Introduction
The podocyte is the key component of the glomerular
slit diaphragm. The podocyte and its associated proteins form the
diaphragm and these cells have an intimate interaction with
capillary endothelial cells, which exhibit an important role in
selective filtration acting as a barrier. Previously, several
studies have identified podocyte-associated proteins as the main
factors in nephropathy demonstrating glomerular basement membrane
alteration, such as in diabetic nephropathy (1,2).
Notably, the blood-brain barrier of the central nervous system,
another representative homeostatic barrier system in the human
body, contains astrocytes, which are similar to podocytes in
structure and function (3,4).
Nephrin is a podocyte-associated protein, a
constituent of the slit diaphragm of the glomerulus, which appears
to have a critical role in maintaining glomerular filtration.
Previously, inactivation of the nephrin gene in the mouse embryo
demonstrated a detrimental loss of the ultrafiltration function of
the kidneys (5).
Similar to the kidney, the placenta is a major organ
of the fetus, which filtrates and exchanges fetal and maternal
blood, enabling the placental cotyledon and the glomerulus to
undertake homeostatic functions. Notably, the expression of the
nephrin gene has been reported in the fetal membranes and placenta
of pregnant Sprague-Dawley rats (6). This led us to hypothesize that
nephrin may be involved in regulating homeostasis at the
maternal-fetal interface. However, thus far, evidence of nephrin
expression in the human placenta is lacking. Therefore, the
objective of the present study was to evaluate the presence and
localization of nephrin, a signature molecule of podocytes
(7), in the human placenta.
Materials and methods
Participants
Females that were pregnant with a single fetus, who
delivered a normal infant between 37 and 40 weeks of gestation by
Cesarean section at Severance Hospital, Yonsei University College
of Medicine (Seoul, Republic of Korea), between January 2011 and
July 2012 were enrolled in the present study. Patients with a
history of active labor prior to the surgery, multiple pregnancies,
prior or current diagnosis of any medical illness (diabetes,
gestational diabetes, hypertension, thyroid disease or infectious
disease), placenta previa, fetal anomaly, oligohydramnios,
hydramnios, fetal aneuploidy, preterm labor or premature rupture of
the membranes during the present pregnancy were excluded from the
present study. The present study was approved by the Institutional
Review Board of Yonsei University College of Medicine and all
patients consented to be involved in the present study.
Sample collection
When the placenta was delivered, the amnion and
chorion were dissected under aseptic conditions. Dissected
membranes were sampled at a size of 1.0×1.0×1.0 cm3.
Villous tissue measuring 1.0×1.0×1.0 cm3 was sampled at
the site near the cord insertion. Following the tissue sampling,
the amnion, chorion and villous tissue were frozen in liquid
nitrogen and stored for further analysis.
Quantification of mature mRNA levels
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
RNA fractions were initially isolated from the
tissue samples using an RNA extraction kit (Intron Biotechnology,
Inc., Gyeonggi, Korea). RNA concentrations were measured using
absorbance at 260/280 nm with a SuperScript First-Strand RT kit
(Invitrogen Life Technologies, Carlsbad, CA, USA). Total RNA (1
µg) was converted to cDNA using oligo-dT as the primer and
reverse transcriptase (Invitrogen Life Technologies). The reaction
mixture (20 µl) was incubated at 65°C for 5 min, 50°C for 50
min and 85°C for 5 min. RT-qPCR was performed with Applied
Biosystems 7500 real-time PCR system (Applied Biosystems Life
Technologies, Foster City, CA, USA) using the Taqman®
Gene Expression Assays kit (Taqman Life Technologies, Foster City,
CA, USA). A total of 20 µl of the RT-qPCR reaction mixture
contained 2 µl RT-qPCR products, 10 µl
Taqman® 2X universal PCR master mix and 1 µl 20X
Taqman® Gene Expression Assay mix (RT-qPCR primers).
