Introduction
Degeneration of the intervertebral disc (IVD) is a
primary cause of lower back pain (LBP) and is a prerequisite for
the occurrence of IVD hernia (1),
which has a high social and economic cost. Regardless, the
pathological mechanism of IVD degeneration remains to be fully
defined. It is commonly accepted that IVD degeneration is
influenced by numerous factors, including age, genetics and
mechanical stimuli, of which the latter is the most important
(2–6). Although mechanical stress is
established to be an important modulator of degeneration, the
underlying molecular mechanism of nucleus pulposus (NP) cells in
the degeneration of the IVDs remains to be fully elucidated.
The IVD is composed of two conspicuous and
interdependent anatomical structures: The surrounding annulus
fibrosus (AF) and the central gelatinous NP. IVD cells,
particularly NP cells, are crucial to maintain the integrity of the
IVD, which occurs through producing type II collagen, aggrecan and
other components involved in extracellular matrix (ECM) metabolism.
A reduced NP cell population and loss of ECM are central features
in the aging and degeneration of IVDs. Previous evidence has
suggested that the NP may be associated with aging and the
initiation of IVD degeneration (7–9). One
study, focusing on the cellular mechanobiology of IVD, suggested
that NP cells possess distinct characteristics necessary for IVD
homeostasis (10). Although the
precise molecular mechanism of IVD degeneration remains unclear,
previous studies have suggested that the apoptosis or programmed
cell death in NP cells may be one of the key factors (11,12).
Apoptosis is an active mode of cell death that is
observed in healthy cells and tumor cells, in physiological and
pathological situations, and is distinct from passive cell death
(necrosis) (13). The signaling
events leading to apoptosis can be divided into two distinct
pathways, either involving the mitochondria or death receptors. In
the mitochondrial pathway, death signals lead to changes in
mitochondrial membrane permeability, and the subsequent release of
pro-apoptotic factors, such as cytochrome c from the
mitochondria. Once in the cytoplasm, cytochrome c catalyzes
the oligomerization of apoptotic protease activating factor-1
(Apaf-1) (14). This promotes the
activation of procaspase-9, which then initiates a caspase cascade
involving the downstream executioner, procaspase-3, which in turn
activates a DNase, termed caspase-activated DNase (15,16).
In the death receptor pathway, apoptosis is triggered by cell
surface death receptors, including Fas and the tumor necrosis
factor (TNF) receptor, which contain death domains. These death
domains recruit adaptors and induce the activation of initiator
caspase-8, followed by cleavage of downstream effector caspase and
various substrates. Park et al (17) established that NP cells participate
in the intrinsic pathway and subsequently undergo apoptotic cell
death through mitochondrial involvement. The cellular commitment to
apoptosis is regulated by the B-cell lymphoma (Bcl)-2 family of
proteins, which consists of apoptosis agonists (Bax, Bak and Bad)
and antagonists (Bcl-2 and Bcl-xl). The balance between
pro-apoptotic proteins, such as Bax, and anti-apoptotic proteins,
such as Bcl-2, is considered to be a crucial factor in the
regulation of apoptosis. Bax and Bcl-2 are mitochondrial proteins,
and have been demonstrated to be associated with the regulation of
mitochondrial membrane permeability. Bax exerts its pro-apoptotic
activity by translocating from the cytoplasm to the mitochondria,
and inducing cytochrome c release from isolated
mitochondria. However, Bcl-2 exerts its anti-apoptotic activity, at
least in part, by inhibiting the translocation of Bax to the
mitochondria.
Carboxymethylated chitosan (CMCS) is a soluble
derivative of chitosan and it possesses numerous desirable
physiochemical and biological features. It has been indicated
previously that CMCS can significantly suppress the degeneration of
cartilage in osteoarthritis and protect chondrocytes from
interleukin-1β-induced catabolism and apoptosis (18,19).
It has been previously observed that CMCS can stimulate
proliferation and the secretion of NGF in cultured Schwann cells
(SCs) by activation of the mitogen-activated protein
kinase/extracellular signal-regulated kinase, phosphatidylinositide
3-kinase/Akt and Wnt/β-catenin signaling cascades (20,21).
The protection of NP cells from apoptosis possesses great potential
for the treatment of IVD degeneration, and the present study aims
to determine whether CMCS serves a similar function in NP cells as
in chondrocytes and SCs.
The aim of the current study was to investigate
whether CMCS is effective in preventing hydrogen peroxide
(H2O2)-induced apoptotic cell death, and to
discuss the potential advantages of this approach in providing a
therapeutic approach to the regulation of IVD degeneration.
Materials and methods
Animals and reagents
24 healthy male Sprague-Dawley (SD) rats with an
average body weight (BW) of 362±35 g were selected as NP cell
donors (obtained from the Center of Experimental Animals of Wuhan
University, Wuhan, China). Dulbecco’s modified Eagle’s medium/Ham’s
F-12 (DMEM/F-12) was obtained from Gibco Life Technologies
(Carlsbad, CA, USA) and fetal bovine serum (FBS) was obtained from
HyClone (Logan, UT, USA). Carboxymethylated chitosan (CMCS, purity
>99%) was supplied by the Institute of Chemistry and
Environmental Science of Wuhan University. A cell counting kit-8
(CCK-8) was purchased from Dojindo Molecular Technologies, Inc.
(Kumamoto, Japan). Primers were provided by Invitrogen Life
Technologies (Carlsbad, CA, USA). Rabbit polyclonal anti-Bcl-2
(#2876) and rabbit monoclonal anti-β-actin (13E5; #4970) antibodies
were obtained from Cell Signaling Technology, Inc. (Beverly, MA,
USA). The anti-inducible nitric oxide synthase rabbit polyclonal
(iNOS; sc-651) antibody was from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Rhodamine 123 (Rho123) and Hoechst 33342 were
obtained from Sigma-Aldrich (St. Louis, MO, USA).
Phosphate-buffered saline (PBS, ×10, ST476) and SDS-PAGE Gel Kit
(P0012A), were obtained from the Beyotime Institute of
Biotechnology (Haimen, China) and were of the highest purity
commercially available.
Cell isolation and culture
5 SD rats (aged 10–12 weeks, weighing 362±35 g) were
enrolled in the present study. Rat NP cells were isolated using a
previously described explant culture method (22). Briefly, rats were euthanized with
an overdose of intravenous pentobarbital (100 mg/kg body weight;
Shanghai Biorui Biological Technological Co., Ltd., Shanghai,
China), and the lumbar IVDs were resected from the spinal column.
The gel-like NP tissue was separated from the AF using a dissection
microscope (Five-Lake Medical Devices Co., Ltd., Wuhan, China)
under aseptic conditions. The gelatinous NP tissues obtained from
each animal were cut into small pieces (<1 mm3)
immediately, then digested with 0.1% type-2 collagenase
(Sigma-Aldrich) in DMEM/F-12 at 37°C in a KYC-100C gyratory shaker
from Shanghai Fuma Laboratory Instrument Company (Shanghai, China)
at 110 rpm. After 4 h, the suspension was filtered through a 70-μm
mesh. The filtered cells were washed with DMEM/F-12 and then seeded
into 25 cm2 culture flasks. The cells were incubated in
DMEM/F-12 with 10% FBS and a penicillin-streptomycin solution
(SV30010; HyClone; 100 U/ml streptomycin and 100 U/ml penicillin)
in a 5% CO2 incubator. The medium was refreshed every 3
days. The NP cells were chondrocyte-like cells, identified by type
II collagen and aggrecan immunohistostaining.
Establishment of apoptotic models of NP
cells
To establish the apoptotic model of cultured NP
cells, H2O2 (Wuhan Boster Biological
Technology Company, Wuhan, China). was used as described previously
(23). Briefly, NP cells (cell
density of 1×106/ml) were cultured overnight at 37°C in
the culture medium as described above. Different concentrations of
H2O2 (100, 200 and 300 μM) were used to
induce the damage to NP cells. NP cells were examined at 6, 12 and
24 h subsequent to the addition of H2O2. To
determine the effects of CMCS on H2O2-induced
apoptosis in NP cells, cell cultures were treated with
H2O2 for 6 h and then the culture medium was
replaced immediately by fresh medium with CMCS. The concentrations
of CMCS were 50, 100 and 200 μg/ml.
