Effects of magnesium sulfate combined with labetalol on inflammatory stress and pregnancy outcome of patients with gestational hypertension
- Authors:
- Published online on: April 26, 2024 https://doi.org/10.3892/etm.2024.12554
- Article Number: 266
-
Copyright: © Gu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Gestational hypertension (GH) is a unique condition that affects pregnant women, which predominantly occurs after 20 weeks of pregnancy and 2 weeks after delivery. Some patients with GH also exhibit proteinuria or edema, and severe cases are associated with headaches, blurred vision, upper abdominal pain, convulsion and coma (1-3). A pregnant woman is considered to have hypertension when their systolic blood pressure (SBP) exceeds 140 mmHg or their diastolic blood pressure (DBP) exceeds 90 mmHg. Being overweight, pre-pregnancy hypertension, pregnancy with multiple births, chronic diseases and/or poor diet (high salt and fat) are all risk factors for GH (4,5). GH not only harms the mother, but can also affect the growth and development of the fetus. Hypofunction of the placenta caused by GH can lead to various complications, such as intrauterine growth retardation, stillbirth, premature delivery or asphyxia, and can damage the organs of newborns to varying degrees (6,7). The fetal nervous system can also be affected, resulting in adverse long-term cognitive outcomes in infants (8). Therefore, if hypertension occurs during pregnancy, pregnant women and their families should pay attention to it and actively receive prenatal examinations, in order to ensure timely detection of the disease and reasonable treatment to minimize harm (9).
GH should be treated actively, whether hypertension is present before or after pregnancy. It is generally considered that if DBP exceeds 12.0 kPa (90 mmHg), in two measurements of the same arm taken >6 h apart, it should be treated in a timely manner. If blood pressure is >21.3/14.6 kPa (160/110 mmHg), this is also an absolute indication for the use of antihypertensive drugs even without clinical symptoms (10-13). Clinical treatment measures generally include a low-salt diet, ensuring a balance between work and rest, elimination of mental over-stress and adequate sleep. The aforementioned general measures can restore the blood pressure of patients with mild hypertension to normal. If the aforementioned measures are ineffective, stepped care can be given according to blood pressure level (14,15). Calcium channel blockers, such as nifedipine, nicardipine and diltiazem, can be applied in the first and second trimesters of pregnancy, but should not be used ~2 weeks before labor. Since calcium channel blockers can inhibit the contractile force of the uterine smooth muscle and affect the progress of labor, they are not suitable for patients in labor. In developed countries, methyldopa (tablets, central sympathoinhibitor) is the first choice of treatment for GH syndrome, and is recognized as the safest and most effective therapeutic drug (16-18). At present, no fetal toxicity has been found in response to methyldopa, and it is the only drug that has been proven to be safe after long-term follow-up into childhood. The only disadvantage is that it has a strong sedative effect, which limits its use (19). Sodium nitroprusside is a powerful vasodilator, which is only suitable for pregnant women in hypertensive crisis for which other antihypertensive drugs are ineffective, and the prenatal application of this drug should not exceed 4 h (20). Labetalol is an α-adrenoceptor blocker, as well as a β-adrenoceptor blocker, which is utilized to treat hypertension. Its principle is to block the adrenoceptor, slow down ventricular rhythm and reduce peripheral vascular resistance (21). Notably, no antihypertensive drugs are completely safe for GH, but allowing blood pressure to rise is considered more dangerous. Thus, for the clinical treatment of GH, antihypertensive drugs targeted at the conditions of different patients should be selected on the basis of fully weighing advantages and disadvantages (22).
