1. Introduction
Millions of people suffer from a variety of ocular
diseases, several of which may lead to vision impairment and even
complete blindness (1,2). In spite of recent progress in
diagnosis and treatment, numerous ocular diseases remain the
leading cause of blindness in adults. At present, there is no
satisfactory treatment available for these disorders; hence, it is
imperative to develop more effective treatments as well as
preventive methods. Gene therapy, which can be defined as the
delivery of nucleic acids into targeted cells to exert a
therapeutic effect, is a promising technology for treating
currently incurable diseases, including malignant tumors and
debilitating genetic disorders. The eye is an immune-privileged
organ and has structural and accessibility properties that make it
an ideal target organ for gene therapies. With increasing insight
into the molecular mechanisms of ocular diseases, gene therapy has
been proposed as a promising therapeutic tool for ocular diseases
(3,4).
Gene vectors are among the most important factors in
gene therapy. Successful gene therapy depends on efficient gene
transfer to targeted cells to warrant stable and prolonged gene
expression with minimal toxicity. Clinical applications of gene
translation are currently hampered due to a lack of a safe,
efficient and non-invasive means to selectively deliver genes to
target cells. With the advances in preparation technology of
microbubbles and innovations in ultrasound imaging, ultrasound is
no longer confined to the detection of tissue perfusion, but
gradually expands to specific molecular imaging and targeted
therapies. In recent years, numerous studies have indicated that
ultrasonic irradiation itself not only promotes gene transfection,
and that ultrasound-mediated microbubble destruction (UTMD) can
further enhance gene transfection efficiency in vitro and
in vivo (3–8). UTMD-mediated gene delivery systems
have been widely used in pre-clinical studies to enhance gene
expression in a site-specific manner in a variety of organs and
tissues (8–10). In the sphere of ophthalmology, the
application of the UTMD-meditated gene therapy has also been proved
to be efficient (11–13). The present article discussed the
current status of gene therapy of ocular diseases and reviewed the
progress in the delivery of genes to ocular by UTMD.
2. Barriers for ocular gene therapy
Effective delivery of bioactive molecules to regions
of pathology is dependent on numerous factors that are often
difficult to control. The major challenge is the site-specific
delivery of the payload to the target tissues and its subsequent
transport across the endothelial barrier. The eye's unique anatomy
and its physiological and anatomical barriers can limit effective
gene delivery into the eye. In ocular gene therapy, one of the
major challenges is to overcome intracellular and extracellular
barriers. Various barriers present at the anterior and posterior
segments of the eye restrict the entry of the gene material.
The cornea, which is an avascular tissue, is a good
target tissue to evaluate gene therapy owing to its simple
histological structure, immune-privileged nature and easy
accessibility. Its primarily consists of external stratified
epithelium, a thick collagenous stroma and a cuboidal monolayer of
epithelial-like cells called endothelium (14). The stratified epithelium is
composed of six to seven layers of stratified epithelial cells with
tight junctions, and the tight junctions create a major barrier to
topical gene delivery. Kamata et al (15) demonstrated that the tight junction
of epithelial and Bowman's membrane constrained viral invasion. The
tight junctions are the main barriers of the anterior segment of
the eye regarding the transport of genes. The collagenous stroma is
mainly composed of the predominant stromal cells and an
extracellular matrix. It is separated from the corneal epithelium
by a condensed collagenous layer, Bowman's membrane, and from the
endothelium by a thin acellular layer, Descemet's membrane
(14). Klausner et al
(16) reported that administration
of viral vectors via the epithelium or endothelium does not result
in efficient transduction of the stromal keratocytes. The
endothelium is the innermost monolayer, forming a leaky barrier
positioned between the stroma and aqueous humour. Kamata et
al (15) also reported that
gene expression was restricted to endothelial cells after the
injection of a viral vector into the anterior chamber of a mouse
eye.
With regard to ocular gene therapy, target cells are
often located in the neuroretina or the retinal pigment epithelium
(RPE). The most convenient way of therapeutic gene delivery to them
would be topical application; however, due to the limited diffusion
of the gene particles through the sclera, the delivery efficiency
is low. For systemic administration, the retina and vitreous are
inaccessible due to the tight blood-retinal barrier (BRB). Topical
application and systemic administration are thus less suitable for
the delivery of gene material to the retina and RPE. Therefore, in
most ocular gene therapy trials, sub-retinal or intravitreal
injection are possible routes of administration for gene complexes.
Although sub-retinal injection has shown encouraging results in
certain studies, this invasive method is not always the first
choice. Intravitreal and topical delivery of liposomes to the eye
have been reported (17,18); however, they have yielded low
transfection efficiency in the retina and RPE. Dalkara et al
(19) reported that the inner
limiting membrane and BRB severely limit the passage of
adeno-associated virus (AAV) after intravitreal delivery.
Intravitreal delivery is an invasive procedure with risk of retinal
detachment, hemorrhage, endophthalmitis and glaucoma (20,21).
Following intravitreal injection, it is difficult for a gene
complex to diffuse through the vitreous. If the target is the RPE,
the neural retina is thought to be another barrier. Peeters et
al (22) and Du et al
(23) found that the neural retina
is a significant barrier for the delivery of non-viral gene
complexes to the RPE.
Vitreous humour is a gel-like material that consists
of collagen, hyaluronan, and proteoglycans containing chondroitin
sulfate and heparan sulfate (24).
Three-dimensional networks of the collagen fibrils are cross-like
with proteoglycan filaments that contain negatively charged
glycosaminoglycans (GAGs) (24).
Size and charge are thought to be the main factors that limit the
movement of the gene carriers in the vitreous humour. The
negatively charged GAGs present in the vitreous humour may bind to
the gene complexes, and gene materials may therefore become stuck
to the gel-like materials in the vitreous humour (25). Considering its structure and
composition, the vitreous humour may decrease the gene transfer
efficiency. Du et al (23)
demonstrated that the biopolymer network in the vitreous humour
decreased the delivery efficiency of nanoparticles loaded with
small interfering (si)RNA to RPE-J cells in vivo. Retinal
gene delivery is a challenging area in the field of ocular gene
delivery. With regard to gene delivery to the posterior segments of
the eye, the BRB, vitreous and neural retina are likely to be the
main barriers.
A series of biological and physiological barriers
associated with almost all aspects of cellular biology are required
to be overcome in order to achieve efficient gene delivery.
Firstly, when systemically injected, the gene vectors are required
to pass through the endothelial barrier of the capillary wall. The
gene complexes face the threat of being rapidly degraded by the
DNAse in the serum or the immune system prior to reaching the
target cells. Nishikawa and Huang (26) demonstrated that the no-viral DNA
vectors are often rapidly cleared from the circulation by
mononuclear phagocyte systems. Manickan et al (27) demonstrated that viral vectors are
rapidly cleared by hepatic Kuppfer cells, which may result in high
deposition in the liver and even liver toxicity. Secondly, it must
be avoided that gene complexes are entrapped into the endosome or
the lysosome, where they are degraded. Thirdly, the gene complexes
are required to penetrate the nuclear membrane to achieve the goal
of gene expression for successful gene therapy. In summary, a
variety of intracellular and extracellular barriers are required to
be overcome for efficient gene delivery.
3. Current status of gene therapy of ocular
disease
As gene therapy begins to produce its first clinical
successes, interest in ocular gene therapy has grown owing to the
favorable safety and efficacy characteristics of the eye as a
target organ for gene delivery. The basic technology of gene
delivery systems is divided into two categories: Viral
vector-mediated methods and a non-viral vector-mediated methods.
Over the last decades, numerous viral and non-viral vector-mediated
gene transfer methods have been tested in a large number of animal
models of ocular diseases.
Viral vectors commonly used for ocular gene transfer
are adenoviral (28),
adeno-associated viral (AAV) (29)
and lentiviral vectors (30).
Viral systems can provide highly efficient delivery into cells with
sustained expression. Recently, Igarashi et al (28) showed that vascular endothelial
growth factor (VEGF)-targeted siRNA can be expressed across the
retina and that long-term suppression of choroidal
neovascularization (CNV) is possible through the use of stable
AAV2/8-mediated VEGF siRNA expression. AAV2/8-mediated VEGF siRNA
expression may be a feasible method to manage CNV in conditions
such as age-associated macular degeneration. Huang et al
(29) conducted a study to
evaluate whether AAV-mediated overexpression of growth-associated
protein-43 (GAP-43) has protective or deleterious effects on
retinal ganglion cell (RGC) survival in laser-induced chronic
intraocular pressure (IOP) elevation injury. The study showed that
AAV mediated the overexpression of the axonal growth-associated
protein GAP-43 in RGCs and severely aggravated RGC death in
experimental glaucomatous injury. At present, the main disadvantage
of viral systems is their potential for uncontrollable and
insertional mutagenesis (31).
Viral vectors evoke immune responses independent of the transgene
constructs used, vector dose or vector preparation, which limits
repetitive regimens (32,33). Furthermore, the transduction of
certain viral vectors occurs with relatively low efficiency, which
limits its therapeutic effects (34). The potential dangers of viral
vectors may hamper their further development for ocular gene
therapy in humans (35). These
limitations have prompted a requirement to develop non-viral
delivery systems with high biosafety and low cytotoxicity.
In the last decade, the development of non-viral
methods for ocular gene therapy has made great progress in cell
lines and animal models. Non-viral delivery approaches are
constituted by chemical methods (mainly involving cationic lipids,
polymers or nanoparticles) and physical methods (mainly involving
administration by gene gun, electroporation, iontophoresis or
microinjection) (36,37). Approaches based on utilization of
non-viral vectors are easily available, cost-effective and do not
evoke any antigen-specific immune and inflammatory responses after
ocular administration (38). The
emergence of nanotechnology may have a profound effect on ocular
biomedical applications, particularly the delivery of drugs to the
posterior of the eye via nanocarriers (39,40).