Cell viability assay
Cell viability was assessed by CCK-8 assay. Cells
were suspended at a final concentration of 2×104
cells/well and cultured in 96-well flatbottomed microplates with
the DMEM/F-12 containing 0.1% FBS. The medium was replaced 24 h
later with DMEM/F-12 containing H2O2, CMCS or
phosphate-buffered saline (PBS; control group). For assessing the
cytotoxic effect of H2O2 with CMCS, cells
were incubated with 100, 200 and 300 μM H2O2
without CMCS, and 300 μM H2O2 with different
concentrations of CMCS (50, 100 and 200 μg/ml) for the indicated
time intervals. For quantitative analysis of the cell
proliferation, 10 μl CCK-8 solution was added to each well of a
96-well flat bottomed microplate containing 100 μl DMEM/F-12, and
the plate was incubated at 37°C for 1 h in a 5% CO2
atmosphere. The optical density, which is proportional to cell
metabolic activity, was measured at 450 nm using an ELx800
Absorbance Microplate Reader (BioTek Instruments, Inc., Winooski,
VT, USA). Cell viability was expressed as a percentage of the
number of control (untreated) cells. Viability in the control group
was designated as 100%. The cell viability of each group was
calculated as follows: Cell viability (% of control) =
[(Ae−Ab)/(Ac−Ab)] × 100. Ae, Ab and Ac represent the A450 of the
experimental, blank and control groups, respectively. All
experiments were performed in triplicate in three independent
experiments.
Annexin V-fluorescein
isothiocyanate(FITC)/propidium iodide (PI) staining
The level of apoptotic death in the NP cells was
determined using flow cytometric analysis. Cellular apoptosis was
observed by annexin V-FITC/PI double staining, performed using an
Annexin V/FITC Apoptosis Detection kit I (no. 556547; BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer’s instructions. Briefly, cells were cultured at a
density of 6×105 cells/ml and seeded in 6-well plates.
The cells were cultured in DMEM/F-12 containing various
concentrations of H2O2 (100, 200 and 300 μM)
for 6, 12 or 24 h, or CMCS at various concentrations (50, 100 and
200 μg/ml) for 3 h followed by the addition of
H2O2 for 24 h for the indicated time. Cells
were harvested by trypsinization (Gibco-BRL, Rockville, MD, USA),
then washed twice with cold PBS and centrifuged at 400 × g.
Approximately 1×105–1×106 cells were then
suspended in 500 μl binding buffer from the apoptosis detection
kit, centrifuged again at 400 × g for 5 min and then the
supernatant was removed. Cells were resuspended in 500 μl binding
buffer and transferred to a sterile flow cytometry glass tube.
Annexin V-FITC (5 μl) and PI (5 μl) were added prior to incubation
in the dark at room temperature. Cells were analyzed with a flow
cytometer (BD Biosciences) at 488 nm. The distribution of cells was
analyzed using Cell Quest Pro software (version 4.01; BD
Biosciences) in the BD FACSVerse™ flow cytometer (BD Biosciences)
within 1 h of staining. Data from 10,000 cells were collected for
each data file. Apoptotic cells were identified as the annexin
V-FITC-positive and PI-negative cells. Finally, the number of cells
in each category was expressed as a percentage of the total number
of stained cells.
Nuclear staining with Hoechst 33342
Apoptotic nuclear morphology was assessed with
Hoechst 33342 (Sigma-Aldrich) staining. To determine whether CMCS
protects from recognized morphological features of apoptosis, such
as H2O2-induced chromatin condensation and
fragmentation, cells were cultured in 6-well plates
(3.0×105 cells/well) with DMEM/F-12 containing 10% FBS,
then treated for 24 h with 300 μM H2O2 and
CMCS at 37°C in a humidified atmosphere of 5% CO2. Cell
apoptosis was evaluated by Hoechst 33342 staining as described
previously (24). Briefly,
following 24 h culture in the DMEM/F-12 medium, the cells were
stained with 10 μg/ml Hoechst 33342 at 37°C for 20 min. The cells
were washed and suspended again in PBS for morphological
observation under a IX51 fluorescence microscope (Olympus
Corporation, Tokyo, Japan) with excitation at 355 nm and emission
at 465 nm. A minimum of 400 cells from six randomly selected fields
per dish were counted, and each treatment was performed in
triplicate.
Measurement of mitochondrial membrane
potential (ΔΨm)
Changes in ΔΨm were estimated by the uptake of
Rho123, a cell-permeant, lipophilic, cationic, fluorescent dye that
permeates easily and interacts with negative charges on the inner
mitochondrial membrane at a low concentration. It accumulates in
normal mitochondria, but a decline in ΔΨm leads to leakage of
Rho123 from the mitochondria, thus the fluorescence intensity is
reduced. Therefore, the effects of H2O2, CMCS
and a combination of the two on ΔΨm were assessed as one of the
markers of mitochondrial function. Briefly,
H2O2 or H2O2/CMCS
treatments were performed for 24 h. At the end of incubation,
treated NP cells were incubated with Rho123 (10 μg/ml) at 37°C for
20 min. Subsequently, they were washed twice with PBS and then
observed with the excitation filter set at 488 nm and the emission
filter at 510 nm under the fluorescence microscope.
Reverse transcription (RT)-quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s protocol. The RNA samples were quantified by
spectrophotometry at 260 and 280 nm (A260/A280 ~2.0; A260 = 40 μg
RNA/ml) by the NanoDrop (ND-8000) Spectrophotometer (Thermo Fisher
Scientific, Braunschweig, Germany). RNA was then
reverse-transcribed to cDNA using a Reverse
Transcription-Polymerase Chain Reaction kit (Takara Biotechnology
Co., Ltd., Dalian, China) according to the manufacturer’s
instructions. The cDNA was analyzed immediately or stored at −20°C.
qPCR amplification was performed with an ABI Prism 7900HT Real-Time
PCR system (Applied Biosystems Life Technologies, Foster City, CA,
USA), and the SYBR Green I fluorescent dye method was used to
quantify cDNA. PCR cycling conditions consisted of an initial
denaturing step for 10 sec at 95°C; then 40 cycles of 5 sec each at
95°C; followed by 30 sec at 60°C. A stable and reliable standard
curve was established by plotting the threshold cycle (Ct) values.
Following amplification, a melting curve analysis was performed in
order to verify the authenticity of the amplified product by its
specific melting temperature (Tm). GAPDH was used as the internal
control. The relative levels of mRNA of the target genes were then
calculated, through which the gene expression level and the trend
of change were determined. The specificity of each reaction was
controlled by melting curve analysis. A negative PCR control
containing water in place of cDNA was prepared. The relative levels
of mRNA were analyzed by the 2−ΔΔCt method. qPCR was
conducted in triplicate in three independent experiments. The
sequences of the primers are presented in Table I.
 | Table IPrimers used for RT-qPCR analysis of
gene expression. |
Table I
Primers used for RT-qPCR analysis of
gene expression.
| Gene | Primer | Sequence | Produce size
(bp) |
|---|
| iNOS | Forward |
5′-GCAGACACATACTTTATGC-3′ | 445 |
| Reverse |
5′-CAATGGCTGGTACATGGGCAC-3′ | |
| Bcl-2 | Forward |
5′-GCGTCAACAGGGAGATGTCA-3 | 225 |
| Reverse |
5′-GGTATGCACCCAGAGTGATG-3′ | |
| Caspase-3 | Forward |
5′-GGCCTGCTTTTTACCTCAGA-3′ | 140 |
| Reverse |
5′-CGTTTCCGCACAGGCTGCTT-3 | |
| Collagen-2 | Forward |
5′-CCCAGAACATCACCTACCAC-3 | 201 |
| Reverse |
5′-GGTACTCGATGATGGTCTTG-3 | |
| Aggrecan | Forward |
5′-GATGTCCCCTGCAATTACCA-3 | 230 |
| Reverse |
5′-TCTGTGCAAGTGATTCGAGG-3 | |
| GAPDH | Forward |
5′-TGTCTCCTGCGACTTCAACAG-3′ | 256 |
| Reverse |
5′-GAGGCCATGTAGGCCATGAG-3′ | |
Western blot analysis
NP cells were treated with
H2O2 in the presence or absence of CMCS.
Samples of the cell cultures were treated with lysis buffer (P0013,
Beyotime Institute of Biotechnology) containing 150 mM NaCl, 10 mM
Tris-HCl, 1 mM EDTA, 1% Triton X-100, 10% glycerol, 1 mM
phenylmethylsulphonyl fluoride, 10 μg/ml leupeptin and 10 μg/ml
aprotinin. Lysates were subsequently centrifuged at 13,000 × g for
15 min and the supernatant was collected for protein analysis.
Sample protein concentration was determined using a commercial
bicinchoninic acid protein assay kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Equal amounts of protein from cell lysates were
resuspended in sample buffer (P0015; Beyotime Institute of
Biotechnology) containing 62 mM Tris-HCl (pH 6.8), 2% sodium
dodecylsulphate (SDS), 10% glycerol, 5% β-mercaptoethanol and 0.04%
bromphenol blue, then resolved by SDS-PAGE and transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billierica, MA,
USA). Following brief washing in Tris-buffered saline with Tween-20
(TBST) [25 mM Tris-HCl (pH 7.5), 50 mM NaCl, 0.1% Tween-20; Beijing
Biosntech Co., Beijing, China], the membrane was blocked with 5%
(w/v) non-fat dried milk in TBST overnight at 4°C. The membrane was
incubated for 3 h with the appropriate primary antibodies.