Nuclease-free water was used to adjust the final volume to 20
µl. The reactions were incubated in a 96-well optical plate
at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and
60°C for 60 sec. Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was used as an endogenous reference gene for
normalizing the results. The relative abundance of each mRNA was
calculated using the comparative cycle threshold
2(−∆∆Ct) method. Individual samples were assayed in
triplicate, with three independent biological replicates. The
primer sequences for the endogenous control (GAPDH) and
nephrin are shown in Table I.
 | Table ISequence of primers for GAPDH
and the human nephrin gene. |
Table I
Sequence of primers for GAPDH
and the human nephrin gene.
| Gene | Primer sequence
(5′–3′) | Size (bp) |
|---|
| GAPDH |
| Sense |
AGGCCAACCGCGAGAAGATGACC | |
| Antisense |
GAAGTCCAGGGCGACGTAGCAC | 320 |
| Human nephrin |
| Sense |
CCAACATCGTTTTCACTTGG | |
| Antisense |
GGGTGGTACGACATCCACAT | 349 |
Western blotting
Nephrin expression was examined using western
blotting analysis of the tissue homogenate samples. Following
isolating each protein using the cell lysis buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA), 30 µg of total protein
loading was used to run 8% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and then transferred to polyvinylidene
difluoride membranes (Millipore, Etten-Leur, The Netherlands). The
blot membranes were blocked with 5% skimmed milk in Tris-buffered
saline-Tween 20 (containing 10 mM Tris, pH 8.0, 150 mM NaCl,
0.0025% Tween-20) at room temperature for 1 h. Subsequently, the
membrane was incubated overnight at 4°C with specific antibodies
against nephrin (polyclonal rabbit anti-human, 1:1,000; #2265;
ProSci, Portway, CA, USA) and β-actin (monoclonal mouse anti-human,
1:2,000; A5441; Sigma-Aldrich, St. Louis, MO, USA). The membrane
was then washed six times with TBS-T for 5 min. Thereafter, the
membrane was incubated with anti-rabbit antibody (IgG, 1:4,000;
ab6802; Abcam, Cambridge, MA, USA) and anti-mouse antibody
conjugated with horseradish peroxidase (IgG, 1:5,000; ab6808;
Abcam) for 1 h at room temperature. The membrane was washed eight
times with TBS-T for 5 min at room temperature. The protein was
visualized on the ImageQuant LAS-4000 imager using enhanced
chemiluminescence (GE Healthcare, Arlington Heights, IL, USA).
Relative densities for nephrin expression were normalized using
tubulin expression for each sample.
Immunofluorescence (IF)
Cells were fixed with acetone for 15 min and washed
three times with PBS-T [PBS/0.1% Tween-20 (v/v)]. Following
blocking with 5% goat serum for 1 h, the cells were incubated with
anti-nephrin antibody (polyclonal rabbit anti-human, 1:1,000;
#2265; ProSci) overnight at 4°C. Negative controls consisted of
cells incubated with rabbit IgG (1:200; cat no. ab27478; Abcam) at
the same concentration as the antibody against nephrin. The cells
were washed three times with PBS-T and incubated with goat
polyclonal secondary antibody to rabbit IgG (1:200,
DyLight® 594; cat no. ab96901; Abcam) for 1 h. The cells
were washed three times in PBS-T and mounted with a fluorescent
mounting medium (Vector Laboratories, Inc., Burlingame, CA, USA).
Fluorescent microscopy was performed using the LSM700 microscope
(Carl Zeiss; Oberkochen, Germany).
Statistical analysis
Statistical analysis was performed using the SPSS
software package, version 18.0 (IBM Inc., Armonk, NY, USA). The
mRNA expression level of the nephrin gene was expressed as the mean
± standard deviation. Descriptive statistics were used for the
baseline characteristics and the linear mixed model for comparing
the mRNA expression of the nephrin gene in the villi, chorion and
amnion. P<0.05 was considered to indicate a statistically
significant difference.
Results
In all, nine healthy pregnant females were enrolled
in the present study. The basal characteristics of the patients are
shown in Table II. Due to the
small sample size, data was expressed as the median and the range.
One participant had a history of a previous cesarean section and
concurrent myomectomy.
 | Table IIClinical characteristics of
pregnancies included in the present study. |
Table II
Clinical characteristics of
pregnancies included in the present study.