A careful decision should be made regarding the clinical medication of patients with GH, because the effects and side effects of drugs differ. Magnesium sulfate (MgSO4) is the most commonly used drug for treating GH and preventing severe eclampsia (23). The clinical effects and toxicity of MgSO4 are related to its concentration in the plasma. The disappearance of the patellar reflex in mothers at plasma concentrations between 3.5-5 mmol/l gives the first warning of impending toxicity. With careful management and monitoring of MgSO4, maternal toxicity is very rare (24). The use of multiple drugs is a common strategy for clinical treatment, such as MgSO4 + nifedipine (25). To identify an effective treatment option, a randomized controlled study of MgSO4 in combination with Labetalol for the treatment of GH was performed. Subsequently, inflammatory factors, hemorheology, pregnancy outcome and perinatal complications of the two groups were assessed, and the effect of MgSO4 + labetalol on the clinical treatment of patients with GH was further discussed. The present study may provide reference for the clinical treatment of GH.
Methods and materials
Research objects
A total of 100 patients with GH, who were registered in the Department of Obstetrics and Gynecology, Taicang TCM Hospital Affiliated to Nanjing University of Chinese Medicine between June 2020 and June 2022, were recruited to the present study. The present study adhered to The Declaration of Helsinki, and was approved and implemented by the Ethics Committee of Taicang Hospital of TCM (approval no. 2022026). All of the patients voluntarily participated and signed informed consent forms prior to the study. During the intervention, the patients' right of privacy and confidentiality was respected, and all information obtained from the patients was kept strictly confidential.
The inclusion criteria were as follows: i) The patients could provide complete clinical data; ii) they were 18-36 years old; iii) they were conscious and were able to communicate normally; iv) they had not received any drug treatment; v) they had a natural pregnancy; and vi) only a single fetus was observed.
The exclusion criteria were as follows: i) Patients had severe cardiac, liver or renal insufficiency; ii) they were severely malnourished; iii) they dropped out of the experiment and did not complete the treatment process; and iv) they had recently suffered from an infection.
Experimental instruments and reagents
The experimental instruments used were as follows: DHM-30D automatic height and weight scale (Zhengzhou Dingheng Electronic Technology Co., Ltd.), diving mercury sphygmomanometer (Yuwell Medical Equipment & Supply Co., Ltd.) and automatic hemorheology analyzer SH210A (Shanghai Langyi Medical Equipment Co., Ltd.).
The experimental reagents included: 5% MgSO4 (Shandong Pingsen Biotechnology Co., Ltd.), 10% glucose solution (Guizhou Tiandi Pharmaceutical Co., Ltd.), 25% MgSO4 (Shandong Pingsen Biotech Co., Ltd.), 5% glucose solution (Guizhou Tiandi Pharmaceutical Co., Ltd.), labetalol (Shanghai Acebright Pharmaceuticals Group Co., Ltd.) and ELISA kits for HMGB1 (cat. no. ml085468), homocysteine (Hcy; cat. no. ml092715) and CysC (cat. no. ml003222) all from Shanghai Enzyme-linked Biotechnology Co., Ltd.
Therapeutic methods
The patients in both groups were given routine basic treatment, including sedation, oxygen inhalation and sodium restriction. The patients' breathing, blood pressure and heart rate were closely monitored, and timely treatment was carried out in case of abnormal conditions of the mothers and infants.
In the Ctrl group, patients were administered MgSO4. Briefly, 15 ml 5% MgSO4 and 20 ml 10% glucose solution were mixed and administered via an intravenous drip (1-2 g/h MgSO4) and, 50 ml 25% MgSO4 and 800 ml 5% glucose solution were mixed and administered via an intravenous drip (1-2 g/h MgSO4) later the same day, this was repeated daily, with a 1-week course of treatment.
In the Expt group, in addition to the drug administered to the Ctrl group, the patients were given an intravenous drip of 100 mg labetalol mixed with 250 ml 5% glucose solution. After the patients' blood pressure was reduced to the expected value (DBP, 90 mmHg) and stabilized, oral labetalol 100 mg was administrated, three times a day until delivery (26).
