Introduction
Gastric cancer is one of the most prevalent
malignancies in Eastern Asia and it is one of the leading causes of
cancer-related deaths worldwide (1). To date, chemotherapy is still an
important treatment for patients with advanced gastric cancer.
However, the efficacy of chemotherapeutic agents is severely
limited due to chemoresistance and adverse side effects. Evidence
has shown that chemotherapy sensitivity and chemoresistance are
tightly correlated with signal transduction pathways and genetic
events. Recently, kinase suppressor of Ras 1 (KSR1) and its
downstream extracellular signal-regulated kinase (ERK) signaling
pathway have received much attention (2).
KSR1 is an essential scaffold protein of the
Ras/Raf/MAPK cascade that facilitates the activation of ERK
(3). Studies have shown that the
expression of KSR1 is upregulated in many types of cancer and is
required for cell proliferation, apoptosis and cell-cycle
reinitiation (4–6). Moreover, KSR1 was confirmed to
contribute to tumorigenesis through the MAPK cascade in a mouse
model (7,8). In addition, a screening experiment
showed that the expression of KSR1 is correlated with cancer cell
sensitivity to anticancer drugs (9). Metastasis suppressor nm23-H1 was found
to bind directly to KSR1 and modulate the scaffold binding
patterns, therefore facilitating its degradation and decreased ERK
activation, resulting in elevated tumor cell sensitivity to cancer
therapeutics (10). Our previous
study also showed that etoposide activated the MAPK/ERK signaling
pathway, which reduced the chemotherapy sensitivity of gastric
cancer cells via suppressing expression of p53 and enhancing
expression of c-Myc (11). These
results indicate a significant role of the KSR1-mediated ERK
signaling pathway in the pathogenesis of tumors and it might be a
potential therapeutic target of chemotherapy resistance. The
identification of an effective agent with few adverse side effects
to suppress the KSR1-mediated ERK signaling pathway may be a
potential method to reverse chemotherapy resistance in gastric
cancer.
Ginkgo biloba extract (EGb), a natural
antioxidant, is a well-known and inexpensive herb that has been
used without side effects for centuries (12). Recently, EGb has attracted
considerable attention for its antitumor properties. EGb was able
to induce cell apoptosis, suppress cell proliferative, migration
and tumor progression of cancer (13–15).
Moreover, it was reported that EGb had chemopreventive effects in
cancer cells and rat models through antiproliferation, antioxidant,
anti-angiogenic and apoptosis-inducing activities (15,16).
Further study indicated that the proliferation, migration and tube
formation of endothelial cells were inhibited by EGb by inhibiting
the Raf-MEK-ERK cascade (17). In
gastric cancer, studies also showed that EGb increased
antioxidative activity and inhibited the progression of gastric
precancerous lesions and gastric cancer via regulation of cell
proliferation and apoptosis (18,19).
Our previous study also revealed that EGb 761 enhanced CDDP and
etoposide-induced apoptosis of gastric cancer cells possibly by
suppressing the protein expression of ERK and p-ERK (20). These findings provide a rational
basis for tumor prevention and adjuvant therapy using EGb 761, and
the KSR1/ERK signaling pathway may play an important role in this
process.
In the present study, correlations of KSR1, p-KSR1,
ERK and p-ERK expression with clinicopathological parameters were
investigated in gastric cancer tissues. Moreover, the effects of
EGb 761 on oxidative stress, the KSR1-mediated ERK signaling
pathway and the chemotherapy sensitivity of gastric cancer cells
and of multidrug resistant gastric cancer cells were investigated.
The aims were to investigate the role of the KSR1-mediated ERK
signaling pathway in tumor progression and development of
chemoresistance, and to explore the potential of EGb 761 in
enhancing the chemotherapeutic sensitivity and reversing the
chemoresistance of gastric cancer.
Materials and methods
Tissue specimens
A total of 62 fresh gastric cancer and matched
distant normal gastric tissues of patients were collected from the
First Affiliated Hospital of Guangxi Medical University, Guangxi,
China. All tissues were obtained from surgery; one-half of each
tissue was snap-frozen immediately in liquid nitrogen and stored at
−80°C and the other half was formalin-fixed and paraffin-embedded.
All patients had not received chemotherapy or radiation therapy
before tumor resection. This study was approved by the Medical
Ethics Committee of The First Affiliated Hospital of Guangxi
Medical University, Guangxi, China. Each patient provided consent
in a written informed consent form and the Ethics Committee
approved the consent procedure.
Immunohistochemical staining
Paraffin-embedded tissue blocks were serially
sectioned at 4 μm. After being deparaffinized and
rehydrated, the sections were treated with 3.0% hydrogen peroxide
in methanol and performed in a microwave for 15 min. Then, the
sections were blocked with normal rabbit serum followed by
incubation overnight at 4°C with rabbit anti-human monoclonal ERK
(1:100) and p-ERK (1:100) primary antibodies (both from Cell
Signaling Technology, Inc., Beverly, MA, USA) or rabbit anti-human
polyclonal KSR1 (1:200) and p-KSR1 (1:200) primary antibodies (both
from Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China).
The sections were washed with PBS and incubated with the secondary
antibody at room temperature for 30 min, and sections were stained
using a streptavidin-peroxidase detection system. Antibody binding
was visualized using diaminobenzidine as chromogen and
counterstained with hematoxylin. The sections incubated with PBS
instead of the primary antibody served as a negative control.
The positive staining of cancer cells was estimated
based on the extent and intensity. i) The extent of positive cells
was scored as: 0, positive-staining cells ≤5%; 1, positive-staining
cells 6–25%; 2, positive-staining cells 26–50%; and 3,
positive-staining cells >50%. ii) The intensity of staining was
scored as: 0, achromatic; 1, light yellow; 2, yellow; and 3, brown.
The scores from i and ii were multiplied to produce a weighted
score for each case, and the staining grade was defined as negative
(−, score ≤1), positive (+, score, ≥2 and <4) or strong positive
(++, score ≥4).
Real-time fluorescent quantitative
PCR
Total RNA was extracted from the gastric cancer and
matched normal tissues using TRIzol reagent (Invitrogen Co.,
Carlsbad, CA, USA). First-strand cDNA was synthesized from 1
μg of total-RNA using PrimeScript® First Strand
cDNA Synthesis kit (Takara Biotechnology Co., Ltd., Dalian,
Liaolin, China) according to the manufacturer’s instructions.
Real-time PCR was performed on the Applied Biosystems StepOne
Real-Time PCR system (Applied Biosystems) using the comparative Ct
quantitation method. The first-strand cDNA was subjected to PCR
amplification with 40 cycles consisting of 95°C for 2 min, 60°C for
30 sec, and 72°C for 5 min using the following primers: KSR1,
5′-AGG GCA TCG TAC ACA AAG ATC TCA-3′ (sense) and 5′-GGG ACA GCT
TTA GCT GGT TCT CAC-3′ (antisense); ERK1, 5′-CGT TGG TAC AGG GCT
CCA GAA-3′ (sense) and 5′-CTG CCA GAA TGC AGC CTA CAGA-3′
(antisense); ERK2, 5′-TCA TCG GCA TCC GAG ACA-3′ (sense) and 5′-TCT
CCA TCA GGT CCT GCA CAA-3′ (antisense); GAPDH, 5′-AAG GTG AAG GTC
GGA GTC AAC-3′ (sense) and 5′-GGG GTCA TTG ATG GCA ACA ATA-3′
(antisense). Ct values for duplicate samples were averaged and the
amounts of mRNA relative to hprt were calculated using the ∆∆Ct
method. All qRT-PCR reactions yielded products with single peak
dissociation curves.
Cell culture and survival analysis
Human gastric cancer SGC-7901 and multidrug
resistant SGC-7901/CDDP cell lines were obtained from the Shanghai
Institute of Cell Biology, Chinese Academy of Sciences Cell Bank.
Cells were cultivated in high glucose Dulbecco’s modified Eagle’s
medium (DMEM; Hyclone Co., Logan, UT, USA) supplemented with 10%
FBS in an atmosphere of 5% CO2 at 37°C. Cell viability
was determined using a colorimetric MTT assay (Sigma-Aldrich Co.,
St. Louis, MO, USA). In brief, the cells seeded in a 96-well plate
from the different groups were treated with CDDP, etoposide and/or
EGb 761. Cells treated with an equal amount of 0.9% NaCl instead of
the drugs served as the control group. Then 20 μl solution
of MTT was added to each well and incubated at 37°C for 4 h. The
solution was carefully removed, and dimethyl sulfoxide (DMSO)
(Invitrogen) was added to each well to solubilize MTT. The
absorbance (A) was measured at 490 nm, and the cell viability was
expressed as A value of the experimental cells/control cells
x100%.
Flow cytometric analysis
Cells were washed twice with PBS and resuspended in
binding buffer at a density of 1×106 cells/ml, and cell
apoptosis was detected using Annexin V-FITC/propidium iodine (PI)
kits (Roche Co. Ltd., Basel, Switzerland). In brief, Annexin V-FITC
was added to the sample and incubated for 20 min at room
temperature in the dark, and then 5 μl PI buffer was added
and incubated for 5 min at 4°C in the dark. Finally, the samples
were evaluated by flow cytometry, and data were analyzed using
CellQuest software.