| Characteristic | Variable |
|---|
| Maternal age
(years) | 34 (30–42) |
| Primigravida (n) | 2 |
| Nulliparity (n) | 4 |
| Gestational days | 38±3 (37±3–39±1) |
| Cesarean section
indication (n) |
| Prior cesarean
section | 6 |
| Prior
myomectomy | 1 |
| Advanced maternal
age | 1 |
| Cephalopelvic
disproportion | 2 |
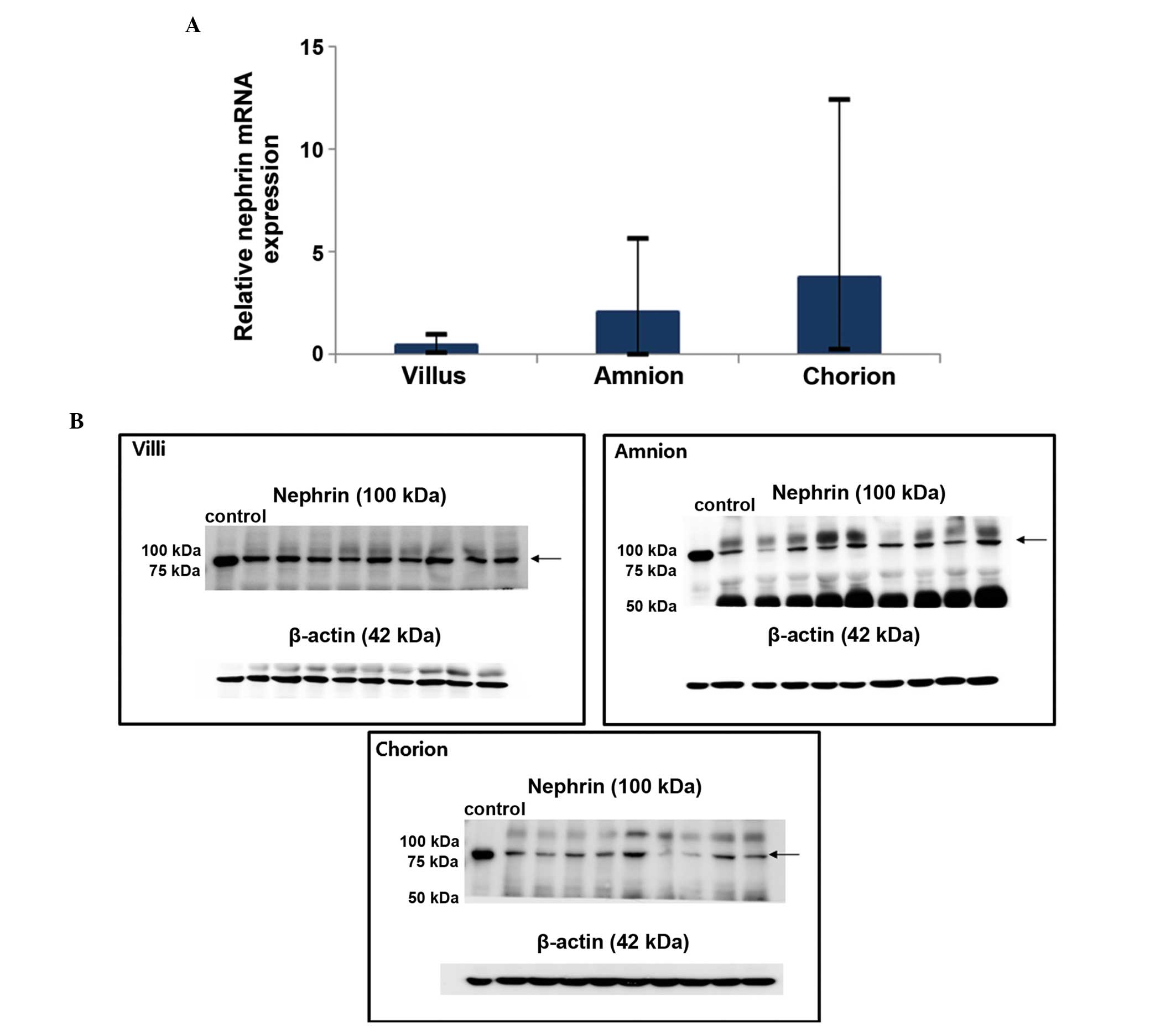
mRNA and protein expression in the
placenta
The expression of nephrin mRNA and protein in the
uteroplacental unit were confirmed using RT-qPCR and western
blotting, respectively (Fig. 1).
The gene expression was relatively higher in the chorion when
compared with the villus and amnion (mean, 2−ΔΔCt
3.79±4.49 vs. 0.50±0.45 and 2.12±2.41); however, the difference was
not statistically significant (P=0.37).
Immunolocalization of nephrin
Marked positive staining for nephrin was observed in
the villi, chorion and amnion in the IF studies. Nephrin expression
was clearly localized to the syncytiotrophoblast and endothelial
cells of the arteries and veins of the chorionic plate, where the
labeling was pronounced at the apical membrane of the
syncytiotrophoblast. In the fetal membranes, intense
immunoreactivity was localized to the cuboidal amniotic epithelium,
particularly at the apical membrane and in the stromal cells
(Fig. 2).
