Effect of naringin on sodium fluoride‑induced neurobehavioral deficits in Wistar rats
- Authors:
- Published online on: April 29, 2024 https://doi.org/10.3892/br.2024.1785
- Article Number: 97
-
Copyright: © Swamy et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Fluoride is a trace element found in drinking water that can be favourable for skeletal and dental growth if ingested within the normal permissible limit (1). Fluoride concentrations in surface and groundwater water in several countries all over the world, as well as in Indian states, have much higher fluoride concentrations in drinking water compared with the maximal permissible limit of 1.5 ppm as advised by the World Health Organisation and 1 mg/l as advised by the Bureau of Indian Standards (1). There has been a substantial surge in the Indian population affected by fluorosis in the last 5 decades. The Czech Republic, Finland and Poland are among the European countries to have levels of fluoride as high as 3 ppm in drinking water (2). In Mexico, ~6% of the population, amounting to 5 million individuals, are affected by groundwater containing fluoride (2). A population exceeding 260 million individuals has been assessed to be affected by fluorosis around the world (3). In India, 85% of the population relies on groundwater for drinking (4). Indian states that have excessive fluoride levels in available drinking water range from 1.54 mg/l in Bihar to 5.98 mg/l in Madhya Pradesh (1). Fluorosis has been found in Karnataka, India, as a result of high fluoride underground water concentrations of >3.06 ppm in Kolar and 5.7 ppm in Koppal (4).
Fluoride can enter the human body by means of food and dietary sources, water and toothpaste; however, the major source of fluoride is drinking water (3). In water, fluoride from the existing compounds entirely dissociates into fluoride ions. Almost 100% of the fluoride ions are imbibed by the gastrointestinal tract and conveyed to the bloodstream (5). In young children, ~80% of fluoride absorbed is retained in the body as compared with 50% in adults (2). In the United states, it is estimated that a population of 1.4 million use drinking water having 1.3-1.9 mg/l of fluoride, 1.4 million use drinking water with 2.0-3.9 mg/l fluoride and ~2 million individuals drink water with ≥4.0 mg/l fluoride concentrations (6) and these concentrations of sodium fluoride may influence the nervous system in terms of depression, behavioural changes and memory deficits, which needs to be investigated.
For this purpose, the animal experiment in the present study was conducted with the aim of finding the concentration of sodium fluoride that could induce depression, behavioural changes and memory deficits. To the best of our knowledge, there is a lack of effective natural treatment options available against the detrimental neurological effects of fluoride, which prompts research to explore and test for alternative, effective and natural products that can provide overall protection to a larger population in fluoride-endemic areas. Therefore, the present experiment was conducted to explore the potential of a flavanone glycoside known as Naringin against fluoride-induced neurobehavioral effects. Naringin is isolated from citrus family plants such as lemons and oranges. Naringin is a 4',5,7-trihydroxyflavanone-7-rhamnoglucoside that tastes bitter and has shown beneficial effects in conditions such as diabetes, hepatic diseases and neurological diseases (7-11). It possesses antioxidant and anti-inflammatory properties, along with hypolipidemic activities (12).
Swamy et al (13) conducted an animal experiment that exhibits the neuroprotective effect of Naringin against 100 ppm of sodium fluoride in adult rodents, ameliorating the stress along with behavioural and memory deficits. However, the study by Swamy et al (13) did not assess the effect of prophylactic administration of naringin on fluoride-induced neurotoxicity, alterations in catalase and acetylcholinesterase (AChE) levels and histology of the medial prefrontal cortex. In the present experiment of this study, 10 ppm of sodium fluoride was selected and used for the experiments.
Thus the present study was carried out with an aim to investigate protective effect of Naringin on behavioural and cognitive changes induced by 10 ppm of sodium fluoride in wistar rats.
Materials and methods
Materials
Details of material procured are as follows. All other chemicals are of standard analytical grade. i) Naringin (cat. no N0073; Tokyo Chemical Industry Co. Ltd.); and ii) Sodium fluoride (cas no. 7681-49-4; EMPLURA®; Merck KGaA).
Animals
After obtaining approval from the Institutional Animal Ethics Committee, Kasturba Medical College, Manipal (approval no. IAEC/KMC/55/2018; dated, 28.07.2018) Manipal, rats were obtained from the central animal research facility (CARF; Manipal, India), and were kept in a 12:12 h light:dark cycle at room temperature and at a humidity of ~81%. A total of 72 adult male Wistar rats, ~90 days old and weighing between 150 to 240 g were used. Food and water was provided ad libitum to all animals. Two separate experiments were conducted.
First experiment
The first experiment was conducted to determine the concentration of fluoride, among 10 and 50 ppm that could induce behavioural and memory deficits in short duration. For this experiment, 24 animals were divided into three groups: i) Normal (NOR) group; ii) sodium fluoride 10 ppm (FLU10) group; and iii) sodium fluoride 50 ppm (FLU50) group. The NOR group received normal tap water with ~0.5 ppm fluoride for 6 months, while the FLU10 and FLU50 groups received drinking water ad libitum with sodium fluoride at 10 and 50 ppm, respectively, for 6 months. Subsequently, each group of animals consisted of eight rats and behavioural studies such as the forced swim test (FST), novel object recognition test (NORT) and open field test (OFT) were conducted in all groups at the end of 15, 30, 60, 90 and 180 days.
Second experiment
The second experiment, which aimed to elucidate the effect of Naringin against sodium fluoride-induced behavioural and memory deficits, consisted of eight groups, with 8 animals in each group. i) NOR; ii) sodium fluoride 10 ppm (FLU10); iii) FLU10 + Naringin 100 mg/kg bw (FLU10NAR100); iv) FLU10 + Naringin 50 mg/kg bw (FLU10NAR50); v) Naringin 100 mg/kg bw (NAR100); vi) Naringin 50 mg/kg bw (NAR50); vii) prophylactic Naringin 100 mg/kg bw + FLU10 (PRONAR100); and viii) prophylactic Naringin 50 mg/kg bw + FLU10 (PRONAR50).