Following washing with TBST, the membranes were incubated with the
respective goat anti-rabbit peroxidase-conjugated secondary (#7074;
Cell Signaling Technology, Inc.) antibodies for 1 h then washed
again with TBST. Immunodetection was accomplished by enhanced
chemiluminescence using an enhanced chemiluminescence detection kit
for HRP (Pierce Biotechnology, Inc.) followed by autoradiography on
Kodak-X-OMAT-AR film (Kodak, Rochester, NY, USA) and performed
using a Geliance 200 Gel Imaging system (PerkinElmer, Inc., Rocky
Hill, NJ, USA) and GeneSnap software, version 6.08.04 (Syngene,
Frederick, MD, USA). Bands were analyzed using the GeneTools
software, version 3.07.04 (Syngene). All data are expressed as the
relative differences between control and treated cells, subsequent
to normalization to the β-actin expression level.
Statistical analysis
Data are presented as the mean ± standard error.
Differences between groups were compared using one-way analysis of
variance on SPSS, version 16.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
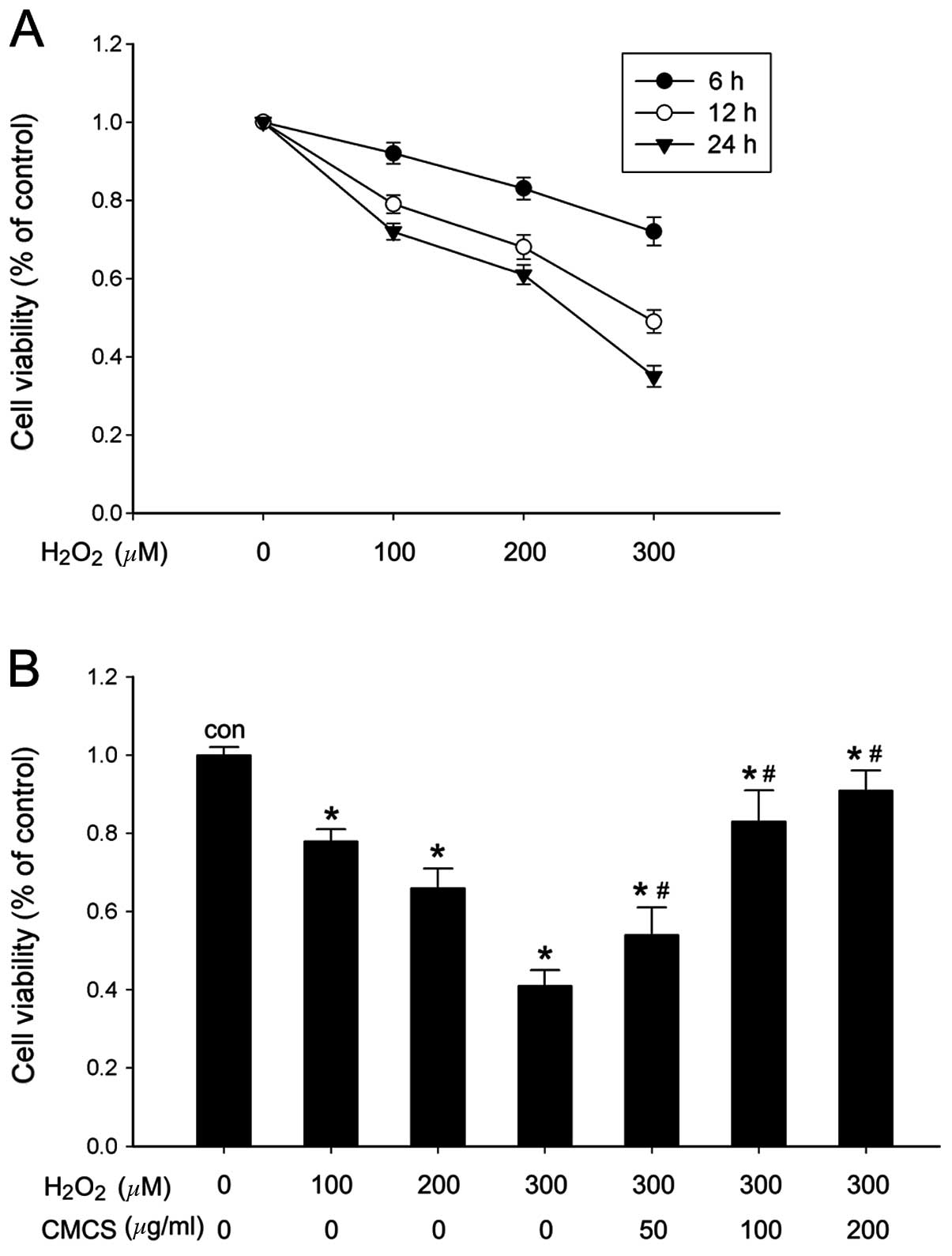
Effect of CMCS on cell viability in
H2O2-treated NP cells
The NP cell viability and metabolic activity were
analyzed by CCK-8 assay. The results indicated that different
concentrations of H2O2 stimulation (100, 200
and 300 μM) were able to reduce the number of metabolically active
cells and viability in a time- and dose-dependent manner (Fig. 1A). A significant reduction in cell
viability was observed at 24 h following 300 μM
H2O2 exposure (Fig. 1B). However, when NP cells were
pretreated with CMCS (50, 100 or 200 μg/ml) for 3 h and then
exposed to H2O2 for 24 h, cell viability was
improved in a dose-dependent manner. The most significant increase
was observed in the 200 μg/ml CMCS-treated group compared with cell
viability following 300 μM H2O2 treatment
(Fig. 1B).
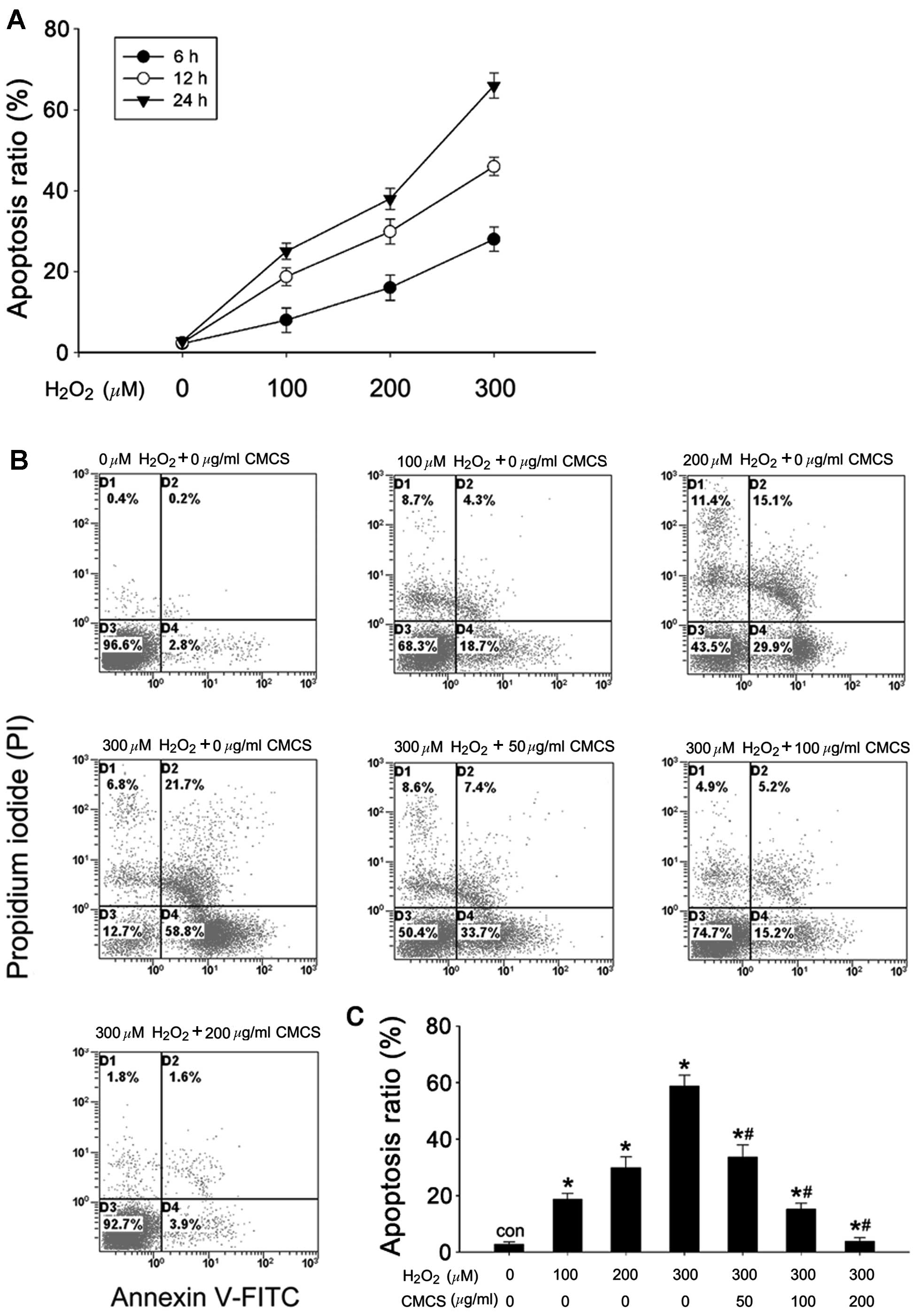
Effect of CMCS on apoptosis in
H2O2-treated NP cells
The rate of apoptosis was quantified using flow
cytometry with annexin V-FITC/PI staining. As presented in Fig. 2A, H2O2
exposure increased the apoptotic rates of the NP cells compared
with the control cells, in a time- and dose-dependent manner. The
apoptotic ratios were 18.7, 29.9 and 58.8% in 100, 200 and 300 μM
H2O2-treated NP cells respectively, while it
was 2.8% in control cells (Fig. 2B and
C). CMCS significantly inhibited
H2O2-induced apoptosis in a dose-dependent
manner; treatment with 50, 100 and 200 μg/ml CMCS in
H2O2-treated NP cells resulted in apoptotic
ratios of 33.7, 15.2 and 3.9%, respectively.
