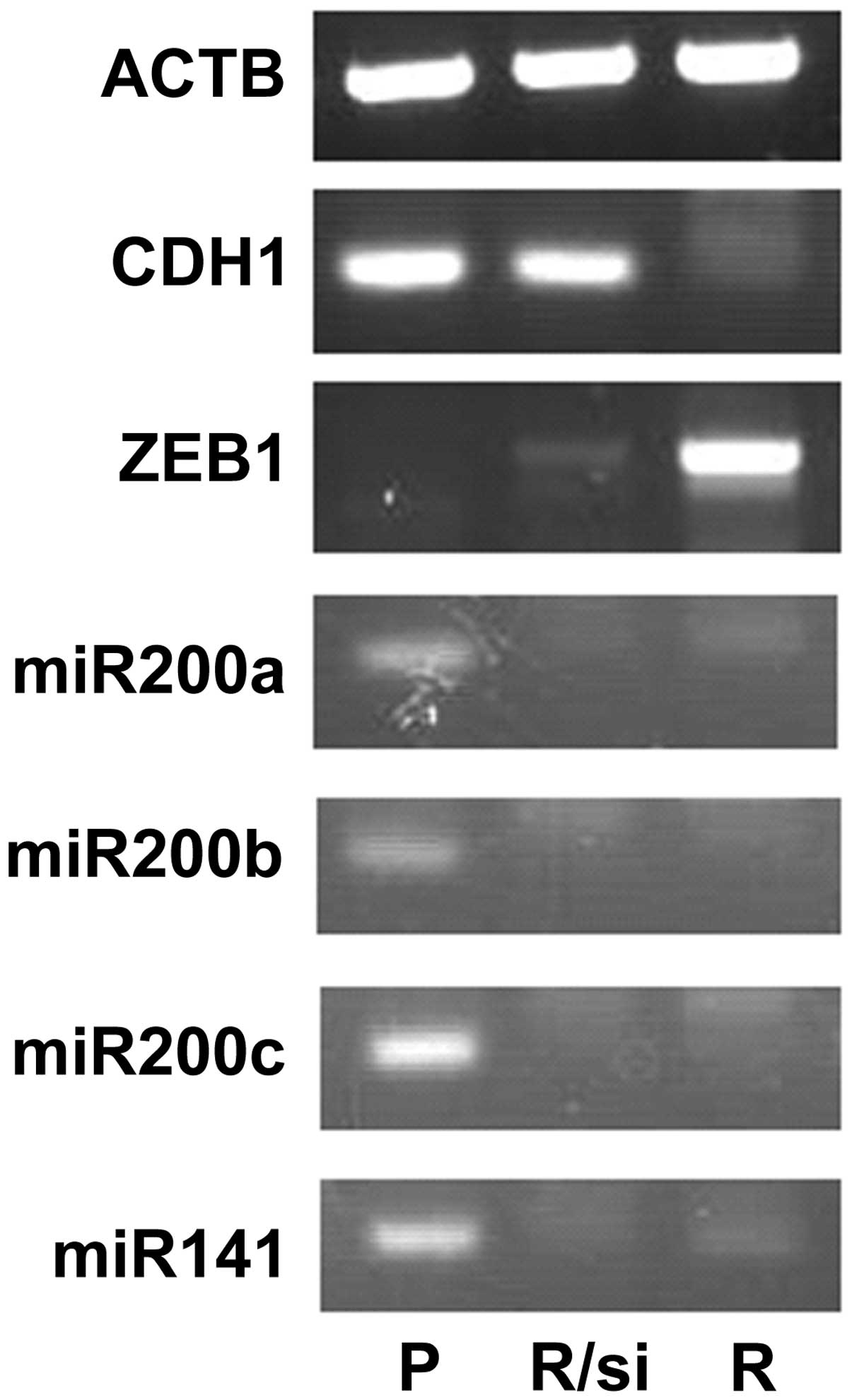
Regulation of ZEB1 expression by the
miR-200 family
Since the miR-200 family was suppressed in Ish/EXM
cells, we measured the expression of these miRNAs in Ishikawa and
Ish/EXM cells, and investigated the effect of transfection with
anti- or pre-miR-200 family (miR-200a, miR-200b, miR-200c and
miR-141) on both transcriptional suppression factor and E-cadherin
expression in Ishikawa or Ish/EXM cells, respectively. Expression
of the miR-200 family in Ish/EXM cells by transfection with the
pre-miR-200 family led to suppression of ZEB1. Moreover, expression
of E-cadherin was observed in Ish/EXM cells transfected with the
pre-miR-200 family (Fig. 7). By
contrast, suppression of the miR-200 family in Ishikawa cells by
transfection with the anti-miR-200 family led to both expression of
ZEB1 and suppression of E-cadherin (Fig. 7). On the other hand, since
suppression of ZEB1 in Ish/EXM cells caused by treatment with its
siRNA did not restore miR200 family expression, the miR200 family
was placed upstream of ZEB1 to regulate the expression (Fig. 8).
