Dandelion root extracts and taraxasterol inhibit LPS‑induced colorectal cancer cell viability by blocking TLR4‑NFκB‑driven ACE2 and TMPRSS2 pathways
- Authors:
- Published online on: April 19, 2024 https://doi.org/10.3892/etm.2024.12544
- Article Number: 256
-
Copyright: © Yang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Colorectal cancer is the fourth leading cause of cancer-related death worldwide, and the incidence of colorectal cancer has increased rapidly in the past few years (1). According to the World Health Organization, >1.9 million new cases of colorectal cancer and approximately 1 million deaths due to colorectal cancer occurred worldwide in 2020. Several risk factors are associated with the initiation and progression of colorectal cancer, including age, sex, ethnicity, dietary habits, colon polyps and long-lasting ulcerative colitis (2,3). There is a growing body of evidence suggesting that the high density of bacteria in the colon serves an important role in colorectal cancer tumorigenesis (4-6). The gut microbiota is vital for human health through the regulation of food digestion and energy metabolism, maintenance of gut homeostasis, and modulation of host immunity (7,8). However, abnormalities in the intestinal microbiota composition caused by lifestyle habits, food intake, age and/or environmental factors may lead to an imbalance in bacterial populations, thus resulting in the overproduction of pro-inflammatory and pro-carcinogenic toxins that promote colorectal carcinogenesis (5,9,10). Lipopolysaccharide (LPS) is a bacterial endotoxin and a highly potent pro-inflammatory molecule, which is often implicated in tumorigenesis (11,12). LPS has been reported to be elevated in patients with colorectal cancer (13-15). The binding of LPS to toll-like receptor 4 (TLR4) in intestinal epithelial cells activates the NFκB pathway, resulting in increased expression and secretion of pro-inflammatory mediators, and contributing to the occurrence, development and metastasis of various tumors, including colorectal cancer and liver tumors (16,17).
The receptor angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) are abundantly expressed in the colon, and have been shown to be upregulated in human colon carcinoma (18-20). LPS and some cytokines (TNFα, IL4 and IL6) can alter the expression levels and activities of ACE2 and TMPRSS2(21). ACE2 and TMPRSS2 are also known as host proteins for the entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into human cells causing COVID-19(22), and blockage of ACE2 or TMPRSS2 can suppress the infection of human epithelial cells with SARS-CoV-2(23). Notably, patients with colorectal cancer are more likely to develop severe gastrointestinal symptoms and experience an increased risk of death compared with healthy individuals infected with SARS-CoV-2 (20,24). These findings indicate a potential association between ACE2 and TMPRSS2 and colorectal cancer.
Dandelions have been used as a popular herbal medicine for centuries, and have been reported to protect against inflammation, digestive diseases and cancer (25,26). Dandelion root extracts have been shown to selectively suppress colorectal cancer cell proliferation but have no detrimental effects on normal colon epithelial cells (27). In addition, dandelion root extract has been shown to protect against stress-induced colitis in mice (28). Phytochemical analysis has identified taraxasterol (TS) as a pharmacologically active compound obtained from dandelion root extracts, which may reduce colorectal cancer cell viability and inhibit colonic epithelial cell inflammation (27,29). In addition, TS has been widely reported to attenuate LPS-induced oxidative stress and inflammation in in vitro cell culture and in vivo animal models (30-32). However, to the best of our knowledge, the regulatory roles of dandelion root extracts and TS in bacteria-driven colorectal cancer cell viability have not been investigated. The present study explored the protective effects of dandelion root extracts and TS on LPS-induced colorectal cancer cell viability, as well as the underlying mechanisms.
Materials and methods
Preparation of dandelion root extracts
Dandelions (Taraxacum officinale) were collected from yards and fields in Sudbury (latitude 46.4917˚ N; longitude 80.9930˚ W), Ontario, Canada. The water extracts of dandelion roots were prepared as described previously (33). Briefly, the air-dried dandelion roots were extracted in boiling water for 4 h. The extracts were first filtered through Whatman filter paper and concentrated. The dried extracts were then weighed and dissolved in distilled water followed by filtration through a 0.2-µm filter. Finally, the aqueous extracts of dandelion roots were stored at 4˚C for the following experiments.
Cell culture
Human colorectal cancer cells (WiDr; cat. no. CCL-218) and normal intestinal epithelial cells (FHC; cat. no. CRL-1831) were purchased from American Type Culture Collection. According to the information from American Type Culture Collection, DNA fingerprinting has shown WiDr cells to be a derivative of the HT-29 cell line (cat. no. HTB-38), which was originally isolated from the colon of a female patient with colorectal adenocarcinoma. The cells were incubated in Dulbecco's modified Eagle's medium (MilliporeSigma) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin (all Thermo Fisher Scientific, Inc.) in a humidified atmosphere of 5% CO2 at 37˚C. The experiments were performed when the cells reached 70-80% confluence.
