Ossifying fibromyxoid tumor of the soft tissue in the left upper arm and a review of the literature: A case report
- Authors:
- Published online on: April 23, 2024 https://doi.org/10.3892/etm.2024.12549
- Article Number: 261
Abstract
Introduction
Ossifying fibromyxoid tumor (OFMT) has relatively clear-cut boundaries and is a mesenchymal neoplasm that commonly occurs subcutaneously (1). This tumor mainly involves adults, particularly middle-aged and elderly individuals, with a median age of ~50 years (1). However, children and newborns can develop OFMT (2,3). The most common site of OFMT is the thigh, while its incidence in the lower extremity is estimated to be >40% (1). Head and neck and trunk are also considered as tumor-prone sites (1-8). The majority of tumors are subcutaneous, with only a few being intramuscular. Rare OFMT sites include mediastinum, spine retroperitoneum and breast (9,10). Clinically, the majority of cases present with a painless, small, well circumscribed and slow-growing mass (1,10). The clinical course of the disease is long, ranging from 1 to 20 years (10). OFMT is often characterized by an entire or incomplete fibrous pseudocapsule (5,11,12). In addition, an incomplete ossification ring is commonly observed in the periphery of the mass, while the boundary of the tumor is clear. However, a small number of patients develop bone invasion and periosteal reaction (1). The size of OFMT is typically 3-5 cm but may reach 14 cm (1). The cut section is usually white or tan, with a hard or firm texture (1). Histologically, OFMT cells are commonly round, elliptic or spindle-shaped, arranged in sheets or trabeculae and commonly accompanied by fibromyxoid stroma and surrounding ossification (1,10). The malignant subtype of OFMT is characterized by high cellularity and nuclear grade, with a mitosis index >2/10 high-power fields (HPFs) (1). The immunostaining pattern of this type of tumor is characterized by S-100 positivity (1). Its histological origin remains unknown. However, previous immunohistochemistry and electron microscopy findings indicate that OFMT may originate from Schwann cells (1). Patients with OFMT are often prone to local recurrence and distant metastasis. However, recurrences usually occur 10-20 years after surgery (1,10). As this type of tumor is characterized by the presence of several histological structures, differentiation can be difficult, particularly for atypical cases. In the present study, the case of 33-year-old female with OFMT in the upper arm was reported, providing a review of this tumor and focusing on its pathological diagnosis.
Case report
Patient information
A 33-year-old female patient was referred to the First Affiliated Hospital of China Medical University (Shenyang, China) with a mass ~1 cm in diameter on the left upper arm in January 2018. The mass was growing slowly. Physical examination revealed a subcutaneous mass ~1.5 cm in size, which was hard in texture and not flexible. The patient felt pressing pain in the site of the tumor but had no other obvious symptoms, including fever or weight loss. The patient underwent preoperative ultrasound examination, which showed an 18x12 mm hypoechoic mass, 3 mm under the epidermis of the left upper arm (Fig. 1). The mass shape was regular and nearly ellipsoidal, with clear boundaries and no significant blood flow signal. According to the ultrasound and intraoperative findings, the tumor was considered as benign and the surgical doctor carefully separated the tumor from the surrounding tissues and it was excised intact with no macroscopic residues. However, the differentiation profile of the tumor and diagnosis remained unclear. The excised tumor was subjected to pathological examination, including hematoxylin and eosin (H&E), and immunohistochemical staining. Light microscopy was used for observation of the morphological features.
The tumor tissues were fixed with 10% formalin at 25˚C for 24 h and embedded in blocks. Subsequently, for histopathological examination, the paraffin-embedded blocks were cut into 4-µm thick sections and stained with hematoxylin and eosin (H&E) (3 min, 25˚C). Additionally, immunohistochemistry was carried out according to the immunohistochemistry test kit manufacturer's instructions (Fuzhou Maixin Biotech Co., Ltd.) and as previously described (13). Antigen retrieval was obtained using a pressure cooker at a heating temperature of 120˚C. Xylene was used for dewaxing. A descending alcohol series was used for rehydration. Endogenous peroxidase activity was blocked with 3% H2O2 (37˚C, 10 min). Non-specific binding was blocked with non-immune calf serum (10%; Sigma-Aldrich; Merck KGaA; 37˚C, 20 min). The sections were incubated with primary antibodies overnight at 4˚C. The primary antibodies were as follows: Anti-actin (1:100, cat. no. M085129-2, Dako; Agilent Technologies, Inc.), anti-CD34 (1:100, cat. no. MEC14.7, Thermo Fisher Scientific, Inc.), anti-cytokeratin (CK; 1:100, cat. no. KRTL/4440R, Thermo Fisher Scientific, Inc.), anti-desmin (1:200, cat. no. R606, Dako; Agilent Technologies, Inc.), anti-human melanoma black 45 (HMB45; 1:200, cat. no. IS052, Dako; Agilent Technologies, Inc.), anti-Ki-67 (1:200, cat. no. 101AP, Thermo Fisher Scientific, Inc.), anti-melanoma A (1:200, cat. no. 4385R, Thermo Fisher Scientific, Inc.), anti-S-100 (1:200, cat. no. 4C4.9, Thermo Fisher Scientific, Inc.) and anti-vimentin (1:100, cat. no. V9, Thermo Fisher Scientific, Inc.). The sections were incubated with biotinylated secondary antibodies (1:200, cat. no. KIT-9710, Fuzhou Maixin Biotech Co., Ltd.) at 37˚C for 30 min. The present study was performed according to the ethical guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of China Medical University (202312). The patient provided informed consent for the publication of their data.
