A long‑term complete response to namodenoson in liver cancer with Child‑Pugh B cirrhosis: A case report
- Authors:
- Published online on: April 24, 2024 https://doi.org/10.3892/etm.2024.12551
- Article Number: 263
-
Copyright: © Ciurescu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
The global burden associated with liver cancer is substantial. In 2020, worldwide, 905,700 new liver cancer cases and 830,200 liver cancer deaths were estimated (1). In 90 countries, liver cancer is among the top 5 causes of cancer death, and in 46 countries it is among the top 3 causes of cancer death (2). Furthermore, with the growth and aging of the world population, the number of liver cancer cases is expected to increase with a predicted 1.3 million liver cancer deaths in 2040(2). Thus, liver cancer, and specifically hepatocellular carcinoma (HCC), which constitutes the majority of liver cancer cases (75-80%), presents a significant global health problem (1).
Considerations in HCC treatment include not only the disease stage, tumor characteristics, and patient's comorbidities but also liver function and the potential hepatotoxicity of the treatment, as the benefits of the treatment should be weighed against a potential deterioration in hepatic function (3,4). Liver function is often categorized by the Child-Pugh (CP) scoring system, which integrates multiple laboratory and clinical criteria. The system classifies patients into 3 categories: Child-Pugh A (CPA; good hepatic function), Child-Pugh B (CPB; moderately impaired hepatic function), and Child-Pugh C (CPC; advanced hepatic dysfunction) (5). For CPB HCC patients, the only curative option includes downstaging followed by liver transplantation. However, this approach is appropriate for only a small proportion of patients and is also limited by the scarcity of livers available for transplantation (6). Thus, CPB HCC patients are often treated with the multi-kinase inhibitor sorafenib, which is approved by the US Food and Drug Administration (FDA) for all advanced HCC patients, irrespective of liver function (7). Notably, all the clinical trials that investigated first-line treatments for advanced HCC focused on patients with CPA cirrhosis (8). Likewise, second-line treatments (following treatment with sorafenib) are also primarily geared toward CPA patients. CPB patients are typically excluded from clinical studies as their prognosis is poor and their expected response rate is low (9). Thus, presently, established treatments for advanced HCC patients with CPB cirrhosis are lacking.
Namodenoson (CF102, also known as Cl-IB-MECA; CAS registry number, 163042-96-4) is an orally available, small molecule, which is a highly selective A3 adenosine receptor (A3AR) agonist. The molecular formula of namodenoson is C18H18ClIN6O4 (molecular weight, 544.73 Da) (Fig. 1). Namodenoson is undergoing clinical development for multiple indications, including HCC, as A3AR was found to be overexpressed in HCC cells and peripheral blood mononuclear cells (PBMC) derived from patients with HCC, but not in healthy tissues (10). The mechanism of action of namodenoson includes direct and indirect effects. The direct effect involves de-regulation of 2 signaling pathways (NF-κB, Wnt), which results in increased levels of pro-apoptotic proteins and Fas-ligand, leading to inhibition of tumor growth (10,11). The indirect effect involves PBMC and specifically, natural killer (NK) cells, as namodenoson was found to activate NK cells and induce IL-2 production, resulting in tumor growth inhibition (12).
Following encouraging results in an open-label phase 1/2 study that investigated namodenoson in advanced HCC (13), a randomized, placebo-controlled, multicenter phase 2 clinical trial was initiated to investigate namodenoson as a second-line treatment for advanced HCC patients with CPB cirrhosis (clinicaltrials.gov identifier: NCT02128958). The findings from this phase 2 trial have been published (14). This trial, which included a total of 78 patients (50 in the namodenoson arm who received 25 mg, BID, and 28 in the placebo arm), demonstrated that namodenoson has a favorable safety profile. No patients withdrew due to adverse events, and no deaths were noted (14). The study did not meet its primary endpoint, which was overall survival (OS). The median OS was 4.1 months in the namodenoson arm vs. 4.3 months in the placebo arm (hazard ratio, 0.82; 95% confidence interval, 0.49-1.38; P=0.46). However, analysis of the subgroup of patients with the least severe cirrhosis in the CPB category (i.e., patients with a CP score of 7), which included 34 patients in the namodenoson arm and 22 in the placebo arm, revealed a statistically significant better 12-month OS in the namodenoson arm vs. placebo (44% vs. 18%, P=0.028) (14).