Cell viability assay
Cell viability was assessed using the MTT assay as described previously (34). In this assay, the living cells can convert MTT to purple formazan crystals. Briefly, equal numbers of cells (1x104) were seeded in each well of a 96-well plate for 24 h. After the cells were treated with dandelion root extracts (0.01-1 mg/ml), TS (0.01-1 µg/ml), LPS (0.1-10 µg/ml) and/or TFNα (1-20 ng/ml) for 24 h at 37˚C, MTT (0.5 mg/ml) was added to each well and the cells were then cultured at 37˚C for an additional 4 h, after which, 100 µl dimethyl sulfoxide was added for 5 min to dissolve the purple formazan. The absorbance at 570 nm was measured using a FLUOstar OPTIMA microplate reader (BMG Labtech GmbH). The control cells without any treatment were considered 100% viable.
Colony formation assay
Human colorectal cancer cells (1x105) were treated with or without 0.5 µg/ml LPS (MilliporeSigma) for 24 h at 37˚C. Subsequently, the medium was discarded, and fresh medium containing root extracts (0.1 mg/ml) or TS (0.1 µg/ml; MilliporeSigma) was added for an additional 14 days at 37˚C. Afterwards, the medium was aspirated and the cells were fixed with cold 100% methanol for 10 min at room temperature. The cells were then stained with 0.01% crystal violet for 60 min at room temperature. After washing with PBS, images of the plates were captured to count the number of colonies formed using ImageJ 1.43 software (National Institutes of Health). Clusters of ≥50 cells were considered a colony.
Detection of protein expression levels by western blotting
After the cells were treated with dandelion root extracts (0.1 mg/ml), TS (0.01 µg/ml), LPS (0.5 µg/ml), TNFα (10 ng/ml) and/or CLI095 (10 µM) for 24 h at 37˚C, the cultured cells were collected for protein analysis by western blotting as described previously (34,35). Briefly, the cells were lysed with radioimmunoprecipitation assay buffer supplemented with a protease inhibitor cocktail (MilliporeSigma) for protein extraction. Protein concentrations were determined with BCA assays (Thermo Fisher Scientific, Inc.). Equal amounts of proteins (90 µg/well) were then separated by SDS-PAGE on 10% gels and were transferred to nitrocellulose membranes (Pall Corporation). The membranes were first blocked with PBS-0.1% Tween 20 containing 3% skim milk at 4˚C overnight. The membranes were then incubated with appropriate primary antibodies for 90 min at room temperature. The following primary antibodies were used: TLR4 (cat. no. 38519; 1:1,000; Cell Signaling Technology, Inc.), NFκB-p65 (cat. no. 8242; 1:1,000; Cell Signaling Technology, Inc.), ACE2 (cat. no. 4355; 1:1,000; Cell Signaling Technology, Inc.), TMPRSS2 (cat. no. PAB11593; 1:1,000; Abnova) and GAPDH (cat. no. 97166; 1:5,000; Cell Signaling Technology, Inc.). After 3 times washing with PBS-0.1% Tween 20 containing 3% skim milk, the membranes were incubated with peroxidase-conjugated secondary antibodies (cat. no. AP132P or AP160P; 1:5,000; MilliporeSigma) for 90 min at room temperature followed by 3 times washing with PBS-0.1% Tween 20. The protein bands in the membranes were then visualized using ECL solution (Bio-Rad Laboratories, Inc.) in a dark room with an X-ray film. The densitometry of the band for each target protein was analyzed using ImageJ 1.43 software by normalizing to the intensity of GAPDH.
Analysis of mRNA expression levels by reverse transcription- quantitative PCR (qPCR)
After the cells were treated with LPS (0.5 µg/ml) with or without dandelion root extracts (0.1 mg/ml) and TS (0.01 µg/ml) for 24 h at 37˚C, the cells were collected for isolation of total RNA using TRI reagent (MilliporeSigma). The RNAs were reversed transcribed to cDNA using a Verso cDNA synthesis kit according to the manufacturer's instructions (Thermo Fisher Scientific, Inc.). The mRNA expression levels were quantified with SYBR Green PCR Master Mix (Thermo Fisher Scientific, Inc.) using a CFX Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.). Samples that were not treated with reverse transcriptase were included as negative controls of genomic DNA contamination. The following primers were used in the present study: TNFα forward, 5'-GGGCTCCAGGCGGTGCTTGTTC-3' and reverse, 5'-GCGGCTGATGGTGTGGGTGAGG-3'; IL4 forward, 5'-ACTGCTTCCCCCTCTGTTCTTCC-3' and reverse, 5'-GAGGTTCCTGTCGAGCCGTTTCA-3'; IL6 forward, 5'-AAAGAGGCACTGGCAGAAAACAAC-3' and reverse, 5'-TTAAAGCTGCGCAGAATGAGATGA-3'; ACE2 forward, 5'-CCGCGGCCAGTTGATTGA-3' and reverse, 5'-ACATTTCCTGGGTCCGTTAGCAT-3'; TMPRSS2 forward, 5'-CACGCAGCCCAAATCCCCATCC-3' and reverse, 5'-GCCGCCCGCCCGTAGTTCTC-3'; and GAPDH forward, 5'-CGGGGCTCTCCAGAACATCAT-3' and reverse, 5'-CCAGCCCCAGCGTCAAAGGTG-3'. The qPCR program was as follows: One cycle at 94˚C for 5 min, 35 cycles at 94˚C for 20 sec, 62˚C for 30 sec and 72˚C for 30 sec, and a final step for melting curve determination (94˚C for 15 sec, increasing from 60˚C to 94˚C in 0.5˚C/15 sec increments). Relative mRNA quantification was performed using the 2-ΔΔCq formula (36) by normalizing to the endogenous reference gene GAPDH.