 | Figure 7Effect of regulation of miR200 family
expression on the expression of ZEB1 and CDH1 (E-cadherin) mRNA (A)
and protein (B) in Ishikawa and Ish/EXM cells by transfection with
the anti- and pre-miR200 family, respectively. Lane 1, Ishikawa
cells; lane 2, Ish/EXM cells; lane 3, non-coding miR-transfected
Ishikawa cells; lane 4, non-coding miR-transfected Ish/EXM cells;
lane 5, anti-miR200 family (miR-200a, miR-200b, miR-200c and
miR-141)-transfected Ishikawa cells; lane 6, pre-miR200 family
(miR-200a, miR-200b, miR-200c and miR-141)-transfected Ish/EXM
cells. |
Discussion
Proteasome inhibitor is a drug with highly
anticipated efficacy as an anticancer agent for clinical use. The
inhibitor, PS-341 (Bortezomib), is already in use for multiple
myeloma (9,10,12,17,21,22).
However, there are scarce data available on the clinical use of a
proteasome inhibitor as an anticancer drug. Very careful use of
this type of agent is necessary, noting any systemic side effect,
and whether cancer cells acquiring resistance to a proteasome
inhibitor reappear after inadequate or incomplete cancer therapy.
When Ishikawa cells acquired resistance to the proteasome inhibitor
epoxomicin (EXM), the cells caused EMT and suppressed E-cadherin.
Induction of EMT was confirmed by suppression of the epithelial
markers CDH1, COL1A2, CNX26 and CTNNB1, and overexpression of the
mesenchymal markers VIN, CDH2, FN1, ZEB1 and ZEB2 in Ish/EXM
cells.
The proteasome inhibitor-resistant cells acquired
invasiveness through chemotherapy and the cells became even more
malignant. EXM-resistant Ish/EXM cells acquired cross-resistance
for MG-132, PSI and PS-341, and other proteasome inhibitors
(Fig. 1A–D). However, Ish/EXM
cells showed a 3-fold resistance to DXR compared to Ishikawa cells
(Fig. 1E). In generation,
DXR-resistant cells exhibited Pgp (MDR mechanism) expression and
DXR was released from the cells using the ATP-dependent
transporter, Pgp. In EXM-resistant cells, it was suggested that
detoxification activity for proteasome inhibitors was enhanced,
because enhanced expression of the Cytochrome P450 family and ALDH
as a detoxification enzyme was observed in Ish/EXM cells (data not
shown).
Expression of ZEB1 among the transcriptional
suppression factors caused suppression of E-cadherin in Ish/EXM
cells. This result demonstrated that E-cadherin was re-expressed in
Ish/EXM cells in which ZEB1 was knocked down by treatment with
siRNA but not by any other transcriptional suppressor factor
(Figs. 4 and 8). ZEB2 also suppressed expression of
E-cadherin in Ish/EXM cells, but the suppression was weaker in
these cells (Fig. 4), possibly
because E-cadherin was partly re-expressed in Ish/EXM cells in
which ZEB2 had been knocked down by treatment with siRNA. Several
studies have demonstrated that transcriptional suppression factors
caused suppression of E-cadherin in various cells (32–37).
Since acquirement of EXM-resistance led to the
disappearance of both E-cadherin mRNA (CDH1) and its protein in
Ish/EXM cells, we studied whether the sensitivity of EXM decreased
with CDH1-supressed Ishikawa cell by transfection of siRNA for
CDH1. Conversely, we studied whether the sensitivity of EXM rose
with Ish/EXM cell by transfection of siRNA for ZEB1. Sensitivity to
EXM against CDH1-supressed Ishikawa cells showed 2-fold resistance
compared to that against Ishikawa cells, and sensitivity to EXM
against ZEB1-supressed Ish/EXM cells showed 1.3-fold higher
susceptibility compared to that against Ish/EXM cells (Fig. 5). Accordingly, the disappearance of
E-cadherin partially participated in acquirement of EXM-resistance.