Determination of basic parameters of patients
First, patients were asked to remove their hats and shoes, and their height and weight were determined according to the unified side-face standard. During the measurement, the patients were requested to stand in the center of the pedal of the DHM-30D automatic height and weight scale, and to maintain a standing position with eyes level ahead, arms down and heels together for 5 sec. Subsequently, the patients were allowed to rest for ~15 min, and their SBP and DBP were measured using the diving mercury sphygmomanometer. Notably, the blood pressure of both upper arms was measured during the first measurement.
In addition, overnight-fasting venous blood was collected from the patients in two groups before and after treatment in the morning, and the supernatant was obtained after centrifugation at 1,500 x g at 4˚C for 15 min. The inflammatory factor indicators, high mobility group box-1 protein (HMGB1), homocysteine (Hcy) and serum cystatin C (CysC), were detected by ELISA according to manufacturer's protocols. The automatic hemorheological analyzer SH210A was used to detect hemorheological indicators, including whole blood viscosity (WBV), plasma viscosity (PV), and hematocrit (HCT), of the two groups before and after treatment. The pregnancy outcomes in the two groups, including cesarean section, spontaneous vaginal delivery and postpartum hemorrhage, were followed up. Perinatal complications, including fetal intrauterine distress (FIUD), placental abruption, neonatal asphyxia, premature birth and neonatal death, were also recorded.
Statistical analysis
The research data were analyzed using SPSS 19.0 (IBM Corp.), with measurement data presented as the mean ± standard deviation and enumeration data as value (%). The patient's basic information was analyzed using unpaired t-tests. Comparison of pregnancy outcomes was analyzed using the χ2 test, whereas comparison of perinatal complications was analyzed using the Fisher's exact test. A mixed ANOVA was performed in every indicator between Expt and Ctrl groups, pre and post treatment. P<0.05 was considered to indicate a statistically significant difference.
Results
Study design and patient enrollment
A total of 100 patients with GH were randomly divided into the Expt group and Ctrl group (n=50 cases/group). In the Expt group, the age of patients was 21-40 years, the mean age was 26.78±5.54 years, the gestational age was 31-38 weeks, and the average gestational age was 35.17±2.05 weeks. There were 32 primiparas and 18 multiparas. In the Ctrl group, the age, average age, gestational age and average gestational age were 20-38 years, 25.59±6.14 years, 30-37 and 36.28±2.24 weeks, respectively. There were 30 primiparas and 20 multiparas in the Ctrl group. There was no significant difference in age, gestational age and pregnancy history between the Expt group and the Ctrl group (P>0.05), indicating comparability between the groups (Table I).
Comparison of blood pressure values between groups before and after treatment
As shown in Figs. 1 and 2, SBP and DBP were 167.29±10.45 and 98.28±6.14 mmHg, respectively, in the Expt group before treatment. SBP and DBP after treatment reached 134.81±12.25 and 82.03±5.77 mmHg, respectively. In the Ctrl group, SBP and DBP were 166.51±11.53 and 98.84±5.93 mmHg before treatment, and 139.72±14.22 and 82.58±6.12 mmHg after treatment, respectively. SBP and DBP in the Expt group before treatment were not significantly different from those in the Ctrl group (P>0.05). In addition, SBP and DBP in the Expt group after treatment were also not significantly different from those in the Ctrl group (P>0.05). Notably, SBP and DBP after treatment were significantly lower than those before treatment in both groups (P<0.05). These data indicated that both SBP and DBP were significantly reduced in patients with GH treated with MgSO4, regardless of whether they were also treated with labetalol or not.
Comparison of inflammatory factor indicators between groups before and after treatment
As shown in Fig. 3, the levels of HMGB1in the Expt group were 9.48±0.75 mg/l before treatment and 1.58±0.23 mg/l after treatment. The levels of HMGB1 in the Ctrl group were 9.57±0.88 mg/l before treatment and 4.02±0.68 mg/l after treatment. No significant difference was observed in HMGB1 levels between the Expt and Ctrl groups before treatment (P>0.05). By contrast, HMGB1 levels were significantly lower in the Expt group than that in Ctrl group after treatment (P<0.05). HMGB1 levels after treatment were also significantly lower than those before treatment in both groups (P<0.05).