Small interfering RNA (siRNA) transfection
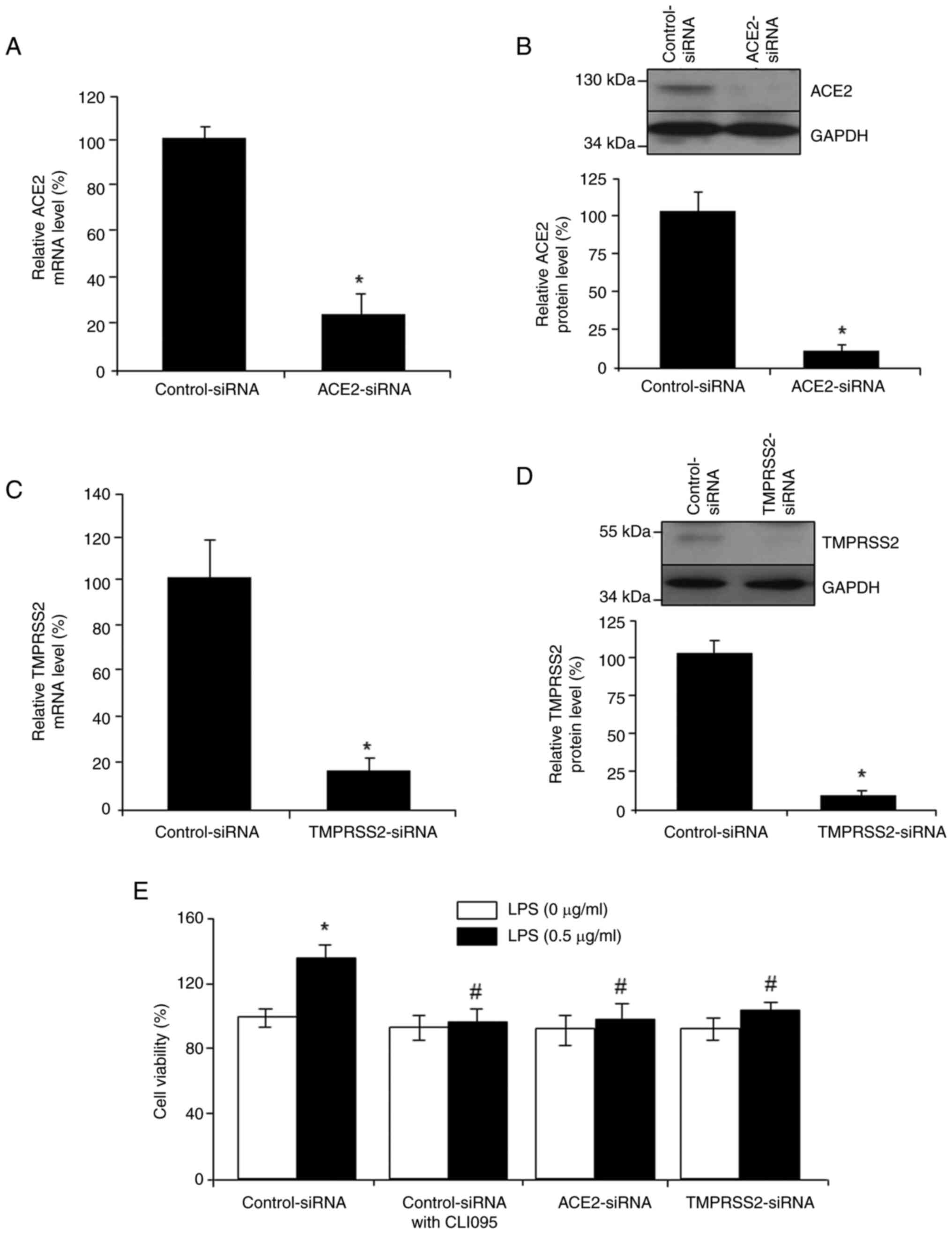
The knockdown of ACE2 and TMPRSS2 was conducted using pre-designed siRNAs from Santa Cruz Biotechnology, Inc. A control-siRNA (cat. no. sc-37007) with a scrambled sequence that would not lead to the specific degradation of any cellular messages acted as a non-targeting control. ACE2-siRNA (cat. no. sc-41400) and TMPRSS2-siRNA (cat. no. sc-41658) consisted of pools of three to five target-specific 19-25 nucleotide sequences. Transfection of the cells with siRNAs was achieved using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Briefly, the cells were plated overnight to form 60-70% confluent monolayers. After colorectal cancer cells were transfected with control siRNA (100 nM) or ACE2 siRNA/TMPRSS2 siRNA (100 nM) for 24 h at 37˚C, the cells were then incubated with LPS (0.5 µg/ml) and/or CLI095 (10 µM) for additional 24 h at 37˚C.