Jayaraman et al (41)
synthesized a nanoformulation consisting of a water-soluble
chitosan conjugated with a peptide (serine-threonine-tyrosine) as a
potential carrier for retinal delivery to treat age-associated
macular degeneration (AMD). In this study, the conjugated
nanochitosan peptide showed evidence of tyrosine kinase activity as
indicated by fluorescent signals under the confocal microscope,
while nanochitosan or peptide alone did not show such activity.
Zhou et al (42) designed a
study for investigating the downregulation of mRNA expression of
VEGF by triamcinolone acetonide acetate (TAA)-loaded chitosan
nanoparticles in human retinal pigment epithelial cells. The study
demonstrated that TAA/loaded deoxycholic acid (DA)-modified
chitosan nanoparticles had a downregulating effect on VEGF mRNA
expression in human retinal pigment epithelial cells with low
cytotoxicity; these are beneficial characteristics suggesting the
suitability of these chitosan-derived nanoparticles to be developed
into therapeutics for diabetic retinopathy. Although non-viral
vector-meditated gene transfer efficiency has improved over the
past decade, it remains relatively low and the expression duration
of the transgene is relatively short (43). Regarding physical methods, their
inherent risks may outweigh their benefits, rendering them
inappropriate for ocular gene transfer, and the invasive nature of
these methods reduces patient compliance for effective therapy
(44). The major obstacle in the
clinical application of gene therapy is not the lack of ideal
genes, but rather the lack of a clinically safe and efficient gene
transfer method (45). Therefore,
it is necessary to develop effective and specific ocular gene
delivery systems.
UTMD-mediated gene delivery systems hold promise to
fulfill this void, particularly with the wide use of ultrasound
contrast agents in clinical diagnostic imaging. UTMD-meditated gene
delivery, with the advantages of low toxicity, a high safety
profile, repetitive applicability and specific tissue targeting,
provides a novel method for gene therapy (46,47).
Lin et al (48) reported
that focused ultrasound with microbubbles was able to effectively
transfer nanoparticles into mouse tumors through altering the
permeability properties of the vasculature and cell membrane. It
was reported that the delivery of the TFPI-2 gene using SonoVue was
able to suppress thrombosis and arterial restenosis, providing a
potential gene therapy approach for atherosclerosis (49). With the extensive research on
ultrasound contrast agents in gene transfer and gene therapy, an
increasing number of researchers are beginning to introduce the
application of UTMD for ocular gene transfection.
4. Mechanisms of ultrasound contrast
agent-mediated gene delivery
Microbubble contrast agents, although typically used
to enhance ultrasound contrast for imaging, are increasingly
gaining attention due to their ability to directly deliver various
classes of bioactive substances to a number of tissue types, and
becoming increasingly popular for targeted gene and drug delivery,
as well as the monitoring thereof. UTMD has evolved as a promising
system for non-invasive, target-specific gene delivery. The low
toxicity and simplicity of its in vivo application make this
technology particularly attractive. Ultrasound contrast agents are
gas-filled spheres remaining completely intravascular when
systemically injected. Microbubbles as cavitation nuclei are able
to volumetrically expand and contract in response to compression
and rarefaction phases of ultrasound waves. When the acoustic
pressure reaches a certain threshold, microbubbles violently
collapse and cause a series of biological effects. The physical
response of microbubbles can mechanically perturb the integrity of
blood vessel walls and cell membranes, thus increasing their
permeability to therapeutic agents, which can thereby penetrate
into the cells (50). Sirsi and
Borden (50) categorized the
mechanisms of the alternation of the vascular permeability by
microbubble cavitation into three different classes: i) Creation of
transient pores in vascular endothelial cells that allow
intracellular macromolecule uptake; ii) disruption of vascular
endothelial integrity; iii) stimulation of endocytotic cellular
uptake.
Due to shock waves and jetting during microbubble
collapse, the inertial cavitation of microbubbles can cause
transient membrane ruptures. This phenomenon termed as 'microbubble
sonoporation', was thought to be the primary mechanism of
intracellular gene delivery (51).
Studies have reported that microbubbles enhanced gene delivery
efficiency by lowering the cavitation threshold and enhance
cavitation erosion (52), and the
'spillover space' on the cell membrane after the microbubble
cavitation lasted for 24 h, which is sufficient for gene entry and
expression (53). Zhou et
al (54) reported that a
single microbubble was able to generate transient pores of a size
proportional to the proximity of the cavitation event to the
membrane, and the membranes returned to normal within 20 sec.
UTMD-meditated pore formation was a highly effective and
controllable approach that transiently disrupted the membrane
integrity to enhance its permeability to circulating agents.
Volumetric expansion of the microbubble in the ultrasound field can
facilitate bubble-vessel interaction, while microbubble oscillation
exerts a longitudinal strain on blood vessels, and can partially
embed them in the endothelium and continue to oscillate, which may
alter vascular permeability (55).
Hauser et al (56) studied
the effects of stable microbubble cavitation on endocytotic
activity in cultured cells, and demonstrated that stable cavitation
of microbubbles increased the number of clatherin-coated pits and
endocytotic vesicles. The study also demonstrated that stable
cavitation of microbubbles can increase endocytotic activity of