The Wistar rats of the NOR group were provided with water sourced from normal tap water containing ~0.5 ppm FLU ad libitum. The FLU10 group received water with 10 ppm FLU. For the FLU10NAR100 and FLU10NAR50 groups, Wistar rats were provided drinking water containing 10 ppm FLU ad libitum along with Naringin 100 and 50 mg/kg bw (MilliporeSigma), respectively given per oral gavage. The NAR100 and NAR50 groups were given Naringin 100 and 50 mg/kg bw per oral gavage, along with normal tap water. The PRONAR100 and PRONAR50 groups received Naringin 100 and 50 mg/kg bw for the first 15 days and then subsequently received sodium fluoride 10 ppm for 60 days (a total of 75 days). After the treatment, all animals were subjected to behavioural tests such as the OFT, FST and NORT. The rats were then euthanized with an excess of intraperitoneal pentobarbital injection (200 mg/kg or 5 times the dose required to induce general anaesthesia) to minimize the pain and discomfort of the rodents. There were no observed behavioural or physical characteristics in rats indicating poor health and none of the rats were required to be euthanised before the study was completed. After euthanasia, the brain was removed, fixed in 10% formalin solution at room temperature for 24 h and subsequently the sections of hippocampus and prefrontal cortex were removed and processed for Cresyl violet staining. Reduced glutathione (GSH), lipid peroxidation (LPO), catalase and AChE levels were estimated in the cerebral hemisphere of the brain. The results of behavioural tests, biochemical tests and neuronal count in the hippocampus and prefrontal area were analysed.
Behavioural tests. OFT
The OFT was conducted according to Bures et al (14) in a rectangular wooden box measuring 100x100 cm floor with 40-cm-high walls. A total of 36 equal squares were drawn on the floor of the apparatus (15). The animals were placed in the apparatus and were observed for 5 min. The total number of peripheral and central squares entered by the rat during these 5 min were recorded. The rat was considered to have entered a given square in case it entered that square with all four paws. After 5 min of exploration, the animal was sent back to its home cage. The floor was thoroughly cleaned after each test with a 75% ethanol solution. The number of lines crossed, number of central squares entered, central square duration, freezing time in sec, number of grooming and rearing times were noted, and further statistical analysis was performed.
NORT
NORT was performed according to Antunes and Biala 2012(16) in the apparatus consisting of a box of 40x 40x40 cm dimension. On the first and second days of the habituation phase, the rats were kept in the box for 10 min for acclimatization to the manipulations and environment. The training phase was on the third day, in which two identical objects were placed in the boxes and rats were allowed to explore for 10 min. The fourth day was the testing phase, and the same procedure was followed as earlier, except that one of the old objects was replaced with a new different novel object. After each test, the objects and the apparatus were thoroughly cleaned with a 75% ethanol solution. The test phase was conducted 24 h after the familiarization phase to test the long-term memory. Time spent exploring the novel and familiar object was noted, the discrimination index and recognition index were calculated and further statistical analysis was performed. Animals that spent more time exploring or observing the novel object are considered to have normal memory, whereas animals that spend excess time exploring the familiar object as compared with the novel object indicated a memory deficit.
FST
To evaluate the depression-like behaviour of rats, the FST test was performed according to Yankelevitch-Yahav et al (17). A transparent cylindrical glass container with a diameter of 20 cm and a height of 50 cm was loaded with tap water at a temperature of 23±1˚C, in such a way that rats were unable to touch the container's bottom with their legs. Initially, a training procedure was performed in which the rat was placed in a cylinder filled with water for 15 min. After 24 h, the same procedure was repeated, but for only 5 min, during which the behaviour of rats was observed and the movements were categorized into three groups: i) Immobile: Except for those necessary movements to keep its nose above water, the rat had no movement and floated; ii) struggling or climbing: The front paws moved quickly enough to break the surface of the water; and iii) swimming: Limb movement was observed in the form of paddling. The mean time for each behavioural category was measured and analysed. The water was changed after every session.
Biochemical tests
Biochemical tests were conducted in the second experiment. After euthanasia, one of the two cerebral hemispheres of the brain were removed quickly and homogenized for 20 min with 0.1 M phosphate-buffered saline with pH 7.4, containing 0.1 mM EDTA at room temperature and stored at -80˚C for biochemical analysis procedures. The homogenates obtained were centrifuged at 4˚C at a rate of 14,000 x g for 30 min and the resultant supernatants were collected for analysis as follows.
Assay of GSH
This method employed an optimized enzymatic GSH reductase recycling procedure for the assessment of GSH. To remove protein, 100 µl of brain homogenate was mixed with 100 µl of metaphosphoric acid at room temperature for 10 min and centrifuged with 2,000 x g for 2 min at 4˚C. To increase the pH, 50 µl of 4 M triethanolamine was mixed with each ml of homogenate. To perform the total GSH assay, a sample of 50 µl was added to 150 µl of a reaction mixture having 0.24 mM NADPH, 2 mM EDTA, 0.1 mM 5,5'-dithiobis-2-nitrobenzoic acid, 0.4 M 2-(N-morpholino) ethane-sulfonic acid, 0.1-unit glutathione reductase and 0.1 M phosphate (pH 6.0). The absorbance at 412 nm was used to determine total glutathione (18).
LPO assay
Brain homogenate of 100 µl was added to 100 µl of saturated methanol (95%) and 1 ml of cold chloroform at 4˚C and mixed thoroughly. LPO was extracted from the chloroform layer after being centrifuged at 2,000 x g for 5 min at 4˚C. Then, 500 µl of chloroform extract was added with chloroform-methanol solvent 450 µl and chromogen 50 µl in a glass tube. After 5 min of incubation at 4˚C, absorbance at 500 nm was measured. Later, LPO was expressed as nmol/g tissue after each of the homogenates was assessed in duplicates (18).
Catalase
Catalase activity was estimated by measuring hydrogen peroxide reduction at 240 nm in a reaction medium having 20 mM H2O2 obtained from MilliporeSigma, 10 mM potassium phosphate buffer at pH 7.0 and 0.1% Triton X-100 (MilliporeSigma). One catalase unit is defined as 1 µmol of H2O2 consumed per min expressed as units/mg protein (19).
AChE
The estimation of AChE activity was performed using Ellman's method (20). In a cuvette, an aliquot of 0.4 ml of homogenate was mixed with 2.6 ml of 0.1 M phosphate buffer at pH 8 and 100 µl of 5, 5'-dithio-bis-(2-nitrobenzoic acid). The contents of the cuvette were mixed with bubbling air, and the absorbance at 412 nm was measured using a LKB spectrophotometer. The basal reading was recorded when the absorbance remained stable. To determine the change in absorbance per minute, 20 µl of acetylthiocholine was added and absorbance change was recorded at intervals of 2 min for a period of 10 min (20).