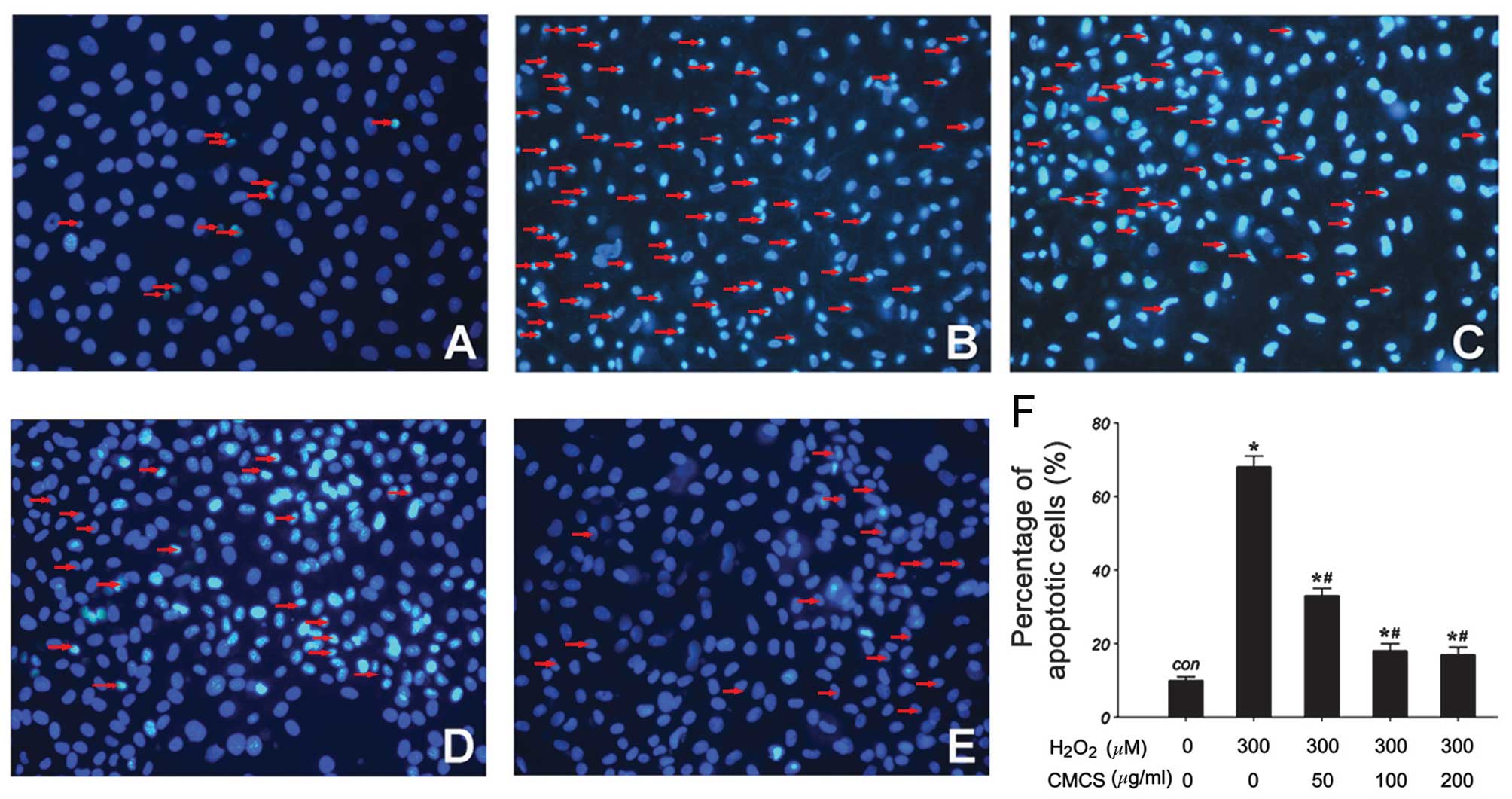
Effect of CMCS on nucleic morphology in
H2O2-treated NP cells
Subsequent to culture with
H2O2 or the H2O2/CMCS
combination, morphological changes in the NP cells were observed by
Hoechst 33342 staining. As presented in Fig. 3, in the control group, NP cell
nuclei were round and stained homogeneously with Hoechst 33342
(Fig. 3A). In
H2O2-treated NP cells, a considerable
proportion of cells displayed characteristics of apoptosis with
condensed and fragmented nuclei (Fig.
3B). Treatment with 50, 100 and 200 μg/ml CMCS led to a
significant reduction in the number of apoptotic cells with
fragmented nuclei (Fig. 3C–F).
These results suggest that CMCS is able to inhibit the
H2O2-induced nucleic morphological changes in
NP cells.