Western blot analysis
Tissue or cell samples were lysed in lysis buffer,
and the lysate was incubated on ice for 20 min and centrifuged at
15,184 × g for 10 min at 4°C. The supernatant was collected for
protein detection, and the concentration of total protein was
evaluated by the BCA method. Then, 20 μg protein of each
sample was separated on SDS-PAGE and electroblotted onto a
nitrocellulose membrane followed by blocking with 5% non-fat milk
in TBST. The membrane was then incubated with rabbit anti-human
monoclonal β-actin (1:8,000) (Cell Signaling Technology), ERK
(1:500) and p-ERK (1:500) primary antibodies or rabbit anti-human
polyclonal KSR1 (1:200) and p-KSR1 (1:200) primary antibodies, and
subsequently incubated with a peroxidase-conjugated secondary
antibody. Finally, the protein signals were visualized using Pierce
enhanced chemiluminescence reaction Western Blotting Substrate
(Pierce Co., Rockford, IL, USA) and exposed to medical X-ray film.
The blotting bands were scanned and quantitated by a densitometer.
The relative expression level was expressed as the relative
absorbance (A) value of the target protein/β-actin.
Immunocytochemistry
Cells were plated onto slides fixed in a culture
dish at a density of 2×104 cells/ml, followed by
treatment with EGb 761 or 0.9% NaCl for 24 h. The slides were fixed
with ice-cold 100% methanol, quenched with 0.3%
H2O2 and blocked with normal goat serum.
After incubation for 30 min with the primary antibodies (the same
as the western blot analysis) and washing, the biotinylated
secondary antibodies were added for 30 min, washed, and followed by
preformed avidin/DH-biotinylated horseradish peroxidase H complex
for 30 min. Slides were then overlaid with DAB, rinsed, dried,
mounted and coverslipped. Image Pro Plus analysis system was used
to analyze the protein expression. Five visual fields of each slide
were selected randomly, and the total area and accumulated optical
density were detected. The expression level was expressed as
average optical density (AOD): AOD = accumulated optical
density/total area.
Analysis of oxidative stress levels in
the gastric cancer cells
Cells were homogenized in PBS and centrifuged at
1,687 × g for 10 min at 4°C. As previously described in detail
(21), the supernatant was obtained
for detection of malondialdehyde (MDA) content and the activities
of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px).
SOD activity was measured by the inhibition of nitroblue
tetrazolium (NBT) reduction by the O2-generated by
xanthine/xanthine oxidase system. One SOD activity unit was defined
as the enzyme causing 50% inhibition in a 1-ml reaction solution/mg
protein and the result was expressed as U/mg protein. GSH-Px
activity was tested by measuring the reduction in glutathione
(GSH)/min on the base of its catalysis. GSH reacts with
5′-dithiobis-p-nitrobenzoic acid (DTNB), and produces
yellow-colored compounds, which are detected at 412 nm and
represent a reduction in GSH. One unit of enzyme activity is
defined as a decrease in 1 μM GSH/min for 1 mg protein after
the decrease in GSH of the non-enzymatic reaction is subtracted and
the result is expressed as U/mg protein. MDA was assayed by the
measurement of thiobarbituric acid reactive substance (TBARS)
levels at 532 nm. The results are expressed as nmol/mg protein. All
above measurements were performed according to the protocol
specified in each kit.
Statistical analysis
Data are presented as mean ± standard deviation
(SD). The significance of the difference between the groups was
assessed by the Student’s two-tailed t-test. The significance
between proteins and clinicopathological characteristics of the
patients was assessed with the χ2 test. The correlation
between KSR1 (p-KSR1) and ERK (p-ERK) was calculated by the method
of Pearson’s correlation coefficient. Differences were considered
significant at P<0.05.
Results
Immunohistochemical analysis of KSR1,
p-KSR1, ERK1/2 and p-ERK1/2 in the gastric cancer and normal
tissues
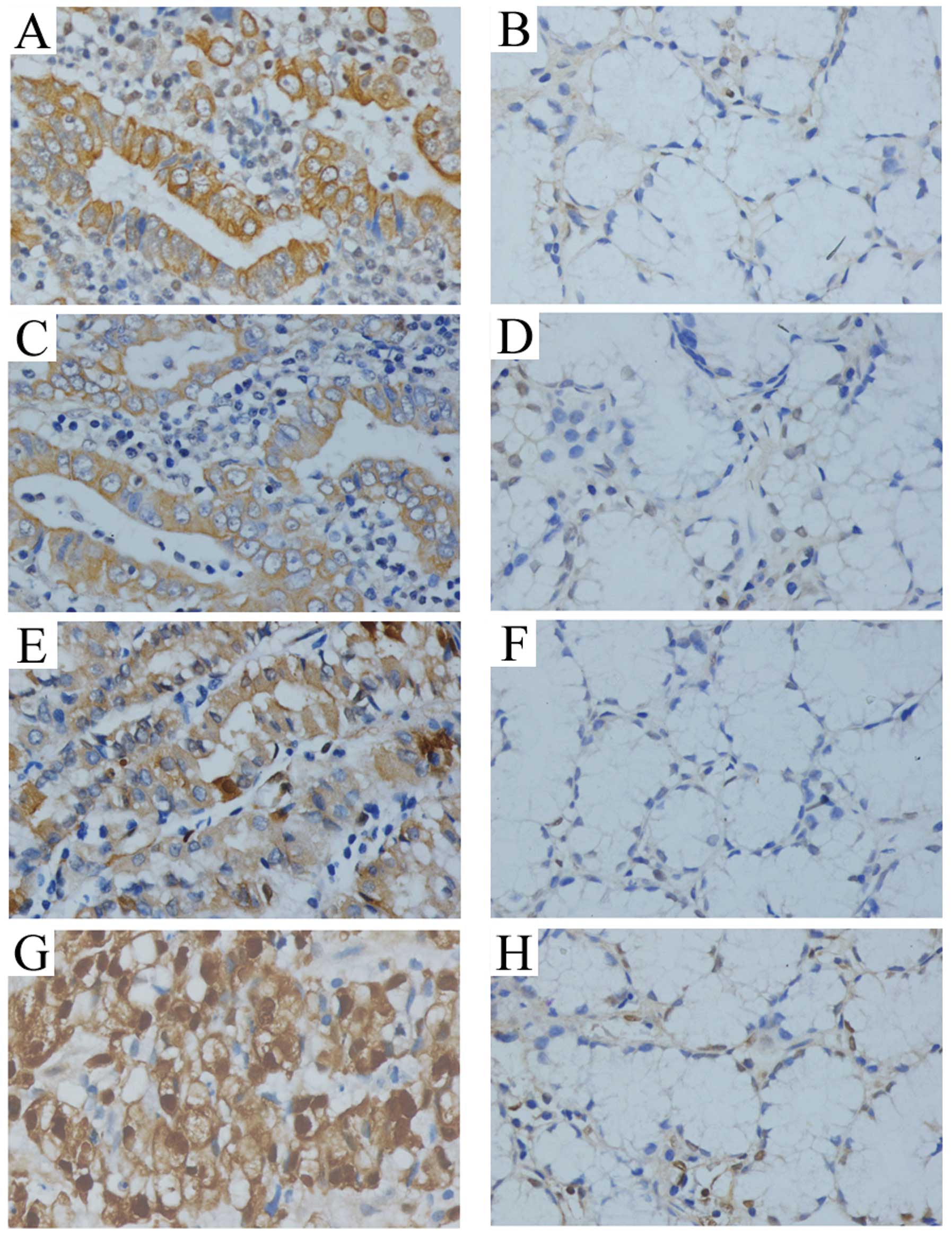
Immunohistochemical staining showed that the
expression of KSR1, p-KSR1, ERK1/2 and p-ERK1/2 was present in the
cytoplasm and staining of KSR1, p-KSR1, ERK1/2 and p-ERK1/2 in the
cancer tissues was significantly stronger than that in the matched
normal tissue (Fig. 1). The
positive staining rates of cancerous tissues were significantly
higher than those of the normal tissues (Table I). Moreover, there was a close
correlation between the expression of KSR1 (p-KSR1) and ERK1/2
(p-ERK1/2) (Table II).
Coexpression of KSR1, p-KSR1, ERK1/2 and p-ERK1/2 was significantly
associated with histological grade, TNM stage, lymph node and
distant metastasis, but there was no correlation between the
expression levels and age or gender (Table III).
 | Table IExpression of KSR1, p-KSR1, EKR1/2
and p-ERK1/2 in gastric cancer and matched normal tissues. |
Table I
Expression of KSR1, p-KSR1, EKR1/2
and p-ERK1/2 in gastric cancer and matched normal tissues.
| Samples | N | KSR1
| p-KSR1
| ERK1/2
| p-ERK1/2
|
|---|
| Positive, n
(%) | P-value | Positive, n (%)
P-value | Positive, n (%)
P-value | Positive, n (%)
P-value |
|---|
| Cancer tissues | 62 | 40 (64.52) | 0.000 | 43 (69.38)
0.000 | 45 (72.58)
0.000 | 42 (67.74)
0.000 |
| Normal tissues | 62 | 14 (22.58) | | 14 (22.58) | 17 (27.42) | 15 (24.19) |
 | Table IICorrelation between the expression of
KSR1, p-KSR1, ERK1/2 and p-ERK1/2 in gastric cancer tissues. |
Table II
Correlation between the expression of
KSR1, p-KSR1, ERK1/2 and p-ERK1/2 in gastric cancer tissues.