As shown in Fig. 4, Hcy levels in the Expt group reached 19.02±1.48 mmol/l before treatment and 12.48±2.08 mmol/l after treatment. Hcy levels in the Ctrl group were 19.58±2.47 mmol/l before treatment and 15.44±2.61 mmol/l after treatment. There was no significant difference in Hcy levels between the groups before treatment (P>0.05). However, Hcy levels were significantly lower in the Expt group than those in the Ctrl group after treatment (P<0.05). Furthermore, Hcy levels after treatment were significantly lower than those before treatment in both groups (P<0.05).
As shown in Fig. 5, the serum CysC levels in the Expt group reached 2.04±0.19 mg/l before treatment and 1.13±0.07 mg/l after treatment. CysC levels in the Ctrl group reached 1.95±0.25 mg/l before treatment and 1.39±0.16 mg/l after treatment. No significant difference was detected in CysC levels between the groups before treatment (P>0.05). Conversely, CysC levels were significantly lower in the Expt group than those in the Ctrl group after treatment (P<0.05). In addition, CysC levels after treatment were significantly lower than those before treatment in both groups (P<0.05). Taken together, these data indicated that the addition of labetalol was more effective in reducing the level of inflammation in patients with GH.
Comparison of hemorheological indicators between groups before and after treatment
As shown in Fig. 6, the WBV of the Expt group was 6.37±0.58 mPa/s before treatment and 4.26±0.33 mPa/s after treatment. The WBV of the Ctrl group was 6.52±0.73 mPa/s before treatment and 5.11±0.48 mPa/s after treatment. There was no significant difference in WBV between the groups before treatment (P>0.05). However, WBV was significantly lower in the Expt group than that in the Ctrl group after (P<0.05). The WBV of both groups was significantly lower after treatment than those before (P<0.05).
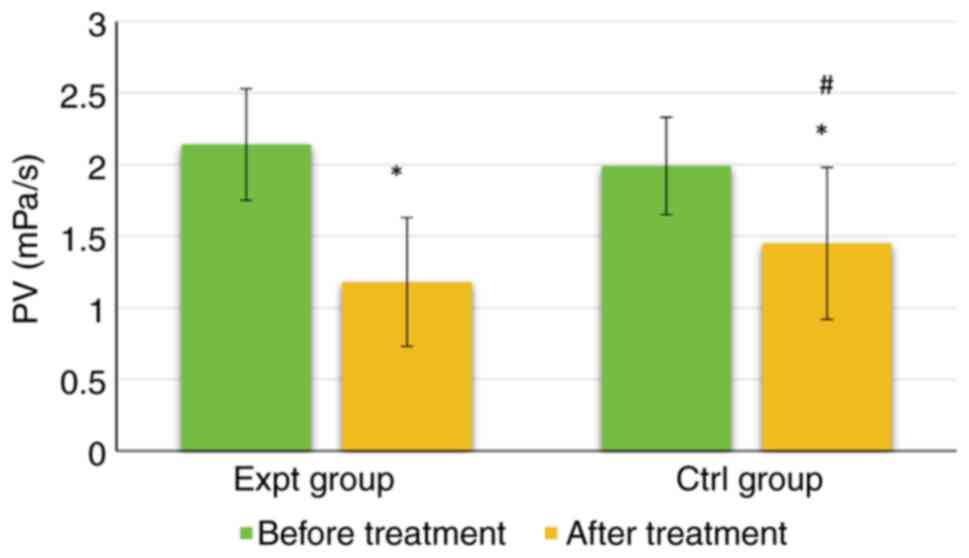
As shown in Fig. 7, the PV of the Expt group reached 2.14±0.39 mPa/s before treatment and 1.18±0.45 mPa/s after treatment. The PV of the Ctrl group was 1.99±0.34 mPa/s before treatment and 1.45±0.53 mPa/s after treatment. Notably, there was no significant difference in PV between the groups before treatment (P>0.05). However, the PV was significantly lower in the Expt group than that in the Ctrl group (P<0.05). Furthermore, the PV of both groups was significantly lower after treatment than before treatment (P<0.05).