cultured cells under low-intensity ultrasound (56). To date, the exact mechanisms
governing the enhancement of UTMD-mediated gene delivery in
vivo have remained to be fully elucidated; however, acoustic
cavitation is thought to be a major contributor. The biological
effects of ultrasound, including microstreaming and other
convective phenomena, are also thought to contribute to the
enhancement of gene delivery.
5. Gene transfer mediated by UTMD in ocular
disease
UTMD-meditated gene delivery provides a novel method
for gene therapy. In recent years, numerous in vitro and
in vivo studies have conformed that ultrasound with
microbubbles significantly enhanced gene transfection efficiency.
UTMD is therefore emerging as a powerful tool for the treatment of
ocular diseases.
UTMD-meditated gene transfer to the
cornea
The cornea is an ideal tissue for studies on gene
transfer, as it is transparent and avascular. Sonoda et al
(9) investigated the practical
efficacy and safety of ultrasound plus microbubble-mediated gene
transfer to cornea in vitro and in vivo. While
treatment with DNA alone did not lead to any gene transfer into the
cultured corneal epithelial cell line RC-1, ultrasound slightly
enhanced gene transfer, and ultrasound plus microbubbles
significantly increased the gene transfer efficiency. In the in
vivo study, ultrasound plus microbubbles markedly increased
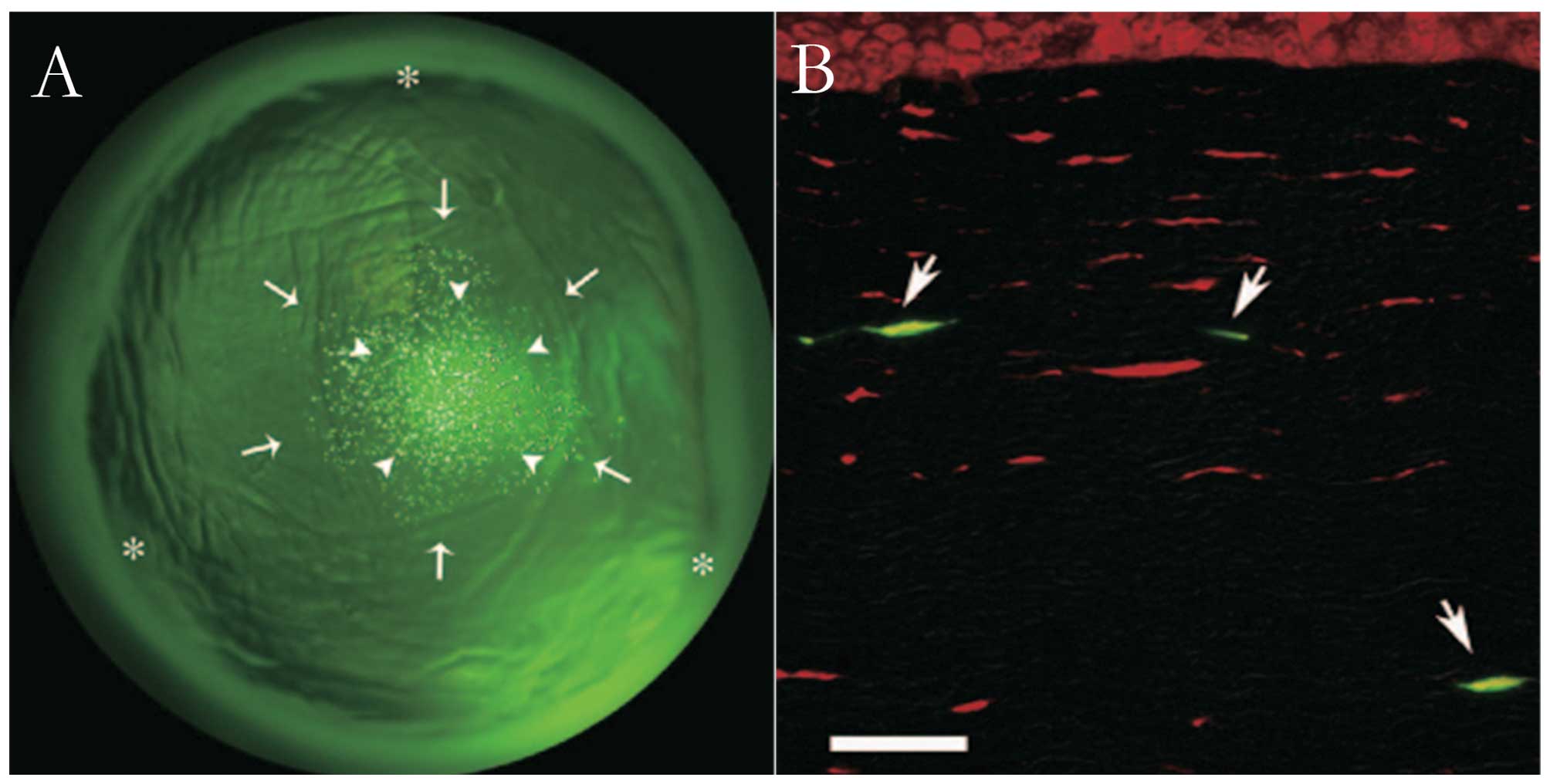
gene transfer efficiency without any apparent tissue damage. Green
fluorescence protein (GFP)-positive cells were observed exclusively
where ultrasound had been applied and GFP was mainly present in
spindle-shaped cells in the targeted regions of the corneal stroma
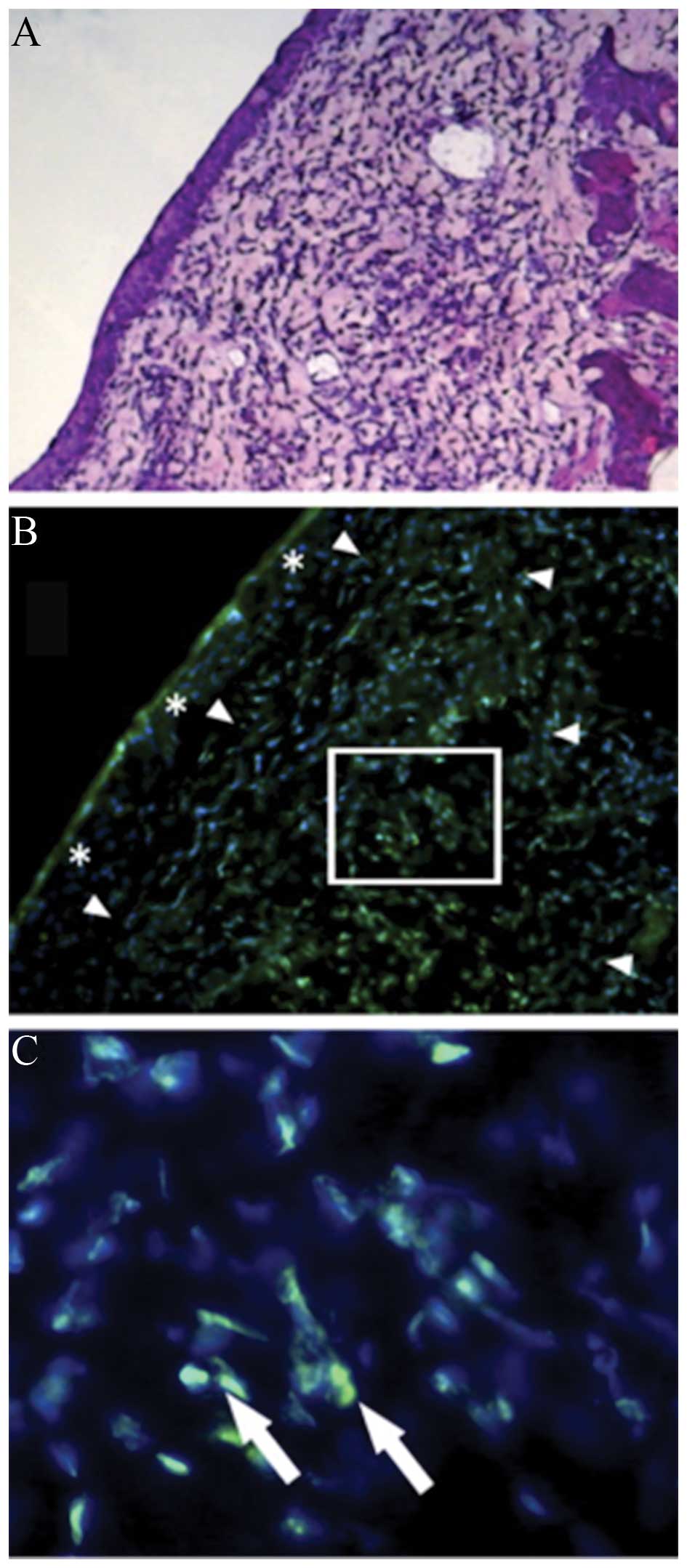
(Fig. 1) (10). Yamashita et al (45) used a novel bubble liposome (BL)
composed of a polyethylenglycol (PEG)-modified liposome containing
perfluoropropane gas, with ultrasound to transport GFP into rabbit
RC-1 cells in vitro and conjunctiva in vivo. The
study showed that BL with US effectively transferred genes into
cultured corneal epithelial cells and rat sub-conjunctival tissue
without causing any apparently adverse effects. Diffuse
fluorescence-positive granules were present in sub-conjunctival
tissues and no tissue damage was observed histologically (Fig. 2).
UTMD-meditated gene transfer to the
retina
Gene transfer provides a novel approach for the
treatment of retinal diseases. Li et al (57) demonstrated that UTMD was able to
safely and effectively deliver plasmids into RGCs in vitro.
Under the optimum parameters, the average transfection rate of p
enhanced (E)GFP-N1 with UTMD was 25%. Compared with the ultrasound
plus plasmid group, the number of transfected cells increased by
28-fold. Another study reported that UTMD-mediated gene transfer of
pigment epithelium-derived factor (PEDF) into retina and chorioids
of rats inhibited the development of CNV (58). The study also demonstrated that in
the short term (7 and 14 days after transfection), the transfection
efficiency mediated by UTMD was not different from that achieved by
liposome-based gene transfer. However, in the long term (28 days
after transfection), the transfection efficiency by UTMD was
significantly higher as compared with that of the liposome
approach. The shock wave of UTMD promoted the delivery of the
plasmid into the cell nucleus, which may partly be explained by the
induction of tight binding of the target plasmid to the cell's
endogenous DNA. UTMD therefore presents a solution for local gene
transfection and reduces the amount of plasmid required. Sonoda
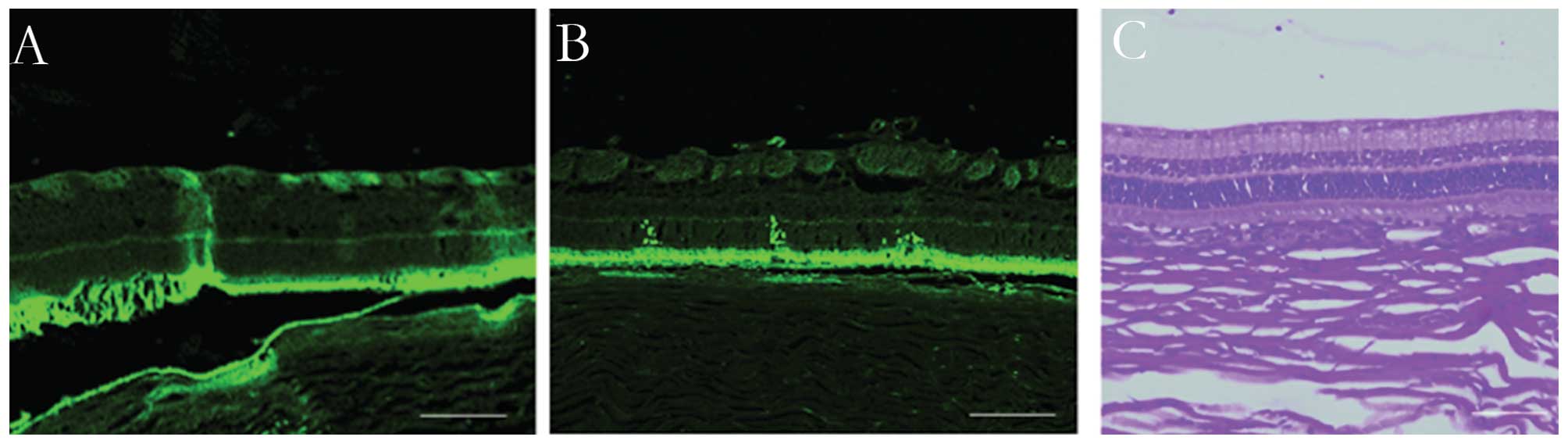
et al (13) used a
miniature ultrasound transducer to evaluate the efficacy of
intravitreal ultrasound (SonoPore 4000) irradiation for selective
GFP plasmid transfer into the rabbit retina. The ultrasound probe,
as small as a 19-gauge needle, was inserted into the vitreous
cavity through a scleral incision. The gene-transfer efficiency was
quantified by counting the number of GFP-positive cells. The study
demonstrated that the retinas that received plasmid with BL and
ultrasound showed a significant increase in the number of
GFP-positive cells without any apparent tissue damage (Fig. 3) (13). GFP-positive cells were observed
exclusively in the area that was exposed to ultrasound, and no
GFP-positive cells were observed in the control eyes that were not
treated with ultrasound. These results indicated that gene delivery
to the retina using intravitreal ultrasound exposure is more
selective than the transcorneal method.
RNA interference is a promising biological strategy
for the treatment of diseases; however, its instability and poor
cellular uptake have limited its application (59). To overcome such limitations, an
siRNA delivery method based on the combined use of nanoparticles
with ultrasound and/or microbubbles was used in a number of
studies. Du et al (23)
designed a study to investigate the efficacy and safety of
ultrasound and/or microbubble-enhanced delivery of monomethoxypoly
(ethylene glycol)-poly(lactic-co-glycolic acid)-poly l-lysine (mPEG-PLGA-PLL)
nanoparticles loaded with platelet-derived growth factor BB
(PDGF-BB) siRNA into rat RPE-J cells. The results showed that
ultrasound and/or microbubbles were able to be used safely to
enhance the delivery of nanoparticles loaded with siRNA to rat
RPE-J cells, and this approach down-regulated the mRNA and protein
expression of PDGF-BB with enhanced efficiency. However, the
combination of ultrasound and microbubbles under the optimal
conditions did not further increase the cellular uptake of
nanoparticles compared to that achieved with either ultrasound or
microbubbles alone. In vitro, the rat RPE-J cells are
fragile and vulnerable, and the bio-effects of UTMD may have been
too aggressive to further increase the delivery of nanoparticles,
which may explain the observations of the study.