Recently, we found that several drug-resistant factors, the
cytochrome P450 family and ALDH family were highly expressed in
Ish/EXM cells (data not shown). These factors may participate in
EXM-resistance.
Since several miRNAs have been found to control EMT,
such as miR-9, miR-10a, miR-10b, miR-21, miR-23a, miR-23b, miR-27a,
miR-27b, miR-34a, miR-141, miR-150, miR-192, miR-200a, miR-200b,
miR-200c, miR-205, miR-206, miR-215, miR-217, miR-221, miR-298,
miR-374b, miR-382, miR-429, miR-508-3p and miR-539 in several cell
types (48–64), we conducted quantitative tests to
compare with the expression of these miRNAs in Ishikawa and Ish/EXM
cells. As shown in Fig. 5, the
miR200 family (miR-200a, miR-200b, miR-200c and miR-141) was
suppressed in Ish/EXM cells.
In initial studies, an inverse correlation between
the miR-200 family and ZEB1 was established in Ishikawa and Ish/EXM
cells (Fig. 8). Suppression of
ZEB1 by the miR-200 family resulted in enhanced expression of the
key epithelial marker, E-cadherin and acquisition of an epithelial
phenotype (Fig. 7). During the
induction of EMT in Ish/EXM cells with acquirement of
EXM-resistance, the miR-200 family and E-cadherin were repressed in
parallel with an increase in ZEB1 expression. The ability to induce
EMT was dependent upon suppression of the miR-200 family and
induction of ZEB1 expression. Conversely, a mesenchymal-epithelial
transition (MET) could be induced by expression of the miR-200
family in cells that were originally mesenchymal in nature
(Fig. 8). These results confirm
that the miR-200 family represses ZEB1 expression and consequently
inhibits the progression of EMT by establishing and maintaining an
epithelial phenotype. The suppression of ZEB1 expression by the
miRNA-200 family is direct, and occurs as a result of the miRNA
binding to the eight and the nine sites in the 30UTRs of ZEB1 (and
ZEB2) mRNA (65,73).
An additional level of miR200-ZEB1/ZEB2 protein
regulation was identified in breast and colon cancer cell lines in
which ZEB1 was constitutively downregulated by shRNA (39). Cells underwent MET, with
corresponding upregulation of the miR-200 family, most notably the
miR-141 and miR-200c transcripts. The miR-141 and miR-200c promoter
contains multiple highly conserved E-boxes which are occupied by
ZEB1 in mesenchymal cells leading to the transcriptional
suppression. This finding was complemented by data showing that
ZEB1-depleted cells retained the epithelial phenotype upon miR-200
inhibition. It was reported that a double-negative feedback loop
controls ZEB1-SIP1 (ZEB2) and miR-200 family expression that
regulates cellular phenotype and has direct relevance to the role
of these factors in tumor progression (64,74–76).
Data suggest that the majority, if not all, epithelial cells
express high levels of the miR-200 family, which directly repress
ZEB1 and ZEB2 and so enable the expression of E-cadherin. However,
if an extracellular signal stimulates the expression of ZEB1, the
miR-200 family is suppressed, thereby allowing EMT to proceed.
Moreover, investigating the effect of transfection
with the anti- and pre-miR-200 family on both transcriptional
suppression factor and E-cadherin expression in Ishikawa and
Ish/EXM cells, respectively, led to an increase in expression of
the miR-200 family in Ish/EXM cells by transfection with
pre-miR-200 and suppression of ZEB1 and re-expression of E-cadherin
(Fig. 7). By contrast, suppression
of the miR-200 family in Ishikawa cells by transfection with
anti-miR-200 family showed both expression of ZEB1 and suppression
of E-cadherin (Fig. 7). Since
suppression of ZEB1 in Ish/EXM cells by treatment with its siRNA
did not restore miR-200 family expression, the miR-200 family was
placed upstream of ZEB1 to regulate the expression (Fig. 8). It was suggested that this
regulatory loop did not control ZEB1 and the miR-200 family in
Ishikawa and Ish/EXM cells.