Histological procedures and quantification of neurons
Histological tests were performed on animals in the second experiment. After euthanasia, the brain was preserved in 10% formalin for 24 h at room temperature. The whole brain was cut coronally 2-4 mm anterior to the midpoint measured from the anterior to the posterior pole of the rat brain for taking sections passing through the hippocampus. For the medial prefrontal cortex, a selected area of the cerebrum was isolated from the other parts of the brain by separating 3/8 of the cerebrum's front parts (21). These cut parts of the brain were used to make a block from which 5-6 mm thick coronal sections were taken and processed for Cresyl violet staining (22). Images were captured from the CA3 area of the dorsal hippocampus and medial prefrontal cortex of each rat brain section were taken at 40X magnifications using a ProgRes CapturePro 2.1, Jenoptik microscopic camera (Jenoptik AG) fitted with an inverted phase contrast light microscope. Numerical quantification of viable/surviving (number of viable neurons/total number of neurons x100) and degenerated CA3 neurons (number of degenerated neurons/total number of neurons x100) in the region of the hippocampus and prefrontal cortex was performed.
Statistical analysis
All data obtained are presented as mean ± SEM. GraphPad Prism 8 software (GraphPad Software, Inc.; Dotmatics) was used to analyse the data. All the parameters were checked for normality using the Kolmogorov-Smirnov test and all the data was distributed normally. A one-way ANOVA test followed by Tukey's multiple comparison test was performed to compare between groups. P<0.05 was considered to indicate a statistically significant difference.
Numerical quantification of viable and degenerated neurons in the region of the prefrontal cortex and CA3 hippocampus was performed. The mean for each group was presented as percent (%). Mean percent values of viable and degenerated neurons of each group with ± SEM were checked for normal distribution using the Kolmogorov-Smirnov test and one-way ANOVA was performed. Tukey's multiple comparison test was used for comparison between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
First experiment
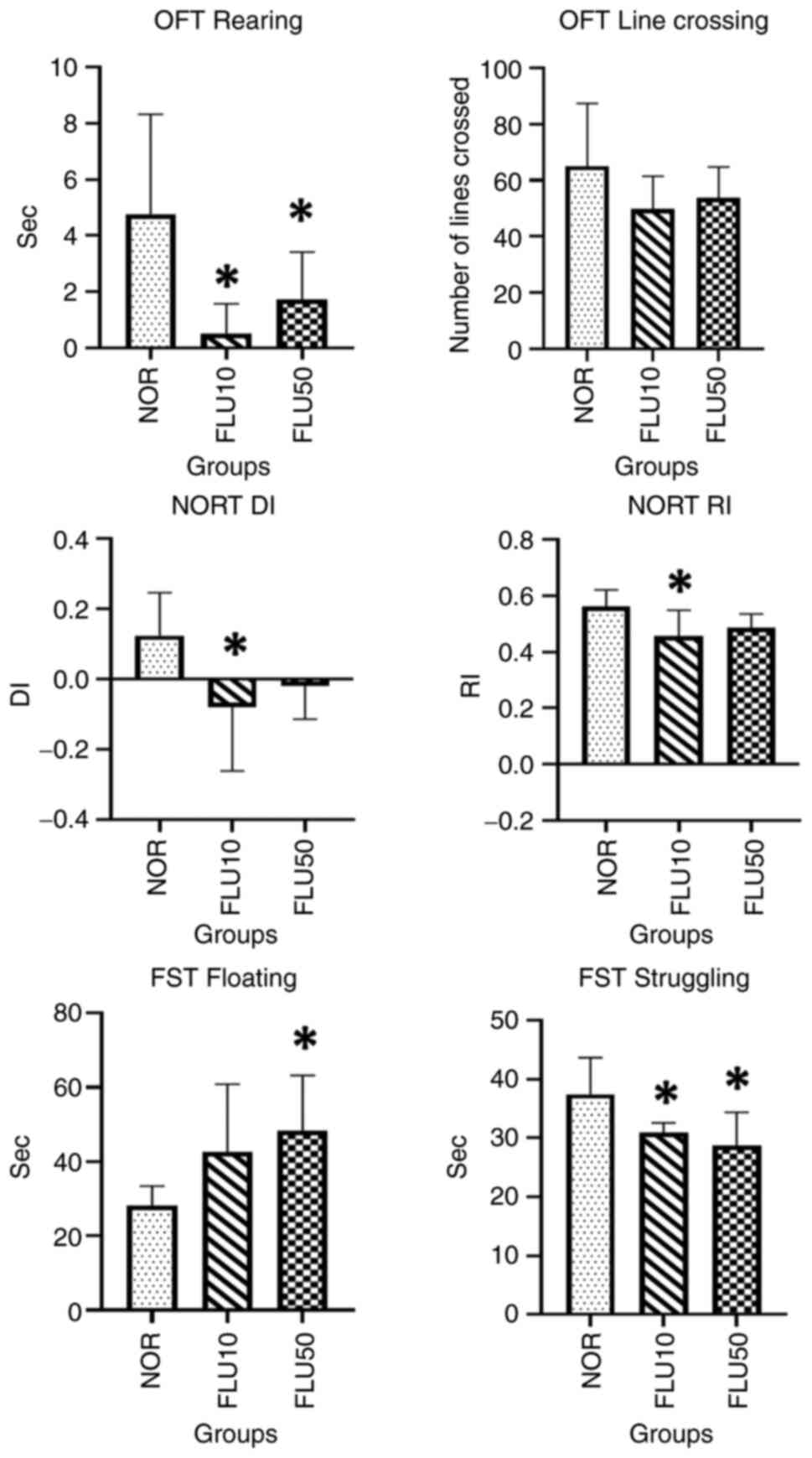
In the first experiment, the data regarding the behavioural tests of OFT, NORT and FST were analysed at the end of 60 days are shown in Fig. 1.
OFT
After 60 days, behavioural tests revealed significant (P<0.05) changes in the rearing aspect of OFT in the FLU10 (0.5±0.3780) and FLU50 (1.714±0.6442) groups compared with the NOR group (4.750±1.264). There was a decrease in the number of lines crossed in both the FLU10 (49.75±4.131) and FLU50 groups (53.71±4.173) compared with the NOR group (65.00±7.919), but this was not statistically significant (Fig. 1).