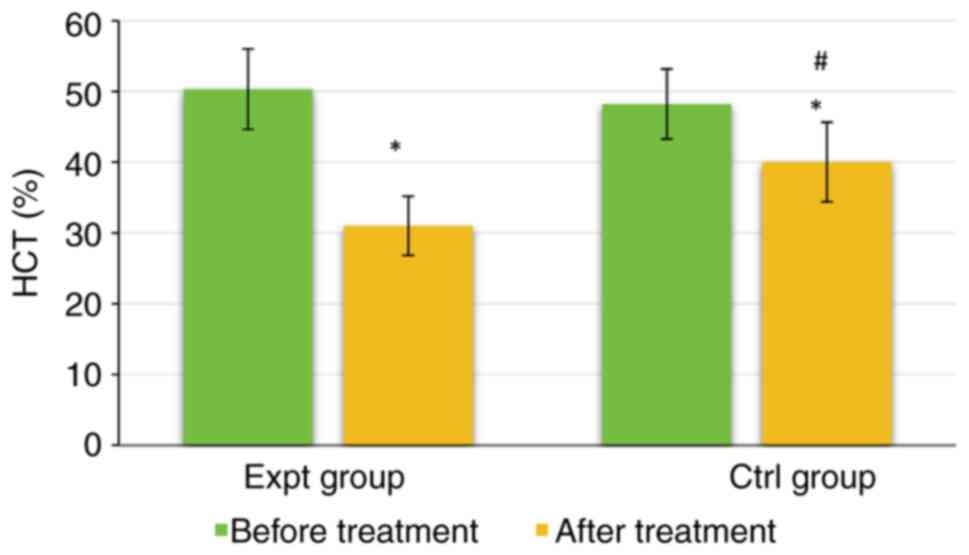
As shown in Fig. 8, the HCT of the Expt group was 50.34±5.68% before treatment and 31.02±4.17% after treatment. The HCT of the Ctrl group was 48.22±4.95% before treatment and 40.02±5.61% after treatment. There were no significant differences in HCT between the two groups before treatment (P>0.05). However, the HCT of the Expt group was significantly lower than that of the Ctrl group after treatment (P<0.05). The HCT in both groups was significantly lower after treatment than those before treatment (P<0.05). Taken together, these data indicated that the addition of labetalol was more effective in improving the hemodynamics of patients and producing antihypertensive effects.
Comparison of pregnancy outcomes between groups
As shown in Table II, there were 40 cases of spontaneous vaginal delivery (80%), 8 cases of cesarean section (16%) and 2 cases of postpartum hemorrhage (4%) in the Expt group. In the Ctrl group, there were 28 cases (56%) of spontaneous vaginal delivery, 15 cases (30%) of cesarean section and 7 cases (14%) of postpartum hemorrhage. The spontaneous vaginal delivery rate was significantly higher in the Expt group than that in the Ctrl group (P<0.05). By contrast, the cesarean section rate and postpartum hemorrhage rate of the Expt group were significantly lower than those in the Ctrl group (P<0.05). These data indicated that the addition of labetalol may improve pregnancy outcome.
Comparison of perinatal complications between groups
As shown in Table III, in the Expt group, there were 2 cases of FIUD (4%), 1 case of placental abruption (2%), 1 case of neonatal asphyxia (2%), 5 cases of premature birth (10%) and 0 cases of neonatal death (0%). In the Ctrl group, there were 4 cases of FIUD (8%), 3 cases of placental abruption (6%), 3 cases of neonatal asphyxia (6%), 12 cases of premature birth (24%) and 1 case of death (2%). The incidence of FIUD, placental abruption, neonatal asphyxia, premature birth and death was significantly lower in the Expt group than that in the Ctrl group. These data indicated that the addition of labetalol may result in fewer cases of perinatal complications.