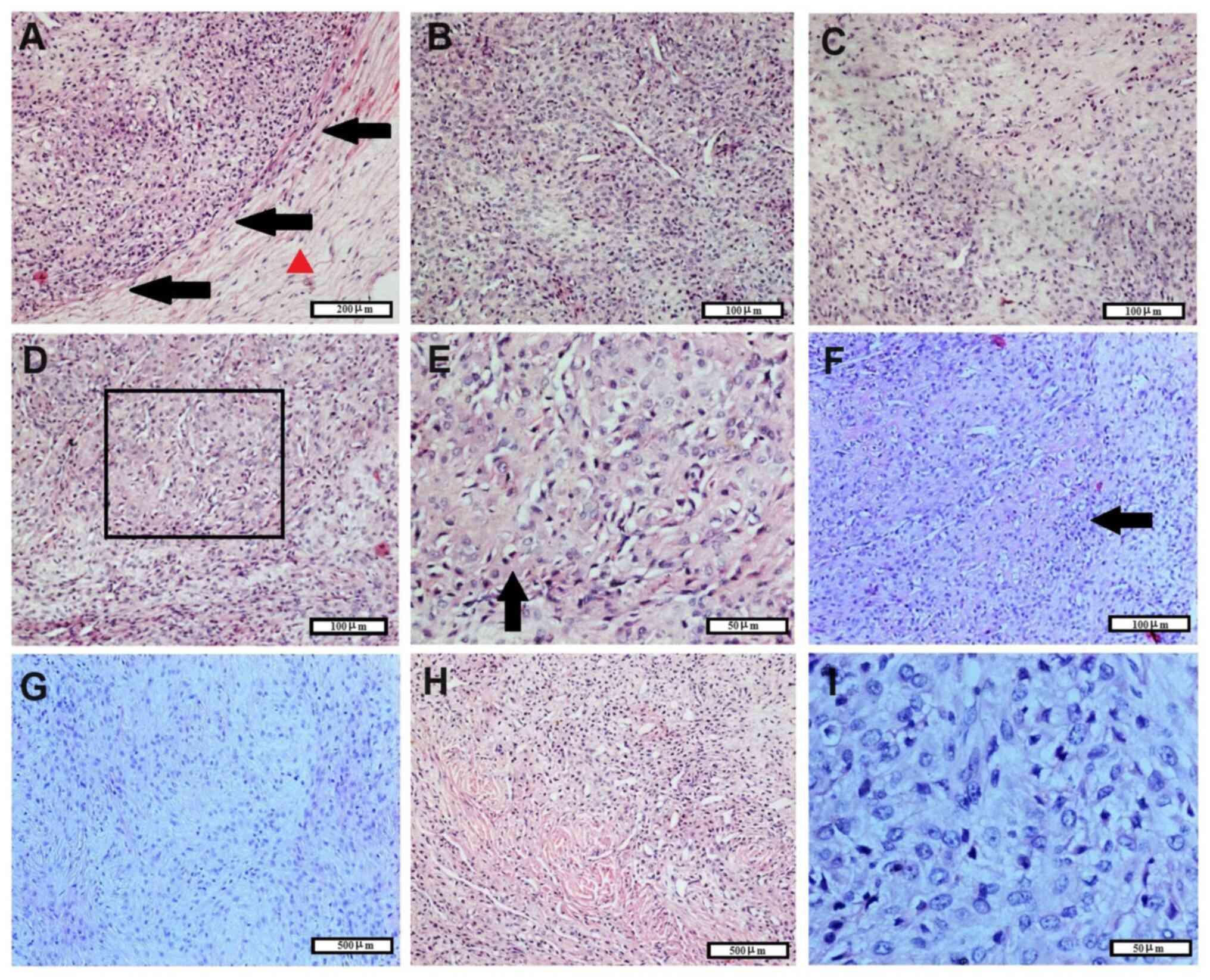
The microscopic histopathological features of the tumor are shown in Fig. 2. The tumor had a clear boundary and showed no invasion to the adjacent tissues (Fig. 2A). A thick fibrous envelope was observed around the tumor. The majority of tumor cells were dense and arranged in sheets (Fig. 2B). Some areas in the tumor tissue had mucous matrix (Fig. 2C), cells with cartilage-like morphology (Fig. 2D-F) or spindle-shaped cells with mucoid (Fig. 2G) or hyalinization matrix (Fig. 2H). Metaplastic bone was not found. Tumor cells were mostly round and medium-sized without obvious atypia, while the cytoplasm was abundant and pale (Fig. 2I). Additionally, tumor cell nuclei were round or oval and pale (Fig. 2I). Nuclear mitosis were counted in 10 hot spots under x400 magnification and the total value was taken. The mitotic index was <1/10 HPF (Fig. 2I).