Statistical analysis
Data are presented as the mean ± standard error of at least three independent experimental repeats. Statistical analyses were conducted using SPSS 21.0 software (IBM Corp.). Unpaired Student's t-test was applied to analyze statistically significant differences between two groups, and one-way analysis of variance followed by the Tukey's test was used to analyze differences among more than two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Dandelion root extracts and TS inhibit the LPS-induced viability of colorectal cancer cells
When both human colorectal cancer cells and normal epithelial cells were incubated with either dandelion root extracts (0-1 mg/ml) or TS (0-1 µg/ml) for 24 h, the cell viabilities were not changed compared with those of the control cells without any treatment (Fig. 1A and B). The cell viability of control cells was set at 100%, and subsequently, it was shown that LPS at a low concentration (0.5 µg/ml) significantly enhanced the viability of colorectal cancer cells by 32%, whereas a lower (0.1 µg/ml) or higher dose (1, 5 and 10 µg/ml) of LPS had no effect on the viability of colorectal cancer cells (Fig. 2A). LPS at all tested doses (0.1, 0.5, 1, 5 and 10 µg/ml) did not affect the viability of normal epithelial cells. The addition of dandelion root extracts at a concentration of >0.1 mg/ml (Fig. 2B) or TS at concentration of >0.05 µg/ml (Fig. 2C) significantly suppressed the stimulatory effect of LPS (0.5 µg/ml) on the viability of colorectal cancer cells. Increasing the concentration of dandelion root extracts to 0.5 and 1 mg/ml, or the concentration of TS to 0.1, 0.5 and 1 µg/ml did not further inhibit LPS-induced cell viability. By contrast, dandelion root extracts at a concentration <0.1 mg/ml or TS at a concentration <0.05 µg/ml had no effect on LPS-induced cell viability (0.5 µg/ml). Therefore, in the following experiments, the concentrations of dandelion root extracts and TS were chosen as 0.1 mg/ml and 0.1 µg/ml, since both doses were able to bring the cell viability to the basal level. It was further observed that the number of colonies formed in LPS (0.5 µg/ml)-treated colorectal cancer cells was 1.6 times that formed in the control cells, which was significantly reduced by co-incubation with root extracts (0.1 mg/ml) or TS (0.1 µg/ml) for 14 days (Fig. 2D). Root extracts or TS alone had no effect on colony formation in comparison with the control cells.
Dandelion root extracts and TS inhibit LPS-stimulated TLR4/NFκB-p65 signaling
LPS (0.5 µg/ml) significantly increased the protein expression levels of TLR4 and NFκB-p65, and the supplementation of dandelion root extracts (0.1 mg/ml) or TS (0.1 µg/ml) decreased the expression levels of NFκB-p65 but not TLR4 (Fig. 3A). The dandelion root extracts or TS alone had no effect on the protein expression levels of TLR4 and NFκB-p65. As shown in Fig. 3B, LPS (0.5 µg/ml) markedly increased the mRNA expression levels of TNFα, IL4 and IL6, which could be significantly inhibited by the supplementation of dandelion root extracts (0.1 mg/ml) or TS (0.1 µg/ml).
Dandelion root extracts and TS suppress LPS-induced ACE2 and TMPRSS2 expression
ACE2 and TMPRSS2 were expressed in both normal epithelial cells and colorectal cancer cells (Fig. 4A). Compared with those in normal epithelial cells, the protein expression levels of ACE2 and TMPRSS2 were significantly higher in colorectal cancer cells. Dandelion root extracts or TS alone had no effect on the protein expression levels of ACE2 and TMPRSS2 in colorectal cancer cells (Fig. 4B). By contrast, LPS (0.5 µg/ml) significantly increased the protein expression levels of ACE2 and TMPRSS2, which could be blocked by the addition of dandelion root extracts (0.1 mg/ml) or TS (0.1 µg/ml) in colorectal cancer cells (Fig. 4C). Similarly, the mRNA expression levels of ACE2 and TMPRSS2 were markedly increased in LPS-treated colorectal cancer cells, whereas co-incubation with dandelion root extracts and TS suppressed the LPS-induced increase in ACE2 and TMPRSS2 transcript levels (Fig. 4D).
Blockage of TLR4/NFκB signaling attenuates LPS-induced ACE2/TMPRSS2 expression and LPS/TNFα-induced cell viability
Incubation of colorectal cancer cells with 10 µM CLI095, a TLR4 signaling inhibitor, significantly reversed the LPS-induced increase in the expression levels of ACE2 and TMPRSS2 (Fig. 5A). By contrast, treatment with TNFα, a downstream molecule of TLR4/NFκB, significantly increased the protein and mRNA expression levels of ACE2 and TMPRSS2 (Fig. 5B and C). Furthermore, in comparison to the control cells, TNFα at 5, 10 and 20 ng/ml, but not 15 ng/ml, significantly stimulated the viability of colorectal cancer cells, which could be reduced by co-incubation with dandelion root extracts (0.1 mg/ml) or TS (0.1 µg/ml) (Fig. 5D). To explore the involvement of ACE2/TMPRSS2 in LPS-induced cancer cell viability, siRNAs were used to knock down ACE2 and TMPRSS2. Knockdown was confirmed at both the mRNA (Fig. 6A and C) and protein levels (Fig. 6B and D). Either siRNA-mediated knockdown of ACE2/TMPRSS2 or blockage of TLR4/NFκB signaling by CLI095 suppressed LPS-stimulated colorectal cancer cell viability (Fig. 6E). These data suggested that ACE2 and TMPRSS2 are the downstream targets of TLR4/NFκB/proinflammatory cytokines, and TLR4/NFκB-driven ACE2/TMPRSS2 pathways may mediate the stimulatory role of LPS in colorectal cancer cell viability.