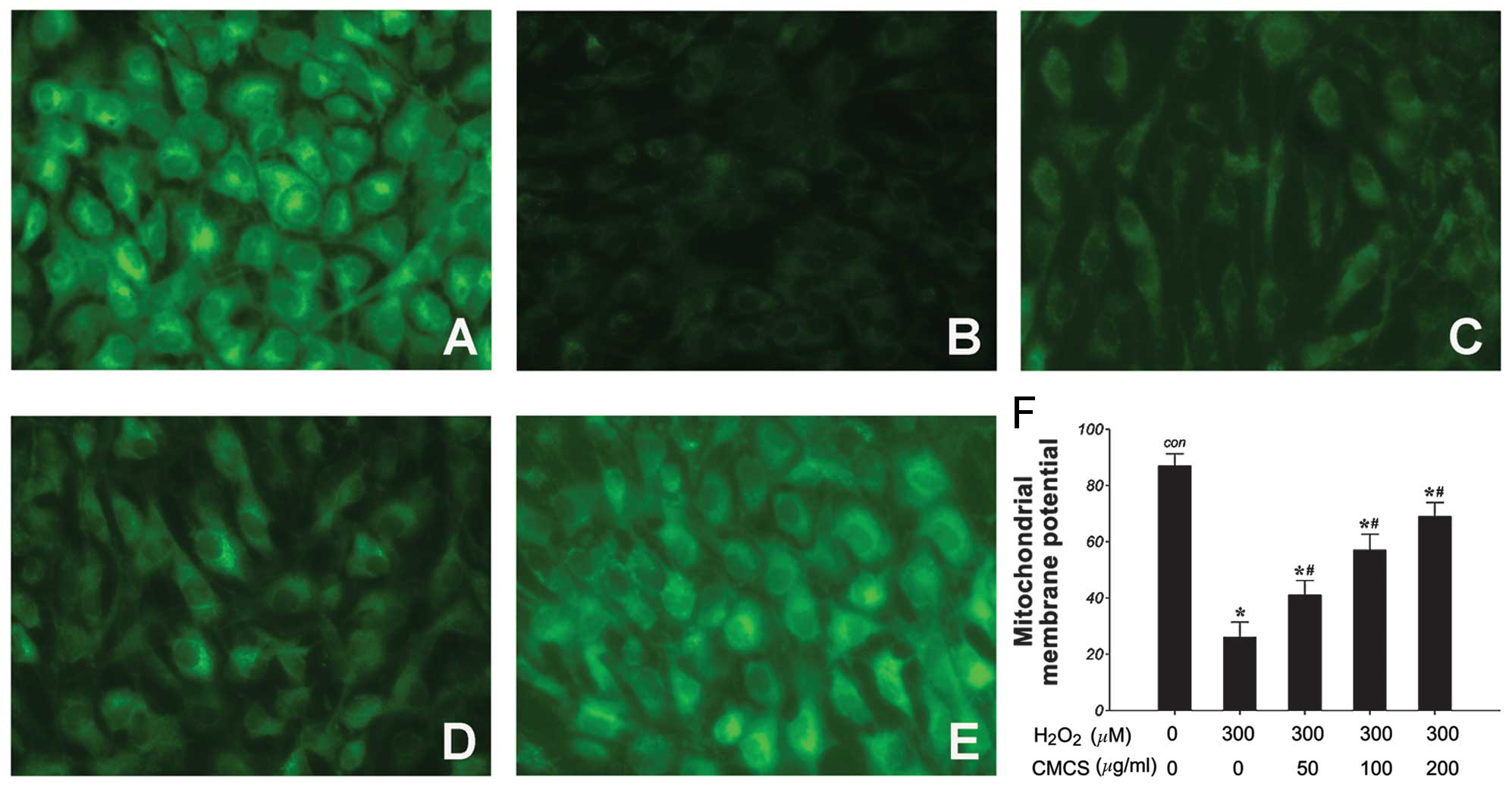
Effects of CMCS on ΔΨm in
H2O2-treated NP cells
It has been reported that CMCS may prevent
mitochondrial oxidative stress. Thus, the effects of
H2O2 and the H2O2/CMCS
combination on ΔΨm were examined as a marker of mitochondrial
function. ΔΨm was assessed using the Rho123 fluorescent dye, the
intensity of which reflects mitochondrial function. As demonstrated
in Fig. 4, 300 μM
H2O2 induced a significant reduction in ΔΨm
following treatment for 24 h compared with the control group
(Fig. 4A and B). Treatment with
CMCS was demonstrated to prevent this reduction in a dose-dependent
manner (Fig. 4C–F). These results
suggest that CMCS may be able to protect mitochondrial function in
NP cells.
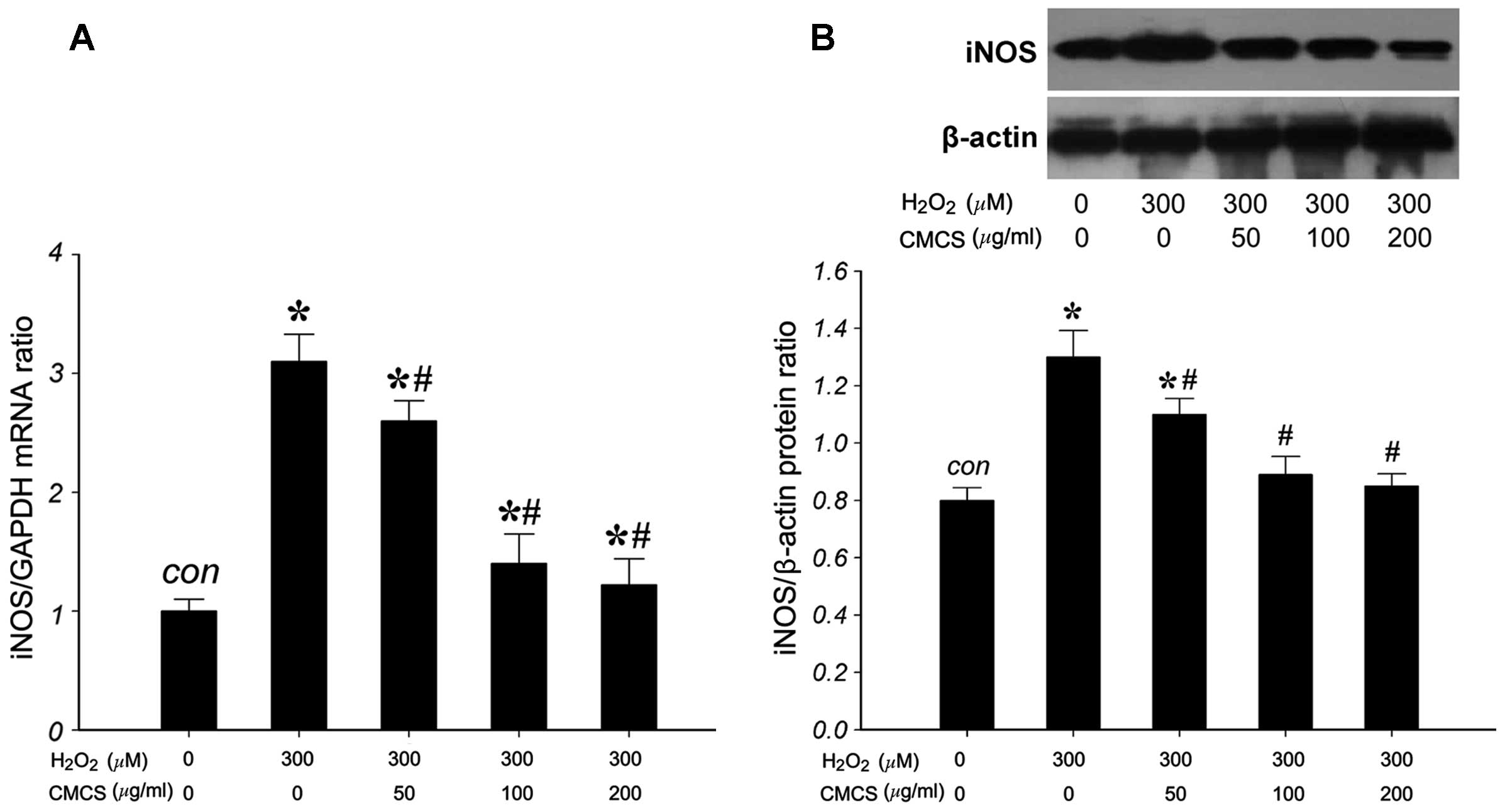
Effect of CMCS on the expression level of
iNOS in H2O2-treated NP cells
To investigate the effects of CMCS on the expression
of iNOS in H2O2-treated NP cells, the mRNA
and protein levels of iNOS were measured. RT-qPCR results indicated
that 300 μM H2O2 significantly increased the
iNOS/GAPDH mRNA ratio compared with that of the control group
(Fig. 5A). However, treatment with
50, 100 and 200 μg/ml CMCS was able to inhibit this increase in a
dose-dependent manner. The expression of iNOS protein (130 kDa) was
detected by western blot analysis (Fig. 5B). H2O2
exposure significantly increased the iNOS protein level compared
with that of the control group, and treatment with 50, 100 and 200
μg/ml CMCS was able to inhibit this increase in a dose-dependent
manner. These results suggest that CMCS is able to inhibit the
H2O2-induced increase in NP cell iNOS mRNA
and protein levels.