The current case report presents a patient who participated in this phase 2 study of namodenoson vs. placebo in HCC with CPB cirrhosis, was assigned to the namodenoson arm and received namodenoson throughout the blinded study, and who, upon unblinding, continued treatment with open-label namodenoson for a total treatment of more than 6 years through the extension program.
Case presentation
The current case involves a patient with advanced HCC and a history of hepatitis B-related cirrhosis, who met the inclusion criteria of the randomized placebo-controlled phase 2 clinical trial investigating namodenoson for the treatment of HCC with CPB cirrhosis (14). This phase 2 study was approved by the relevant local Institution Review Boards (IRBs). For the site in which the patient described herein participated, the study was approved by the National Bioethics Committee for Medicine and Medical Devices in Bucharest Romania (approval date, Oct 29, 2014; approval code, 116S).
The patient enrolled to the study on Nov 28, 2016, and was treated at the S.C. Pelican Impex S.R.L.-Oncology Department in Oradea, Romania. This patient, who was 61 years old at enrollment, was a Caucasian woman with metastatic HCC that was histology-confirmed (data not shown) and had received 5 cycles of sorafenib therapy as first-line treatment for her disease. The patient was categorized as having CPB cirrhosis with a CP score of 7: She had ascites but no encephalitis, her total bilirubin level was 0.23 mg/dl (normal range: 0.3-1.0 mg/dl), her albumin level was 3.2 g/dl (normal range: 3.5-5.5 g/dl), and her international normalized ratio (INR) was 1.0 (normal range: 0.9-1.2). Also at enrollment, the patient had Barcelona Clinic Liver Cancer (BCLC) stage C, and Eastern Cooperative Oncology Group (ECOG) performance status of 1.
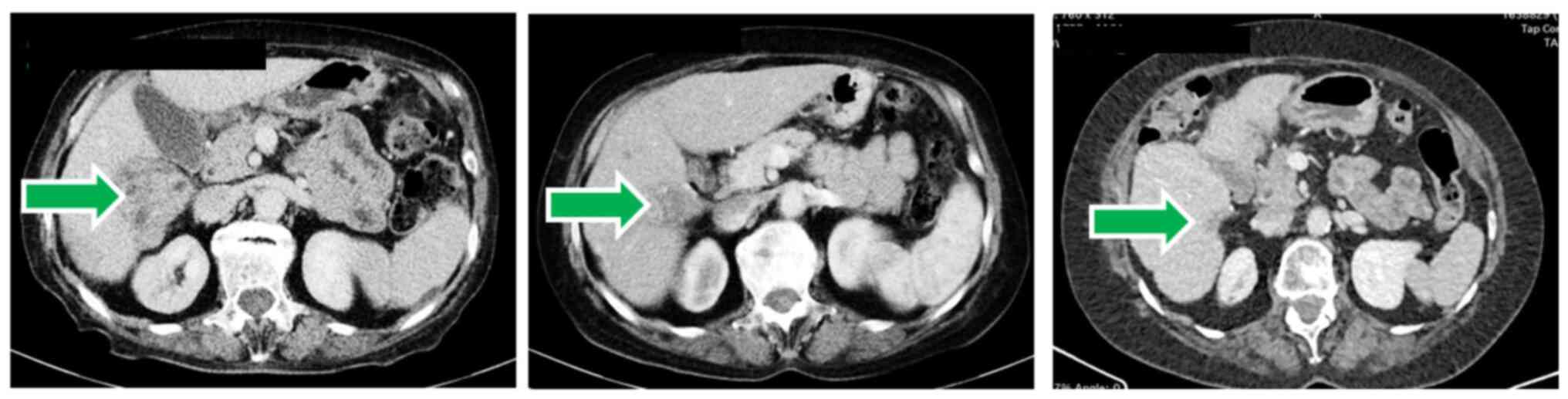
Computed tomography (CT) scans demonstrated that at baseline, the patient had 2 HCC lesions in the liver. The larger lesion was observed in the right lobe (segments VI and VII) and its longest diameter was 91 mm. A smaller liver lesion was observed in segment VI of the right lobe and its longest diameter was 55 mm. In addition, 2 lesions were noted in the abdominal wall (a pelvic peritoneal nodule with a longest diameter of 20 mm, and a subumbilical peritoneal nodule with a longest diameter of 18 mm), and another nodule was noted in the interaortocaval lymph node (longest diameter, 16 mm). The sum of the longest diameters of all target lesions at baseline was 200 mm. In addition, peritoneal carcinomatosis was noted.