| Expression | ERK1/2 expression
| p-ERK1/2 expression
|
|---|
| − | + | ++ | Correlation | P-value | − | + | ++ | Correlation | P-value |
|---|
| KSR1 |
| − | 10 | 8 | 4 | 0.435 | 0.000 | 11 | 8 | 3 | 0.414 | 0.001 |
| + | 5 | 5 | 7 | | | 6 | 5 | 6 | | |
| ++ | 2 | 6 | 15 | | | 3 | 7 | 13 | | |
| p-KSR1 |
| − | 9 | 7 | 3 | 0.426 | 0.001 | 10 | 7 | 2 | 0.417 | 0.001 |
| + | 5 | 6 | 7 | | | 6 | 6 | 6 | | |
| ++ | 3 | 6 | 16 | | | 4 | 7 | 14 | | |
 | Table IIIClinicopathological characteristics
and their association with the protein expression in the gastric
cancer tissues. |
Table III
Clinicopathological characteristics
and their association with the protein expression in the gastric
cancer tissues.
| Clinicopathological
characteristicss | N | KSR1
| P-value | p-KSR1
| P-value | ERK1/2
| P-value | p-ERK1/2
| P-value |
|---|
| + | − | + | − | + | − | + | − |
|---|
| Gender |
| Male | 37 | 25 | 12 | 0.541 | 27 | 10 | 0.452 | 30 | 7 | 0.068 | 28 | 9 | 0.104 |
| Female | 25 | 15 | 10 | | 16 | 9 | | 15 | 10 | | 14 | 11 | |
| Age (years) |
| ≤50 | 22 | 13 | 9 | 0.508 | 16 | 6 | 0.669 | 17 | 5 | 0.539 | 16 | 6 | 0.533 |
| >50 | 40 | 27 | 13 | | 27 | 13 | | 28 | 12 | | 26 | 14 | |
| Histological
grade |
| Well and
moderately differentiated | 29 | 11 | 18 | 0.000 | 13 | 16 | 0.000 | 14 | 15 | 0.000 | 16 | 13 | 0.047 |
| Poorly
differentiated | 33 | 29 | 4 | | 30 | 3 | | 31 | 2 | | 26 | 7 | |
| TNM stage |
| I+II | 23 | 9 | 14 | 0.001 | 10 | 13 | 0.001 | 11 | 12 | 0.001 | 12 | 11 | 0.044 |
| III+IV | 39 | 31 | 8 | | 33 | 6 | | 34 | 5 | | 30 | 9 | |
| Lymph node
metastasis |
| Positive | 42 | 22 | 20 | 0.004 | 25 | 17 | 0.032 | 26 | 16 | 0.006 | 24 | 18 | 0.010 |
| Negative | 20 | 18 | 2 | | 18 | 2 | | 19 | 1 | | 18 | 2 | |
| Distant
metastasis |
| Positive | 44 | 27 | 17 | 0.048 | 27 | 17 | 0.033 | 28 | 16 | 0.031 | 26 | 18 | 0.023 |
| Negative | 18 | 15 | 3 | | 16 | 2 | | 17 | 1 | | 16 | 2 | |
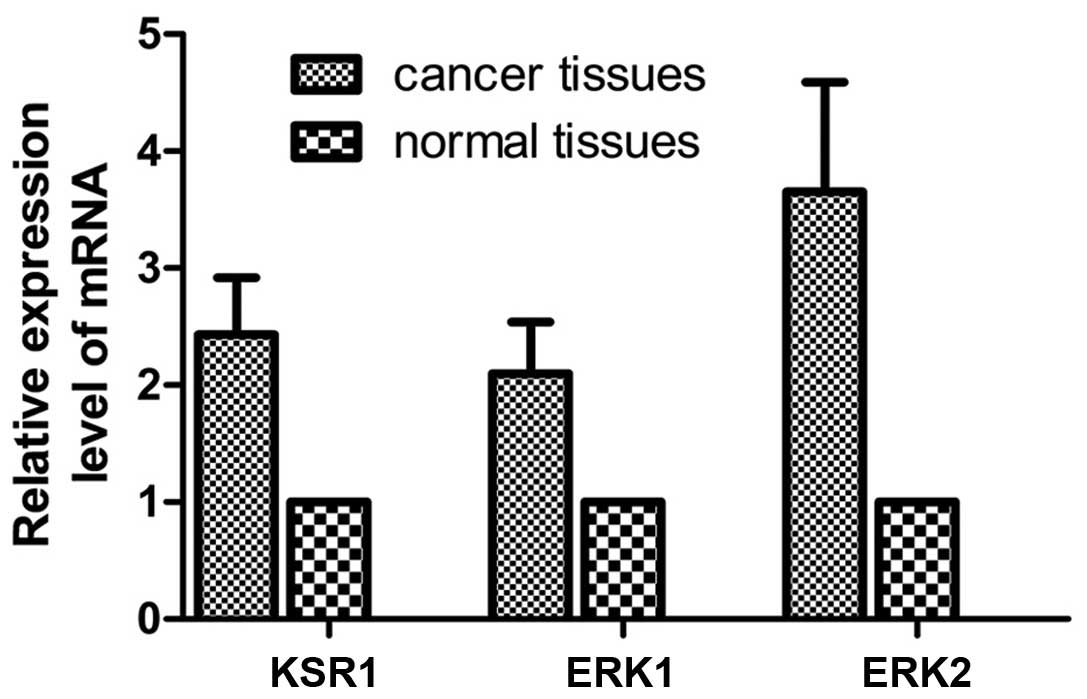
Real-time fluorescent quantitative PCR
detection of KSR1, ERK1 and ERK2 in the gastric cancer and normal
tissues
The mRNA expression level was detected in 62 paired
gastric cancer and matched normal tissues. Compared with the normal
tissues, the relative mRNA copy values of KSR1, ERK1 and ERK2 were
2.43±0.49, 2.10±0.44 and 3.65±0.94 in the cancer tissues (Fig. 2).
Effects of CDDP and etoposide on the
proliferation and apoptosis of SGC-7901 and SGC-7901/CDDP
cells
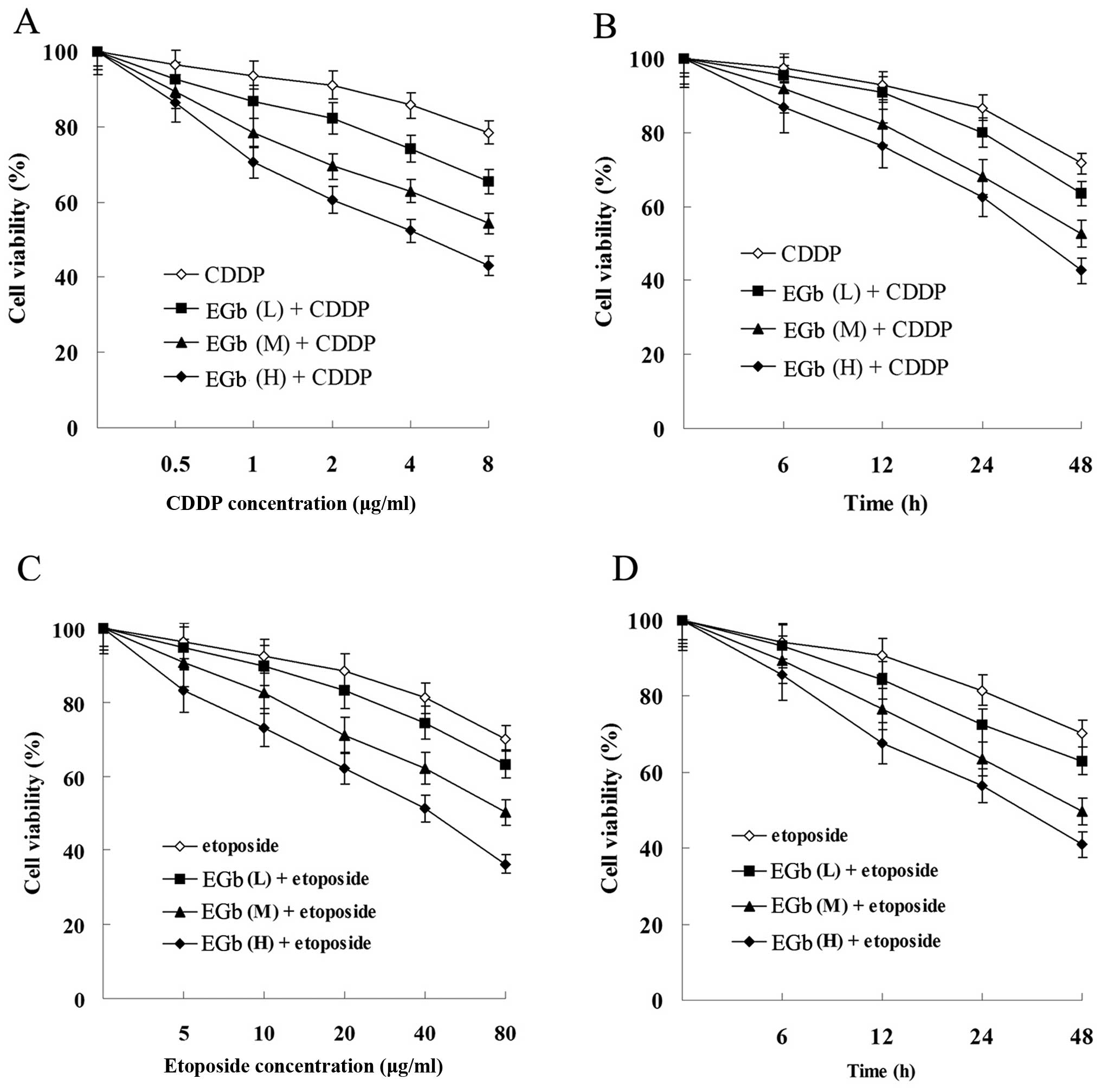
As showed in Fig. 3,
the proliferation of SGC-7901 and SGC-7901/CDDP cells was
suppressed by CDDP and etoposide in a time- and dose-dependent
manner. Moreover, the proliferation suppression level of SGC-7901
cells was more significant than that of SGC-7901/CDDP cells.