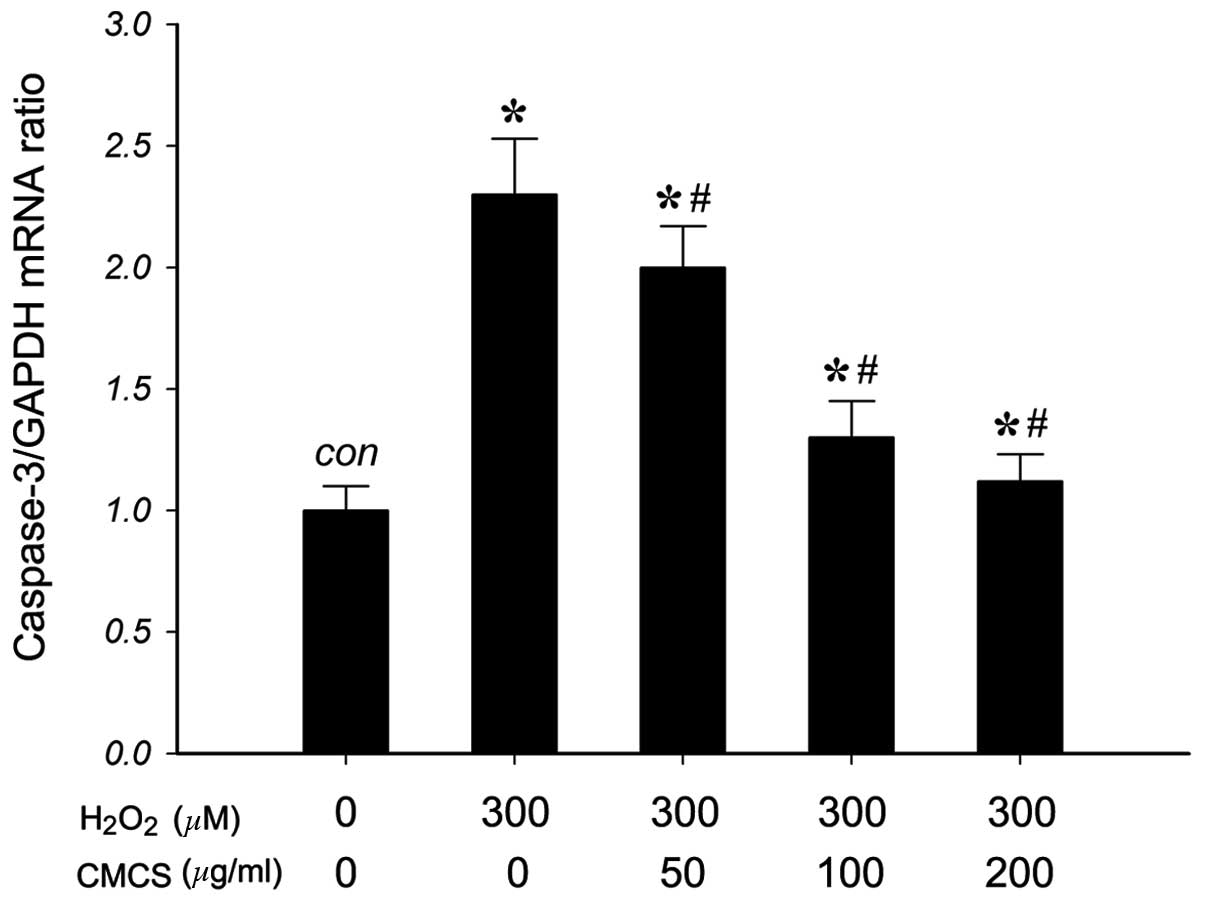
Effect of CMCS on caspase-3 mRNA
expression in H2O2-treated NP cells
To investigate the effects of CMCS on the expression
of caspase-3 (a mediator of apoptosis) in
H2O2-treated NP cells, the mRNA levels of
caspase-3 were measured. As presented in Fig. 6, RT-qPCR results indicated that 300
μM H2O2 exposure significantly increased the
level of caspase-3 mRNA compared with that of the control group.
Treatment with 50, 100 and 200 μg/ml CMCS was able to inhibit this
increase in a dose-dependent manner. These results suggested that
CMCS can inhibit the H2O2-induced increase in
the level of caspase-3 in NP cells.
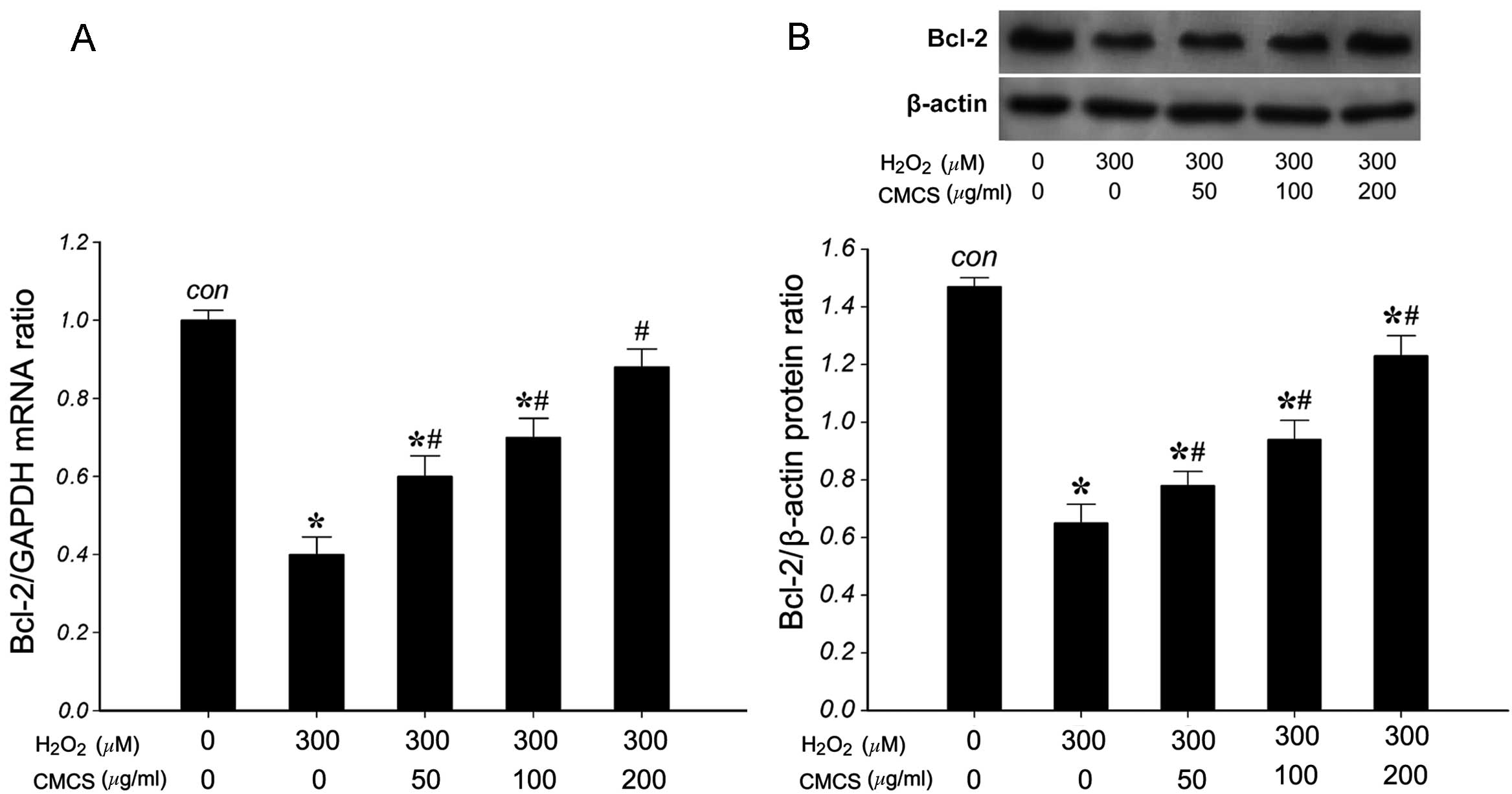
Effect of CMCS on the expression levels
of Bcl-2 in H2O2-treated NP cells
To investigate the effects of CMCS on the expression
of Bcl-2 in H2O2-treated NP cells, the mRNA
and protein levels of Bcl-2 were measured. RT-qPCR results
indicated that 300 μM H2O2 significantly
reduced the level of Bcl-2 mRNA compared with that of the control
group (Fig. 7A). However,
treatment with 50, 100 and 200 μg/ml CMCS was able to inhibit this
reduction in a dose-dependent manner. The expression of Bcl-2
protein (26 kDa) was detected by western blot analysis. As
presented in Fig. 7B, 300 μM
H2O2 significantly reduced the level of Bcl-2
protein compared with that in the control group. However, treatment
with 50, 100 and 200 μg/ml CMCS was able to inhibit the reduction
in Bcl-2 protein in H2O2-treated NP cells in
a dose-dependent manner. These results suggest that CMCS is able to
inhibit the H2O2-induced reduction of Bcl-2
mRNA and protein in NP cells.