The patient was randomized to the namodenoson arm of the study, and thus received 25 mg namodenoson BID for the duration of study. Her treatment started on Dec 7, 2016 (approximately 6 weeks after discontinuing sorafenib) and participation in the blinded study, where she had a follow up every 2 weeks, continued until March 29, 2019. Upon unblinding of the treatment assignments in the phase 2 study, the patient continued treatment with open-label namodenoson at the same dose for a total of more than 6 years under an extension program (treatment is ongoing).
After 2 treatment cycles (e.g., approximately 7 weeks) the CT scans revealed a smaller tumor mass, consistent with a partial response. Within 4 years of treatment, the tumors, as well as ascites, and peritoneal carcinomatosis disappeared, consistent with a complete response by RECIST 1.1 and mRECIST (Fig. 2). This complete response is ongoing.
A secondary objective of the study was an evaluation of liver function. At baseline, albumin levels were lower than normal and normalized within 1 treatment cycle (4 weeks) (Fig. 3). Bilirubin levels were within the normal range at baseline and remained within this range throughout (Fig. 3).
Also, at baseline, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST) levels were reported (68 U/l and 44 U/l, respectively). Within 1 treatment cycle (4 weeks), both ALT and AST levels normalized and were stable for more than 6 years (Fig. 4). Also, the serum α-fetoprotein level, which was elevated at baseline (47 ng/ml) normalized after 5 cycles of treatment (20 weeks) and was 1.3 ng/ml at time of complete response. The patient experienced no treatment-emergent adverse events. At the time of reporting this case (more than 6 years from namodenoson treatment initiation), the patient is alive, on namodenoson treatment (25 mg BID), and her response is ongoing as manifested by imaging studies and liver function evaluation.
 | Figure 4ALT and AST levels over time (every cycle represents 4 weeks of treatment with namodenoson). |
Discussion
This report of a patient who participated in the phase 2 study of namodenoson in advanced HCC with CPB cirrhosis demonstrates that namodenoson treatment is safe and well tolerated and can result in a long-term complete response and improved liver function in HCC with CPB cirrhosis and a CP score of 7. The improvement in liver function is notable, as it is consistent with the hepatoprotective properties of namodenoson [reviewed in (15)]. In contrast, sorafenib (the first-line therapy received by the patient), similar to some of the other tyrosine-kinase inhibitors, is associated with hepatotoxicity (16).
The current case report provides only a low level of evidence (LOE) for the clinical utility of namodenoson, with respect to both its efficacy and safety, as it is limited by the inherent characteristics of the design (i.e., a sample size of one patient, lack of generalizability, lack of comparison). It is, though, an example of a long-term response to namodenoson in one patient that represents a population for whom no effective treatments are available. This example could be used to complement findings from prospective randomized trials that, by nature, provide a higher LOE. The results of the phase 2 clinical trial which included the patient described in the current case report have been published (beyond the current case report, the data from the open-label extension program do not merit publication) (14).
The encouraging results of this phase 2 study (14), particularly in the subgroup of patients with a CP score of 7 prompted the initiation of a pivotal phase 3 clinical study (LIVERATION) investigating namodenoson (25 mg BID) in HCC and CPB cirrhosis with a CP score of 7, which is currently recruiting patients (ClinicalTrials.gov identifier: NCT05201404) (17). The patient described in the current case report is not enrolled in the phase 3 study and will continue to receive namodenoson as part of the phase 2 extension program until disease progression or unaccepted toxicity. Data from the phase 3 trial which will include a total of 471 patients will be reported once the study is concluded and the data become available.
Acknowledgements
Not applicable.
Funding
Funding: The Phase 2 study was sponsored by Can-Fite BioPharma Ltd.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
PF, SMS, MF, ZH, AB-S, and MHS conceived and designed the manuscript. IAC and RL performed data acquisition. AB-S and PF wrote the manuscript. All authors read and approved the final manuscript. PF, IAC, and MF confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The phase 2 study in which the case described therein participated was approved by all relevant national regulatory authorities and local Ethics Committees/Institutional Review Boards, was conducted in accordance with the Declaration of Helsinki, and a written informed consent was obtained from all patients [as described in Stemmer et al (14)]. For the site in which the patient described herein participated, the study was approved by the National Bioethics Committee for Medicine and Medical Devices in Bucharest Romania (approval date, Oct 29, 2014; approval no. 116S).