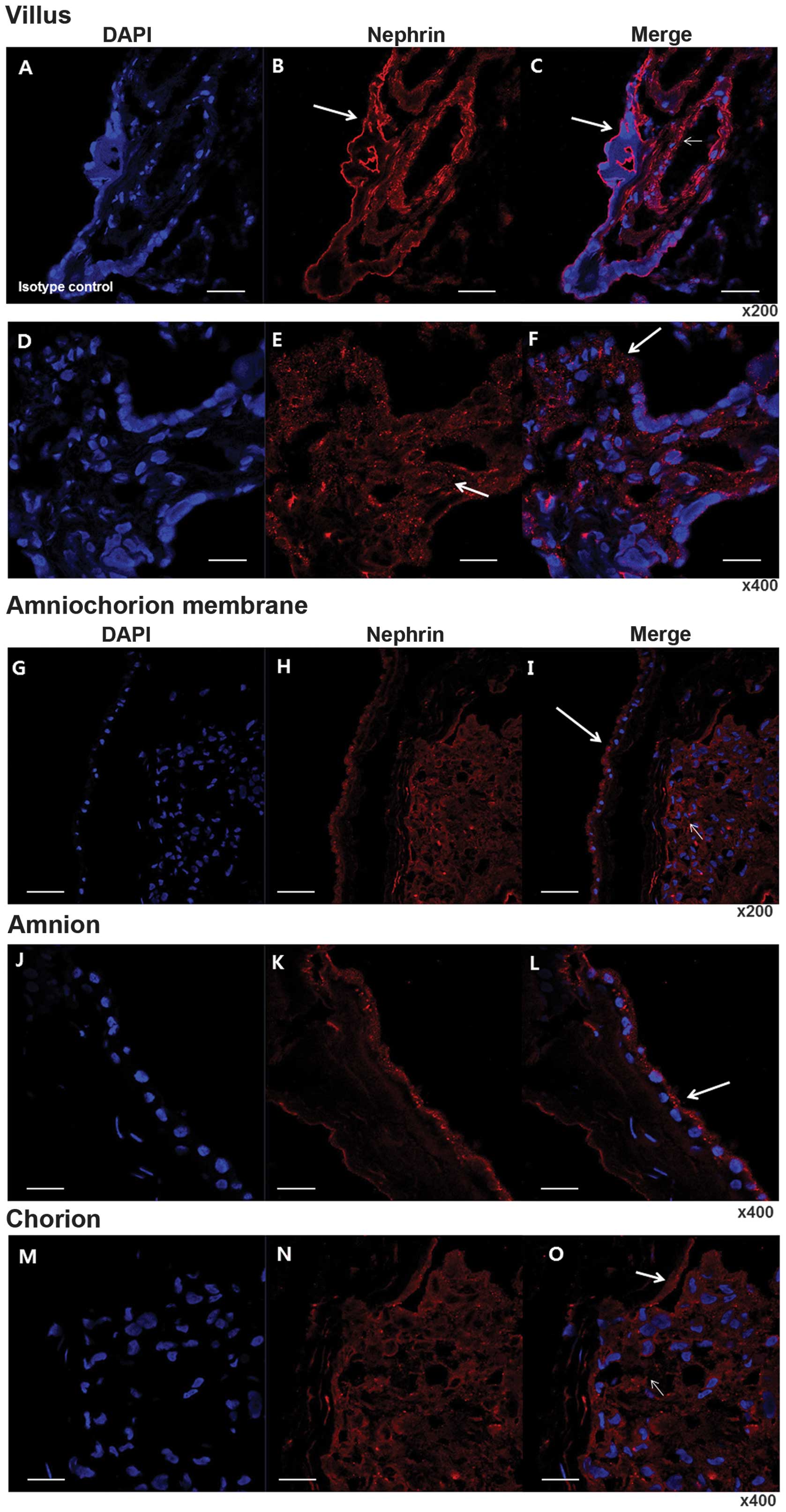 | Figure 2Immunolocalization of nephrin in the
placenta using immunofluorescence. (A–C) Immunofluorescence
staining of isotype control with DAPI and nephrin in the villi.