The immunostaining features of tumor cells are shown in Fig. 3. Tumor cells were negative for smooth muscle actin, CD34, CK, desmin, HMB45 and melanoma A. In addition, the blood vessels in tumor tissues showed positive immunostaining for smooth muscle actin and CD34, while weak immunostaining was detected for S-100 expression. The Ki67 index was generally low (~1%), with a few areas showing a Ki67 index of ~5%. Finally, tumor cells were diffusely and strongly positive for vimentin expression. Based on the aforementioned pathological examination combined with ultrasound examination and the patient's medical history, the tumor was diagnosed as OFMT. The last follow-up was in January 2024; no tumor recurrence was reported 6 years after surgery.
Discussion
OFMT was first reported by Enzinger et al (14) in 1989. According to the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone, OFMT is a mesenchymal neoplasm of uncertain differentiation, whose histological origin cannot be determined (1). Emerging evidence has suggested that OFMT may have a Schwannian, neuronal or chondroid origin (15-17). However, its exact origin remains to be confirmed. A study by Min et al (18) using immunohistochemical and electron microscopic examination indicated that OFMT displays a myoepithelial histogenesis. The uncertainty regarding the histological origin of this type of tumor stems from the multipotentiality of its differentiation. In the present case, the tumor tissue showed various differentiation patterns, including chondroid, mucinous and fibrous, with hyaline degeneration. Although OFMT is defined by an International Classification of Diseases-10 code of 0, it exhibits the potential of recurrence and metastasis (1,10). Therefore, this type of cancer should be more appropriately classified as an intermediate type of tumor. The histopathological features of OFMT include fibromyxoid matrix and the peripheral partial shell of the metaplastic bone (1).
The age range of OFMT onset is wide. However, the majority of patients are adults, aged ~50 years old (1). OFMT is more common in males than females, with a ratio of ~1.5:1.0(1). The lower extremity is the most common site of OFMT (1). OFMTs in the head and neck region are relative common. These sites include the submandibular gland, retroauricular perimastoid region, the retromolar trigone, the face, the scalp, the nasal septum and ethmoid sinus (2,5-8,16,19). A previous study by Mesinkovska et al (20) showed that the median tumor size in 26 patients with OFMT was 2.3 cm. However, Graham et al (21) reported a median tumor size of 5.4 cm (46 cases). Τhe general features of patients with OFMT reported in the literature, including age, sex and tumor size are summarized in Table I (2,5-9,11,12,15-17,19,22-40).
Histologically, OFMT tissue contains areas with different differentiation status. Osseous metaplasia at the margin of the tumor tissue is a histopathological feature of this type of cancer (1). However, the presence of metaplastic bone can be rare and difficult to detect in OFMT tissues (23). In the study by Mesinkovska et al (20) peripheral ossification was recorded in only half of patients with OFMT (13/26). However, in the study by Folpe and Weiss (41), bone was present in ~63% (44/70) of tumors. In the present case, although the histological features were consistent with OFMT, no ossification was detected in the tumor tissue. Additionally, consistent with the present case, focal chondroid metaplasia can also be identified in OFMT (22). OFMT can have lipomatous areas (23). Fisher et al (42) reported the presence of microcysts in tumor tissues, formed by the accumulation of myxoid stroma. Other reports of cystic changes mainly reflect the wrong clinical impression prior to histological diagnosis, including physical or imaging examination (22,43). OFMT often shows high vasculature (23), while nuclear pseudoinclusions have been described (32). Hemorrhage and necrosis are rare in OFMT (1,22,32). By contrast, necrosis is more common in the malignant subtype of this tumor (44). Ahmed et al (24) reported, using fine-needle aspiration, several cytological features of OFMT, including an epithelioid morphology lacking obvious malignant characteristics, round nuclei, fine chromatin and background with fibromyxoid stroma fragments. Additionally, Min et al (18) described electron microscopic findings from three OFMT cases, such as centrally located round to oval nuclei, varying amounts of cytoplasm, few cytoplasmic organelles and absence of tonofilaments or actin filaments. Other studies also detected few mitotic cells in OFMT samples, particularly <1/10 HPF (22,23), while the Ki67 index is generally low (~1%) (8,22). Currently, there