As we recently found that several drug-resistant
factors, the cytochrome P450 family and ALDH family, and the ERK
signal-regulating factors were highly expressed in Ish/EXM cells,
further study will attempt to confirm the relationship between
proteasome inhibitor resistance and EMT induction.
Acknowledgements
This study was partly supported by The Jikei
University Research Fund.
References
|
1
|
Varshavsky A: The ubiquitin system. Trends
Biochem Sci. 22:383–387. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ciechanover A: The ubiquitin-proteasome
pathway: On protein death and cell life. EMBO J. 17:7151–7160.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hershko A and Ciechanover A: The ubiquitin
system. Annu Rev Biochem. 67:425–479. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Voges D, Zwickl P and Baumeister W: The
26S proteasome: A molecular machine designed for controlled
proteolysis. Annu Rev Biochem. 68:1015–1068. 1999. View Article : Google Scholar
|
|
5
|
Hershko A, Ciechanover A and Varshavsky A:
Basic Medical Research Award. The ubiquitin system. Nat Med.
6:1073–1081. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ciechanover A and Schwartz AL:
Ubiquitin-mediated degradation of cellular proteins in health and
disease. Hepatology. 35:3–6. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naujokat C and Hoffmann S: Role and
function of the 26S proteasome in proliferation and apoptosis. Lab
Invest. 82:965–980. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee DH and Goldberg AL: Proteasome
inhibitors: Valuable new tools for cell biologists. Trends Cell
Biol. 8:397–403. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adams J, Palombella VJ, Sausville EA,
Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S and
Elliott PJ: Proteasome inhibitors: A novel class of potent and
effective antitumor agents. Cancer Res. 59:2615–2622.
1999.PubMed/NCBI
|
|
10
|
Meng L, Mohan R, Kwok BH, Elofsson M, Sin
N and Crews CM: Epoxomicin, a potent and selective proteasome
inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl
Acad Sci USA. 96:10403–10408. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hideshima T, Richardson P, Chauhan D,
Palombella VJ, Elliott PJ, Adams J and Anderson KC: The proteasome
inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes
drug resistance in human multiple myeloma cells. Cancer Res.
61:3071–3076. 2001.PubMed/NCBI
|
|
12
|
Shah SA, Potter MW and Callery MP:
Ubiquitin proteasome inhibition and cancer therapy. Surgery.
131:595–600. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adams J: Preclinical and clinical
evaluation of proteasome inhibitor PS-341 for the treatment of
cancer. Curr Opin Chem Biol. 6:493–500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adams J: Proteasome inhibitors as new
anticancer drugs. Curr Opin Oncol. 14:628–634. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Almond JB and Cohen GM: The proteasome: A
novel target for cancer chemotherapy. Leukemia. 16:433–443. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garber K: On the eve of protein
destruction: Ubiquitin research begins to pay off. J Natl Cancer
Inst. 94:550–552. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hernandez AA and Roush WR: Recent advances
in the synthesis, design and selection of cysteine protease
inhibitors. Curr Opin Chem Biol. 6:459–465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ling YH, Liebes L, Ng B, Buckley M,
Elliott PJ, Adams J, Jiang JD, Muggia FM and Perez-Soler R: PS-341,
a novel proteasome inhibitor, induces Bcl-2 phosphorylation and
cleavage in association with G2-M phase arrest and apoptosis. Mol
Cancer Ther. 1:841–849. 2002.PubMed/NCBI
|
|
19
|
Sakamoto KM: Ubiquitin-dependent
proteolysis: Its role in human diseases and the design of
therapeutic strategies. Mol Genet Metab. 77:44–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith DM, Wang Z, Kazi A, Li LH, Chan TH
and Dou QP: Synthetic analogs of green tea polyphenols as
proteasome inhibitors. Mol Med. 8:382–392. 2002.PubMed/NCBI
|
|
21
|
Yu R, Ren SG and Melmed S: Proteasome
inhibitors induce apoptosis in growth hormone- and
prolactin-secreting rat pituitary tumor cells. J Endocrinol.
174:379–386. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
LeBlanc R, Catley LP, Hideshima T, et al:
Proteasome inhibitor PS-341 inhibits human myeloma cell growth in
vivo and prolongs survival in a murine model. Cancer Res.