NORT
The FLU10 group had statistically significant changes in the discrimination index (DI) (0.07875±0.06443) and recognition index (RI) (0.4563±0.03278) of the NORT compared with the NOR group, with DI 0.1238±0.04342 and RI 0.5613±0.02133, respectively. The FLU50 group showed increased floating time (48.29±5.656) and reduced struggling time (28.71±2.146), while the FLU10 group showed reduced struggling time (30.88±0.6105) as compared with the NOR group floating time of 28.14±2.005 and struggling time of 37.43±2.369, respectively, with statistical significance (P<0.05) as seen in Fig. 1.
Although similar and enhanced changes were observed in FLU50 group, the present study selected the FLU10 group at the end of 60 days for the second experiment, as this was the concentration and duration that could induce significant behavioural changes along with memory deficits.
From the above results of the first experiment, the FLU10 group with a 60-day duration was selected for the second experiment to explore the protective and ameliorative effects of Naringin 50 and 100 mg/kg bw on sodium fluoride-induced behavioural changes.
Second experiment. OFT
An OFT was conducted, and the results were analysed. Parameters such as lines crossed, central square entry, central square duration, freezing time, rearing and grooming were analysed between the groups. In FLU10 group, there was decrease in the number of lines crossed (54.00±7.26), number of central square entry (3.5±0.84) and central square duration (5.0±1.48) as compared with other groups, but was statistically not significant. Rearing (5.0±1.06), grooming (1.41±0.94) and freezing (3.5±0.84) in FLU10 group did not show statistically significant changes as compared to other groups.
NORT
In this test, the Recognition Index (RI) and Discrimination Index (DI) of the Novel object recognition test (NORT) for the FLU10 group were 0.39±0.01 and -0.21±0.03 respectively, which was statistically significant as compared with all other groups except the PRONAR100 and PRONAR50 groups.
The RI and DI showed statistically significant changes between the FLU10 and NOR groups, and the FLU10NAR100 and FLU10NAR50 groups compared with FLU10, suggesting that the memory deficit induced by sodium fluoride at 10 ppm was ameliorated by the treatment group of FLU10NAR100 as well as the FLU10NAR50 group. The NAR100 and NAR50 groups also showed statistically significant enhanced memory as compared with the FLU10 group. However, the PRONAR100 and PRONAR50 groups, even after marginal improvement in RI and DI failed to show a statistically significant difference as compared with FLU10 as shown in Fig. 2.
FST
In the FST the struggling time for the FLU10 group was 61.17±9.71 sec, which was the lowest struggling time as compared to all other groups. The floating time was 99.83±11.65 sec, which was the highest floating time as compared with all other groups, and floating time was increased and struggling time was decreased in FLU10 group with statistically significant changes as compared with all other groups as shown in Fig. 3. This suggested that the stress and depression induced by FLU10 was ameliorated by the treatment group of the FLU10NAR100 and FLU10NAR50 groups, along with the PRONAR100 and PRONAR50 prophylactic groups (Fig. 3).
Biochemical test results. LPO
LPO in the NOR group was 346.3±6.38 nm/mg of protein, which was statistically significant compared with that of the FLU10 group at 1,104±1.46 nm/mg of protein (P<0.05). The combination of Naringin 50 and 100 mg/kg bw with sodium fluoride 10 ppm (FLU10NAR 50 and FLU10NAR100 groups, respectively) showed 244.6±2.10 and 297.8±3.49 nm/mg of protein LPO, respectively; this indicated a significant reduction in LPO compared with the FLU10 group. Other groups, such as NAR50, NAR100, PRONAR50 and PRONAR100 also showed a signification reduction in LPO compared with the FLU10 group. All groups showed a significant change of P<0.05 as compared with the FLU10 group (Fig. 4).
Catalase
The catalase activity of the FLU10 group was 20.12±2.67 µmol of H2O2 decomposed/mg of protein, which showed significant (P<0.05) changes as compared with the NOR (68.31±8.72), NAR50 (64.51±5.57), NAR100 (74.17±6.41), PRONAR50 (68.67±8.27) and PRONAR100 (89.20±2.34) groups. Catalase activity was increased in the FLU10NAR50 (33.49±4.27) and FLU10NAR100 (40.36±7.13) groups compared with the FLU10 group but was statistically insignificant, as seen in Fig. 4.
GSH and AChE
GSH of the FLU10 group was 26.54±1.70 nm/mg of protein, which was lower compared with the NOR group (39.12±7.80), as well as to all other groups such as NAR50 (32.88±2.00), NAR100 (33.38±1.07), FLU10NAR50 (33.26±2.11), FLU10NAR100 (35.72±2.21), PRONAR50 (32.60±1.87), PRONAR100 (37.58±1.54), but it was statistically insignificant as seen in Fig. 5.
AChE of NOR group was 0.001202±0.0003 (micromoles of acetylcholine iodide hydrolysed/mg of protein), FLU10 (0.0007015±0.05), NAR50 (0.0008399±0.06), NAR100 (0.001042±0.04), FLU10NAR50 (0.0009300±0.08), FLU10NAR100 (0.0009822±0.07), PRONAR50 (0.0009677±0.10), PRONAR100 (0.001001±0.03). These levels were not statistically significant with normal as well as fluoride groups as seen in Fig. 5.
Histology. Hippocampus CA3 region
In the hippocampus CA3 region, the viable neuronal count percent of the FLU10 group was (52.85±01.25), which was significantly reduced (P<0.05) compared with the viable neuron count of NAR100 group (61.53±01.45). The NOR group had 59.5% viable neurons, which was increased compared with the FLU10 group, but not statistically significant. The treatment groups of FLU10NAR100 (58.50±01.64) and FLU10NAR50 (57.11±01.88) did not appear to have any statistically significant changes as compared with the FLU10 group (Fig. 6).
The FLU10 (47.14±01.23) group had a significantly (P<0.05) higher percentage of degenerated neurons compared with the NAR100 (38.46±02.01) group. The treatment groups of degenerated FLU10NAR100 (41.50±01.76) and FLU10NAR50 (42.88±03.19) did not appear to have any significant changes as compared with the degenerated FLU10 group. However, in both viable and degenerated neuron counts, there was no statistical significance between NOR and FLU10 (Fig. 6).