AAV vectors possess a number of advantages over
other vectors, which render them suitable for transfection studies,
in particular their ability to transfect cells in a stable and
long-term manner and their relative lack of pathogenicity (1). Viral vectors are usually delivered
systemically, which may lead to anti-viral immune responses of the
host. Due to the immunoreaction and the limits of the endothelial
barrier, the transduction of these viral vectors occurs with
relatively low efficiency, which limits its therapeutic effects.
Increasing viral vector transduction may produce improved
therapeutic effects (34).
UTMD-meditated local gene therapy has the potential not only for
plasmid-DNA transfer, but also virus-mediated gene transfer. A
study demonstrated that a microbubble can load and protect an
adenoviral vector, and that the delivery system comprising the
vector incorporated into the microbubble was able to improve
site-specific targeting of GFP (60). The study also proved that the
microbubble was able to reduce the degradation rate of the viral
vectors after intravenous injection. In analogy with this, Geers
et al (61) found that UTMD
can specifically and effectively increase recombinant
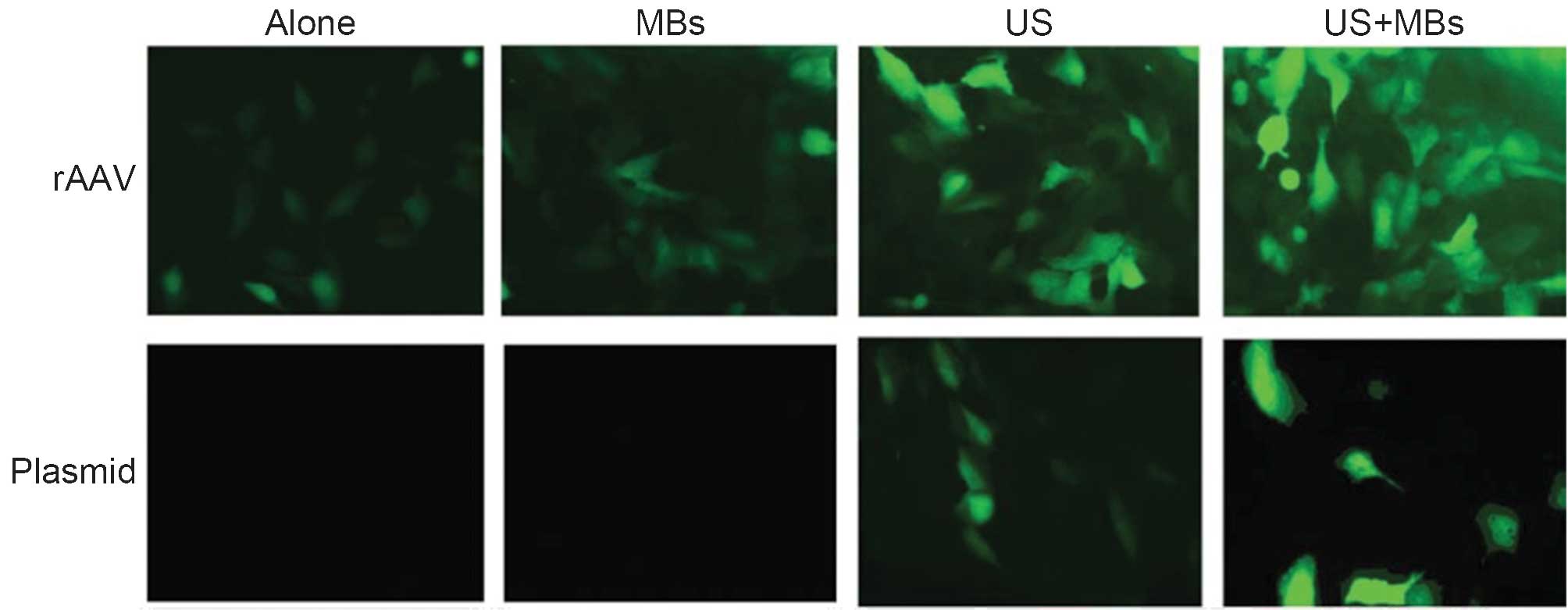
(r)AAV-mediated gene delivery. Li et al (62) demonstrated that UTMD enhanced rAAV2
transfer efficiency into less permissive hRCC cells by two- to
three-fold without decreasing cell viability. Polymerase chain
reaction analysis showed that with the use of UTMD, a more than
nine-fold enhancement of rAAV2 genomic DNA copies was achieved
compared with that using rAAV2 alone in vitro, and a more
than two-fold enhancement in vivo. In the in vivo
study, UTMD not only amplified rAAV2 transduction, but also
inhibited tumor growth.
Compared to other viral vectors that have recently
been investigated, rAAV has the advantages of low immunogenicity
and stable long-term transgene expression (63), which has been widely studied in
retinal diseases. Ultrasound-meditated microbubble destruction was
able to enhance rAAV-mediated gene delivery into retina cells
(11,12). Li et al (11) reported that UTMD enhanced rAAV2
transfection efficiency in human RPE cells in vitro and in
Wistar rat retina in vivo. In this study, UTMD induced
rAAV2-mediated EGFP expression earlier after injection and
substantially increased gene expression prior to the peak (35 days)
with no evident tissue damage. Fluorescence microscopic analysis of
a tissue-stretched preparation showed that the number of
EGFP-positive cells in the group treated with AAV, microbubbles and
ultrasound was higher than that in the AAV and normal saline
groups, and that EGFP expression mainly appeared in the layer of
RPE cells and neural retina (Fig.
4). Recently, a study using mouse models of proliferative
vitreoretinopathy demonstrated that UTMD produced a therapeutic
effect by facilitating the insertion of rAAV2-conjugated genes into
tumors (64). Xie et al
(12) investigated the efficiency
and safety of UTMD-mediated delivery of rAAV2-EGFP into RGCs of
rats and demonstrated that EGFP expression in the group treated
with rAAV2-EGFP, ultrasound and microbubbles was the highest, and
that the number of transfected RGCs was the largest compared to
that in the other groups. No obvious damage was observed by
histopathological analysis. Zheng et al (65) investigated the feasibility of
UTMD-enhanced rAAV or plasmid-mediated transfection into the human
RPE cell line ARPE-19. The result showed that the transfection
efficiency of rAAV and plasmid in ARPE-19 cells was enhanced by
UTMD without any adverse effects on cell viability (Fig. 5). The transfection efficiency of
rAAV was higher than that of plasmid. UTMD-enhanced rAAV-mediated
transfection was therefore thought to be an appropriate method for
retinal gene therapy.
UTMD combined with viral vectors offers numerous
benefits: First, under ultrasound irradiation, microbubbles may
improve the site-specific release of the viral vector (66). Second, UTMD can mechanically
enhance the permeability of the blood vessel walls and cell
membranes, and thus improve the transfer efficiency of the viral
vector; and finally, the ultrasound contrast agent can
simultaneously impose restrictions on the immune response to the
viruses, thus allowing for intravascular administration and
repetitive injection (60). Jin
et al (67) showed that
UTMD stimulated the formation of clathrin-coated pits (CPs) as well
as uncoated pits (nCPs), and facilitated the uptake of viral
particles into the cytoplasm and nucleus for long periods of time,
mainly by stimulating endocytosis. The combination of UTMD with
viral vectors may represent a novel gene delivery system with high
specificity and low invasiveness for patients with retinal
diseases.
UTMD-meditated drug or gene transfer to
retinoblastoma
In recent years, with the developments in molecular
biology, gene therapy has been gaining importance in cancer
therapy. Retinoblastoma (RB) is the most common malignant
intraocular tumor in children. In spite of advances in enucleation
and conservative treatments, there has been no improvement in the
five-year survival rate in children. The treatment of
retinoblastoma has been increasingly focused on localizing the
therapy to the eye. UTMD-meditated drug or gene delivery to ocular
tumors is regarded to be a non-invasive gene transfer technology
and provides a novel means of gene therapy for retinal disease.
Microbubble destruction by ultrasound exposure
generates microstreams or microjets that create shear stress on
cells and open transient pores in cell membranes, which has the
capability of transiently enhancing cell membrane permeability
(68). The use of ultrasound with
diagnostic microbubbles in cancer treatment to increase the
efficiency of chemotherapy through passive, localized delivery has
been an emerging area of research. Numerous studies have
demonstrated that optimized UTMD-mediated therapy has the potential
to improve cancer response to therapy via increased localized drug
uptake and targeted therapeutics, which may lead to a lowering of
chemotherapeutic drug dosages and systemic toxicity (69,70).