Discussion
GH mainly refers to patients with normal blood pressure before pregnancy, whose blood pressure rises above normal values during or after pregnancy, with a series of related symptoms. GH is mainly associated with increased blood pressure, as well as edema, headache, dizziness and other phenomena in some patients, and even convulsions in severe cases (27,28). Long-term high blood pressure can result in serious harm to the fetus and mother, and even prove fatal. Most patients exhibit symptoms of systemic arteriolar spasm, which can cause insufficiency of blood supply in the microcirculation of organs throughout the body. In severe cases, GH can even lead to failure and necrosis of all organs (29). At present, effective control of blood pressure is the key to clinical treatment of GH, and spasmolysis and pressure reduction are the basic principles of treatment. Both labetalol and MgSO4 are commonly used drugs for treating GH (30-33). Therefore, the present study recruited 100 patients with GH, and randomly split them into the Ctrl and Expt groups. The Ctrl group was treated with MgSO4, whereas the Expt group was administered MgSO4 + labetalol. There were no statistically significant differences in age, gestational age and pregnancy history between the groups (P>0.05), which indicates that the groupings used were reasonable.
SBP and DBP of the Expt group after treatment were not statistically different compared with those in the Ctrl group (P>0.05); however, SBP and DBP in both groups were significantly lower after treatment than before treatment (P<0.05). The results of the present study are consistent with the findings of an open-label, randomized controlled trial (34). However, recent studies have reported the effect of labetalol on blood pressure control (35,36), though its potency has not been elucidated. Thus, the effectiveness of labetalol in controlling blood pressure needs to be evaluated in a separate study.
Further comparisons of inflammatory factors indicated that HMGB1, Hcy and serum CysC levels in the two groups were significantly lower after treatment, and those in the Expt group were also markedly lower than those in the Ctrl group after treatment (P<0.05). HMGB1 is a crucial late-stage pro-inflammatory factor, which has greater clinical significance than tumor necrosis factor (TNF), interleukin-1 and other early-stage immediate inflammatory factors (37). In addition, numerous studies have identified the close connection between HMGB1 and TNF-α (38,39). Hcy is a sulfur-containing amino acid in the human body, which is an intermediate metabolite of methionine and cysteine. Increased levels of Hcy can damage endothelial cells, disrupt the release and secretion balance of vasoactive substances, and promote the occurrence and development of hypertension (40). Serum CysC is a new endogenous biomarker which is regulated by transforming growth factor-β1(41). As a sensitive and accurate indicator of early renal function damage, it also has been reported that high serum CysC levels are associated with abnormal cardiac diastolic properties in elderly Chinese patients with heart failure with a preserved ejection fraction (42), CysC also has the potential of tumor indication (43). Studies on the regulation of the inflammatory response by labetalol are rare. Xu et al (44,45) conducted several studies, which reported that labetalol can improve the TNF-α-induced separation of HTR-8/SVneo trophoblast cells from the endothelial cell network (44); furthermore, labetalol has been shown to reduce inflammatory factor inducible nitric oxide synthase levels by increasing the expression of endothelial nitric oxide synthase (45), thereby demonstrating that CysC was a key factor in inflammation. Notably, the present findings indicated that MgSO4 + labetalol could effectively improve inflammatory stress in patients with GH compared with single MgSO4 treatment.