62:4996–5000. 2002.PubMed/NCBI
|
|
23
|
Orlowski RZ, Stinchcombe TE, Mitchell BS,
et al: Phase I trial of the proteasome inhibitor PS-341 in patients
with refractory hematologic malignancies. J Clin Oncol.
20:4420–4427. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohkawa K, Asakura T, Aoki K, Shibata S,
Minami J, Fujiwara C, Sai T, Marushima H and Kuzuu H: Establishment
and some characteristics of epoxomicin (a proteasome inhibitor)
resistant variants of the human squamous cell carcinoma cell line,
A431. Int J Oncol. 24:425–433. 2004.PubMed/NCBI
|
|
25
|
Duband JL, Monier F, Delannet M and
Newgreen D: Epithelium-mesenchyme transition during neural crest
development. Acta Anat (Basel). 154:63–78. 1995. View Article : Google Scholar
|
|
26
|
Viebahn C: Epithelio-mesenchymal
transformation during formation of the mesoderm in the mammalian
embryo. Acta Anat (Basel). 154:79–97. 1995. View Article : Google Scholar
|
|
27
|
Birchmeier W and Behrens J: Cadherin
expression in carcinomas: Role in the formation of cell junctions
and the prevention of invasiveness. Biochim Biophys Acta.
1198:11–26. 1994.PubMed/NCBI
|
|
28
|
Bussemakers MJG, van Bokhoven A, Völler M,
Smit FP and Schalken JA: The genes for the calcium-dependent cell
adhesion molecules P- and E-cadherin are tandemly arranged in the
human genome. Biochem Biophys Res Commun. 203:1291–1294. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berx G, Cleton-Jansen AM, Nollet F, de
Leeuw WJ, van de Vijver M, Cornelisse C and van Roy F: E-cadherin
is a tumour/invasion suppressor gene mutated in human lobular
breast cancers. EMBO J. 14:6107–6115. 1995.PubMed/NCBI
|
|
30
|
Takeichi M: Cadherins in cancer:
Implications for invasion and metastasis. Curr Opin Cell Biol.
5:806–811. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Christofori G and Semb H: The role of the
cell-adhesion molecule E-cadherin as a tumour-suppressor gene.
Trends Biochem Sci. 24:73–76. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bolós V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with Snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Comijn J, Berx G, Vermassen P, Verschueren
K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D and van Roy
F: The two-handed E box binding zinc finger protein SIP1
downregulates E-cadherin and induces invasion. Mol Cell.
7:1267–1278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perez-Moreno MA, Locascio A, Rodrigo I,
Dhondt G, Portillo F, Nieto MA and Cano A: A new role for E12/E47
in the repression of E-cadherin expression and
epithelial-mesenchymal transitions. J Biol Chem. 276:27424–27431.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berx G, Raspé E, Christofori G, Thiery JP
and Sleeman JP: Pre-EMTing metastasis? Recapitulation of
morphogenetic processes in cancer. Clin Exp Metastasis. 24:587–597.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Burk U, Schubert J, Wellner U, Schmalhofer
O, Vincan E, Spaderna S and Brabletz T: A reciprocal repression
between ZEB1 and members of the miR-200 family promotes EMT and
invasion in cancer cells. EMBO Rep. 9:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Korpal M and Kang Y: The emerging role of
miR-200 family of microRNAs in epithelial-mesenchymal transition
and cancer metastasis. RNA Biol. 5:115–119. 2008. View Article : Google Scholar
|
|
42
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Peter ME: Let-7 and miR-200 microRNAs:
Guardians against pluripotency and cancer progression. Cell Cycle.
8:843–852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Spaderna S, Brabletz T and Opitz OG: The
miR-200 family: central player for gain and loss of the epithelial
phenotype. Gastroenterology. 136:1835–1837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee Y, Ahn C, Han J, et al: The nuclear
RNase III Drosha initiates microRNA processing. Nature.
425:415–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yi R, Qin Y, Macara IG and Cullen BR:
Exportin-5 mediates the nuclear export of pre-microRNAs and short
hairpin RNAs. Genes Dev. 17:3011–3016. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hutvágner G, McLachlan J, Pasquinelli AE,
Bálint E, Tuschl T and Zamore PD: A cellular function for the
RNA-interference enzyme Dicer in the maturation of the let-7 small
temporal RNA. Science. 293:834–838. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wienholds E, Kloosterman WP, Miska E,
Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz HR, Kauppinen
S and Plasterk RH: MicroRNA expression in zebrafish embryonic
development. Science. 309:310–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yi R, O’Carroll D, Pasolli HA, Zhang Z,
Dietrich FS, Tarakhovsky A and Fuchs E: Morphogenesis in skin is
governed by discrete sets of differentially expressed microRNAs.