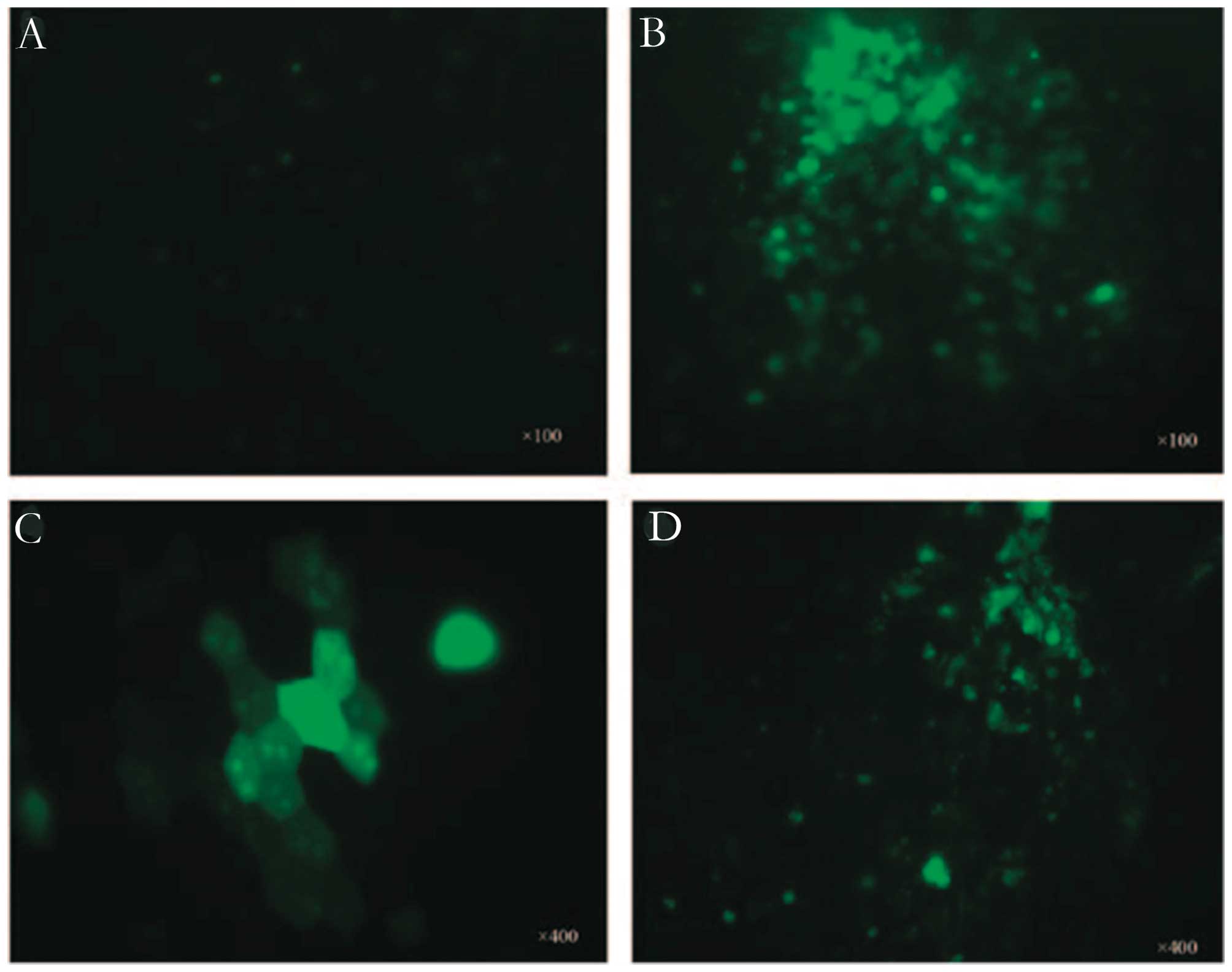
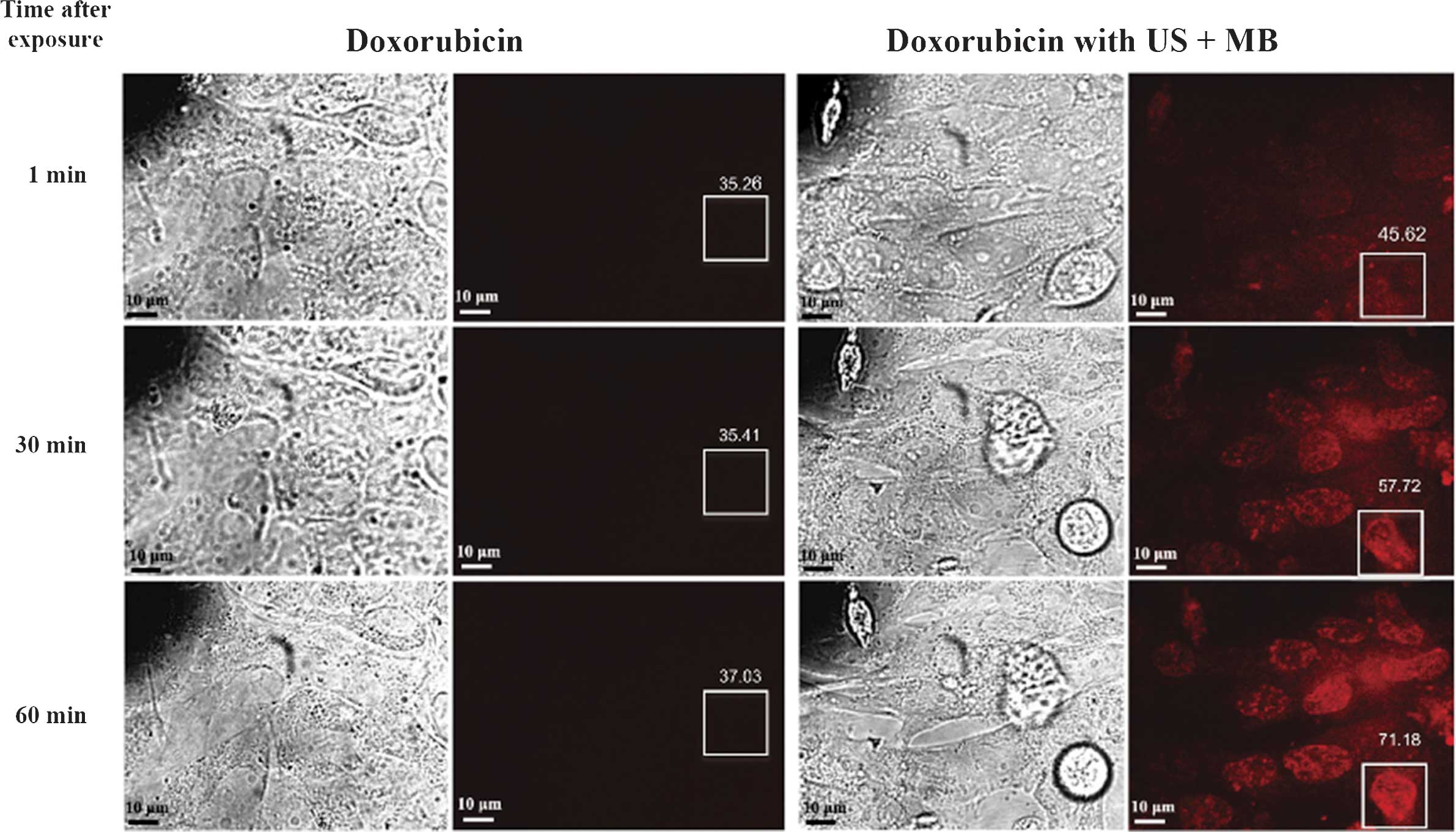
Lee et al (71) proved that
using low-intensity (0.3 W/cm2) and low-frequency (1
MHz) ultrasound with microbubbles for 10 sec enhanced the
chemotherapeutic efficacy of doxorubicin against retinoblastoma Y79
cells in vitro. Cells exposed to ultrasound and microbubbles
showed earlier and higher trace intracellular fluorescence than
that of cells treated with doxorubicin alone (Fig. 6). There is a significant decrease
in cell viability in cells treated with this method compared with
cells treated with chemotherapy alone. To investigate the duration
and underlying mechanism of increased permeability, the study used
scanning electron microscopy to image cells exposed to ultrasound +
microbubbles (for 10 or 60 sec). Pores were identified in cells
exposed to ultrasound + microbubbles for 60 sec but not in those
exposed for 10 sec. However, in vitro fluorescence showed
that doxorubicin uptake significantly increased immediately after
exposure to ultrasound + microbubbles for 10 sec. These results
suggested that the presence of physical pores may not be a
pre-requisite for enhanced drug entry into the cells. It is
possible that transient electrical changes, endocytosis or other
unidentified mechanisms contributed to the enhanced drug uptake.
UTMD may become a valuable adjuvant to chemotherapy of RB, whose
treatment is often limited by challenges in drug delivery, and may
lead to more effective chemotherapy treatments with less damage and
side effects to ocular tissues.
Luo et al (46) explored the efficiency of
wild-type53 (wtp53) plasmid transfection into Y79 RB cells and RB
xenograft tumor tissue meditated by ultrasound with microbubbles,
and demonstrated that the wtp53 gene was successfully transfected
into solid tumors in the plasmid with microbubbles and ultrasound
group. Flow cytometry showed that apoptosis was higher in the
microbubbles and ultrasound group (25.58%) compared with that in
the plasmid with liposomes group (19.50%) and the other two groups
(<10%). Another study used the same method to explore the
transfection of the recombinant expression plasmid pEGFP-C1/RB94
into the human RB cell line HXO-Rb44 and examined the efficiency of
RB94 in the inhibition of the growth of RB cells (72). The results showed that UTMD
enhanced the transfection efficiency of RB94, which had an obvious
impact on the growth inhibition of the RB cells. UTMD-meditated
gene therapy may be a useful method for application in the gene
therapy of RB.
UTMD-meditated reversible BRB
disruption
The major challenge in delivering systemically
administered substances to specific retinal locations is the
existence of the BRB, which is formed by a complex tight junction
of the retinal endothelial cells and the RPE. The BRB prevents most
systemically administered drugs from reaching the retina. Almost
98% of clinically validated drugs are not able to cross the BRB
(73). Multiple administration
routes are currently used to deliver bioactive materials to the
retina. However, topical, systemic and periocular approaches are
limited by the BRB and other ocular barriers. Though sub-retinal
and intravitreal injections can provide direct access for genes to
the retina, these invasive methods are not the first choice for the
treatment of the diseases of the eye due to the risk of the retinal
detachment and hemorrhage (20).
In addition, for certain chronic diseases requiring repeated
administration, the risks multiply. Therefore, the development of
minimally invasive and efficient methods that can bypass the BRB
and enhance the delivery of therapeutic materials to the retina is
desired.