are no clear and accepted criteria for diagnosis of malignant subtypes of OFMT. However, in a study including 70 patients with OFMT, Folpe and Weiss (41) suggested that tumors with high nuclear grade or high cellularity and mitotic activity of >2 mitotic figures/50 HPF were more likely to recur and metastasize and should be therefore considered as malignant OFMT. Invasive growth is another key feature of OFMT malignancy, which was not highlighted in the aforementioned study (41). Atypical OFMT has been proposed by several authors (3,44-46). However, diagnostic criteria are still lacking. In the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone, atypical OFMTs are described as tumors with higher mitotic cell count compared with typical OFMTs, but not as high as in the malignant subtype (1). However, this group of OFMTs should be defined by more features and the subclassification should involve more histopathological characteristics, including pleomorphism, hypercellularity and nuclear grade. Therefore, differential diagnosis of OFMT should include all mesenchymal neoplasms with myxoid or fibromyxoid matrix. Myxoid content is seen in the majority of mesenchymal neoplasms, mainly in fibroblastic or myofibroblastic tumors. Myxoid content, which also serves a key role in the differential diagnosis of OFMT, is common in schwannoma (47). Thway et al (48) reported a case of low-grade fibromyxoid sarcoma with a bony shell, mimicking OFMT. OFMT in the breast can also be mistaken as fibroadenoma (9). OFMT also needs to be differentiated from bone and cartilage tumors. Ogose et al (49) reported a case resembling parosteal osteosarcoma. Collagen fibers are commonly detected between tumor cells in OFMT and sometimes tumor cells can be spindle-shaped. Therefore, it is necessary to distinguish OFMT from desmoid tumors. Histologically, desmoid tumors are primarily composed of spindle cells and lack bone and cartilage formation. Nuclear staining of β-catenin is detected in the majority of desmoid tumors, but not in OFMTs (1). Neuschwannoma needs to be differentiated from this tumor. Histologically, schwannomas have typical fascicular and reticular regions. Dermal nerve sheath myoxoma (DNSM) is another important tumor that needs to be differentiated from OFMT. Tumor cells in DNSM are primarily spindle shaped and multinucleated cells are common. As aforementioned, the differential spectrum of OFMT is extensive. However, OFMTs are characterized by marked ossification at the mass periphery accompanied by clear boundaries, which is also a common characteristic of benign tumors. The aforementioned features can be therefore used to distinguish OFMTs. However, cytological diagnosis can be difficult (29). A previous study reported a case of OFMT at a prethyroidal location, which was misdiagnosed as follicular neoplasia using fine needle aspiration (50). When biopsy material is insufficient for pathological diagnosis, imaging techniques can be helpful, while it is more realistic to evaluate the nature of the tumors than determine their names.
OFMTs commonly show positive staining for S-100 and vimentin (1,8), as in the present case. However, not all OFMT cases are positive for S-100 (16,20,21,41), potentially due to the enhanced histological malignancy (21). Two other common markers for OFMT are neuron-specific enolase and Leu7(23). OFMTs can be positive for glial fibrillary acidic protein (1,23), pan-CK, smooth muscle actin and desmin (41). CD10 positivity and mosaic loss of integrase interactor 1 (INI-1) has been reported in typical and atypical OFMTs (26,46). Graham et al (21) demonstrated using fluorescence in situ hybridization that ~71% (5/7) of OFTMs display INI-1 deletion. PHD finger protein (PHF) gene rearrangements are common in typical, but not in malignant, OFMT (1,51). In addition, EP400-PHF1 gene fusions have been detected in OFMTs (26,52). This genetic alteration is of great significance in the diagnosis and comprehension of the molecular abnormalities in this type of cancer.
Imaging techniques are key for detection and preliminary evaluation of OFMTs, and are commonly used to detect a well-defined mass (27,32). Here, the patient underwent ultrasound examination, which showed a mass with clear boundaries and regular shape. Calcification is an imaging feature of OFMTs (8,27). Computer tomography can visualize the ossification at the periphery of the tumors (27), while magnetic resonance imaging can detect myxoid content (25). Abdessayed et al (22) reported a case of OFMT mimicking hydatid cyst in radiological assessment. In the absence of ossification, OFMT imaging lacks characteristic features to distinguish it from other types of mesenchymal neoplasms (53).