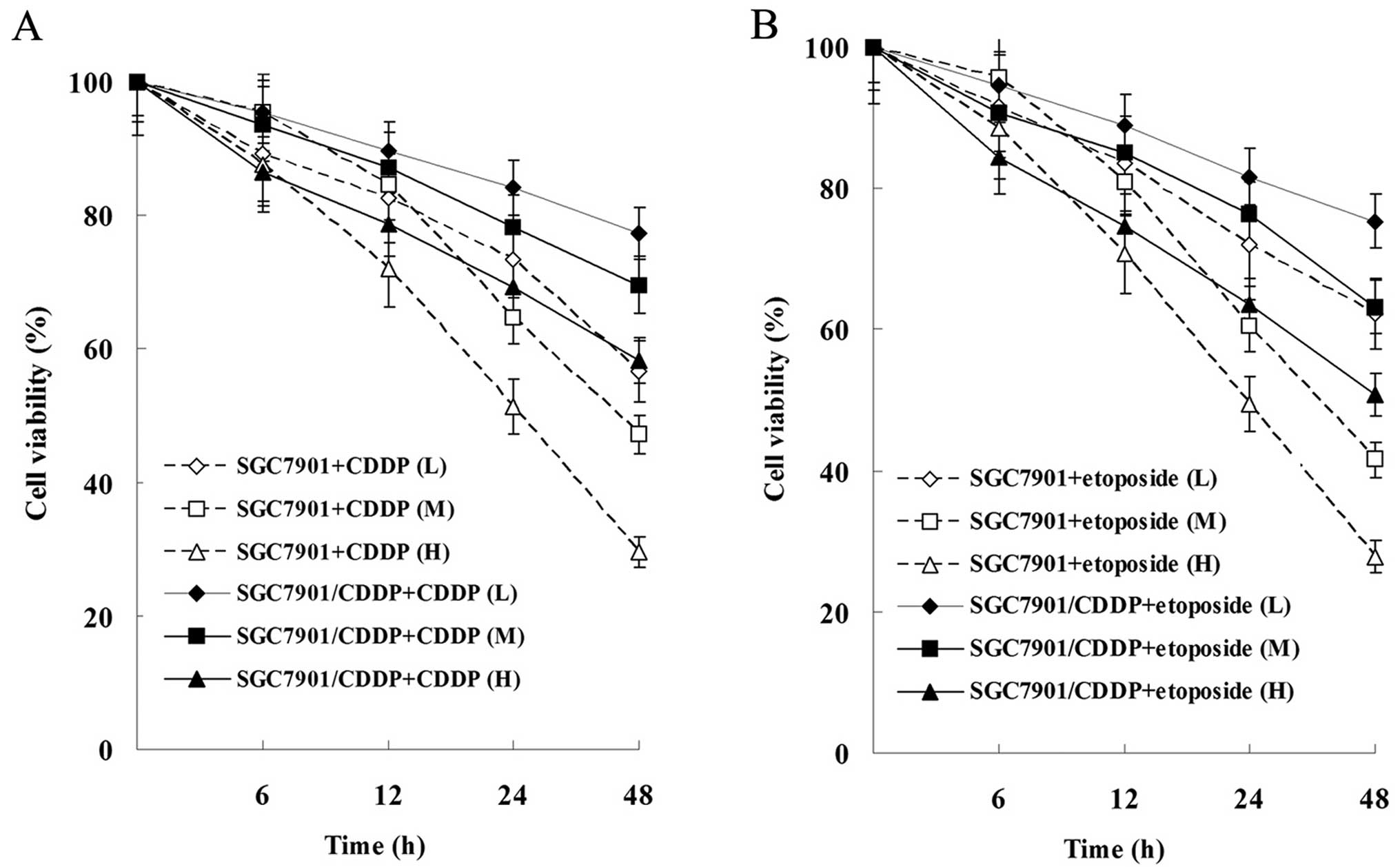 | Figure 3Effects of CDDP and etoposide on tumor
cell proliferation. (A) Effect of CDDP on the cell viability of
SGC-7901 and SGC-7901/CDDP cells. (B) Effect of etoposide on the
cell viability of SGC-7901 and SGC-7901/CDDP cells. Cells were
treated with 1 μg/ml (L, low dose), 2 μg/ml (M,
medium dose), 4 μg/ml (H, high dose) CDDP or 5 μg/ml
(L), 10 μg/ml (M), 20 μg/ml (H) etoposide for 0, 6,
12, 24 and 48 h. All results shown are the mean ± SD of 3
independent experiments. CDDP, cisplatin. |
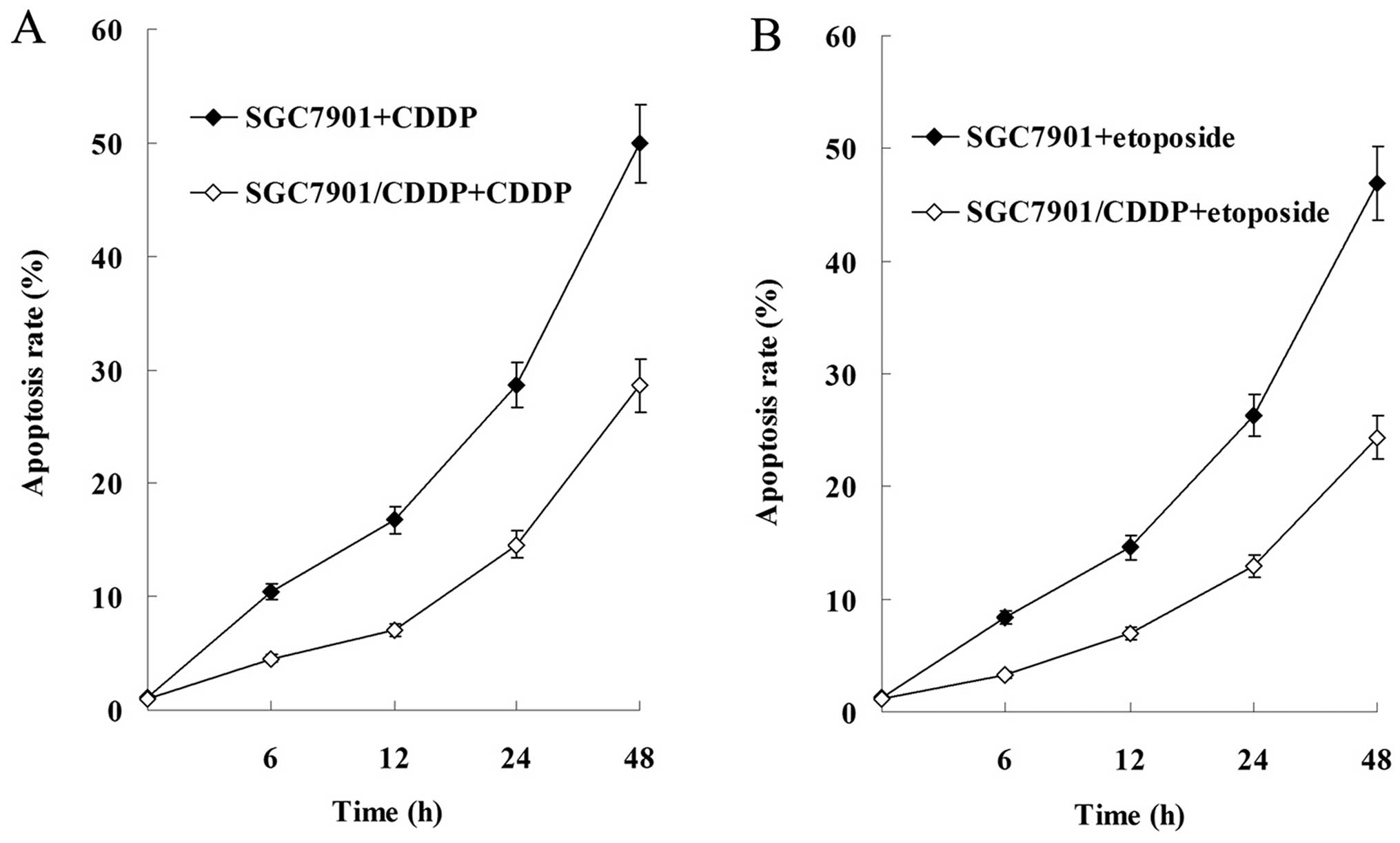
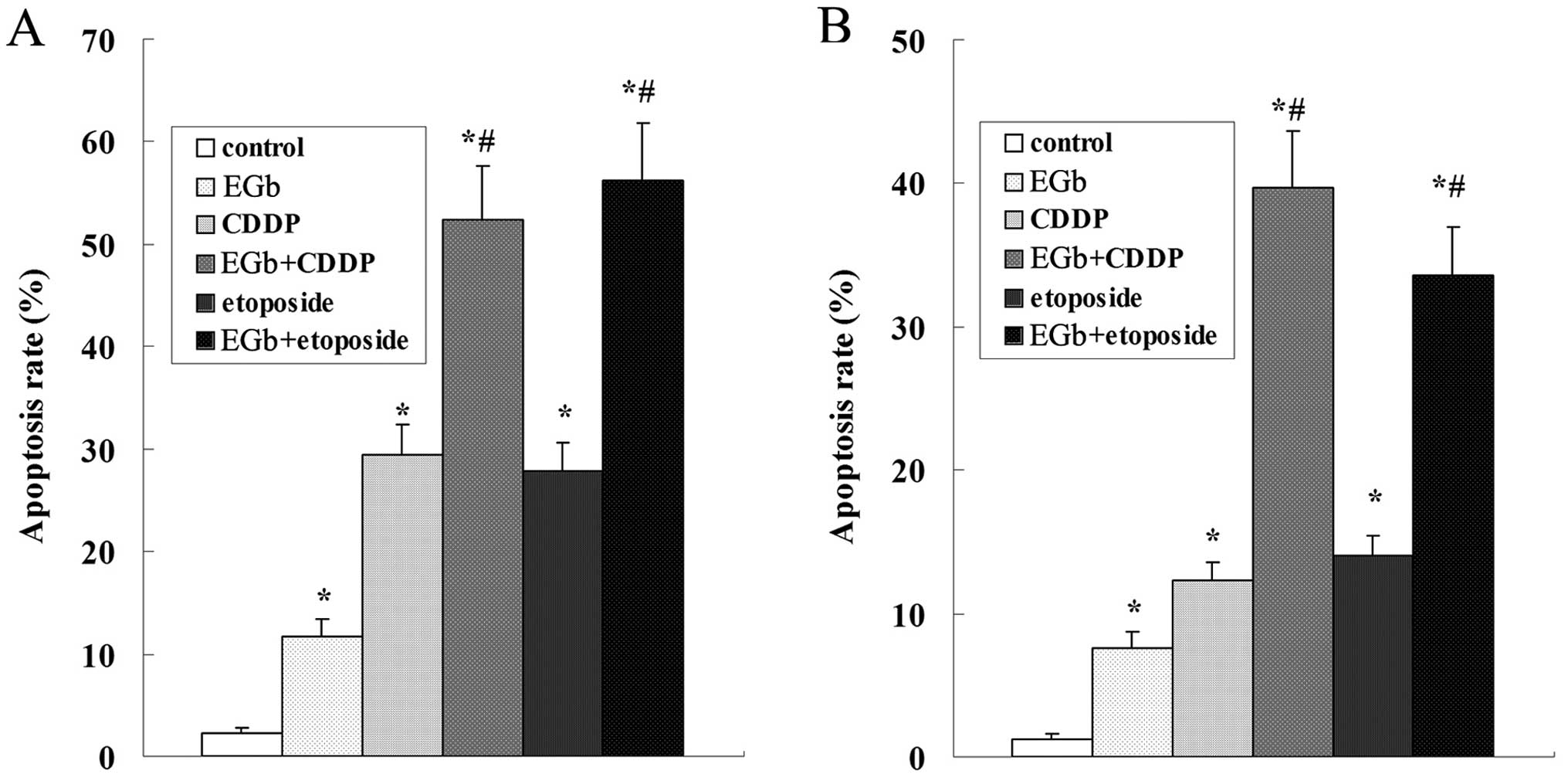
Results of the flow cytometric analysis showed that
cell apoptosis was induced by CDDP and etoposide in a
time-dependent manner, and the apoptosis rate of SGC-7901 cells was
significantly higher than that of the SGC-7901/CDDP cells (Fig. 4).
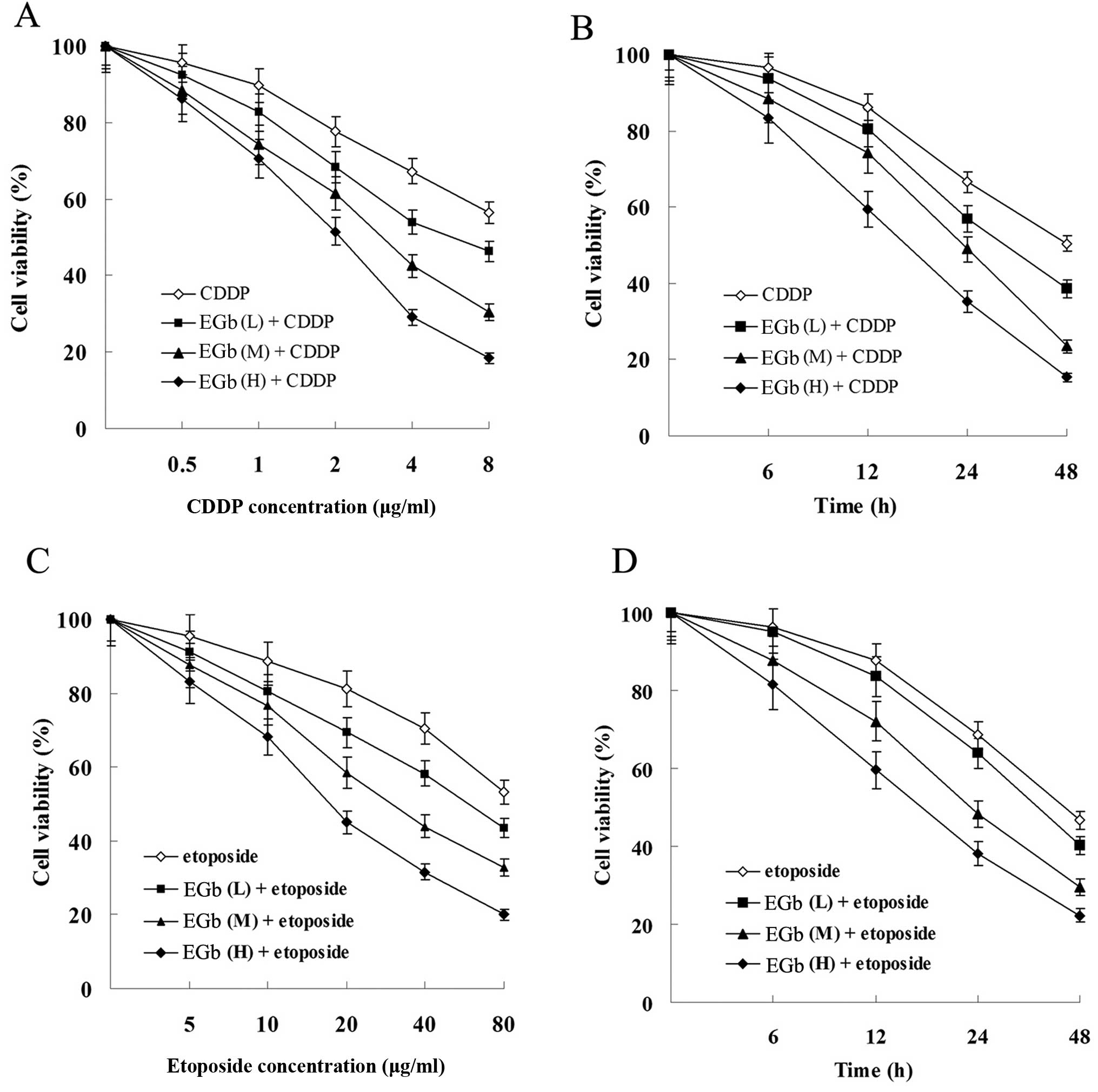
EGb 761 enhances the antiproliferation
and apoptosis-inducing effect of CDDP and etoposide in cancer
cells
Compared with the groups treated with CDDP and
etoposide, combined treatment with EGb 761 reduced the viability of
the SGC-7901 (Fig. 5) and
SGC-7901/CDDP cells (Fig. 6) in a
time- and dose-dependent manner.
The apoptosis of the SGC-7901 and SGC-7901/CDDP
cells was strikingly induced by treatment with EGb 761, CDDP and