Discussion
Colorectal cancer is one of the major causes of cancer mortality worldwide. The pathogenesis of colorectal cancer is highly diverse and complex (2,3). Evidence has demonstrated that abnormalities in intestinal bacteria may contribute to the initiation or progression of colorectal cancer (1,6,10). Dandelion roots have been used as a traditional herbal medicine for thousands of years, and have been reported to protect against inflammation, digestive diseases and cancer (25,26). The present study explored the protective effects of dandelion root extracts, and the major active component TS, against LPS-stimulated colorectal cancer cell viability.
The colon is continuously exposed to bacteria, and alterations in the gut microbiota and bacteria-derived LPS endotoxin may have a direct effect on the initiation and progression of colorectal cancer by inducing chronic inflammation, DNA damage and genetic instability (11,37). It has been demonstrated that circulating levels of LPS are markedly higher in patients with colorectal cancer (14,15). Using an MTT assay, the present study revealed that the viability of 0.5 µg/ml LPS-treated cells (132%) was significantly higher than that of the control cells (100%), suggesting that LPS at 0.5 µg/ml may promote cell viability. Notably, the increased cell viability does not necessarily indicate cancer induction. To further explore this, a clonogenic assay was conducted to explore whether LPS at 0.5 µg/ml could promote a single cell to grow into a colony during a period of 2 weeks. It was confirmed that LPS at 0.5 µg/ml induced colony formation, indicating the carcinogenic role of LPS.
The present study subsequently demonstrated that dandelion root extracts or TS could inhibit LPS-induced colorectal cancer cell viability and colony formation. TS is a major pharmacologically active compound in dandelion root extracts. Dandelion extracts and TS have been extensively studied for their anti-inflammatory and anticancer effects (25,26,30-32). In addition, as determined in the present study, incubation of human colorectal cancer cells and normal colon epithelial cells with dandelion root extracts at concentrations ≤1 mg/ml for 24 h did not affect their viability. Ovadje et al (27) observed that colorectal cancer cell proliferation was only inhibited following 96-h treatment with dandelion root extracts at concentrations ≥1.5 mg/ml, indicating that the inhibitory effect of dandelion root extracts on cancer cell viability is time- and dose-dependent. Dandelion root extracts have also been shown to protect against dextran sodium sulfate-induced colonocyte damage, thus indicating the anti-colitis potential of dandelion root extracts (28). In addition, dandelion root extracts and TS have been reported to attenuate the antibiotic resistance of bacteria, which may also contribute to the inhibition of colorectal cancer cell proliferation due to a lower level of colorectal cancer-driving bacteria and genotoxins (38). These data suggested that regular intake of dietary dandelion may be helpful for gut health and inhibition of colorectal cancer development.
The present study also assessed the mechanisms underlying LPS-induced colorectal cancer cell viability. TLR4/NFκB signaling is often involved in the cell immune response, tumorigenesis and cancer progression (39). Interventions in TLR4/NFκB signaling have been demonstrated to be effective in cancer prevention and treatment (40,41). A growing body of evidence has shown that the TLR4/NFκB pathway is primarily responsible for the initiation of pro-inflammatory responses by LPS (16,42). The present study also confirmed that LPS activated TLR4/NFκB signaling and induced the transcription of several pro-inflammatory cytokines, including TNFα, IL4 and IL6. Similar to LPS, the presence of TNFα could induce increased colorectal cancer cell viability. By contrast, blockage of the TLR4/NFκB pathway by CLI095 markedly abolished the stimulatory effect of LPS on colorectal cancer cell viability. These data suggested that LPS-activated TLR4/NFκB signaling may contribute to increased colorectal cancer cell viability. Future studies should identify the molecular mechanisms underlying the inhibition of the LPS-induced TLR4/NFκB pathway by dandelion root extracts or TS.
The present study further revealed that ACE2 and TMPRSS2 were more highly expressed in colorectal cancer cells than in normal epithelial cells, and their expression levels could be upregulated by LPS. Both ACE2 and TMPRSS2 also mediated the inhibitory roles of dandelion root extracts and TS on LPS-induced colorectal cancer cell viability, as knockdown of either ACE2 or TMPRSS2 suppressed LPS-induced cell viability. The regulatory effects of LPS on ACE2 have been widely reported. ACE2 expression has been shown to be increased in a mouse model of LPS-induced lung fibrosis, whereas the ACE2 activator diminazene aceturate reduced pulmonary fibrosis, indicating the potential value of targeting ACE2 for healing lung fibrosis (43). Abdelhamid et al (13) revealed that xanthenone treatment reversed LPS-induced acute respiratory distress syndrome in mice by modulating ACE2 expression. ACE2 and TMPRSS2 have also been observed to be highly expressed in various types of cancer, including colorectal, stomach, pancreatic and prostate cancer (44). ACE2 expression was also upregulated in patients with gastric cancer who had different stages of tumor invasion depth (24,45). However, the functional roles of ACE2 and TMPRSS2 in tumorigenesis remain unclear and require further mechanistic studies. In addition, patients with colorectal cancer may be particularly susceptible to SARS-CoV-2 infection due to higher expression levels of ACE2 and TMPRSS2 (20,24). Notably, Tran et al (46) reported that dandelion leaf aqueous extracts can block the protein-protein interaction between the SARS-CoV-2 spike protein and the ACE2 receptor. It is thus predicted that dandelion extracts may be used for prevention of SARS-CoV-2 infection.