Clinically, the majority of patients do not experience OFMT-associated symptoms (1,8). The clinical course of the disease is commonly indolent (1). In the present case, the tumor grew slowly and the patient had no other obvious symptoms, thus indicating the indolent behavior of the tumor. The biological behavior of OFMT is not consistent; most cases are cured after resection, but there are also some cases that exhibit recurrence or even metastasis. Although the majority of OFMTs are benign, they can be malignant, however, without clear histological features of malignancy (23,54,55). Cha et al (30) reported a case of OFMT adjacent to the L5 vertebral body, which invaded the cortex of the vertebral body and the spinal canal. Surgery is the primary treatment strategy for OFMT. For the malignant subtype of OFMT, no standard therapeutic approach is currently available, other than basal resection of the tumor (51). Chemotherapy with epirubicin and ifosfamide and perfusion with human recombinant tumor necrosis factor and melphalan was applied in a patient with malignant OFMT with lung metastasis. The aforementioned patient responded well to this therapy and partial response after chemotherapy was observed (51). In another case of malignant tumor near the bone, the patient was treated with chemotherapy combined with radiotherapy, showing a significant therapeutic effect (49). To decrease risk of recurrence, postoperative adjuvant radiotherapy is commonly used for malignant tumors that cannot be completely removed. However, for some cases of spinal malignancy, following surgery and postoperative radiotherapy combined with chemotherapy, recurrence was recorded, suggesting that these conventional treatment methods still have limitations in controlling these types of tumor (30). Currently, there is a lack of d EDITED ETM-21149-305351.docx ata on targeted therapy for OFMT. However, several gene mutations have been identified in this type of tumor, providing the basis for future targeted therapy. Gene fusions involving PHF1 or BCL6-corepressor (BCOR) can be detected in the majority of OFMTs. Other gene fusions found in these tumors include CREBBP-BCORL1 and KDM2A-WWTR1(56). The KDM2A protein is a histone demethylase targeting Lys-36 of histone H3. WWTR1 acts as a regulatory partner in the Hippo signaling pathway. The postoperative recurrence of OFMT is rare (8). However, this may be underestimated due to the short follow-up time. Usually, OFMT recurrence is reported a long time after the initial resection, commonly up to 5 or >10 years (1,32). Lastra et al (57) reported a case of a patient with OFMT in the left ankle, which metastasized to the lung and thyroid gland 12 years following surgery. It has been suggested that the most common site of metastasis in patients with OFMT is the lungs (19,51,58), while tumors can recur multiple times (19,33,47). OFMT recurrence is associated with the particular site and the failure to complete surgical resection (33). Emerging evidence has also indicated that OFMTs can be transformed into malignant subtype after recurrence (59,60). A previous study reported a rare case of extraosseous osteosarcoma secondary to OFMT (59). Furthermore, Soldano et al (32) demonstrated that metaplastic bone formation became more extensive in recurrent tumor, although no malignant transformation was detected. According to Folpe and Weiss (41) the factors that affect prognosis of patients with OFMT mainly include cellularity, mitotic rate and nuclear grade. However, Miettinen et al (61) suggested that mitotic cell count, but not necrosis and tumor size, is the main risk factor for OFMT recurrence. Additionally, complete OFMT excision is considered as the most useful treatment strategy for this type of tumor (10,14,41). However, the safe distance of the surgical edge from the tumor was not analyzed in detail in the aforementioned studies. Currently, the factors affecting OFMT prognosis primarily focus on histological morphology. However, it has been reported that tumors with benign microscopic findings can recur (1). Therefore, it is necessary to investigate the importance of surgical treatment methods, including the safe distance of the surgery margins. Due to the presence of gene fusion mutations, particularly those involving either PHF1 or BCOR (56,62,63), genetic testing can be considered as a key diagnostic tool for cases that are difficult to diagnose histologically. In addition, these mutations may also provide a basis for the application of targeted therapy in future.
In summary, OFMT of soft parts is a mesenchymal neoplasm of uncertain histogenesis. Histologically, this neoplasm is characterized by a variety of structures, including fibromyxoid stroma and ossification. The slow growth and clear boundaries of the cancerous mass and the absence of obvious symptoms are key features for evaluating the indolence of this tumor. Differentiation from other types of mesenchymal tumor and malignant subtypes is essential for providing the appropriate therapy, while close follow-up serves a key role in the timely detection of recurrence and metastasis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural Science Foundation of China (grant no. 81472599) and the Project Funded by China Postdoctoral Science Foundation (grant no. 2019M661171).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
CF designed the study and revised the manuscript. NL and CF evaluated histopathological findings. NL, YJ and JD reviewed the literature and analyzed the patient data. NL drafted the manuscript. CF and NL confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the local Ethics Committee of China Medical University (Shenyang, China; approval no. 202312), and consent was obtained from the patient.