Prefrontal cortex
In the prefrontal region, the viable neuron count percent of the FLU10 group (51.50±01.31) was significantly (P<0.05) reduced compared with the NAR100 (60.66±01.85) and NAR50 groups (59.33±01.66). The treatment groups of FLU10NAR100 (54.00±02.17) and FLU10NAR50 (57.11±01.88) were not significantly changed compared with the FLU10 group (Fig. 7).
The FLU10 group had an increased number of degenerated neurons percent (48.50±01.31) compared with the NAR100 (39.33±01.25) and NAR50 groups (40.67±01.40) with a statistical significance of P<0.05. The treatment groups of FLU10NAR100 (46.00±01.31) and FLU10NAR50 (44.00±02.12) did not have any statistically significant changes compared with the FLU10 group (Fig. 7).
Discussion
A fluoride concentration of 0.2 to 3.5 ppm is sufficient to induce dental fluorosis (2). According to the data published from 1990 to 2012, it has been reported that the prevalence of fluorosis in South Africa is 47%, while in Iran it is 61% (23). Approximately 82.4% of Sri Lanka's population lives in the low fluoride region with ~80% of children experiencing mild to severe forms of dental fluorosis (24).
Intake of fluoride in an excessive amount above the safe limit is the chief cause of fluorosis and various neurological manifestations in the form of depression and decreased intelligence quotient (IQ), which has been observed in children (25,26). In Mexico, 132 children between the age group of 6 to 10 years from rural communities drink water that contains high fluoride content and thus exhibit reduced IQ scores (27). Fluoride concentrations from 1.2 to 3 ppm in drinking water have an inverse correlation with the IQ of 10-12-year-old children in Karnataka (26). Attention deficit hyperactivity disorder was observed at a higher rate in children aged 4 to 17 years in the United States of America in national surveys conducted in 2003, 2007 and 2011, where a greater portion of the population received fluoridated water (28).
Moreover, in adults, ~50% of the ingested fluoride accumulates in the tissues of the body and only 50% is excreted per day through the kidneys, while in infants and children, approximately 80-90% of the absorbed fluoride accumulates in the tissues (29).
In animal experiments, adult Wistar rats provided with 20 ppm of sodium fluoride have shown behavioural and memory changes as observed by rota rod and hot plate tests (30). Young adult rats had inhibited learning ability and memory due to 50 ppm of sodium fluoride given in drinking water (31). In rodents, chronic fluoride exposure can lead to neurotoxicity due to oxidative stress, inflammation and mitochondrial dysfunction leading to programmed cell death (32). Even at 5 ppm, concentrated sodium fluoride leads to deficits in memory and spatial learning in rats with fluorosis along with decreased expression levels of phosphorylated (p)-ERK1/2, α4 and α7 nAChR subunits and increased expression levels of p-MEK1/2, p-ERK1/2 and total-ERK1/2 in the brains of the rats (33).
Fluoride concentration in the rat hippocampus has been found to be negatively correlated with AChE activities (34). Wistar rats were provided with 5, 15, 50 ppm fluoride in their drinking water for 60 days during their gestation and lactation. This resulted in significantly elevated levels of AChE in the cerebral synaptic membranes of maternal rats by 30.0-67.6% in a dose-dependent manner, along with an increase in the AChE activity of their offspring 80 days after birth in cerebral synaptic membranes by 8.7-28.7% (35).
The animal experiment in the present study was conducted to evaluate the behavioural and memory deficits induced by 10 ppm concentration of fluoride in adult rodents. At the end of 60 days, significant changes in the rearing aspect of OFT, DI and RI of NORT and an increase in floating time with reduced struggling time in FST was observed in the FLU10 group as compared with the NOR group. Thus, the FLU10 group was selected for the second experiment.
Some of the natural non-polyphenolic phytochemicals, such as curcumin, resveratrol, quercetin, fisetin and berberine, have potential to ameliorate and prevent the toxic effects of fluoride (32).
Naringin, a glycoside, was selected to evaluate its neuroprotective effect against 10 ppm sodium fluoride. Naringin, even at 2.5, 5 and 10 mg/kg doses administered for 7 days by intraperitoneal injection in mice, showed antioxidant and antidepressant effects by increased mobility time in the FST, anxiolytic effect and increased cognitive performance. It has significantly increased the activities of GST, catalase, superoxide dismutase and decreased nitrite contents, malondialdehyde and AChE activity (36). Naringenin, an active metabolite of Naringin, also has potential inhibitory activity against AChE (37,38).
In the second experiment, the NORT test, which was conducted at the end of 60 days, showed considerable memory deficits in the sodium fluoride 10 ppm (FLU10) group. Naringin 100 mg/kg bw (NAR100) and Naringin 50 mg/kg bw (NAR50) along with the NOR group did not show any memory deficits as indicated by the RI and DI. However, the FLU10NAR100 with RI 0.54±0.02 and DI 0.08±0.04 and FLU10NAR50 groups with RI 0.52±0.02 and DI 0.04±0.04 recovered from memory deficits compared with the FLU10 group with RI 0.39±0.01 and DI-0.21±0.03. This demonstrated the protective effect of Naringin in sodium fluoride-induced memory deficits. A similar effect was found in both prophylactic groups of PRONAR100 and PRONAR50, but without significant statistical changes.
The FST test results showed that the FLU10 group floated longer and had a decreased struggle time compared with all other groups, including the NOR group. The FLU10NAR100 and FLU10NAR50 groups showed recovered floating time and struggling time, as compared with the FLU10 group. This indicated that Naringin was able to protect the Wistar rats from sodium fluoride-induced stress and depression.
However, the FLU10 group showed only marginal changes as compared with the NOR group in number of lines crossed, central square entry and duration, indicating a low level of anxiety and fear in the FLU10 group. In addition, anxiety and fear seen in FLU10 group was reduced in treatment groups of FLU10NAR100 and FLU10NAR50, but was statistically insignificant.
Thus, Naringin prevented the neurobehavioral deficits such as stress, depression-like behaviour along with protection against memory deficits caused by 10 ppm sodium fluoride in treatment groups (FLU10NAR50 and FLU10NAR100) as observed in FST and NORT tests. Moreover, it prevented stress and depression-like behaviour as seen in the FST in the prophylactic groups (PRONAR100, PRONAR50).