A non-invasive, reversible and targeted technique
that combines low-energy ultrasound bursts with a microbubble
ultrasound contrast agent to temporarily induce blood-brain barrier
(BBB) disruption was identified (74). The barrier can be restored without
significant side effects, and the method was shown to improve
therapeutic outcomes in animal disease models (75). In principle, similar techniques may
be used to deliver drugs or genes to the retina. Using a rat model,
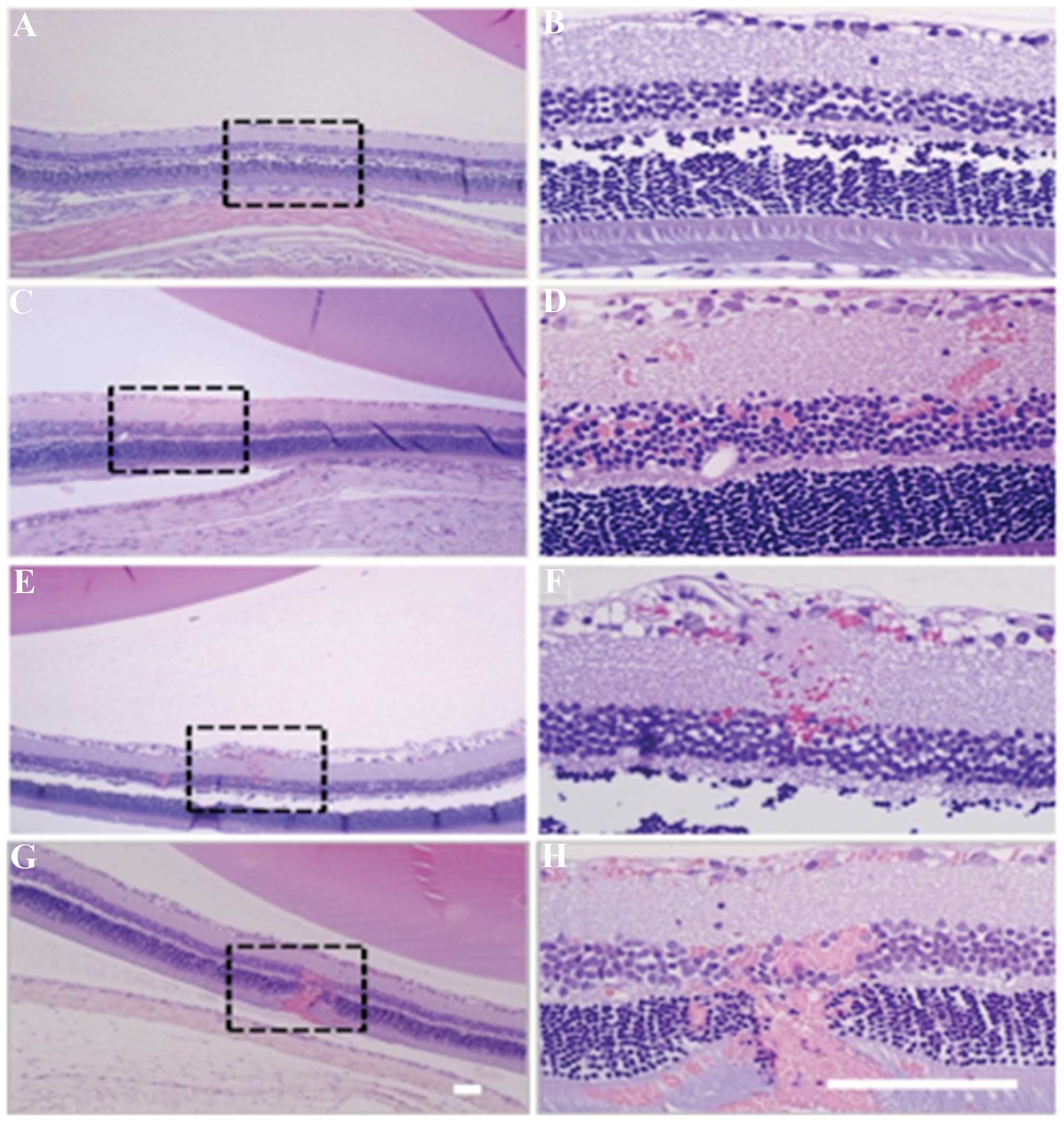
Park et al (76)
demonstrated that burst ultrasound together with an intravenously
(i.v.) administered microbubble agent was able to induce transient
increases of retinal vascular permeability for ocular drug
delivery. For BRB disruption, 10-msec bursts were applied at 1 Hz
for 60 sec with different peak rarefactional pressure amplitudes
(0.81, 0.88 and 1.1 MPa). To evaluate BRB disruption, a magnetic
resonance imaging contrast agent gadolinium
diethylenetriaminepentaacetic acid (Gd-DTPA; Magnevist) was
injected i.v. immediately after the last sonication, and serial
T1-weighted magnetic resonance images were acquired at up to 30
min. No signal enhancement was observed, suggesting that no Gd-DTPA
leakage into the retina or vitreous humor was present in the
non-sonicated animals. All of the animals that received ultrasound
and microbubbles showed detectable signal enhancement. Though the
maximum signal enhancement was greatest after sonication at 1.1
MPa, the retinal damage was severe (Fig. 7). Increased petechaie and retinitis
were observed after sonication at 1.1 MPa. No significant retinal
damage was identified by histological analysis at the two lower
acoustic pressure amplitudes tested, and the barrier was found to
be restored 3 h after sonication. The study demonstrated that
appropriately powered focused ultrasound combined with microbubble
induced a temporary and reversible disruption of the BRB in rats
without any significant side effects. The BRB appeared to be
restored within a few hours, which provided a suitable time-window
for ocular pharmaceutical agent delivery while avoiding undesired
effects that may result from long-term BRB disruption (76).
Ultrasound with microbubbles may offer a
non-invasive, localized and repeatable means to reversibly disrupt
the BRB for ocular substance delivery. To date, the mechanisms of
the UTMD meditated BRB disruption have not been fully elucidated.
The mechanical stimulation that induces a temporary widening of the
tight junctions and the active transport may partly explain the
beneficial effects of UTMD on gene delivery and drug uptake
(77).
UTMD-meditated gene transfer to the
ocular ciliary muscle
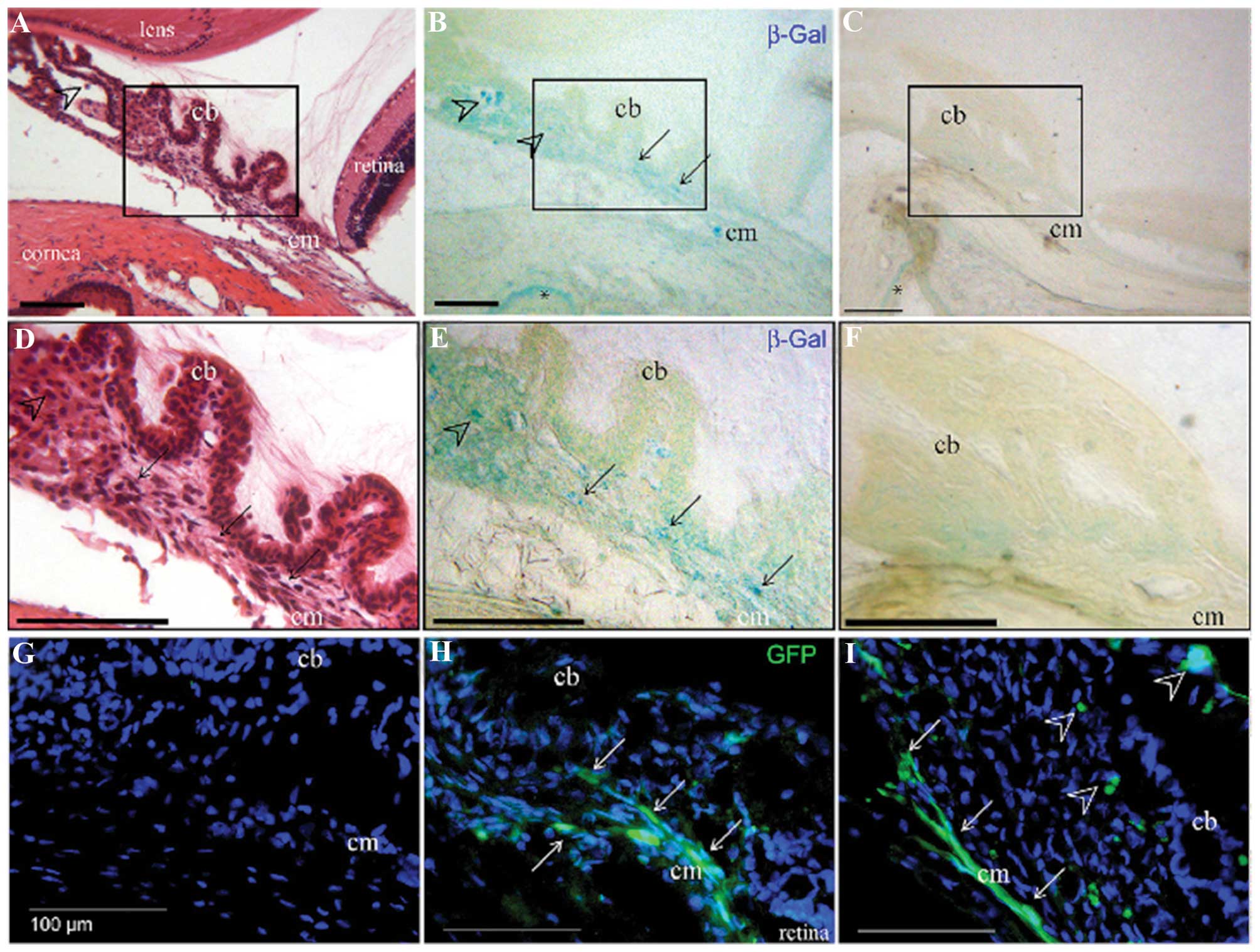
Kowalczuk et al (78) assessed the application of low-
intensity ultrasound combined with commercial microbubbles to
transfect the ciliary muscle of rat eyes. The ultrasound settings
applied were as follows: 1 MHz, 2 W/cm2 and a 50% duty
cycle of 2 min. At 1 week, the ultrasound + microbubble treatment
produced a significant increase in luminescence compared with that
in the control eyes injected with plasmid only, with or without
microbubbles. The reporter proteins were localized in the ciliary
muscle as indicated by histochemical analysis (Fig. 8). At 1 month, all groups showed a
significant decrease in luciferase activity. A rise in lens and
ciliary muscle temperature was detected during the procedure;
however, this did not result in any observable damage at 1 and 8
days. This study demonstrated that the ocular ciliary muscle can be
targeted by DNA sonoporation, allowing for protein secretion into
the ocular sphere. Sonoporation targeted to ciliary muscles has
potential as a non-viral gene delivery procedure for the treatment
of various ocular diseases.