Patient consent for publication
The patient provided written consent for the publication of data and accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
|
Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: World Health Organization Classifiction of Tumors of Soft Tissue and Bone. IARC Press, Lyon, 2013. | |
|
Al-Mazrou KA, Mansoor A, Payne M and Richardson MA: Ossifying fibromyxoid tumor of the ethmoid sinus in a newborn: Report of a case and literature review. Int J Pediatr Otorhinolaryngol. 68:225–230. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Ghosal N, Rudrappa S, Tandon AS, Rao P and Gopal S: Atypical ossifying fibromyxoid tumor in left maxillo-ethmoid sinus with intracranial extension in a child. Clin Neuropathol. 35:329–332. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Sharif MA, Mushtaq S, Mamoon N and Khadim MT: Ossifying fibromyxoid tumor of oral cavity. J Coll Physicians Surg Pak. 18:181–182. 2008.PubMed/NCBI | |
|
Kondylidou-Sidira A, Kyrgidis A, Antoniades H and Antoniades K: Ossifying fibromyxoid tumor of head and neck region: Case report and systematic review of literature. J Oral Maxillofac Surg. 69:1355–1360. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Titsinides S, Nikitakis NG, Tasoulas J, Daskalopoulos A, Goutzanis L and Sklavounou A: Ossifying fibromyxoid tumor of the retromolar trigone: A case report and systematic review of the literature. Int J Surg Pathol. 25:526–532. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Velasco IA, Zhang R, Li T and Wang D: Ossifying Fibromyxoid tumor of soft parts in head and neck: Case report and literature review. Diagn Pathol. 13(21)2018.PubMed/NCBI View Article : Google Scholar | |
|
Varakliotis T, Bellocchi G, Eibenstein A, Acquaviva G and Casorati F: A rare case report of a typical variant ossifying fibromyxoid tumor (OFMT), located in the retroauricular perimastoid region. Int J Surg Case Rep. 44:16–19. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Asirvatham JR, Shah A, Carreon CK, Bhuiya TA, Kahn LB, Kostroff K and Morgenstern NJ: Ossifying fibromyxoid tumor of the breast mimicking fibroadenoma: A case report and differential diagnoses. Arch Pathol Lab Med. 138:1098–1100. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Bakiratharajan D and Rekhi B: Ossifying fibromyxoid tumor: An update. Arch Pathol Lab Med. 140:371–375. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Choi JH, Park JS, Jin W, Park YK and Ryu KN: Sonographic features of an ossifying fibromyxoid tumor of the buttock. J Ultrasound Med. 27:809–812. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Nonaka CF, Pacheco DF, Nunes RP, de A Freitas R and Miguel MC: Ossifying fibromyxoid tumor in the mandibular gingiva: Case report and review of the literature. J Periodontol. 80:687–692. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Fan C, Yu J, Yang L, Lin X and Wang E: Clear cell sarcoma of soft tissue in right parapharyngeal region: Report of a rare case. Int J Clin Exp Pathol. 8:10935–10940. 2015.PubMed/NCBI | |
|
Enzinger FM, Weiss SW and Liang CY: Ossifying fibromyxoid tumor of soft parts. A clinicopathological analysis of 59 cases. Am J Surg Pathol. 13:817–827. 1989.PubMed/NCBI View Article : Google Scholar | |
|
Saadat P, Pullarkat S, Kelly L and Vadmal M: Ossifying fibromyxoid tumor of the skin: A report of 2 cases with light microscopic, immunohistochemical, and electron microscopic characterization. J Am Acad Dermatol. 52:644–647. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Blum A, Back W, Naim R, Hörmann K and Riedel F: Ossifying fibromyxoid tumor of the nasal septum. Auris Nasus Larynx. 33:325–327. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Kawashima H, Ogose A, Umezu H, Hotta T, Tohyama T, Tsuchiya M and Endo N: Ossifying fibromyxoid tumor of soft parts with clonal chromosomal aberrations. Cancer Genet Cytogenet. 176:156–160. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Min KW, Seo IS and Pitha J: Ossifying fibromyxoid tumor: Modified myoepithelial cell tumor? Report of three cases with immunohistochemical and electron microscopic studies. Ultrastruct Pathol. 29:535–548. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Sarraj A, Duarte J, Dominguez L and Pun YW: Resection of metastatic pulmonary lesion of ossifying fibromyxoid tumor extending into the left atrium and ventricle via pulmonary vein. Eur J Echocardiogr. 8:384–386. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Mesinkovska NA, Buehler D, McClain CM, Rubin BP, Goldblum JR and Billings SD: Ossifying fibromyxoid tumor: A clinicopathologic analysis of 26 subcutaneous tumors with emphasis on differential diagnosis and prognostic factors. J Cutan Pathol. 42:622–631. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Graham RP, Dry S, Li X, Binder S, Bahrami A, Raimondi SC, Dogan A, Chakraborty S, Souchek JJ and Folpe AL: Ossifying fibromyxoid tumor of soft parts: A clinicopathologic, proteomic, and genomic study. Am J Surg Pathol. 35:1615–1625. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Abdessayed N, Mestiri S, Ammar H, Bdioui A, Chhaidar A, Toumi O, Mhamdi N, Gupta R, Guerfela M and Mokni M: Ossifying fibromyxoid tumor of the trunk mimicking hydatid cyst: A case report. Int J Surg Case Rep. 39:80–83. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Dere Y, Çelik SY, Çelik Öİ and Dere Ö: A rare soft tissue tumor located in the trunk: Ossifying fibromyxoid tumor. Turk J Surg. 33:299–301. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Ahmed OI, Qasem SA and Salih ZT: Ossifying fibromyxoid tumor: Report of a case with cytomorphologic description. Diagn Cytopathol. 43:646–649. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Sharma K, Hughes D and Harper RD: Ossifying fibromyxoid tumor (OFMT)-A rare cause of a painful thumb. Int J Surg Case Rep. 7C:93–95. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Endo M, Kohashi K, Yamamoto H, Ishii T, Yoshida T, Matsunobu T, Iwamoto Y and Oda Y: Ossifying fibromyxoid tumor presenting EP400-PHF1 fusion gene. Hum Pathol. 44:2603–2608. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Ideta S, Nishio J, Aoki M, Ishimatsu T, Nabeshima K, Iwasaki H and Naito M: Imaging findings of ossifying fibromyxoid tumor with histopathological correlation: A case report. Oncol Lett. 5:1301–1314. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Alvarez-Rodríguez F, Jiménez-Heffernan J, Salas C, Pastrana M and Sanz E: Cytological features of ossifying fibromyxoid tumor of soft parts. J Cytol. 29:205–207. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Goyal P, Sehgal S, Agarwal R, Singh S, Gupta R and Kumar A: Ossifying fibromyxoid tumor-Diagnostic challenge for a cytopathologist. Cytojournal. 9(17)2012.PubMed/NCBI View Article : Google Scholar | |
|
Cha JH, Kwon JW, Cho EY, Lee CS, Yoon YC and Choi SH: Ossifying fibromyxoid tumor invading the spine: A case report and review of the literature. Skeletal Radiol. 37:1137–1140. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Al-Brahim N and Dashti MA: Ossifying fibromyxoid tumor of soft parts. Report of a rare tumor in Kuwait. Med Princ Pract. 17:340–342. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Soldano AC, Vazquez-Martul E, Romero JA and Prieto VG: Subcutaneous ossifying fibromyxoid tumor. J Cutan Pathol. 33:749–753. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Park DJ, Miller NR and Green WR: Ossifying fibromyxoid tumor of the orbit. Ophthalmic Plast Reconstr Surg. 22:87–91. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Nishio J, Iwasaki H, Ohjimi Y, Ishiguro M, Isayama T, Naito M, Okabayashi H, Kaneko Y and Kikuchi M: Ossifying fibromyxoid tumor of soft parts. Cytogenetic findings. Cancer Genet Cytogenet. 133:124–128. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Ijiri R, Tanaka Y, Misugi K, Sekido K and Nishi T: Ossifying fibromyxoid tumor of soft parts in a child: A case report. J Pediatr Surg. 34:1294–1296. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Sovani V, Velagaleti GV, Filipowicz E, Gatalica Z and Knisely AS: Ossifying fibromyxoid tumor of soft parts: Report of a case with novel cytogenetic findings. Cancer Genet Cytogenet. 127:1–6. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Motoyama T, Ogose A and Watanabe H: Ossifying fibromyxoid tumor of the retroperitoneum. Pathol Int. 46:79–83. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Nakayama F and Kuwahara T: Ossifying fibromyxoid tumor of soft parts of the back. J Cutan Pathol. 23:385–388. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Velasco-Pastor AM, Martínez-Escribano J, del Pino Gil-Mateo M, Quecedo-Estébanez E, Fortea-Baixauli JM and Aliaga-Boniche A: Ossifying fibromyxoid tumor of soft parts. J Cutan Pathol. 23:381–384. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Yang P, Hirose T, Hasegawa T, Gao Z and Hizawa K: Ossifying fibromyxoid tumor of soft parts: A morphological and immunohistochemical study. Pathol Int. 44:448–453. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Folpe AL and Weiss SW: Ossifying fibromyxoid tumor of soft parts: A clinicopathologic study of 70 cases with emphasis on atypical and malignant variants. Am J Surg Pathol. 27:421–431. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Fisher C, Hedges M and Weiss SW: Ossifying fibromyxoid tumor of soft parts with stromal cyst formation and ribosome-lamella complexes. Ultrastruct Pathol. 18:593–600. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Seykora JT, Kutcher C, van de Rijn M, Dzubow L, Junkins-Hopkins J and Ioffreda M: Ossifying fibromyxoid tumor of soft parts presenting as a scalp cyst. J Cutan Pathol. 33:569–572. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Dantey K, Schoedel K, Yergiyev O, McGough R, Palekar A and Rao UNM: Ossifying fibromyxoid tumor: A study of 6 cases of atypical and malignant variants. Hum Pathol. 60:174–179. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Squillaci S, Tallarigo F, Cazzaniga R and Capitanio A: Ossifying fibromyxoid tumor with atypical histological features: A case report. Pathologica. 101:248–252. 2009.PubMed/NCBI | |