The present study also examined how LPS enhanced ACE2 and TMPRSS2 expression. It was revealed that TLR4/NFκB signals may act upstream of ACE2/TMPRSS2, which was supported by two pieces of evidence. First, blockage of TLR4 with CLI095 significantly reversed the stimulatory effect of LPS on the expression levels of ACE2 and TMPRSS2. Second, TNFα, a downstream molecule of TLR4/NFκB, markedly increased the expression levels of ACE2 and TMPRSS2 at both the mRNA and protein levels. By contrast, Zhang et al (47) reported that high levels of ACE2 can protect against LPS-induced inflammation in bovine mammary epithelial cells by inhibiting the NFκB pathway. It has also been reported that LPS caused inflammatory damage in porcine intestinal epithelial cells (IPEC-J2) by inactivating ACE2 and stimulating pro-inflammatory cytokines (TNFα, IL1β and IL8), whereas knockdown of the ACE2 gene increased TLR4 expression and aggravated the inflammatory response (43). These data suggested that the LPS-induced interaction between TLR4/NFκB and ACE2 is complex, and ACE2 may serve a dual role in regulating the inflammatory response depending on the cell type, and the dose and duration of LPS treatment. The identification of the missing links between TLR4/NFκB/inflammatory cytokines and the ACE2/TMPRSS2 cascade will lead to an improved understanding of the association between ACE2/TMPRSS2 and the pathogenesis of colorectal cancer.
In conclusion, the present study revealed that LPS (0.5 µg/ml) had a significant stimulatory effect on the viability of human colorectal cancer cells but did not affect normal epithelial cells. Dandelion root extracts at concentrations ≥0.1 mg/ml or TS at concentrations ≥0.05 µg/ml were able to reverse the LPS-induced increase in colorectal cancer cell viability and colony formation via interruption of TLR4/NFκB/inflammatory cytokines/ACE2/TMPRSS2 pathways. Thus, the in vitro results from the present study suggested that dandelion root extracts and TS could be used as preventative strategies for reversing bacteria-increased colorectal cancer cell viability (Fig. 7). Further research using animal models and a human trial to assess the clinical relevance of dandelion root extracts should be encouraged.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
KY and YW were responsible for the study design, and acquisition and interpretation of data. KY and YW wrote the article. Each author agrees to the author's own contributions and to the integrity and accuracy of the work. KY and YW confirm the authenticity of all the raw data. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Reis SAD, da Conceição LL and Peluzio MDCG: Intestinal microbiota and colorectal cancer: Changes in the intestinal microenvironment and their relation to the disease. J Med Microbiol. 68:1391–1407. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Maryam S, Krukiewicz K, Haq IU, Khan AA, Yahya G and Cavalu S: Interleukins (cytokines) as biomarkers in colorectal cancer: Progression, detection, and monitoring. J Clin Med. 12(3127)2023.PubMed/NCBI View Article : Google Scholar | |
|
Hofseth LJ, Hebert JR, Chanda A, Chen H, Love BL, Pena MM, Murphy EA, Sajish M, Sheth A, Buckhaults PJ and Berger FG: Early-onset colorectal cancer: Initial clues and current views. Nat Rev Gastroenterol Hepatol. 17:352–364. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Collins D, Hogan AM and Winter DC: Microbial and viral pathogens in colorectal cancer. Lancet Oncol. 12:504–512. 2011.PubMed/NCBI View Article : Google Scholar | |
|
de Waal GM, de Villiers WJS, Forgan T, Roberts T and Pretorius E: Colorectal cancer is associated with increased circulating lipopolysaccharide, inflammation and hypercoagulability. Sci Rep. 10(8777)2020.PubMed/NCBI View Article : Google Scholar | |
|
Tjalsma H, Boleij A, Marchesi JR and Dutilh BE: A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat Rev Microbiol. 10:575–582. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Lucas C, Barnich N and Nguyen HTT: Microbiota, inflammation and colorectal cancer. Int J Mol Sci. 18(1310)2017.PubMed/NCBI View Article : Google Scholar | |
|
Chu FF, Esworthy RS, Chu PG, Longmate JA, Huycke MM, Wilczynski S and Doroshow JH: Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 64:962–968. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Pandey H, Tang DWT, Wong SH and Lal D: Gut microbiota in colorectal cancer: Biological role and therapeutic opportunities. Cancers (Basel). 15(866)2023.PubMed/NCBI View Article : Google Scholar | |
|