Magnification, ×200. (D–F) Magnification, ×400. Note that
immunoreactive staining is distributed over almost all villous
cytotrophoblasts (arrows in B, E and F) and endothelium of
intravillous vessels (small arrow in E). (G–I) Immunofluorescence
staining of isotype control with DAPI and nephrin in fetal
membranes. Magnification, ×200. (J–L) Immunofluorescence staining
of isotype control with DAPI and nephrin in amnion. Magnification,
×400. (M–O) Immunofluorescence staining of isotype control with
DAPI and nephrin in chorion. Magnification, ×400. A specific
distribution at membranous lining (arrows in I, L and O), in
addition to in fibroblasts in the chorion (small arrow in I and O).
Note that specifically in the amnion, the outer lining of
amniocytes was markedly positive (arrows in I and L). Bar =50
µm. DAPI, 4′6-diamidino-2-phenylindole. |
Discussion
The main role of the placenta is the separation of
maternal and fetal circulation, while also facilitating the
transport of nutrients and substances from the mother to the fetus.
As the placenta has extensive contact between the mother and the
fetus in structures, such as the villus, amnion-chorion and the
chorion-decidua, it is hypothesized that a filtering, barrier-like
structure exists within the placenta at such interfaces. As
hypothesized, evidence of the presence of nephrin in cells at the
maternal-fetal interface was identified and immunoreactivity to
nephrin was most prominent at the apical membrane of the
syncytiotrophoblasts and amniotic epithelium, and in the stromal
cells of the chorion. The present findings suggest the existence of
a barrier-like structure formed by nephrin in the placenta similar
to that of the glomerulus.
Although the present study did not investigate the
function of nephrin in the placenta, it is likely that in the
placenta, the protein may have a role similar to that observed in
other organs (8–13). Its presence in astrocytes, which
interact with the capillaries in the blood-brain barrier of the
central nervous system suggested it exhibits a similar role as in
the glomerular slit membrane (13). The role for nephrin as a barrier
system has also been suggested in the testis due to the observation
of nephrin in the Sertoli cells colocalized with zona occludens-1
along the basement membrane of the seminiferous tubule in the
testis (9). However, a previous
study demonstrated that nephrin is expressed in the radial glial
cells, which are involved in the directional migration of neurons
and development of glial cell lineages (14), and that it binds with glutamate
receptors and scaffolding molecules of the primary neuronal cells
(5). Furthermore, its active role
in the vesicle and actin interaction involved in insulin release
was described in the pancreatic β islet cells (11,12).
These findings suggest that nephrin not only functions as a
barrier, but also exhibits functional roles, such as in cell
maturation and development, cell-to-cell interaction and signaling
(15–19).
It has been hypothesized that nephrin, expressed in
the villi and the membranes holds such functions as well as other
organs. Nephrin may be involved in the seclusion of fetal materials
from the maternal circulation and thereby provide an
immunologically anergic environment, which may be crucial to the
exchange of nutrients between the mother and the fetus or it may
have a role in placental development. If so, impairment of
placental nephrin may result in an adverse pregnancy outcome, such
as preeclampsia, restricted fetal growth or abnormal placental
growth. As serum from a patient with preeclampsia has been revealed
to damage the podocyte and shed nephrin via endothelin-mediated
endothelin-1 (20), theoretically,
nephrin in the syncytiotrophoblasts or the chorioamniotic membranes
in close contact with the maternal serum may also be affected or
disrupted via a similar mechanism. This may partly contribute to
the pathophysiological mechanism of adverse fetal outcomes.
To the best of our knowledge, the present study is
the first to provide evidence of the presence of nephrin in the
human placenta and placental membranes. The identification of
podocyte-associated nephrin expression in the placenta,
particularly at the maternal-fetal interface, provides novel
insight into the molecular basis of the selective permeability of
the placental barrier, which requires further elucidation. The
present findings highlight the requirement for further
investigation into the placental expression of podocyte-associated
proteins in pregnancies with complications and their specific roles
in the human placenta.
Acknowledgments
This study was supported by the National Research
Foundation of Korea funded by the Korean Government (grant no.
2011-0004069/7-2011-0208).
References
|
1
|
Tryggvason K and Wartiovaara J: Molecular
basis of glomerular permselectivity. Curr Opin Nephrol Hypertens.