etoposide. The apoptosis rate of SGC-7901 was higher than that of
the SGC-7901/CDDP cells. Compared with the CDDP and etoposide
groups, the apoptosis rate was obviously elevated following
simultaneous treatment with EGb 761 both in the SGC-7901 and
SGC-7901/CDDP cells (Fig. 7).
EGb 761 reduces the oxidative stress
level of the SGC-7901 and SGC-7901/CDDP cells
Compared with the control, CDDP and etoposide
groups, the activities of SOD and GSH-Px were notably increased,
while the content of MDA was obviously decreased in the EGb 761,
EGb 761+CDDP and EGb 761+etoposide groups (Table IV and V).
 | Table IVEffects of EGb761 on SOD, GSH-Px and
MDA in the SGC-7901 cells. |
Table IV
Effects of EGb761 on SOD, GSH-Px and
MDA in the SGC-7901 cells.
| Treatment | SOD (U/mg
prot) | GSH-Px (U/mg prot)
(nmol/mg prot) | MDA |
|---|
| Control | 16.57±3.20 | 22.18±4.36 | 2.46±0.38 |
| EGb | 25.96±3.57a | 33.59±5.64a | 1.42±0.26a |
| CDDP | 17.36±3.13 | 23.98±3.35 | 2.27±0.39 |
| EGb+CDDP | 27.35±4.84a,c | 35.78±6.56a,c | 1.39±0.25b,c |
| Etoposide | 16.23±2.79 | 22.87±4.34 | 2.33±0.45 |
| EGb+etoposide | 26.40±4.27a,d | 35.33±5.90a,d | 1.40±0.23b,d |
 | Table VEffects of EGb 761 on SOD, GSH-Px and
MDA in the SGC-7901/CDDP cells. |
Table V
Effects of EGb 761 on SOD, GSH-Px and
MDA in the SGC-7901/CDDP cells.