Patient consent for publication
Written informed consent for publication was obtained from the patient.
Competing interests
MF, ZH, MHS, and PF are Can-Fite BioPharma, Ltd employees. SMS received research grant and stock options from Can-Fite BioPharma Ltd. AB-S is a consultant for Can-Fite BioPharma Ltd. The remaining authors declare no competing interests. Can-Fite BioPharma sponsored the phase 2 study; however, this sponsorship had no bearing on the results of the study or their scientific interpretation.
References
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA and Soerjomataram I: Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 77:1598–1606. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Ramadori G, Schleyer E and Armbrust T: Safety of imatinib in patients with liver cirrhosis and hepatocellular carcinoma. J Clin Oncol. 22:4244. 2004. | |
|
Shannon AH, Ruff SM and Pawlik TM: Expert insights on current treatments for hepatocellular carcinoma: Clinical and molecular approaches and bottlenecks to progress. J Hepatocell Carcinoma. 9:1247–1261. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Tsoris A and Marlar CA: Use of the child Pugh score in liver disease. In: StatPearls. Treasure Island (FL), 2021. | |
|
Granito A and Bolondi L: Non-transplant therapies for patients with hepatocellular carcinoma and Child-Pugh-Turcotte class B cirrhosis. Lancet Oncol. 18:e101–e112. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Sorafenib (package insert). Whippany, NJ: Bayer Health Care Pharmaceuticals Inc; 2017. | |
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, et al: Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 100:698–711. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, Barer F, Zabutti A, Perez-Liz G, Del Valle L and Fishman P: The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-kappaB signal transduction pathways. Int J Oncol. 33:287–295. 2008.PubMed/NCBI | |
|
Cohen S, Stemmer SM, Zozulya G, Ochaion A, Patoka R, Barer F, Bar-Yehuda S, Rath-Wolfson L, Jacobson KA and Fishman P: CF102 an A3 adenosine receptor agonist mediates anti-tumor and anti-inflammatory effects in the liver. J Cell Physiol. 226:2438–2447. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Harish A, Hohana G, Fishman P, Arnon O and Bar-Yehuda S: A3 adenosine receptor agonist potentiates natural killer cell activity. Int J Oncol. 23:1245–1249. 2003.PubMed/NCBI | |
|
Stemmer SM, Benjaminov O, Medalia G, Ciuraru NB, Silverman MH, Bar-Yehuda S, Fishman S, Harpaz Z, Farbstein M, Cohen S, et al: CF102 for the treatment of hepatocellular carcinoma: A phase I/II, open-label, dose-escalation study. Oncologist. 18:25–26. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Stemmer SM, Manojlovic NS, Marinca MV, Petrov P, Cherciu N, Ganea D, Ciuleanu TE, Pusca IA, Beg MS, Purcell WT, et al: Namodenoson in advanced hepatocellular carcinoma and Child-Pugh B cirrhosis: Randomized placebo-controlled clinical trial. Cancers (Basel). 13(187)2021.PubMed/NCBI View Article : Google Scholar | |
|
Fishman P, Stemmer SM, Bareket-Samish A, Silverman MH and Kerns WD: Targeting the A3 adenosine receptor to treat hepatocellular carcinoma: Anti-cancer and hepatoprotective effects. Purinergic Signal. 19:513–522. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Shah RR, Morganroth J and Shah DR: Hepatotoxicity of tyrosine kinase inhibitors: Clinical and regulatory perspectives. Drug Saf. 36:491–503. 2013.PubMed/NCBI View Article : Google Scholar | |
|
National Library of Medicine (NLM): Description of the ‘Namodenoson in the treatment of advanced hepatocellular carcinoma in patients with Child-Pugh Class B7 cirrhosis (LIVERATION)’ study (NCT05201404). Clinical Trials.gov ID, NCT05201404. NLM, Bethesda, MD, 2023. https://clinicaltrials.gov/study/NCT05201404?intr=namodenoson&rank=2&tab=table. Accessed February 20, 2024. |