6. Conclusion
UTMD has evolved as a promising method for
non-invasive, target-specific gene delivery. The low toxicity and
simplicity of in vivo application make this technology
particularly attractive. The combination of UTMD and viral vectors
in gene delivery not only enhanced the efficiency, but also
abolished immunogenicity. UTMD is a promising technique for ocular
gene delivery. However, the application of UTMD is still in its
infancy stage and far from ready to be used in clinical
applications. There remain certain unresolved issues. The
ultrasound exposure parameters, frequency, mechanical index and
amount of the plasmid DNA used should be optimized. The microbubble
size and surface architecture should be optimized to prolong
circulation time and gene-loading efficiency.
With regard to ocular gene therapy, most available
studies only evaluated the ocular tissue structure damage after
gene therapy, but the impact on vision has not been sufficiently
investigated. Further evaluation of the impact on vision is of high
importance, particularly in view of eventual clinical application.
Further research is required prior to the clinical application of
UTMD. In spite of several problems remaining to be solved, UTMD is
a promising system for ocular gene delivery.
Acknowledgments
The present review was supported by grants from the
Natural Science Foundation of Shanghai Science Commission (grant
no. 11ZR1421100), the Scientific Effort Project of Shanghai Science
and Technology committee (grant no. 1441968200) and the Natural
Science Foundation of China (grant no. 81200700).
References
|
1
|
Surace EM and Auricchio A: Versatility of
AAV vectors for retinal gene transfer. Vision Res. 48:353–359.
2008. View Article : Google Scholar
|
|
2
|
Zhang L, Li X, Zhao M, He P, Yu W, Dong J,
et al: Antisense oligonucleotide targeting c-fos mRNA limits
retinal pigment epithelial cell proliferation; a key step in the
progression of proliferative vitreoretinopathy. Exp Eye Res.
83:1405–1411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuliszewski MA, Kobulnik J, Lindner JR,
Stewart DJ and Leong-Poi H: Vascular gene transfer of SDF-1
promotes endothelial progenitor cell engraftment and enhances
angiogenesis in ischemic muscle. Mol Ther. 19:895–902. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li HL, Zheng XZ, Wang HP, Li F, Wu Y and
Du LF: Ultrasound-targeted microbubble destruction enhances
AAV-mediated gene transfection in human RPE cells in vitro and rat
retina in vivo. Gene Ther. 16:1146–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhigang W, Zhiyu L, Haitao R, et al:
Ultrasound-mediated microbubble destruction enhances VEGF gene
delivery to the infarcted myocardium in rats. Clin Imaging.
28:395–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Q, Wang Z, Ran H, et al: Enhanced
gene delivery into skeletal muscles with ultrasound and microbubble
techniques. Acad Radiol. 13:363–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren JL, Wang ZG, Zhang Y, et al:
Transfection efficiency of TDL compound in HUVEC enhanced by
ultrasound-targeted microbubble destruction. Ultrasound Med Biol.
34:1857–1867. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen S, Shimoda M, Chen J and Grayburn PA:
Stimulation of adult resident cardiac progenitor cells by durable
myocardial expression of thymosin beta 4 with ultrasound-targeted
microbubble delivery. Gene Ther. 20:225–233. 2013. View Article : Google Scholar
|
|
9
|
Sonoda S, Tachibana K, Uchino E, et al:
Gene transfer to corneal epithelium and keratocytes mediated by
ultrasound with microbubbles. Invest Ophthalmol Vis Sci.
47:558–564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen S, Shimoda M, Wang MY, et al:
Regeneration of pancreatic islets in vivo by ultrasound-targeted
gene therapy. Gene Ther. 17:1411–1420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li HL, Zheng XZ, Wang HP, Li F, Wu Y and
Du LF: Ultrasound-targeted microbubble destruction enhances
AAV-mediated gene transfection in human RPE cells in vitro and rat
retina in vivo. Gene Ther. 16:1146–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie W, Liu S, Su H, Wang Z, Zheng Y and Fu
Y: Ultrasound microbubbles enhance recombinant adeno-associated
virus vector delivery to retinal ganglion cells in vivo. Acad
Radiol. 17:1242–1248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sonoda S, Tachibana K, Yamashita T, et al:
Selective gene transfer to the retina using intravitreal ultrasound
irradiation. J Ophthalmol. 2012:4127522012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hippert C, Ibanes S, Serratrice N, et al:
Corneal transduction by intra-stromal injection of AAV vectors in
vivo in the mouse and ex vivo in human explants. PLoS One.
7:e353182012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamata Y, Okuyama T, Kosuga M, et al:
Adenovirus-mediated gene therapy for corneal clouding in mice with
mucopolysac-charidosis type VII. Mol Ther. 4:307–312. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klausner EA, Peer D, Chapman RL, Multack
RF and Andurkar SV: Corneal gene therapy. J Control Release.
124:107–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu HA, Liu YL, Ma ZZ, Wang JC and Zhang
Q: A lipid nanoparticle system improves siRNA efficacy in RPE cells
and a laser-induced murine CNV model. Invest Ophthalmol Vis Sci.
52:4789–4794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shafaa MW, El Shazly LH, El Shazly AH, El
gohary AA and El hossary GG: Efficacy of topically applied
liposome-bound tetracycline in the treatment of dry eye model. Vet
Ophthalmol. 14:18–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dalkara D, Kolstad KD, Caporale N, et al:
Inner limiting membrane barriers to AAV-mediated retinal
transduction from the vitreous. Mol Ther. 17:2096–2102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peyman GA, Lad EM and Moshfeghi DM:
Intravitreal injection of therapeutic agents. Retina. 29:875–912.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu H and Chen TC: The effects of
intravitreal ophthalmic medications on intraocular pressure. Semin
Ophthalmol. 24:100–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peeters L, Lentacker I, Vandenbroucke RE,
et al: Can ultrasound solve the transport barrier of the neural
retina? Pharm Res. 25:2657–2665. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du J, Shi QS, Sun Y, et al: Enhanced
delivery of monomethoxypoly(ethylene
glycol)-poly(lactic-co-glycolic acid)-poly l-lysine nanoparticles
loading platelet-derived growth factor BB small interfering RNA by
ultrasound and/or microbubbles to rat retinal pigment epithelium
cells. J Gene Med. 13:312–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bishop P: The biochemical structure of
mammalian vitreous. Eye (Lond). 10(Pt 6): 664–670. 1996. View Article : Google Scholar
|
|
25
|
Peeters L, Sanders NN, Braeckmans K, et
al: Vitreous: a barrier to nonviral ocular gene therapy. Invest
Ophthalmol Vis Sci. 46:3553–3561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishikawa M and Huang L: Nonviral vectors
in the new millennium: delivery barriers in gene transfer. Hum Gene
Ther. 12:861–870. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manickan E, Smith JS, Tian J, et al: Rapid
Kupffer cell death after intravenous injection of adenovirus
vectors. Mol Ther. 13:108–117. 2006. View Article : Google Scholar
|
|
28
|
Igarashi T, Miyake N, Fujimoto C, et al:
Adeno-associated virus type 8 vector-mediated expression of siRNA
targeting vascular endothelial growth factor efficiently inhibits
neovascularization in a murine choroidal neovascularization model.
Mol Vis. 20:488–496. 2014.PubMed/NCBI
|
|
29
|
Huang C, Cen LP, Liu L, et al:
Adeno-associated virus-mediated expression of growth-associated
protein-43 aggravates retinal ganglion cell death in experimental
chronic glaucomatous injury. Mol Vis. 19:1422–1432. 2013.PubMed/NCBI
|
|
30
|
Ikeda Y, Yonemitsu Y, Miyazaki M, et al:
Acute toxicity study of a simian immunodeficiency virus-based
lentiviral vector for retinal gene transfer in nonhuman primates.
Hum Gene Ther. 20:943–954. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cavazzana-Calvo M and Fischer A: Gene
therapy for severe combined immunodeficiency: are we there yet? J
Clin Invest. 117:1456–1465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Storb R, Lee D, et al: Immune
responses to AAV in canine muscle monitored by cellular assays and
noninvasive imaging. Mol Ther. 18:617–624. 2010. View Article : Google Scholar :
|
|
33
|
Wilson JM: Lessons learned from the gene
therapy trial for ornithine transcarbamylase deficiency. Mol Genet
Metab. 96:151–157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu J, Zhang S, Wu X, et al: Enhanced
transduction and improved photoreceptor survival of retinal
degeneration by the combinatorial use of rAAV2 with a lower dose of
adenovirus. Vision Res. 48:1648–1654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Provost N, Le Meur G, Weber M, et al:
Biodistribution of rAAV vectors following intraocular
administration: evidence for the presence and persistence of vector
DNA in the optic nerve and in the brain. Mol Ther. 11:275–283.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park HJ, Yang F and Cho SW: Nonviral
delivery of genetic medicine for therapeutic angiogenesis. Adv Drug
Deliv Rev. 64:40–52. 2012. View Article : Google Scholar
|
|
37
|
Nayerossadat N, Maedeh T and Ali PA: Viral
and nonviral delivery systems for gene delivery. Adv Biomed Res.