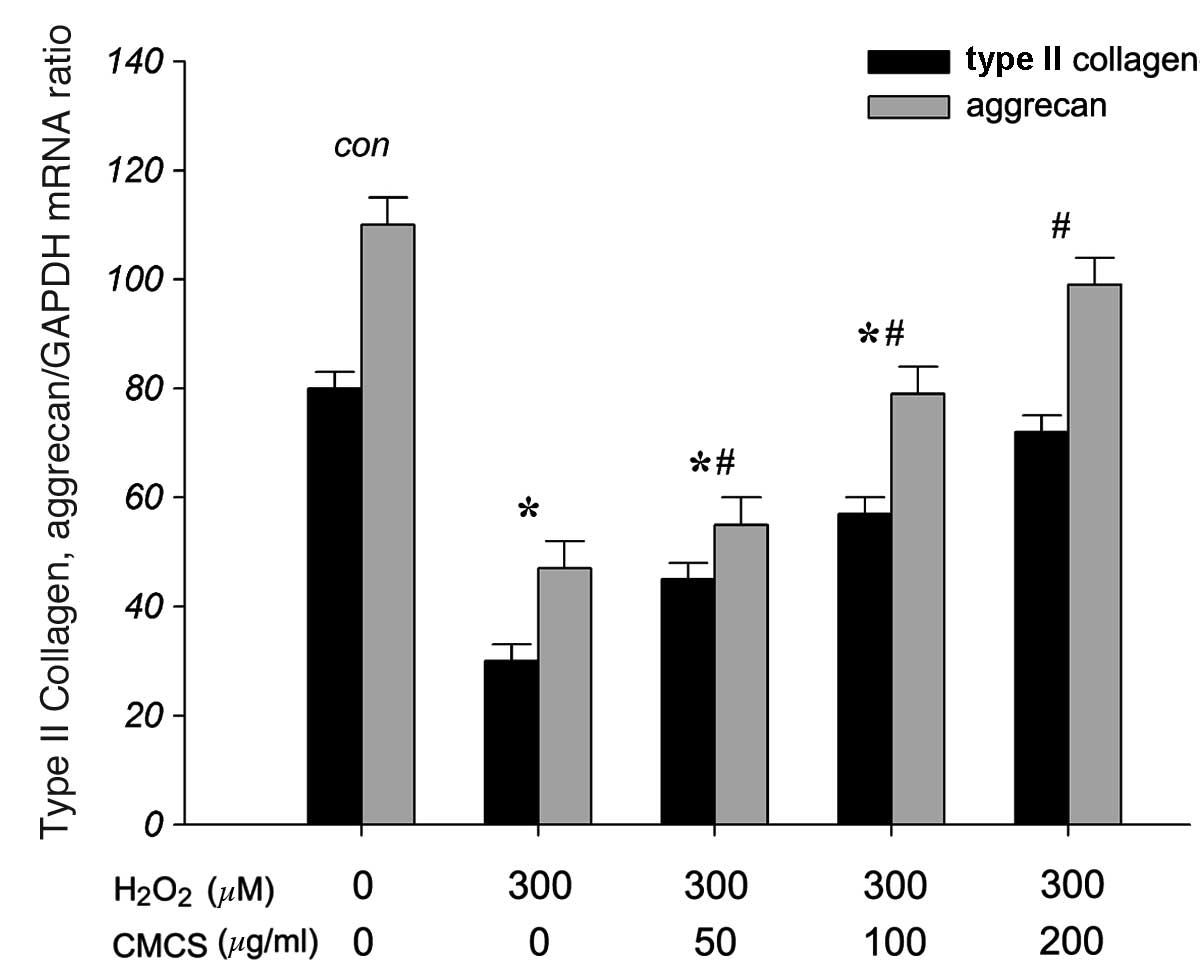
CMCS increases ECM production in
H2O2-treated NP cells
To investigate the effects of CMCS on the secretion
of ECM components, including collagen type II and aggrecan, in
H2O2-exposed NP cells, the mRNA levels of
type II collagen and aggrecan were measured. As presented in
Fig. 8, RT-qPCR results indicated
that 300 μM H2O2 significantly reduced the
levels of type II collagen and aggrecan mRNA compared with those of
the control group. However, treatment with 50, 100 and 200 μg/ml
CMCS was able to inhibit this reduction in a dose-dependent manner.
These results suggest that CMCS is able to protect the secretion of
type II collagen and aggrecan in the apoptotic environment.
Discussion
The present study demonstrated that CMCS can protect
or rescue NP cells in vitro from undergoing apoptosis
following H2O2 exposure, and that the
mechanisms of this protection may involve caspase-3 and Bcl-2
activation and mitochondrial function.
IVD degeneration is considered to be associated with
genetic factors, in addition to excessive mechanical loading, which
together alter the biomechanical properties of the IVD. Although
the precise mechanism of disc degeneration remains unclear, it has
been suggested in previous studies that apoptosis or programmed
cell death of IVD cells may be one of the key steps in disc
degeneration (25).
In the present study, it was observed that treatment
of the NP cells with CMCS prior to exposure to
H2O2 resulted in significantly increased cell
survival. This was accompanied by the finding that CMCS,
prophylactively added to the NP cell cultures, demonstrated a
protective effect on the NP cells regarding the
H2O2-induced reduction in viability. These
results were consistent with previous observations that CMCS and
chitosan were able to protect chondrocytes, endometriotic cells,
vein endothelial cells and astrocytes from apoptosis (19,26–28).
There are numerous factors involved in apoptotic
cascades. Caspases, the ‘key executioners’ of apoptosis, are a
family of cysteine proteases capable of cleaving essential cellular
substrates with aspartate residues (29). Caspases-8, -9 and -10 are involved
in the initiation and amplification of apoptosis, while caspases-3,
-6 and -7 are involved in executing the apoptotic program and cell
death (30). Caspase-3, the most
prominent effective caspase, is localized downstream in the caspase
cascade and represents the main effective molecule in apoptosis. It
irreversibly executes programmed cell death. Caspase-3 is located
in the cellular cytoplasm in its inactive form in the normal
microenvironment, but it is auto-proteolytically cleaved into an
active form of the enzyme under apoptotic conditions (31,32).
Apoptosis is triggered by several stimuli resulting in several
apoptotic pathways. Rannou et al (33) demonstrated that mechanical overload
induces disc degeneration via a caspase-9-dependent apoptotic
pathway, suggesting that disc cell apoptosis is the primary cause
of disc degeneration. Others have indicated that caspase-3 acts as
the main apoptosis effector, and it may be the therapeutic target
for regulation of IVD degeneration (34). In the present study,
H2O2-exposed NP cells exhibited an increased
level of intracellular caspase-3 mRNA and caspase-3 activity.
Treatment with CMCS significantly inhibited the caspase-3 activity
generated by H2O2. The results of the current
study suggest that CMCS inhibits caspase-3 activity and the
anti-apoptotic effect of CMCS is at least partly mediated via
caspase-3 enzymatic inhibition.
There are two major signaling pathways controlling
the initiation of apoptosis in mammals. The extrinsic pathway
involves engagement of cell-surface death receptors by ligands that
belong to the TNF receptor superfamily and the consequent
activation of caspase-8. The intrinsic pathway involves caspase-9
as the initiator, and originates from the mitochondria. Stressed
mitochondria release a set of molecules, including
cytochrome-c and Apaf-1, to form the apoptosome molecular
cluster that activates caspase-9 and its downstream effector,
caspase-3. Park et al (17)
examined human herniated lumbar disc tissues with the use of
immunohistochemical staining and western blot analysis to determine
the presence of several proteins associated with apoptosis. They
established that the proteins associated with the intrinsic pathway
were stained positive in all samples. The results of their study
suggest that disc cells participate in the intrinsic pathway, and
subsequently undergo apoptotic cell death through mitochondrial
involvement.
In the intrinsic pathway, Bcl-2 prevents or delays
apoptotic induction by a large variety of stimuli in various cell
types (35). Molecular
intervention at the level of Bcl-2 in the apoptotic pathway,
therefore, has the potential to enhance cell survival. Although the
apoptotic cascade remains to be fully elucidated, overexpression of
Bcl-2 has previously been demonstrated to prevent the release of
apoptotic induction factors and the subsequent activation of
caspase-3 (36). Sudo and Minami
(37) indicated that Bcl-2
overexpression in IVD cells effectively prevented in vitro
apoptotic cell death.
Mitochondria are complex organelles that oxidize a
wide range of metabolic intermediates, and their impairment has
been linked to various disorders (38). Changes in the permeability and
structure of the mitochondrial membrane may lead to apoptosis.
An impaired ΔΨm reflects the malfunction of
mitochondria subsequent to H2O2 exposure. It
also implies the decoupling of oxidative phosphorylation,
accumulation of reactive oxygen species, and a reduction in
cytoplasmic ATP levels. Mitochondria synthesize ATP to maintain the
vital metabolism conducted in eukaryotic cells. In the early stages
of cell apoptosis, the breakdown of ΔΨm regulation is one of the
earliest features preceding nuclear condensation and apoptotic body
formation. Data from the current study demonstrate that the ΔΨm was
lower in the H2O2-treated NP cells compared
with the control group, and this effect is partly abolished by CMCS
in a dose-dependent manner. This indicates that the inhibitory
effect of CMCS on NP cell apoptosis is associated with its
protection of mitochondrial function. CMCS may protect
mitochondrial function through inhibiting the reduction of ΔΨm, and
thus, promote the synthesis of ATP, inhibited by
H2O2 exposure.
In agreement with previous studies, the current
study identified a restorative effect of CMCS on
H2O2-induced ECM reduction in disc cells
in vitro. The anti-apoptotic (anticatabolic) effects of CMCS
and its potential to enhance ECM production (anabolic effect)
potentially make it an excellent molecular candidate to break the
cycle of degenerative cytokines that lead to further progression of
IVD degeneration.