WBV, PV and HCT of both groups were significantly lower after treatment than those before treatment, whereas WBV, PV and HCT after treatment were significantly lower in the Expt group than those in the Ctrl group (P<0.05). These were similar to the findings of Tooher et al (46), suggesting that MgSO4 + labetalol could effectively improve the hemodynamics of patients and produce antihypertensive effects. Labetalol is a combined α- and β-adrenoceptor blocker, which is a non-selective antagonist of β-adrenoceptors and a competitive antagonist of postsynaptic α1-adrenoceptors. Labetalol acts more strongly on human β-adrenergic receptors than on α1-adrenergic receptors; the ratio of β-α antagonism is 3:1 and 6.9:1 after oral and intravenous administration, respectively (47). Unlike conventional β-adrenergic receptor blocking agents, which do not have intrinsic sympathomimetic activity, labetalol reduces peripheral vascular resistance and blood pressure during acute administration with minimal effect on heart rate or cardiac output (47). The potential mechanism by which labetalol improves blood pressure is that labetalol inhibits sympathetic nerve excitation, reduces catechol secretion, dilates blood vessels and reduces peripheral resistance by selectively blocking the effects of α- and β-adrenoceptor. Therefore, the hemodynamic indicators of patients can be improved (48-50).
Regarding pregnancy outcome, the spontaneous vaginal delivery rate was much higher in the Expt group than that in the Ctrl group, whereas the cesarean section rate and postpartum hemorrhage rate were markedly lower than those in the Ctrl group (P<0.05). These findings demonstrated that MgSO4 + labetalol could markedly improve the pregnancy outcomes of patients with GH. In addition, the incidences of FIUD, placental abruption, neonatal asphyxia, premature birth and death of perinatal infants in the Expt group were markedly lower than those in the Ctrl group (P<0.05). This further indicated that MgSO4 + labetalol could not only improve pregnancy outcomes of patients, but also reduce the harm of drugs to both mothers and fetuses (51).
In conclusion, in the present study, compared with single MgSO4 treatment, MgSO4 + labetalol effectively improved the inflammatory stress and hemodynamics of patients with GH during pregnancy, and exhibited a marked antihypertensive effect. Furthermore, MgSO4 + labetalol could improve pregnancy outcomes and reduce perinatal complications. However, there were some limitations in the present study. The sample size of selected patients was quite small and all patients were recruited from a single source. In addition, there was no comparison of treatment effect in patients with different conditions, such as complications of diabetes mellitus. Therefore, more patients with GH should be included in a future study to explore the clinical application value of MgSO4 + labetalol. In summary, the results of the present study offered a reference for drug treatment of patients with GH.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated and/or analysed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
ZG, WG and LZ conceived and designed the study. ZG, WG and GZ performed research. YT, MW and YG analyzed data. ZG, WG and LZ confirm the authenticity of all the raw data. MW and YG validated data. ZG and WG wrote the paper. WG and LZ reviewed the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study adhered to The Declaration of Helsinki, and was approved by the Ethics Committee of Taicang Hospital of TCM (approval no. 2022026). Patients provided written informed consent.
Patient consent for publication
All participants in this study consented for publication.
Competing interests
The authors declare that they have no competing interests.
References
|
Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, Kenny LC, McCarthy F, Myers J, Poon LC, et al: The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 27:148–169. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Corrigan L, O'Farrell A, Moran P and Daly D: Hypertension in pregnancy: Prevalence, risk factors and outcomes for women birthing in Ireland. Pregnancy Hypertens. 24:1–6. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G and Ishaku S: International Society for the Study of Hypertension in Pregnancy (ISSHP). The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 13:291–310. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Tan JS, Liu NN, Guo TT, Hu S and Hua L: Genetic predisposition to COVID-19 may increase the risk of hypertension disorders in pregnancy: A two-sample Mendelian randomization study. Pregnancy Hypertens. 26:17–23. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Ma XL, Zhai X, Liu JW, Xue XX, Guo SZ, Xie H, Chen JX, Zhao HH and Wang W: Study on the biological basis of hypertension and syndrome with liver-fire hyperactivity based on data mining technology. World J Tradit Chin Med. 4:176–180. 2018. | |