Mandal P: Molecular mechanistic pathway of colorectal carcinogenesis associated with intestinal microbiota. Anaerobe. 49:63–70. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, Tameda M, Shiraki K, Ito M, Takei Y and Takase K: Comparison of human gut microbiota in control subjects and patients with colorectal carcinoma in adenoma: Terminal restriction fragment length polymorphism and next-generation sequencing analyses. Oncol Rep. 35:325–333. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Pitari GM, Zingman LV, Hodgson DM, Alekseev AE, Kazerounian S, Bienengraeber M, Hajnóczky G, Terzic A and Waldman SA: Bacterial enterotoxins are associated with resistance to colon cancer. Proc Natl Acad Sci USA. 100:2695–2699. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Abdelhamid AM, Deeb ME and Zaafan MA: The protective effect of xanthenone against LPS-induced COVID-19 acute respiratory distress syndrome (ARDS) by modulating the ACE2/Ang-1-7 signaling pathway. Eur Rev Med Pharmacol Sci. 26:5285–5296. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Seo GS: The role of NF-kappaB in colon cancer. Korean J Gastroenterol. 57:3–7. 2011.PubMed/NCBI View Article : Google Scholar : (In Korean). | |
|
O'Leary DP, Bhatt L, Woolley JF, Gough DR, Wang JH, Cotter TG and Redmond HP: TLR-4 signalling accelerates colon cancer cell adhesion via NF-κB mediated transcriptional up-regulation of Nox-1. PLoS One. 7(e44176)2012.PubMed/NCBI View Article : Google Scholar | |
|
Killeen SD, Wang JH, Andrews EJ and Redmond HP: Bacterial endotoxin enhances colorectal cancer cell adhesion and invasion through TLR-4 and NF-kappaB-dependent activation of the urokinase plasminogen activator system. Br J Cancer. 100:1589–1602. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Ranneh Y, Akim AM, Hamid HA, Khazaai H, Fadel A and Mahmoud AM: Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr Metab (Lond). 16(15)2019.PubMed/NCBI View Article : Google Scholar | |
|
An X, Lin W, Liu H, Zhong W, Zhang X, Zhu Y, Wang X, Li J and Sheng Q: SARS-CoV-2 host receptor ACE2 protein expression atlas in human gastrointestinal tract. Front Cell Dev Biol. 9(659809)2021.PubMed/NCBI View Article : Google Scholar | |
|
Bernardi S, Zennaro C, Palmisano S, Velkoska E, Sabato N, Toffoli B, Giacomel G, Buri L, Zanconati F, Bellini G, et al: Characterization and significance of ACE2 and Mas receptor in human colon adenocarcinoma. J Renin Angiotensin Aldosterone Syst. 13:202–209. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Liu C, Wang K, Zhang M, Hu X, Hu T, Liu Y, Hu Q, Wu S and Yue J: High expression of ACE2 and TMPRSS2 and clinical characteristics of COVID-19 in colorectal cancer patients. NPJ Precis Oncol. 5(1)2021.PubMed/NCBI View Article : Google Scholar | |
|
Reindl-Schwaighofer R, Eskandary F, Bartko J, Heinzel A, Jilma B, Hecking M and Schoergenhofer C: Corticosteroid treatment prevents lipopolysaccharide-induced increase of ACE2 and reduces fibrin degradation products in bronchoalveolar lavage fluid. Front Med (Lausanne). 9(856891)2022.PubMed/NCBI View Article : Google Scholar | |
|
Burgueño JF, Reich A, Hazime H, Quintero MA, Fernandez I, Fritsch J, Santander AM, Brito N, Damas OM, Deshpande A, et al: Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis. 26:797–808. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Zabiegala A, Kim Y and Chang KO: Roles of host proteases in the entry of SARS-CoV-2. Anim Dis. 3(12)2023.PubMed/NCBI View Article : Google Scholar | |
|
Ahmadi M, Pashangzadeh S, Mousavi P, Saffarzadeh N, Habibi MA, Hajiesmaeili F and Rezaei N: ACE2 correlates with immune infiltrates in colon adenocarcinoma: Implication for COVID-19. Int Immunopharmacol. 95(107568)2021.PubMed/NCBI View Article : Google Scholar | |
|
Hfaiedh M, Brahmi D and Zourgui L: Hepatoprotective effect of Taraxacum officinale leaf extract on sodium dichromate-induced liver injury in rats. Environ Toxicol. 31:339–349. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Sigstedt SC, Hooten CJ, Callewaert MC, Jenkins AR, Romero AE, Pullin MJ, Kornienko A, Lowrey TK, Slambrouck SV and Steelant WF: Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int J Oncol. 32:1085–1090. 2008.PubMed/NCBI | |
|
Ovadje P, Ammar S, Guerrero JA, Arnason JT and Pandey S: Dandelion root extract affects colorectal cancer proliferation and survival through the activation of multiple death signalling pathways. Oncotarget. 7:73080–73100. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Ding A and Wen X: Dandelion root extract protects NCM460 colonic cells and relieves experimental mouse colitis. J Nat Med. 72:857–866. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Sharma K and Zafar R: Occurrence of taraxerol and taraxasterol in medicinal plants. Pharmacogn Rev. 9:19–23. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Liu B, He Z, Wang J, Xin Z, Wang J, Li F and Fu Y: Taraxasterol inhibits LPS-induced inflammatory response in BV2 microglia cells by activating LXRα. Front Pharmacol. 9(278)2018.PubMed/NCBI View Article : Google Scholar | |