In conclusion, the current study demonstrated that
CMCS can protect NP cells from H2O2-induced
cell apoptosis. The mechanism of CMCS in protecting NP cells from
apoptosis remains unknown, but appears to be partly mediated via
caspase-3 enzymatic inhibition/Bcl-2 activation, in addition to
diminishing nitric oxide production and protecting mitochondrial
function. These data suggest one possible mechanism of CMCS rescue
in IVD degeneration, and support the therapeutic rationale for CMCS
utilization in human disc degeneration.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81301056 and
30801166).
References
|
1
|
Chen WH, Liu HY, Lo WC, Wu SC, Chi CH,
Chang HY, et al: Intervertebral disc regeneration in an ex vivo
culture system using mesenchymal stem cells and platelet-rich
plasma. Biomaterials. 30:5523–5533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalichman L and Hunter DJ: The genetics of
intervertebral disc degeneration. Familial predisposition and
heritability estimation. Joint Bone Spine. 75:383–387. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schultz DS, Rodriguez AG, Hansma PK and
Lotz JC: Mechanical profiling of intervertebral discs. J Biomech.
42:1154–1157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao CQ, Wang LM, Jiang LS and Dai LY: The
cell biology of intervertebral disc aging and degeneration. Ageing
Res Rev. 6:247–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao CQ, Jiang LS and Dai LY: Programmed
cell death in intervertebral disc degeneration. Apoptosis.
11:2079–2088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Setton LA and Chen J: Cell mechanics and
mechanobiology in the intervertebral disc. Spine (Phila Pa 1976).
29:2710–2723. 2004. View Article : Google Scholar
|
|
7
|
Aguiar DJ, Johnson SL and Oegema TR:
Notochordal cells interact with nucleus pulposus cells: regulation
of proteoglycan synthesis. Exp Cell Res. 246:129–137. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwashina T, Mochida J, Miyazaki T,
Watanabe T, Iwabuchi S, Ando K, Hotta T and Sakai D: Low-intensity
pulsed ultrasound stimulates cell proliferation and proteoglycan
production in rabbit intervertebral disc cells cultured in
alginate. Biomaterials. 27:354–361. 2006. View Article : Google Scholar
|
|
9
|
Vonk LA, Kroeze RJ, Doulabi BZ,
Hoogendoorn RJ, Huang C, Helder MN, Everts V and Bank RA: Caprine
articular, meniscus and intervertebral disc cartilage: an integral
analysis of collagen network and chondrocytes. Matrix Biol.
29:209–218. 2010. View Article : Google Scholar
|
|
10
|
Hsieh AH and Twomey JD: Cellular
mechanobiology of the intervertebral disc: new directions and
approaches. J Biomech. 43:137–145. 2010. View Article : Google Scholar :
|
|
11
|
Gruber HE and Hanley EN Jr: Analysis of
aging and degeneration of the human intervertebral disc. Comparison
of surgical specimens with normal controls. Spine (Phila Pa 1976).
23:751–757. 1998. View Article : Google Scholar
|
|
12
|
Kim KW, Ha KY, Lee JS, Rhyu KW, An HS and
Woo YK: The apoptotic effects of oxidative stress and antiapoptotic
effects of caspase inhibitors on rat notochordal cells. Spine
(Phila Pa 1976). 32:2443–2448. 2007. View Article : Google Scholar
|
|
13
|
Nosseri C, Coppola S and Ghibelli L:
Possible involvement of poly(ADP-ribosyl) polymerase in triggering
stress-induced apoptosis. Exp Cell Res. 212:367–373. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang B, Xiao W, Shi Y, Liu M and Xiao X:
Heat shock pretreatment inhibited the release of Smac/DIABLO from
mitochondria and apoptosis induced by hydrogen peroxide in
cardiomyocytes and C2C12 myogenic cells. Cell Stress Chaperones.
10:252–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagata S: Apoptotic DNA fragmentation. Exp
Cell Res. 256:12–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh M, Sharma H and Singh N: Hydrogen
peroxide induces apoptosis in HeLa cells through mitochondrial
pathway. Mitochondrion. 7:367–373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JB, Lee JK, Park SJ, Kim KW and Riew
KD: Mitochondrial involvement in fas-mediated apoptosis of human
lumbar disc cells. J Bone Joint Surg Am. 87:1338–1342. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu SQ, Qiu B, Chen LY, Peng H and Du YM:
The effects of carboxymethylated chitosan on metalloproteinase-1,
-3 and tissue inhibitor of metalloproteinase-1 gene expression in
cartilage of experimental osteoarthritis. Rheumatol Int. 26:52–57.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Q, Liu SQ, Du YM, Peng H and Sun LP:
Carboxymethyl-chitosan protects rabbit chondrocytes from
interleukin-1beta-induced apoptosis. Eur J Pharmacol. 541:1–8.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He B, Liu SQ, Chen Q, Li HH, Ding WJ and
Deng M: Carboxymethylated chitosan stimulates proliferation of
Schwann cells in vitro via the activation of the ERK and Akt
signaling pathways. Eur J Pharmacol. 667:195–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tao HY, He B, Liu SQ, Wei AL, Tao FH, Tao
HL, Deng WX, Li HH and Chen Q: Effect of carboxymethylated chitosan
on the biosynthesis of NGF and activation of the Wnt/β-catenin
signaling pathway in the proliferation of Schwann cells. Eur J
Pharmacol. 702:85–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Risbud MV, Guttapalli A, Stokes DG,
Hawkins D, Danielson KG, Schaer TP, Albert TJ and Shapiro IM:
Nucleus pulposus cells express HIF-1 alpha under normoxic culture
conditions: a metabolic adaptation to the intervertebral disc
microenvironment. J Cell Biochem. 98:152–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng YH, Yang SH and Lin FH:
Themosensitive chitosan-gelatin-glycerol phosphate hydrogel as a
controlled release system of ferulic acid for nucleus pulposus
regeneration. Biomaterials. 32:6953–6961. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tonomura H, Takahashi KA, Mazda O, Arai Y,
Inoue A, Terauchi R, Shin-Ya M, Kishida T, Imanishi J and Kubo T:
Glutamine protects articular chondrocytes from heat stress and
NO-induced apoptosis with HSP70 expression. Osteoarthritis
Cartilage. 14:545–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim KW, Ha KY, Lee JS, Rhyu KW, An HS and
Woo YK: The apoptotic effects of oxidative stress and antiapoptotic
effects of caspase inhibitors on rat notochordal cells. Spine
(Phila Pa 1976). 32:2443–2448. 2007. View Article : Google Scholar
|
|
26
|
Wang YC, Fu RH, Hsieh HJ, Chao HT and Kao
SH: Polyglycolic acid/chitosan glue and apoptosis of endometriotic
cells. Fertil Steril. 84:75–81. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu HT, Li WM, Xu G, Li XY, Bai XF, Wei P,
Yu C and Du YG: Chitosan oligosaccharides attenuate hydrogen
peroxide-induced stress injury in human umbilical vein endothelial
cells. Pharmacol Res. 59:167–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koo HN, Jeong HJ, Hong SH, Choi JH, An NH
and Kim HM: High molecular weight water-soluble chitosan protects
against apoptosis induced by serum starvation in human astrocytes.
J Nutr Biochem. 13:245–249. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar
|
|
30
|
Ruest LB, Khalyfa A and Wang E:
Development-dependent disappearance of caspase-3 in skeletal muscle
is post-transcriptionally regulated. J Cell Biochem. 86:21–28.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thornberry NA and Lazebnik Y: Caspases:
enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rannou F, Lee TS, Zhou RH, Chin J, Lotz
JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A and Shyy JY:
Intervertebral disc degeneration: the role of the mitochondrial
pathway in annulus fibrosus cell apoptosis induced by overload. Am
J Pathol. 164:915–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sudo H and Minami A: Caspase 3 as a
therapeutic target for regulation of intervertebral disc
degeneration in rabbits. Arthritis Rheum. 63:1648–1657. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao H, Yenari MA, Cheng D, Sapolsky RM
and Steinberg GK: Bcl-2 overexpression protects against neuron loss
within the ischemic margin following experimental stroke and
inhibits cytochrome c translocation and caspase-3 activity. J
Neurochem. 85:1026–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sudo H and Minami A: Regulation of
apoptosis in nucleus pulposus cells by optimized exogenous Bcl-2
overexpression. J Orthop Res. 28:1608–1613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takei N and Endo Y: Ca2+
ionophore-induced apoptosis on cultured embryonic rat cortical
neurons. Brain Res. 652:65–70. 1994. View Article : Google Scholar : PubMed/NCBI
|