10:543–549. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cooper ME, Mundel P and Boner G: Role of
nephrin in renal disease including diabetic nephropathy. Semin
Nephrol. 22:393–398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hennessy A and Makris A: Preeclamptic
nephropathy. Nephrology (Carlton). 16:134–143. 2011. View Article : Google Scholar
|
|
4
|
Son GH, Kim JH, Hwang JH, Kim YH, Park YW
and Kwon JY: Urinary excretion of nephrin in patients with severe
preeclampsia. Urinary nephrin in preeclampsia. Hypertens Pregnancy.
30:408–413. 2011. View Article : Google Scholar
|
|
5
|
Putaala H, Soininen R, Kilpeläinen P,
Wartiovaara J and Tryggvason K: The murine nephrin gene is
specifically expressed in kidney, brain and pancreas: inactivation
of the gene leads to massive proteinuria and neonatal death. Hum
Mol Genet. 10:1–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beall MH, Amidi F, Gayle DA, Wang S,
Beloosesky R and Ross MG: Placental and fetal membrane Nephrin and
Neph1 gene expression: response to inflammation. J Soc Gynecol
Investig. 12:298–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Welsh GI and Saleem MA: Nephrin-signature
molecule of the glomerular podocyte? J Pathol. 220:328–337.
2010.
|
|
8
|
Aström E, Rinta-Valkama J, Gylling M,
Ahola H, Miettinen A, Timonen T and Holthöfer H: Nephrin in human
lymphoid tissues. Cell Mol Life Sci. 63:498–504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Aya K, Tanaka H, Shimizu J, Ito S
and Seino Y: Nephrin is an important component of the barrier
system in the testis. Acta Med Okayama. 55:161–165. 2001.PubMed/NCBI
|
|
10
|
Putaala H, Sainio K, Sariola H and
Tryggvason K: Primary structure of mouse and rat nephrin cDNA and
structure and expression of the mouse gene. J Am Soc Nephrol.
11:991–1001. 2000.PubMed/NCBI
|
|
11
|
Fornoni A, Jeon J, Varona Santos J, et al:
Nephrin is expressed on the surface of insulin vesicles and
facilitates glucose-stimulated insulin release. Diabetes.
59:190–199. 2010. View Article : Google Scholar
|
|
12
|
Rastaldi MP, Armelloni S, Berra S,
Calvaresi N, Corbelli A, Giardino LA, Li M, Wang GQ, Fornasieri A,
Villa A, et al: Glomerular podocytes contain neuron-like functional
synaptic vesicles. FASEB J. 20:976–978. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buniatian GH, Hartmann HJ, Traub P,
Wiesinger H, Albinus M, Nagel W, Shoeman R, Mecke D and Weser U:
Glial fibrillary acidic protein-positive cells of the kidney are
capable of raising a protective biochemical barrier similar to
astrocytes: expression of metallothionein in podocytes. Anat Rec.
267:296–306. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Armelloni S, Ikehata M, Corbelli A,
Pesaresi M, Calvaresi N, Giardino L, Mattinzoli D, Nistico F,
Andreoni S, et al: Nephrin expression in adult rodent central
nervous system and its interaction with glutamate receptors. J
Pathol. 225:118–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grant SG, O'Dell TJ, Karl KA, Stein PL,
Soriano P and Kandel ER: Impaired long-term potentiation, spatial
learning, and hippocampal development in fyn mutant mice. Science.
258:1903–1910. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huber TB and Benzing T: The slit
diaphragm: a signaling platform to regulate podocyte function. Curr
Opin Nephrol Hypertens. 14:211–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li M, Armelloni S, Edefonti A, Messa P and
Rastaldi MP: Fifteen years of research on nephrin: what we still
need to know. Nephrol Dial Transplant. 28:767–770. 2013. View Article : Google Scholar
|
|
18
|
Verma R, Wharram B, Kovari I, Kunkel R,
Nihalani D, Wary KK, Wiggins RC, Killen P and Holzman LB: Fyn binds
to and phosphorylates the kidney slit diaphragm component Nephrin.
J Biol Chem. 278:20716–20723. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wagner N, Morrison H, Pagnotta S, Michiels
JF, Schwab Y, Tryggvason K, Schedl A and Wagner KD: The podocyte
protein nephrin is required for cardiac vessel formation. Hum Mol
Genet. 20:2182–2194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collino F, Bussolati B, Gerbaudo E, et al:
Preeclamptic sera induce nephrin shedding from podocytes through
endothelin-1 release by endothelial glomerular cells. Am J Physiol
Renal Physiol. 294:F1185–F1194. 2008. View Article : Google Scholar : PubMed/NCBI
|