Nat Genet. 38:356–362. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Johnson SM, Grosshans H, Shingara J, Byrom
M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack
FJ: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang Q, Gumireddy K, Schrier M, et al:
The microRNAs miR-373 and miR-520c promote tumour invasion and
metastasis. Nat Cell Biol. 10:202–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tavazoie SF, Alarcón C, Oskarsson T, Padua
D, Wang Q, Bos PD, Gerald WL and Massagué J: Endogenous human
microRNAs that suppress breast cancer metastasis. Nature.
451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar
|
|
57
|
Akao Y, Nakagawa Y and Naoe T: let-7
microRNA functions as a potential growth suppressor in human colon
cancer cells. Biol Pharm Bull. 29:903–906. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Calin GA, Dumitru CD, Shimizu M, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar
|
|
59
|
Johnson CD, Esquela-Kerscher A, Stefani G,
et al: The let-7 microRNA represses cell proliferation pathways in
human cells. Cancer Res. 67:7713–7722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Takamizawa J, Konishi H, Yanagisawa K, et
al: Reduced expression of the let-7 microRNAs in human lung cancers
in association with shortened postoperative survival. Cancer Res.
64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J and Tanaka T:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
He L, Thomson JM, Hemann MT, et al: A
microRNA polycistron as a potential human oncogene. Nature.
435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Voorhoeve PM, le Sage C, Schrier M, et al:
A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in
testicular germ cell tumors. Cell. 124:1169–1181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bracken CP, Gregory PA, Kolesnikoff N,
Bert AG, Wang J, Shannon MF and Goodall GJ: A double-negative
feedback loop between ZEB1-SIP1 and the microRNA-200 family
regulates epithelial-mesenchymal transition. Cancer Res.
68:7846–7854. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Spaderna S, Schmalhofer O, Hlubek F, Jung
A, Kirchner T and Brabletz T: Epithelial-mesenchymal and
mesenchymal-epithelial transitions during cancer progression. Verh
Dtsch Ges Pathol. 91:21–28. 2007.
|
|
67
|
Cano A and Nieto MA: Non-coding RNAs take
centre stage in epithelial-to-mesenchymal transition. Trends Cell
Biol. 18:357–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gregory PA, Bracken CP, Bert AG and
Goodall GJ: MicroRNAs as regulators of epithelial-mesenchymal
transition. Cell Cycle. 7:3112–3118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Savagner P: Leaving the neighborhood:
Molecular mechanisms involved during epithelial-mesenchymal
transition. BioEssays. 23:912–923. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Brabletz T, Jung A, Reu S, Porzner M,
Hlubek F, Kunz-Schughart LA, Knuechel R and Kirchner T: Variable
beta-catenin expression in colorectal cancers indicates tumor
progression driven by the tumor environment. Proc Natl Acad Sci
USA. 98:10356–10361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fuchs IB, Lichtenegger W, Buehler H,
Henrich W, Stein H, Kleine-Tebbe A and Schaller G: The prognostic
significance of epithelial-mesenchymal transition in breast cancer.
Anticancer Res. 22:3415–3419. 2002.
|
|
73
|
Huang HN, Chen SY, Hwang SM, et al:
miR-200c and GATA binding protein 4 regulate human embryonic stem
cell renewal and differentiation. Stem Cell Res (Amst). 12:338–353.
2014. View Article : Google Scholar
|
|
74
|
Moes M, Le Béchec A, Crespo I, Laurini C,
Halavatyi A, Vetter G, Del Sol A and Friederich E: A novel network
integrating a miRNA-203/SNAI1 feedback loop which regulates
epithelial to mesenchymal transition. PLoS One. 7:e354402012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Brabletz S and Brabletz T: The ZEB/miR-200
feedback loop - a motor of cellular plasticity in development and
cancer? EMBO Rep. 11:670–677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hill L, Browne G and Tulchinsky E:
ZEB/miR-200 feedback loop: At the crossroads of signal transduction
in cancer. Int J Cancer. 132:745–754. 2013. View Article : Google Scholar
|