| Treatment | SOD (U/mg
prot) | GSH-Px (U/mg
prot) | MDA (U/mg
prot) |
|---|
| Control | 15.48±2.63 | 20.67±5.90 | 2.67±0.45 |
| EGb | 23.28±3.36a | 36.49±6.62a | 1.53±0.24a |
| CDDP | 15.86±3.52 | 21.42±4.08 | 2.92±0.46 |
| EGb+CDDP | 24.56±4.36a,b | 39.52±5.09a,c | 1.68±0.22a,b |
| Etoposide | 13.34±2.53 | 18.73±3.64 | 2.81±0.37 |
| EGb+etoposide | 24.12±4.05a,d | 36.45±5.13a,e | 1.51±0.31a,e |
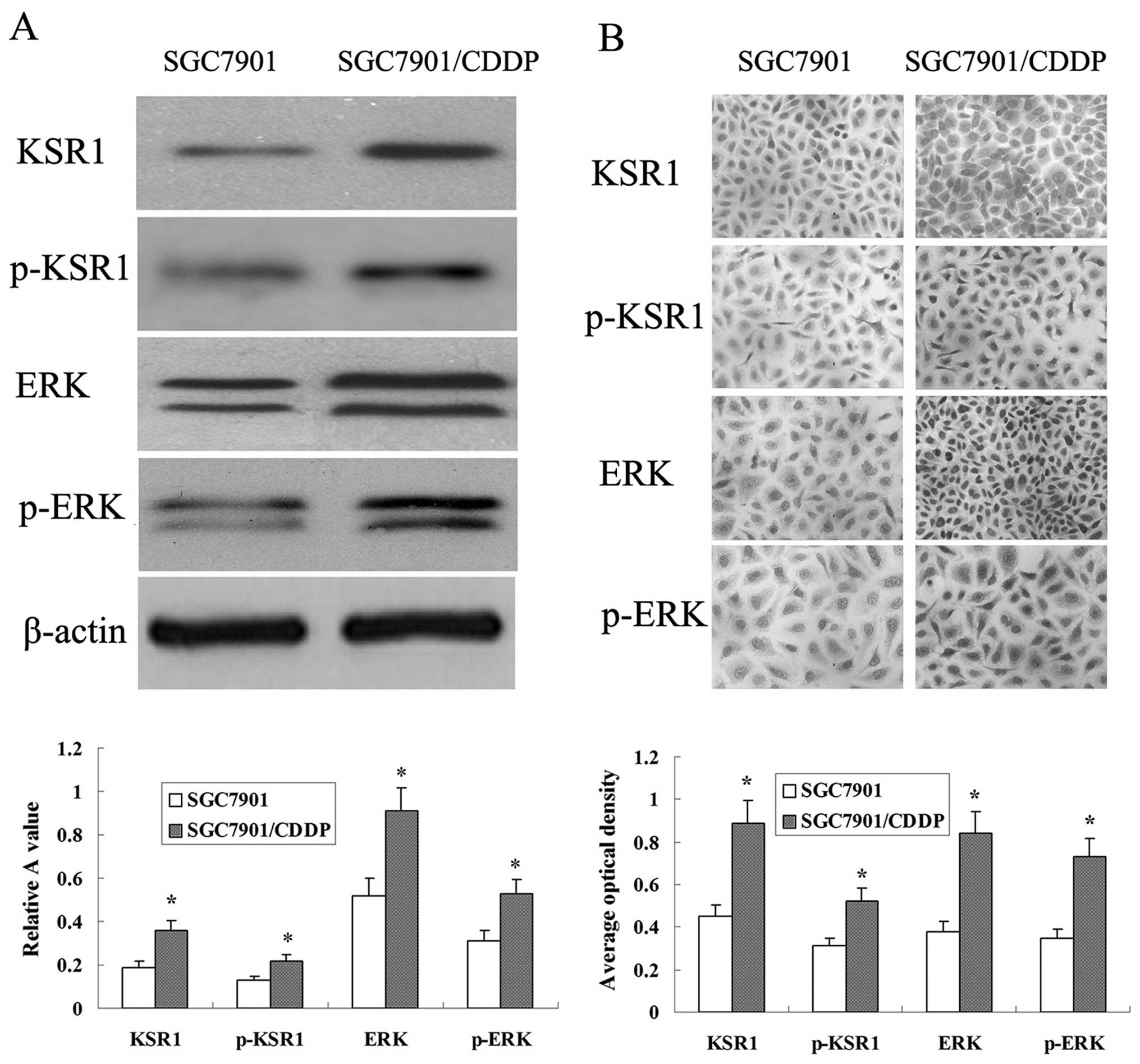
Expression of KSR1, p-KSR1, ERK1/2 and
p-ERK1/2 in the SGC-7901 and SGC-7901/CDDP cells
Western blot analysis determined that the expression
levels of KSR1, p-KSR1, ERK1/2 and p-ERK1/2 in the SGC-7901/CDDP
cells were higher than those levels in the SGC-7901 cells (Fig. 8A). Protein expression was also
detected using immunocyto-chemical analysis. KSR1, p-KSR1, ERK1/2
and p-ERK1/2 expression in the SGC-7901/CDDP cells was much higher
than that in the SGC-7901 cells (Fig.
8B).
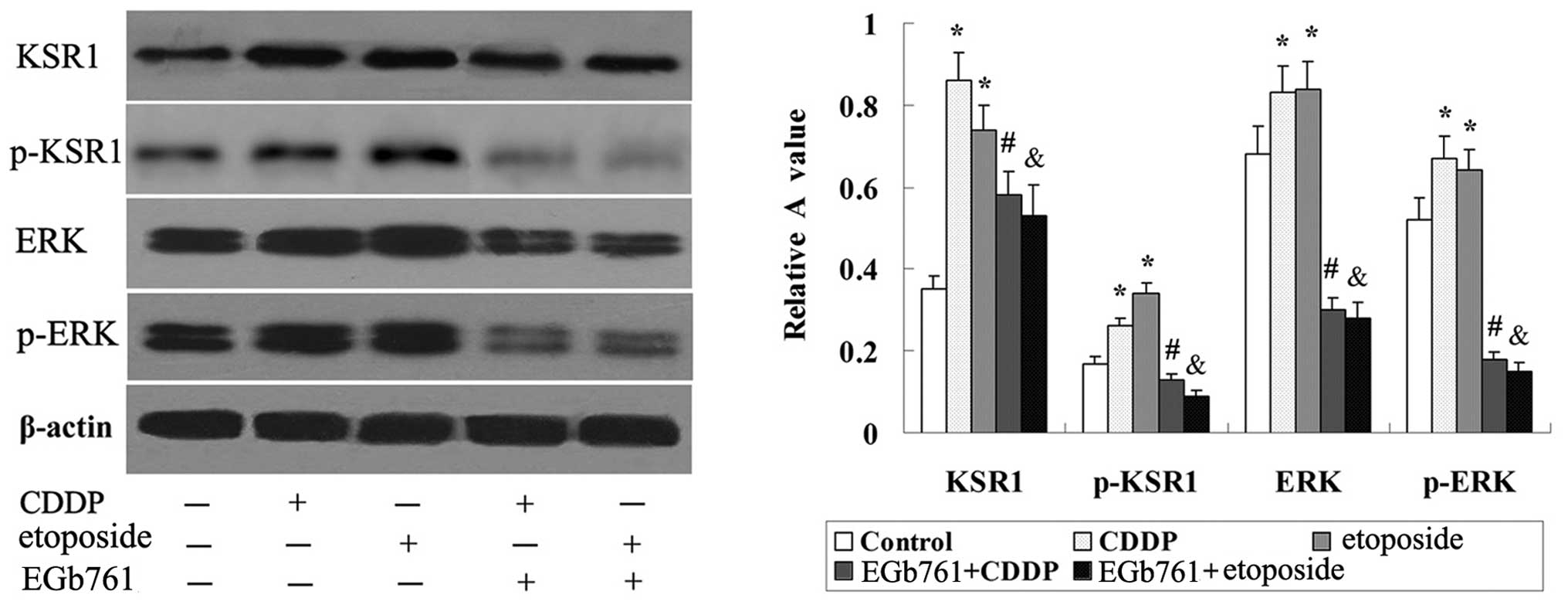
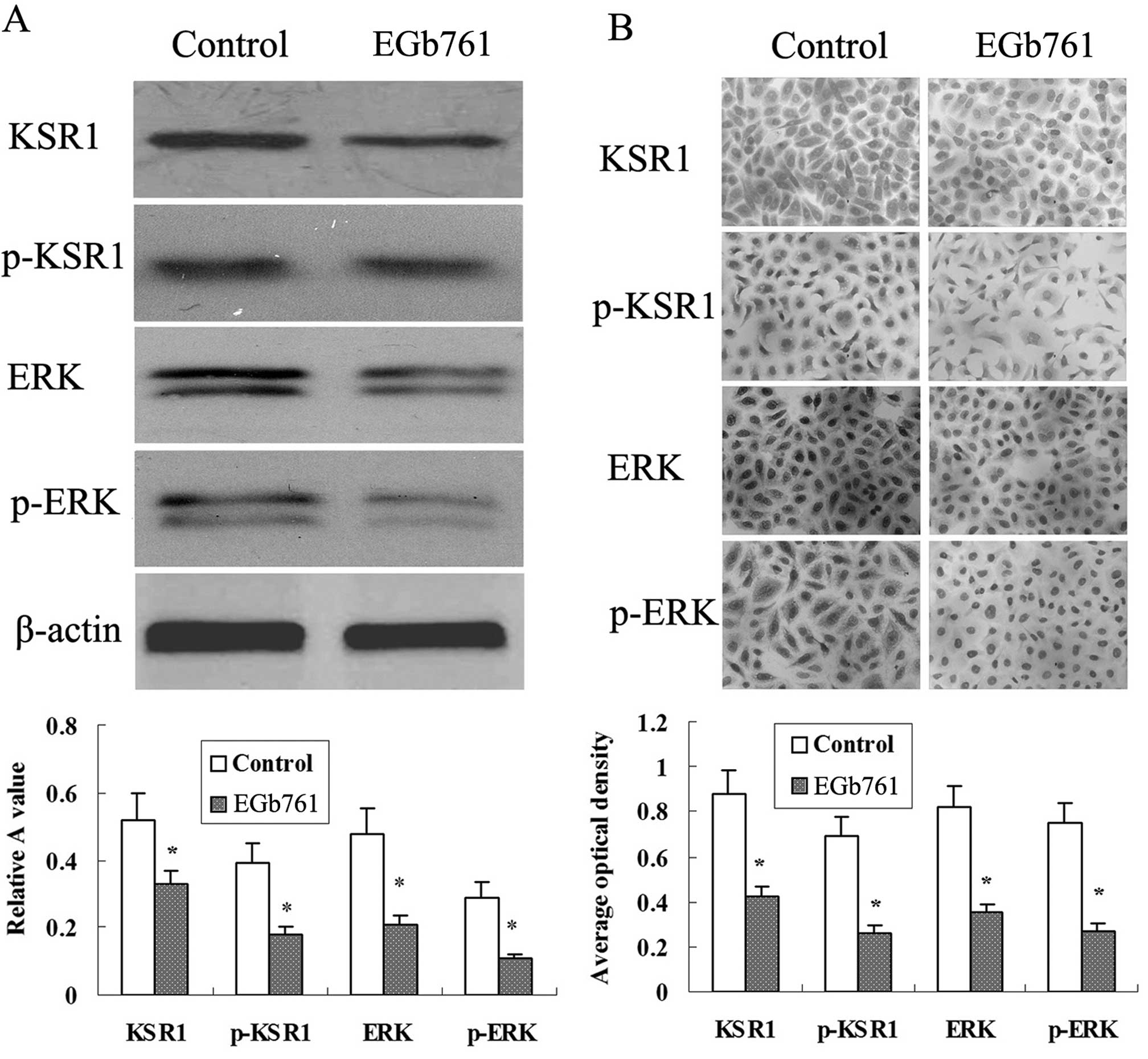
EGb 761 suppresses the expression of
KSR1, p-KSR1, ERK1/2 and p-ERK1/2 induced by CDDP and etoposide in
the SGC-7901 cells
As shown in Fig. 9,
there was basic expression of KSR1, p-KSR1, ERK1/2 and p-ERK1/2 in
the SGC-7901 cells, and the expression of KSR1, p-KSR1, ERK1/2 and
p-ERK1/2 was induced by CDDP and etoposide. Further study indicated
that the expression of KSR1, p-KSR1, ERK1/2 and p-ERK1/2 induced by
CDDP and etoposide in the SGC-7901 cells was strikingly reduced
following combined treatment with EGb 761.
Expression of KSR1, p-KSR1, ERK1/2 and
p-ERK1/2 is suppressed by EGb 761 in the SGC-7901/CDDP cells
Western blot analysis determined that the expression
of KSR1, p-KSR1, ERK1/2 and p-ERK1/2 in the SGC-7901/CDDP cells was
significantly suppressed by EGb 761 (Fig. 10A). Immunocytochemical analysis
revealed that the KSR1, p-KSR1, ERK1/2 and p-ERK1/2 expression in
the SGC-7901/CDDP cells was strikingly reduced following combined
treatment with EGb 761 (Fig.
10B).
Discussion
As KSR1 is an essential scaffold protein of the
Ras/Raf/MAPK cascade (3), one of
the well-known oncogenic pathways, studies are beginning to explore
the biological characteristics of KSR1 in different types of
cancer. There are reports that the expression of KSR1 is
upregulated in various types of tumors (5). The present research confirmed that
KSR1 (dephosphorylated KSR1) and p-KSR1 (phosphorylated KSR1-S392)
were overexpressed in gastric cancer tissues. Evidence showed that
KSR-1 locates in the cytosol, is phosphorylated on S297 and S392,
and is held in an inactive state in quiescent cells. The activation
of RAS stimulates the dephosphorylation of KSR-1 on S392 and
results in its translocation to the plasma membrane where KSR-1
potentiates the MAPK signaling pathway (8). Thus, the expression and activation of
KSR1 are both elevated in gastric cancer. Additionally, the
overexpression of KSR1 and p-KSR1 was found to correlate with TNM
stage, histological grade, lymph node metastasis and distant
metastasis, indicating that KSR1 may contribute to the
carcinogenesis and metastasis of gastric cancer.
Evidence also showed that the cell proliferative and
oncogenic potential were induced by introduction of KSR1 into
KSR1−/− mouse embryonic fibroblasts (MEFs). In contrast,
the cell transformation was suppressed by the removal of KSR1
(22). Moreover, KSR1 was shown to
contribute to the tumorigenesis of B-cell tumors, and skin and
pancreatic cancer through the regulation of the MAPK cascade in a
mouse model (7,8,23). In