In addition, it was observed that 50 mg/kg bw of Naringin used in treatment and prophylactic groups decreased the levels of catalase compared with 100 mg/kg bw; thus, decreased level of catalase indicates Naringin's dose dependent action as an antioxidant that mitigates oxidative stress (39). No other substantial difference was observed in the neuroprotective effect of the NAR50, FLU10NAR50, PRONAR50 groups compared with the NAR100, FLU10NAR100 and PRONAR100 groups.
The neurons of the brain are metabolically active and demand high oxygen consumption along with optimal creation of reactive oxygen species (ROS) (39).
ROS are by-products produced from the cellular metabolism that predominantly occurs in the mitochondria. ROS are formed by the combination of O2-derived free radicals such as hydroxyl radicals, hydrogen peroxide, superoxide anions, alkoxyl and peroxyl. A significant amount of intracellular ROS is produced in the mitochondrial membrane, particularly at complex I and complex III (40). Fluoride alters the potential of a mitochondrial inner membrane, occludes the transmission of the mitochondrial electron transport chain, hinders the procedure of the respiratory chain complex, disrupts the equilibrium between fusion and fission, decreases adenosine triphosphate, enhances the levels of Ca2+ and damages to morphology of cell mitochondria, ultimately leading to cell dysfunction and apoptosis (32).
Antioxidants keep substantial amounts of ROS under control. If antioxidants under certain conditions fail to reduce the levels of ROS, it will result in neuronal oxidative stress (40). These excessive ROS are detrimental to neurons and exert their toxic effects by oxidising essential macromolecules such as cytoskeletal proteins and enzymes of neurons (39). Polyunsaturated fatty acids of neurons are affected by free radicals or ROS through LPO to form highly reactive electrophilic aldehydes, such as malondialdehyde and secondary products responsible for extensive cellular damage and damage to neuronal membranes (41). Peroxidation of membrane lipids leads to variation in functions, resulting in marked rigidity of membrane, decreased activity of membrane-bound enzymes, altered permeability and damage to membrane receptors, which contribute to modified membrane integrity, resulting in (32) pathogenesis of neurodegenerative disorders (40). Thus, excessive ROS affects the neuronal circuitry necessary for memory formation, leading to decreased performance in cognitive function, impaired learning and memory processes (41,42).
The LPO level of the FLU10 group was significantly higher compared with the NOR group as well as to all other groups, including the FLU10NAR50 and FLU10NAR100 groups. LPO levels in the FLU10NAR100 and FLU10NAR50 groups indicated a strong resistance to LPO in the treatment group induced by fluoride 10 ppm administered to rats. This may indicate that Naringin is a potent protective alkaloid effective against ROS-induced LPO by sodium fluoride.
Catalase activity of the FLU10 group decreased significantly when compared with the NOR, NAR50, NAR100, PRONAR50 and PRONAR100 groups. Catalase activity was increased in the FLU10NAR50 and FLU10NAR100 groups compared with the FLU10 group but was statistically insignificant. This indicated that Naringin exhibited antioxidant properties against fluoride-induced ROS, without statistically significant changes.
GSH levels in the FLU10 group compared with the NOR group, as well as to all other groups, were not significantly different, including the treatment groups of FLU10NAR50 and FLU10NAR100. These levels of GSH marginally indicate Naringin's antioxidant properties against the fluoride-induced ROS, which were statistically insignificant.
Alteration in activity of AChE has been associated with neurobehavioral and cognitive deficits, as seen in neurodegenerative diseases such as Alzheimer's disease and intellectual disability with lower intelligence quotients and thinking and memory impairment (42,43). AChE was low in the FLU10 compared with all other groups but was not statistically significant, indicating a weak action of Naringin on AChE activity in the treatment group, which can be attributed to changes in some parameters of OFT and NORT.
Catalase levels were increased, while LPO levels were decreased in treatment as well as prophylactic groups compared with the fluoride group. Catalase is one of the crucial antioxidant enzymes that mitigates oxidative stress by converting cellular hydrogen peroxide to produce water (32). LPO is a metabolic process that causes oxidative deterioration of lipids by ROS. This process can degrade the lipids within the cell membrane leading to cell damage and eventually, cell death (32,40,43). This indicates the proposed pathway of the neuroprotective effect of Naringin is by reducing ROS caused by fluoride, as shown in Fig. 8.
In the histological analysis of the hippocampus CA3 region, the percentage of degenerated neurons was 38.46% in the NAR100 group and over 47.14% in the FLU10 group, indicating that FLU10 induced neurodegeneration in the FLU10 group compared with the NAR100 group only. The fluoride with naringin groups (FLU10NAR100 and FLU10NAR50) and prophylactic groups (PRONAR50 and PRONAR100) groups showed minor changes with pyknotic neurons, but neurodegeneration was not significant compared with the fluoride group.
In the prefrontal region, fluoride with naringin (FLU10NAR100 and FLU10NAR50) and prophylactic groups (PRONAR50 and PRONAR100) showed minor changes in the number of degenerated neurons with pyknosis and viable neurons, but the difference was not statistically significant as compared with the FLU10 group.
Neurons of fluoride with naringin groups (FLU10NAR100 and FLU10NAR50) and prophylactic groups (PRONAR100 and PRONAR50) showed reduced pyknosis as compared with the FLU10 group in hippocampal as well as medial prefrontal regions.
The aforementioned histological changes could be associated with the results of biochemical tests, especially the LPO, which was reduced in all the other groups except the FLU10 group. In addition, the catalase activity and GSH were lower in the FLU10 group compared with the NAR100, NAR50, PRONAR100, PRONAR50 groups and was marginally decreased in the FLU10NAR100 and FLU10NAR50 treatment groups.
Moreover, in NORT DI and RI, the values for the NAR50 and NAR100 groups showed enhanced memory results compared with the FLU10NAR50, FLU10NAR100, PRONAR50 and PRONAR100 groups.
In FST, floating time was increased, and struggling time was reduced in FLU10NAR50, FLU10NAR100, PRONAR50 and PRONAR100 groups compared with the NAR50 and NAR100 groups. Catalase was increased in the NAR50 and NAR100 groups compared with FLU10NAR50 and FLU10NAR100 groups.