|
Che L, Li Y, Song R, Qin C, Hao W, Wang B, Yang L, Peng P and Xu F: Anti-inflammatory and anti-apoptosis activity of taraxasterol in ulcerative colitis in vitro and in vivo. Exp Ther Med. 18:1745–1751. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Zhang X, Xiong H, Li H and Cheng Y: Protective effect of taraxasterol against LPS-induced endotoxic shock by modulating inflammatory responses in mice. Immunopharmacol Immunotoxicol. 36:11–16. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Cho SY, Park JY, Park EM, Choi MS, Lee MK, Jeon SM, Jang MK, Kim MJ and Park YB: Alternation of hepatic antioxidant enzyme activities and lipid profile in streptozotocin-induced diabetic rats by supplementation of dandelion water extract. Clin Chim Acta. 317:109–117. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Barrow K, Wang Y, Yu R, Zhu J and Yang G: H2S protects from oxidative stress-driven ACE2 expression and cardiac aging. Mol Cell Biochem. 477:1393–1403. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Li L, Li M, Li Y, Sun W, Wang Y, Bai S, Li H, Wu B, Yang G, Wang R, et al: Exogenous H2S contributes to recovery of ischemic post-conditioning-induced cardioprotection by decrease of ROS level via down-regulation of NF-κB and JAK2-STAT3 pathways in the aging cardiomyocytes. Cell Biosci. 6(26)2016.PubMed/NCBI View Article : Google Scholar | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Yang Y, Yang L, Jiang S, Yang T, Lan J, Lei Y, Tan H and Pan K: HMGB1 mediates lipopolysaccharide-induced inflammation via interacting with GPX4 in colon cancer cells. Cancer Cell Int. 20(205)2020.PubMed/NCBI View Article : Google Scholar | |
|
Yang K and Zhang Y: Reversal of heavy metal-induced antibiotic resistance by dandelion root extracts and taraxasterol. J Med Microbiol. 69:1049–1061. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Sun Q, Liu Q, Zheng Y and Cao X: Rapamycin suppresses TLR4-triggered IL-6 and PGE(2) production of colon cancer cells by inhibiting TLR4 expression and NF-kappaB activation. Mol Immunol. 45:2929–2936. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Ying J, Zhou H, Wang Z, You Q, Chen J, Lu H and Zhang J: Aspirin increases chemosensitivity of colorectal cancer cells and inhibits the expression of toll-like receptor 4. Cancer Cell Int. 23(6)2023.PubMed/NCBI View Article : Google Scholar | |
|
Zhuo Q, Yu B, Zhou J, Zhang J, Zhang R, Xie J, Wang Q and Zhao S: Lysates of Lactobacillus acidophilus combined with CTLA-4-blocking antibodies enhance antitumor immunity in a mouse colon cancer model. Sci Rep. 9(20128)2019.PubMed/NCBI View Article : Google Scholar | |
|
Li Z, Wang K, Ji X, Wang H and Zhang Y: ACE2 suppresses the inflammatory response in LPS-induced porcine intestinal epithelial cells via regulating the NF-κB and MAPK pathways. Peptides. 149(170717)2022.PubMed/NCBI View Article : Google Scholar | |
|
Lin X, Lin W, Zhuang Y and Gao F: Angiotensin-converting enzyme 2 inhibits lipopolysaccharide-caused lung fibrosis via downregulating the transforming growth factor β-1/Smad2/Smad3 pathway. J Pharmacol Exp Ther. 381:236–246. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Cheng J, Zhou J, Fu S, Fu J, Zhou B, Chen H, Fu J and Wei C: Prostate adenocarcinoma and COVID-19: The possible impacts of TMPRSS2 expressions in susceptibility to SARS-CoV-2. J Cell Mol Med. 25:4157–4165. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Klooster JPT, Bol-Schoenmakers M, van Summeren K, van Vliet ALW, de Haan CAM, van Kuppeveld FJM, Verkoeijen S and Pieters R: Enterocytes, fibroblasts and myeloid cells synergize in anti-bacterial and anti-viral pathways with IL22 as the central cytokine. Commun Biol. 4(631)2021.PubMed/NCBI View Article : Google Scholar | |
|
Tran HTT, Gigl M, Le NPK, Dawid C and Lamy E: In vitro effect of Taraxacum officinale leaf aqueous extract on the interaction between ACE2 cell surface receptor and SARS-CoV-2 spike protein D614 and four mutants. Pharmaceuticals (Basel). 14(1055)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhang X, Jia F, Ma W, Li X and Zhou X: DAD3 targets ACE2 to inhibit the MAPK and NF-κB signalling pathways and protect against LPS-induced inflammation in bovine mammary epithelial cells. Vet Res. 53(104)2022.PubMed/NCBI View Article : Google Scholar |