1:272012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bloquel C, Bourges JL, Touchard E, Berdugo
M, BenEzra D and Behar-Cohen F: Non-viral ocular gene therapy:
potential ocular therapeutic avenues. Adv Drug Deliv Rev.
58:1224–1242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thrimawithana TR, Young S, Bunt CR, Green
C and Alany RG: Drug delivery to the posterior segment of the eye.
Drug Discov Today. 16:270–277. 2011. View Article : Google Scholar
|
|
40
|
Gaudana R, Ananthula HK, Parenky A and
Mitra AK: Ocular drug delivery. AAPS J. 12:348–360. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jayaraman MS, Bharali DJ, Sudha T and
Mousa SA: Nano chitosan peptide as a potential therapeutic carrier
for retinal delivery to treat age-related macular degeneration. Mol
Vis. 18:2300–2308. 2012.PubMed/NCBI
|
|
42
|
Zhou H, Yang L, Li H, et al:
Downregulation of VEGF mRNA expression by triamcinolone acetonide
acetate-loaded chitosan derivative nanoparticles in human retinal
pigment epithelial cells. Int J Nanomedicine. 7:4649–4660.
2012.PubMed/NCBI
|
|
43
|
Bainbridge JW, Tan MH and Ali RR: Gene
therapy progress and prospects: the eye. Gene Ther. 13:1191–1197.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han S, Mahato RI, Sung YK and Kim SW:
Development of biomaterials for gene therapy. Mol Ther. 2:302–317.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamashita T, Sonoda S, Suzuki R, et al: A
novel bubble liposome and ultrasound-mediated gene transfer to
ocular surface: RC-1 cells in vitro and conjunctiva in vivo. Exp
Eye Res. 85:741–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luo J, Zhou X, Diao L and Wang Z:
Experimental research on wild-type p53 plasmid transfected into
retinoblastoma cells and tissues using an ultrasound microbubble
intensifier. J Int Med Res. 38:1005–1015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Daigeler A, Chromik AM, Haendschke K, et
al: Synergistic effects of sonoporation and taurolidin/TRAIL on
apoptosis in human fibrosarcoma. Ultrasound Med Biol. 36:1893–1906.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin CY, Liu TM, Chen CY, et al:
Quantitative and qualitative investigation into the impact of
focused ultrasound with microbubbles on the triggered release of
nanoparticles from vasculature in mouse tumors. J Control Release.
146:291–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Y, Zhou J, Zhang Y, Wang X and Chen
J: Delivery of TFPI-2 using SonoVue and adenovirus results in the
suppression of thrombosis and arterial re-stenosis. Exp Biol Med
(Maywood). 235:1072–1081. 2010. View Article : Google Scholar
|
|
50
|
Sirsi SR and Borden MA: Advances in
ultrasound mediated gene therapy using microbubble contrast agents.
Theranostics. 2:1208–1222. 2012. View Article : Google Scholar
|
|
51
|
Liang HD, Tang J and Halliwell M:
Sonoporation, drug delivery and gene therapy. Proc Inst Mech Eng H.
224:343–361. 2010. View Article : Google Scholar
|
|
52
|
Lawrie A, Brisken AF, Francis SE, et al:
Ultrasound enhances reporter gene expression after transfection of
vascular cells in vitro. Circulation. 99:2617–2620. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Taniyama Y, Tachibana K, Hiraoka K, et al:
Development of safe and efficient novel nonviral gene transfer
using ultrasound: enhancement of transfection efficiency of naked
plasmid DNA in skeletal muscle. Gene Ther. 9:372–380. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou Y, Yang K, Cui J, Ye JY and Deng CX:
Controlled permeation of cell membrane by single bubble acoustic
cavitation. J Control Release. 157:103–111. 2012. View Article : Google Scholar :
|
|
55
|
Chen H, Kreider W, Brayman AA, Bailey MR
and Matula TJ: Blood vessel deformations on microsecond time scales
by ultrasonic cavitation. Phys Rev Lett. 106:0343012011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hauser J, Ellisman M, Steinau HU, Stefan
E, Dudda M and Hauser M: Ultrasound enhanced endocytotic activity
of human fibroblasts. Ultrasound Med Biol. 35:2084–2092. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li W, Liu S, Ren J, Xiong H, Yan X and
Wang Z: Gene transfection to retinal ganglion cells mediated by
ultrasound microbubbles in vitro. Acad Radiol. 16:1086–1094. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou XY, Liao Q, Pu YM, et al:
Ultrasound-mediated microbubble delivery of pigment
epithelium-derived factor gene into retina inhibits choroidal
neovascularization. Chin Med J (Engl). 122:2711–2717. 2009.
|
|
59
|
Lee SY, Huh MS, Lee S, et al: Stability
and cellular uptake of polymerized siRNA
(poly-siRNA)/polyethylenimine (PEI) complexes for efficient gene
silencing. J Control Release. 141:339–346. 2010. View Article : Google Scholar
|
|
60
|
Howard CM, Forsberg F, Minimo C, Liu JB,
Merton DA and Claudio PP: Ultrasound guided site specific gene
delivery system using adenoviral vectors and commercial ultrasound
contrast agents. J Cell Physiol. 209:413–421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Geers B, Lentacker I, Alonso A, et al:
Elucidating the mechanisms behind sonoporation with
adeno-associated virus-loaded microbubbles. Mol Pharm. 8:2244–2251.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li F, Jin L, Wang H, et al: The dual
effect of ultrasound-targeted microbubble destruction in mediating
recombinant adeno-associated virus delivery in renal cell
carcinoma: transfection enhancement and tumor inhibition. J Gene
Med. 16:28–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mueller C and Flotte TR: Clinical gene
therapy using recombinant adeno-associated virus vectors. Gene
Ther. 15:858–863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zheng X, Du L, Wang H and Gu Q: A novel
approach to attenuate proliferative vitreoretinopathy using
ultrasound-targeted microbubble destruction and recombinant
adeno-associated virus-mediated RNA interference targeting
transforming growth factor-beta2 and platelet-derived growth
factor-B. J Gene Med. 14:339–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zheng XZ, Wu Y, Li HL, Du LF, Wang HP and
Gu Q: Comparative analysis of the effects of ultrasound-targeted
microbubble destruction on recombinant adeno-associated virus-and
plasmid-mediated transgene expression in human retinal pigment
epithelium cells. Mol Med Rep. 2:937–942. 2009.PubMed/NCBI
|
|
66
|
Müller OJ, Schinkel S, Kleinschmidt JA,
Katus HA and Bekeredjian R: Augmentation of AAV-mediated cardiac
gene transfer after systemic administration in adult rats. Gene
Ther. 15:1558–1565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jin LF, Li F, Wang HP, Wei F, Qin P and Du
LF: Ultrasound targeted microbubble destruction stimulates cellular
endocytosis in facilitation of adeno-associated virus delivery. Int
J Mol Sci. 14:9737–9750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
van Wamel A, Kooiman K, Harteveld M, et
al: Vibrating microbubbles poking individual cells: drug transfer
into cells via sonoporation. J Control Release. 112:149–155. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sorace AG, Warram JM, Umphrey H and Hoyt
K: Microbubble-mediated ultrasonic techniques for improved
chemotherapeutic delivery in cancer. J Drug Target. 20:43–54. 2012.
View Article : Google Scholar :
|
|
70
|
Heath CH, Sorace A, Knowles J, Rosenthal E
and Hoyt K: Microbubble therapy enhances anti-tumor properties of
cisplatin and cetuximab in vitro and in vivo. Otolaryngol Head Neck
Surg. 146:938–945. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lee NG, Berry JL, Lee TC, et al:
Sonoporation enhances chemotherapeutic efficacy in retinoblastoma
cells in vitro. Invest Ophthalmol Vis Sci. 52:3868–3873. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zheng MM, Zhou XY, Wang LP and Wang ZG:
Experimental research of RB94 gene transfection into retinoblastoma
cells using ultrasound-targeted microbubble destruction. Ultrasound
Med Biol. 38:1058–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Campbell M, Nguyen AT, Kiang AS, et al: An
experimental platform for systemic drug delivery to the retina.
Proc Natl Acad Sci USA. 106:17817–17822. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Baseri B, Choi JJ, Tung YS and Konofagou
EE: Multi-modality safety assessment of blood-brain barrier opening
using focused ultrasound and definity microbubbles: a short-term
study. Ultrasound Med Biol. 36:1445–1459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu HL, Hua MY, Chen PY, et al:
Blood-brain barrier disruption with focused ultrasound enhances
delivery of chemotherapeutic drugs for glioblastoma treatment.
Radiology. 255:415–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Park J, Zhang Y, Vykhodtseva N, Akula JD
and McDannold NJ: Targeted and reversible blood-retinal barrier
disruption via focused ultrasound and microbubbles. PLoS One.
7:e427542012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sheikov N, McDannold N, Sharma S and
Hynynen K: Effect of focused ultrasound applied with an ultrasound
contrast agent on the tight junctional integrity of the brain
microvascular endothelium. Ultrasound Med Biol. 34:1093–1104. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kowalczuk L, Boudinet M, El Sanharawi M,
et al: In vivo gene transfer into the ocular ciliary muscle
mediated by ultrasound and microbubbles. Ultrasound Med Biol.
37:1814–1827. 2011. View Article : Google Scholar : PubMed/NCBI
|