|
Tajima S and Koda K: Atypical ossifying fibromyxoid tumor unusually located in the mediastinum: Report of a case showing mosaic loss of INI-1 expression. Int J Clin Exp Pathol. 8:2139–2145. 2015.PubMed/NCBI | |
|
Martinez-Rodriguez M, Subramaniam MM, Calatayud AM, Ramos D, Navarro S and Llombart-Bosch A: Ossifying fibromyxoid tumor of soft parts mimicking a schwannoma with uncommon histology: A potential diagnostic pitfall. J Cutan Pathol. 36:71–73. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Thway K, Chisholm J, Hayes A, Swansbury J and Fisher C: Pediatric low-grade fibromyxoid sarcoma mimicking ossifying fibromyxoid tumor: Adding to the diagnostic spectrum of soft tissue tumors with a bony shell. Hum Pathol. 46:461–466. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Ogose A, Otsuka H, Morita T, Kobayashi H and Hirata Y: Ossifying fibromyxoid tumor resembling parosteal osteosarcoma. Skeletal Radiol. 27:578–580. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Lax S and Langsteger W: Ossifying fibromyxoid tumor misdiagnosed as follicular neoplasia. A case report. Acta Cytol. 41 (Suppl 4):S1261–S1264. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Provenzano S, Raimondi A, Bertulli RM, Colia V, Renne SL, Collini P, Dagrada G, Callegaro D, Fiore M, Greco FG and Casali PG: Response to isolated limb perfusion and chemotherapy with epirubicin plus ifosfamide in a metastatic malignant ossifying fibromyxoid tumor. Clin Sarcoma Res. 7(20)2017.PubMed/NCBI View Article : Google Scholar | |
|
Gebre-Medhin S, Nord KH, Möller E, Mandahl N, Magnusson L, Nilsson J, Jo VY, Vult von Steyern F, Brosjö O, Larsson O, et al: Recurrent rearrangement of the PHF1 gene in ossifying fibromyxoid tumors. Am J Pathol. 181:1069–1077. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Thompson J, Castillo M, Reddick RL, Smith JK and Shockley W: Nasopharyngeal nonossifying variant of ossifying fibromyxoid tumor: CT and MR findings. AJNR Am J Neuroradiol. 16:1132–1134. 1995.PubMed/NCBI | |
|
Yoshida H, Minamizaki T, Yumoto T, Furuse K and Nakadera T: Ossifying fibromyxoid tumor of soft parts. Acta Pathol Jpn. 4:480–486. 1991.PubMed/NCBI View Article : Google Scholar | |
|
Binesh F, Akhavan A and Navabii H: Ossifying fibromyxoid tumour: A rare soft tissue tumour of intermediate malignancy. BMJ Case Rep. 2011(bcr0820103263)2011.PubMed/NCBI View Article : Google Scholar | |
|
Kao YC, Sung YS, Zhang L, Chen CL, Huang SC and Antonescu CR: Expanding the molecular signature of ossifying fibromyxoid tumors with two novel gene fusions: CREBBP-BCORL1 and KDM2A-WWTR1. Genes Chromosomes Cancer. 56:42–50. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Lastra RR, Newman JG, Brooks JS and Huang JH: Ossifying fibromyxoid tumor metastatic to the thyroid: A case report and review of the literature. Ear Nose Throat J. 93:221–223. 2014.PubMed/NCBI | |
|
Schaffler G, Raith J, Ranner G, Weybora W and Jeserschek R: Radiographic appearance of an ossifying fibromyxoid tumor of soft parts. Skeletal Radiol. 26:615–618. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Shelekhova KV, Kazakov DV and Michal M: Extraosseous osteosarcoma arising in recurrent ossifying fibromyxoid tumor of soft tissue: A case report. Arkh Patol. 75:24–28. 2013.PubMed/NCBI | |
|
Ohta K, Taki M, Ogawa I, Ono S, Mizuta K, Fujimoto S, Takata T and Kamata N: Malignant ossifying fibromyxoid tumor of the tongue: Case report and review of the literature. Head Face Med. 9(16)2013.PubMed/NCBI View Article : Google Scholar | |
|
Miettinen M, Finnell V and Fetsch JF: Ossifying fibromyxoid tumor of soft parts-a clinicopathologic and immunohistochemical study of 104 cases with long-term follow-up and a critical review of the literature. Am J Surg Pathol. 32:996–1005. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Lu Q and Ho CL: A case of an 82-year-old man with a spinal extradural malignant ossifying fibromyxoid tumor. Am J Case Rep. 24(e939408)2023.PubMed/NCBI View Article : Google Scholar | |
|
Argani P, Dickson BC, Gross JM, Matoso A, Baraban E and Antonescu CR: Ossifying fibromyxoid tumor of the genitourinary tract: Report of 4 molecularly confirmed cases of a diagnostic pitfall. Am J Surg Pathol. 47:709–716. 2023.PubMed/NCBI View Article : Google Scholar |