In the hippocampal CA3 and prefrontal region of the rat brain, the number of viable neurons in the NAR50 and NAR100 group was increased compared with the FLU10NAR50, FLU10NAR100, PRONAR50 and PRONAR100 groups. The numbers of degenerated neurons in the NAR50 and NAR100 group were decreased compared with the FLU10NAR50, FLU10NAR100, PRONAR50 and PRONAR100 groups. Thus, neurobehavioral outcomes in the groups without sodium fluoride (NAR50, NAR100) exposure were improved compared with the naringin-treated groups (FLU10NAR50, FLU10NAR100, PRONAR50 and PRONAR100).
Histological staining of neurons with Fluoro-Jade B may be crucial in revealing neurons undergoing degeneration, and we expect researchers to include this in forthcoming research projects. The results of the present study shows that Naringin may be protective for nervous system as well as other body organs against the ROS produced by fluoride as well as other toxic substances that humans or any other species may encounter.
In conclusion, sodium fluoride 10 ppm in drinking water induced ROS, stress, depression and memory deficits and these were ameliorated by Naringin 100 and 50 mg/kg bw per oral administration as observed by the NORT, FST and insignificant minor changes in OFT and supported with biochemical tests of LPO. Naringin may be useful as a nutraceutical and possibly as an ameliorative drug to overcome the neurological ill effects of fluoride in adult Wistar rats. Thus, Naringin can be used as a protective agent in fluoride endemic areas that may protect and mitigate the ROS induced by fluoride ingestion, thus avoiding the behavioural and cognitive changes in the human population.
Acknowledgements
Not applicable.
Funding
Funding: This research was funded by the Indian Council of Medical Research (ICMR) (grant no. 5/8-4/6/Env/2020-NCD-II; dated, 15/02/2022).
Availability of data and materials
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
RSS performed the experiments, collected the data and performed its analysis. NiK was major contributor in conception and design, analysing and interpreting the data and in writing the manuscript. SS contributed substantially in conception and design along with analysis and interpretation of data. NaK made substantial contributions to conception, design and writing the manuscript. VR contributed for acquisition, analysis and interpretation of data. All authors read and approved the final manuscript. RSS and NiK confirmed the authenticity of all the raw data.
Ethics approval and consent to participate
The animal experiments were conducted as per animal ethics standards after obtaining approval from the Institutional Animal Ethics Committee (IAEC) (Manipal Academy of Higher Education, Karnataka, India; approval no. IAEC/KMC/55/2018; dated, 28.07.2018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Authors' information
Dr Ravindra Shantakumar Swamy, ORCID-0000-0002-2163-3355; Dr Nitesh Kumar, ORCID-0000-0002-4929-3954; Professor Dr Smita Shenoy, ORCID-0000-0002-7578-1855; Dr Naveen Kumar, ORCID-0000-0003-2805-779X; Ms Vanishree Rao, SCOPUS ID-57699031200.
References
|
Ali S, Fakhri Y, Golbini M, Thakur SK, Alinejad A, Parseh I, Shekhar S and Bhattacharya P: Concentration of fluoride in groundwater of India: A systematic review, meta-analysis and risk assessment. Groundw Sustain Dev. 9(100224)2019. | |
|
Ayoob S and Gupta AK: Fluoride in drinking water: A review on the status and stress effects. Crit Rev Environ Sci Technol. 36:433–487. 2006. | |
|
Amini M, Mueller K, Abbaspour KC, Rosenberg T, Afyuni M, Møller KN, Sarr M and Johnson CA: Statistical modeling of global geogenic fluoride contamination in groundwaters. Environ Sci Technol. 42:3662–3668. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Chowdhury CR, Shahnawaz K, Kumari D, Chowdhury A, Bedi R, Lynch E, Harding S and Grootveld M: Spatial distribution mapping of drinking water fluoride levels in Karnataka, India: Fluoride-related health effects. Perspect Public Health. 136:353–360. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Chowdhury A, Adak MK, Mukherjee A, Dhak P, Khatun J and Dhak D: A critical review on geochemical and geological aspects of fluoride belts, fluorosis and natural materials and other sources for alternatives to fluoride exposure. J Hydrol. 574:333–359. 2019. | |
|
DenBesten P and Li W: Chronic fluoride toxicity: Dental fluorosis. Monogr Oral Sci. 22:81–96. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Shirani K, Yousefsani BS, Shirani M and Karimi G: Protective effects of naringin against drugs and chemical toxins induced hepatotoxicity: A review. Phytother Res. 34:1734–1744. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Mani VM and Sadiq AMM: Naringin modulates the impairment of memory, anxiety, locomotor, and emotionality behaviors in rats exposed to deltamethrin; a possible mechanism association with oxidative stress, acetylcholinesterase and ATPase. Biomed Prev Nutr. 4:527–533. 2014. | |
|
Gindri Dos Santos B, Peres Klein C, Scortegagna Crestani M, Moura Maurmann R, Mateus Hözer R, Dos Santos Rodrigues K, Maciel August P and Matté C: Naringin supplementation during pregnancy induces sex and region-specific alterations in the offspring's brain redox status. Int J Environ Res Public Health. 18(4805)2021.PubMed/NCBI View Article : Google Scholar | |
|
Ahmed S, Khan H, Aschner M, Hasan MM and Hassan STS: Therapeutic potential of naringin in neurological disorders. Food Chem Toxicol. 132(110646)2019.PubMed/NCBI View Article : Google Scholar | |
|
Bharti S, Rani N, Krishnamurthy B and Arya DS: Preclinical evidence for the pharmacological actions of naringin: A review. Planta Med. 80:437–451. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Jung UJ and Kim SR: Effects of naringin, a flavanone glycoside in grapefruits and citrus fruits, on the nigrostriatal dopaminergic projection in the adult brain. Neural Regen Res. 9:1514–1517. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Swamy RS, Kumar N, Shenoy S, Cheruku SP, Rao V, Kumar N, Kumar S and Ravichandiran V: Neuroprotective effect by naringin against fluorosis-induced neurodegeneration in adult Wistar rats. Neuroreport. 34:449–456. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Bures J, Buresova O and Huston JP: Techniques and basic experiments for the study of brain and behavior. 2nd edition. Elsevier, Amsterdam, pp326, 1983. | |
|