gastric cancer, much attention has focused on the ERK signaling
pathway, and accumulated data reveal that the MAPK/ERK signaling
pathway not only regulates tumor progression but is also involved
in the development of chemotherapy resistant (11,24).
In gastric cancer tissues, the expression of KSR1 and p-KSR1 was
confirmed to be closely related with the expression of ERK1/2 and
p-ERK1/2. Moreover, the expression and activation of KSR1 and
ERK1/2 in the multidrug resistance gastric cancer SGC-7901/CDDP
cells were higher than these parameters in the SGC-7901 cells.
Therefore, the KSR1-mediated ERK1/2 signaling pathway may play an
important role in tumorigenesis, metastasis and development of
chemoresistance in gastric cancer.
It was reported that continuous infusion of
phosphorothioate antisense ODNs targeting KSR1 reduced the tumor
growth of human PANC-1 pancreatic and A549 non-small cell lung
carcinoma xenografts in nude mice (25), and blocking the activation of the
ERK1/2 signaling pathway enhanced the proliferation-suppressing and
apoptosis-inducing capacity of chemotherapy reagents in gastric
cancer (11). Hence, suppression of
the KSR1-mediated ERK1/2 signaling pathway may be a potential
therapeutic for enhancing chemotherapy sensitivity and reversing
chemoresistance in gastric cancer.
EGb, a natural plant material, has been used as a
medicine for centuries with little side effects in China. EGb 761
is a standardized concentrated extract of Ginkgo biloba,
containing 24% flavone glycosides, 6% terpene lactones and less
than 5 ppm ginkgolic acid (26).
EGb 761 has been registered as a prescription medicine in many
countries. Considerable studies indicate that EGb is beneficial in
the prevention and therapy of diseases and degenerative processes
associated with oxidative stress (27). In recent years, a number of
experimental and clinical evidence has demonstrated that EGb
possesses antitumor activities and it has been used to treat
several types of solid tumors and malignancies of the blood system
(13,27). EGb 761 can inhibit tumor cell
proliferation and induce cell apoptosis of colon, pancreatic and
oral cavity cancer (14,28,29).
However, the anticancer effects of EGb on gastric cancer have not
yet been confirmed. One study showed that EGb can reduce the
incidence of mild to severe intestinal metaplasia and dysplasia in
rat gastric mucosa induced by oral administration of
N-methyl-N’-nitro-N-nitrosoguanidine, and its mechanism may be
related to the regulation of cell proliferation and apoptosis
(18). In the present study, EGb
761 inhibited cell growth and enhanced the antiproliferation and
apoptosis-inducing activities of CDDP and etoposide in SGC-7901 and
SGC-7901/CDDP cells. These findings suggested that EGb 761 was be
able to prevent the development, enhance the chemotherapy
sensitivity and reverse the chemoresistance of gastric cancer.
There is evidence that the proliferation, migration
and tube formation in vitro and the angiogenesis in
vivo of endothelial cells was suppressed by EGb 761 through
inhibiting the ERK signaling pathway (17). In the present study, the
antiproliferation and apoptosis-inducing activities of CDDP and
etoposide were elevated with the suppression of the KSR1-mediated
ERK1/2 signaling pathway by EGb 761. These findings indicate that
EGb 761 enhanced the chemotherapy sensitivity and reversed the
chemoresistance of gastric cancer cells via suppressing the
activation of the KSR1-mediated ERK signaling pathway. However, the
molecular mechanisms remain to be determined.