El-Lethey H, Kamel M and Shaheed I: Neurobehavioral toxicity produced by sodium fluoride in drinking water of laboratory rats. J Am Sci. 6:54–63. 2010. | |
|
Antunes M and Biala G: The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn Process. 13:93–110. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Yankelevitch-Yahav R, Franko M, Huly A and Doron R: The forced swim test as a model of depressive-like behavior. J Vis Exp. 97(52587)2015.PubMed/NCBI View Article : Google Scholar | |
|
Zhu Y, Carvey PM and Ling Z: Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 1090:35–44. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Aebi H: Catalase in vitro. Methods Enzymol. 105:121–126. 1984.PubMed/NCBI View Article : Google Scholar | |
|
Pohanka M, Hrabinova M, Kuca K and Simonato JP: Assessment of acetylcholinesterase activity using indoxylacetate and comparison with the standard Ellman's method. Int J Mol Sci. 12:2631–2640. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Paxinos G and Watson C: The Rat Brain in Stereotaxic Coordinates. Academic Press, New York, NY, 1997. | |
|
Madhyastha S, Somayaji SN, Rao MS, Nalini K and Bairy KL: Hippocampal brain amines in methotrexate-induced learning and memory deficit. Can J Physiol Pharmacol. 80:1076–1084. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Azami-Aghdash S, Ghojazadeh M, Pournaghi Azar F, Naghavi-Behzad M, Mahmoudi M and Jamali Z: Fluoride concentration of drinking waters and prevalence of fluorosis in iran: A systematic review. J Dent Res Dent Clin Dent Prospects. 7:1–7. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Ranasinghe N, Kruger E and Tennant M: Spatial distribution of groundwater fluoride levels and population at risk for dental caries and dental fluorosis in Sri Lanka. Int Dent J. 69:295–302. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Nagarajappa R, Pujara P, Sharda AJ, Asawa K, Tak M, Aapaliya P and Bhanushali N: Comparative assessment of intelligence quotient among children living in high and low fluoride areas of Kutch, India-a pilot study. Iran J Public Health. 42:813–818. 2013.PubMed/NCBI | |
|
Aravind A, Dhanya RS, Narayan A, Sam G, Adarsh VJ and Kiran M: Effect of fluoridated water on intelligence in 10-12-year-old school children. J Int Soc Prev Community Dent. 6 (Suppl 3):S237–S242. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Rocha-Amador D, Elena Navarro M, Carrizales L, Morales R and Calderón J: Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad Saude Publica. 23 (Suppl 4):S579–S587. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Malin AJ and Till C: Exposure to fluoridated water and attention deficit hyperactivity disorder prevalence among children and adolescents in the United States: An ecological association. Environ Health. 14(17)2015.PubMed/NCBI View Article : Google Scholar | |
|
Bartos M, Gumilar F, Gallegos CE, Bras C, Dominguez S, Mónaco N, Esandi MDC, Bouzat C, Cancela LM and Minetti A: Alterations in the memory of rat offspring exposed to low levels of fluoride during gestation and lactation: Involvement of the α7 nicotinic receptor and oxidative stress. Reprod Toxicol. 81:108–114. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Manusha S, Sudhakar K and Reddy KP: Protective effects of allium sativum extract against sodium fluoride induced neurotoxicity. Int J Pharm Sci Res. 10:625–633. 2019. | |
|
Dong YT, Wei N, Qi XL, Liu XH, Chen D, Zeng XX and Guan ZZ: Attenuating effect of vitamin E on the deficit of learning and memory of rats with chronic fluorosis: The mechanism may involve muscarinic acetylcholine receptors. Fluoride. 50:354–364. 2017. | |
|
Kumar S, Shenoy S, Swamy RS, Ravichandiran V and Kumar N: Fluoride-induced mitochondrial dysfunction and approaches for its intervention. Biol Trace Elem Res. 202:835–849. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Liu YJ, Gao Q, Wu CX and Guan ZZ: Alterations of nAChRs and ERK1/2 in the brains of rats with chronic fluorosis and their connections with the decreased capacity of learning and memory. Toxicol Lett. 192:324–329. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Zhai JX, Guo ZY, Hu CL, Wang QN and Zhu QX: Studies on fluoride concentration and cholinesterase activity in rat hippocampus. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 21:102–104. 2003.PubMed/NCBI(In Chinese). | |
|
Zhao Q, Niu Q, Chen J, Xia T, Zhou G, Li P, Dong L, Xu C, Tian Z, Luo C, et al: Roles of mitochondrial fission inhibition in developmental fluoride neurotoxicity: Mechanisms of action in vitro and associations with cognition in rats and children. Arch Toxicol. 93:709–726. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Ben-Azu B, Nwoke EE, Aderibigbe AO, Omogbiya IA, Ajayi AM, Olonode ET, Umukoro S and Iwalewa EO: Possible neuroprotective mechanisms of action involved in the neurobehavioral property of naringin in mice. Biomed Pharmacother. 109:536–546. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Tran TH, Vo TTH, Vo TQN, Cao TCN and Tran TS: Synthesis and evaluation of the acetylcholinesterase inhibitory activities of some flavonoids derived from naringenin. ScientificWorldJournal. 2021(4817900)2021.PubMed/NCBI View Article : Google Scholar | |
|
Heo HJ, Kim MJ, Lee JM, Choi SJ, Cho HY, Hong B, Kim HK, Kim E and Shin DH: Naringenin from Citrus junos has an inhibitory effect on acetylcholinesterase and a mitigating effect on amnesia. Dement Geriatr Cogn Disord. 17:151–157. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Massaad CA and Klann E: Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 14:2013–2054. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Sultana R, Perluigi M and Butterfield DA: Lipid peroxidation triggers neurodegeneration: A redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med. 62:157–169. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Kumar S, Chhabra V, Mehra M, K S, Kumar B H, Shenoy S, Swamy RS, Murti K, Pai KSR and Kumar N: The fluorosis conundrum: Bridging the gap between science and public health. Toxicol Mech Methods. 34:214–235. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Ren C, Li HH, Zhang CY and Song XC: Effects of chronic fluorosis on the brain. Ecotoxicol Environ Saf. 244(114021)2022.PubMed/NCBI View Article : Google Scholar | |
|
Adedara IA, Abolaji AO, Idris UF, Olabiyi BF, Onibiyo EM, Ojuade TD and Farombi EO: Neuroprotective influence of taurine on fluoride-induced biochemical and behavioral deficits in rats. Chem Biol Interact. 261:1–10. 2017.PubMed/NCBI View Article : Google Scholar |