It is true that EGb is a potent antioxidant and has
been showed to have hydroxyl scavenging property, lipid
peroxidation restraining capacity and antioxidant enzyme-like
activity (12,21). In gastric cancer cells, the
antioxidative activity of EGb 761 was confirmed to be related to
the suppression of the KSR1-mediated ERK signaling pathway. Indeed,
phosphorylation of ERK1/2 was induced by H2O2
treatment in lymphocytes and the peak activity was at ~10 min after
ROS exposure (30). Additionally,
the growth and proliferation of human cervical cancer cells were
enhanced by cancer-derived immunoglobulin G (IgG) via inducing the
production of low level ROS. Inversely, the growth of IgG-deficient
cancer cells was inhibited by ROS scavengers through suppressing
the MAPK/ERK signaling pathway induced by a low level of
intracellular ROS (31). Thus, ROS
may play a role in the activation of the ERK1/2 signaling pathway
in some cancer cells. EGb 761 may enhance the chemotherapeutic
sensitivity and reverse the chemoresistance via suppressing ROS
induced by the KSR1-mediated ERK1/2 signaling pathway in gastric
cancer. However, there is a report that a high level of glucose
contributes to the oxidative stress and activated ERK signaling
pathway. Inhibitor of the ERK signaling pathway impaired the
production of ROS in pancreatic cancer cells (32), suggesting that the ERK signaling
pathway may be involved in the regulation of the production of ROS.
The exact mechanism of the KSR1-mediated ERK1/2 signaling pathway
and ROS requires further investigation.
In summary, activation of the KSR1-mediated ERK1/2
signaling pathway may contribute to tumorigenesis, metastasis and
chemoresistance of gastric cancer. EGb 761 may enhance the
chemotherapeutic sensitivity and reverse the chemoresistance
through suppressing the activation of the KSR1-mediated ERK1/2
signaling pathway in gastric cancer cells, and the underlying
mechanism may be related to its antioxidative activity.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81460380), the Natural
Science Foundation of Guangxi (no. 2011GXNSFA018182) and the
Project Foundation from the Health Department of Guangxi, China
(no. Z2012103).
Abbreviations:
|
KSR1
|
kinase suppressor of Ras 1
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
EGb
|
Ginkgo biloba extract
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen CH, Yuan P, Perez-Lorenzo R, Zhang Y,
Lee SX, Ou Y, Asara JM, Cantley LC and Zheng B: Phosphorylation of
BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation.
Mol Cell. 52:161–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang H, Koo CY, Stebbing J and Giamas G:
The dual function of KSR1: a pseudokinase and beyond. Biochem Soc
Trans. 41:1078–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kortum RL, Fernandez MR, Costanzo-Garvey
DL, Johnson HJ, Fisher KW, Volle DJ and Lewis RE: Caveolin-1 is
required for kinase suppressor of Ras 1 (KSR1)-mediated
extracellular signal-regulated kinase 1/2 activation,
H-RasV12-induced senescence, and transformation. Mol Cell Biol.
34:3461–3472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llobet D, Eritja N, Domingo M, Bergada L,
Mirantes C, Santacana M, Pallares J, Macià A, Yeramian A, Encinas
M, et al: KSR1 is overexpressed in endometrial carcinoma and
regulates proliferation and TRAIL-induced apoptosis by modulating
FLIP levels. Am J Pathol. 178:1529–1543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Razidlo GL, Johnson HJ, Stoeger SM, Cowan
KH, Bessho T and Lewis RE: KSR1 is required for cell cycle
reinitiation following DNA damage. J Biol Chem. 284:6705–6715.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lozano J, Xing R, Cai Z, Jensen HL,
Trempus C, Mark W, Cannon R and Kolesnick R: Deficiency of kinase
suppressor of Ras1 prevents oncogenic ras signaling in mice. Cancer
Res. 63:4232–4238. 2003.PubMed/NCBI
|
|
8
|
Cullis J, Meiri D, Sandi MJ, Radulovich N,
Kent OA, Medrano M, Mokady D, Normand J, Larose J, Marcotte R, et
al: The RhoGEF GEF-H1 is required for oncogenic RAS signaling via
KSR-1. Cancer Cell. 25:181–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stoeger SM and Cowan KH: Characterization
of kinase suppressor of Ras-1 expression and anticancer drug
sensitivity in human cancer cell lines. Cancer Chemother Pharmacol.
63:807–818. 2009. View Article : Google Scholar
|
|
10
|
Salerno M, Palmieri D, Bouadis A,
Halverson D and Steeg PS: Nm23-H1 metastasis suppressor expression
level influences the binding properties, stability, and function of
the kinase suppressor of Ras1 (KSR1) Erk scaffold in breast
carcinoma cells. Mol Cell Biol. 25:1379–1388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu SQ, Yu JP, Yu HG, Lv P and Chen HL:
Activation of Akt and ERK signalling pathways induced by etoposide
confer chemoresistance in gastric cancer cells. Dig Liver Dis.
38:310–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ude C, Schubert-Zsilavecz M and Wurglics
M: Ginkgo biloba extracts: A review of the pharmacokinetics of the
active ingredients. Clin Pharmacokinet. 52:727–749. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsai JR, Liu PL, Chen YH, Chou SH, Yang
MC, Cheng YJ, Hwang JJ, Yin WH and Chong IW: Ginkgo biloba extract
decreases non-small cell lung cancer cell migration by
down-regulating metastasis-associated factor heat-shock protein 27.
PLoS One. 9:e913312014. View Article : Google Scholar
|
|
14
|
Chen XH, Miao YX, Wang XJ, Yu Z, Geng MY,
Han YT and Wang LX: Effects of Ginkgo biloba extract EGb761 on
human colon adenocarcinoma cells. Cell Physiol Biochem. 27:227–232.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El Mesallamy HO, Metwally NS, Soliman MS,
Ahmed KA and Abdel Moaty MM: The chemopreventive effect of Ginkgo
biloba and Silybum marianum extracts on hepatocarcinogenesis in
rats. Cancer Cell Int. 11:382011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park YJ, Kim MJ, Kim HR, Yi MS, Chung KH
and Oh SM: Chemopreventive effects of Ginkgo biloba extract in
estrogen-negative human breast cancer cells. Arch Pharm Res.
36:102–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koltermann A, Liebl J, Fürst R, Ammer H,
Vollmar AM and Zahler S: Ginkgo biloba extract EGb 761 exerts
anti-angiogenic effects via activation of tyrosine phosphatases. J
Cell Mol Med. 13:2122–2130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang XY, Qian LP, Zheng XJ, Xia YY, Jiang
YB and Sun Y: Interventional effect of Ginkgo biloba extract on the
progression of gastric precancerous lesions in rats. J Dig Dis.
10:293–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu AH, Chen HS, Sun BC, Xiang XR, Chu YF,
Zhai F and Jia LC: Therapeutic mechanism of Ginkgo biloba exocarp
polysaccharides on gastric cancer. World J Gastroenterol.
9:2424–2427. 2003.PubMed/NCBI
|
|
20
|
Mao YB, Liu SQ, Tan L, Zhou Q and Huang
JA: EGb761 enhances cisplatin and etoposide-induced apoptosis of
human gastric cancer SGC-7901 cells. Shijie Huaren Xiaohua Zazhi.
21:3330–3337. 2013.In Chinese.
|
|
21
|
Liu SQ, Yu JP, Chen HL, Luo HS, Chen SM
and Yu HG: Therapeutic effects and molecular mechanisms of Ginkgo
biloba extract on liver fibrosis in rats. Am J Chin Med. 34:99–114.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kortum RL and Lewis RE: The molecular
scaffold KSR1 regulates the proliferative and oncogenic potential
of cells. Mol Cell Biol. 24:4407–4416. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gramling MW and Eischen CM: Suppression of
Ras/Mapk pathway signaling inhibits Myc-induced lymphomagenesis.
Cell Death Differ. 19:1220–1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fukui H, Zhang X, Sun C, Hara K, Kikuchi
S, Yamasaki T, Kondo T, Tomita T, Oshima T, Watari J, et al: IL-22
produced by cancer-associated fibroblasts promotes gastric cancer
cell invasion via STAT3 and ERK signaling. Br J Cancer.
111:763–771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xing HR, Cordon-Cardo C, Deng X, Tong W,
Campodonico L, Fuks Z and Kolesnick R: Pharmacologic inactivation
of kinase suppressor of ras-1 abrogates Ras-mediated pancreatic
cancer. Nat Med. 9:1266–1268. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith JV and Luo Y: Studies on molecular
mechanisms of Ginkgo biloba extract. Appl Microbiol Biotechnol.
64:465–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mohanta TK, Tamboli Y and Zubaidha PK:
Phytochemical and medicinal importance of Ginkgo biloba L. Nat Prod
Res. 28:746–752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Chen AY, Li M, Chen C and Yao Q:
Ginkgo biloba extract kaempferol inhibits cell proliferation and
induces apoptosis in pancreatic cancer cells. J Surg Res.
148:17–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang JW, Kim JH, Song K, Kim SH, Yoon JH
and Kim KS: Kaempferol and quercetin, components of Ginkgo biloba
extract (EGb 761), induce caspase-3-dependent apoptosis in oral
cavity cancer cells. Phytother Res. 24(Suppl 1): S77–S82. 2010.
View Article : Google Scholar
|
|
30
|
Akhiani AA, Werlenius O, Aurelius J,
Movitz C, Martner A, Hellstrand K and Thorén FB: Role of the ERK
pathway for oxidant-induced parthanatos in human lymphocytes. PLoS
One. 9:e896462014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Lin D, Peng H, Huang Y, Huang J
and Gu J: Cancer-derived immunoglobulin G promotes tumor cell
growth and proliferation through inducing production of reactive
oxygen species. Cell Death Dis. 4:e9452013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Wu Z, Ma Q, Liu J, Xu Q, Han L, Duan
W, Lv Y, Wang F, Reindl KM, et al: Hyperglycemia regulates
TXNIP/TRX/ROS axis via p38 MAPK and ERK pathways in pancreatic
cancer. Curr Cancer Drug Targets. 14:348–356. 2014. View Article : Google Scholar : PubMed/NCBI
|