|
Thombre Kulkarni M, Holzman C, Wasilevich E, Luo Z, Scheid J and Allswede M: Pregnancy hypertension and its associations with pre-pregnancy depression, anxiety, antidepressants, and anxiolytics. Pregnancy Hypertens. 16:67–74. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Chen L, Shortreed SM, Easterling T, Cheetham TC, Reynolds K, Avalos LA, Kamineni A, Holt V, Neugebauer R, Akosile M, et al: Identifying hypertension in pregnancy using electronic medical records: The importance of blood pressure values. Pregnancy Hypertens. 19:112–118. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ives CW, Sinkey R, Rajapreyar I, Tita ATN and Oparil S: Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J Am Coll Cardiol. 76:1690–1702. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ishikuro M, Murakami K, Yokozeki F, Onuma T, Noda A, Ueno F, Obara T and Kuriyama S: Hypertension in pregnancy as a possible factor for child autistic behavior at two years old. Pregnancy Hypertens. 25:88–90. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Avorgbedor F, Silva S, McCoy TP, Blumenthal JA, Merwin E, Seonae Y and Holditch-Davis D: Hypertension and infant outcomes: North Carolina pregnancy risks assessment monitoring system data. Pregnancy Hypertens. 28:189–193. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Ukah UV, De Silva DA, Payne B, Magee LA, Hutcheon JA, Brown H, Ansermino JM, Lee T and von Dadelszen P: Prediction of adverse maternal outcomes from pre-eclampsia and other hypertensive disorders of pregnancy: A systematic review. Pregnancy Hypertens. 11:115–123. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Cheu LA, Drexler K and Kominiarek MA: Using m-Health apps to diagnose hypertension in pregnancy. Pregnancy Hypertens. 22:99–100. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Sahni S, Palkar AV, Rochelson BL, Kępa W and Talwar A: Pregnancy and pulmonary arterial hypertension: A clinical conundrum. Pregnancy Hypertens. 5:157–164. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Fletcher B, Chappell LC, Lavallee L, Wilson HM, Stevens R, Mackillop L, McManus RJ and Tucker KL: Changes to management of hypertension in pregnancy, and attitudes to self-management: An online survey of obstetricians, before and following the first wave of the COVID-19 pandemic. Pregnancy Hypertens. 26:54–61. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Du Q, Jovanović S, Tulić L, Tulić I and Jovanović A: Pregnancy-induced hypertension is associated with down-regulation of Kir6.1 in human myometrium. Pregnancy Hypertens. 18:96–98. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Badon SE, Dublin S, Nance N, Hedderson MM, Neugebauer R, Easterling T, Cheetham TC, Chen L, Holt VL and Avalos LA: Gestational weight gain and adverse pregnancy outcomes by pre-pregnancy BMI category in women with chronic hypertension: A cohort study. Pregnancy Hypertens. 23:27–33. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Naeh A and Ray JG: Pregnancy hypertension: An international journal of women's cardiovascular health-Reply: Letter to the editor. Pregnancy Hypertens. 30(96)2022.PubMed/NCBI View Article : Google Scholar | |
|
Lu CQ, Lin J, Yuan L, Zhou JG, Liang K, Zhong QH, Huang JH, Xu LP, Wu H, Zheng Z, et al: Pregnancy induced hypertension and outcomes in early and moderate preterm infants. Pregnancy Hypertens. 14:68–71. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Myerscough PR: Infant growth and development after treatment of maternal hypertension. Lancet. 1(883)1980.PubMed/NCBI View Article : Google Scholar | |
|
Vidyasagar S, Kumar S and Morton A: Screening for primary aldosteronism in pregnancy. Pregnancy Hypertens. 25:171–174. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Nash CM and Shetty N: Current state of affairs: A study regarding diagnosis, treatment and use of home blood pressure monitoring for hypertension in pregnancy. Pregnancy Hypertens. 24:96–99. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Gasnier R, Valério EG, Vettorazzi J, Martins-Costa SH, Barros EG and Ramos JG: Calcium-to-creatinine ratio in pregnancy-induced hypertension. Pregnancy Hypertens. 2:59–64. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Lu JF and Nightingale CH: Magnesium sulfate in eclampsia and pre-eclampsia: Pharmacokinetic principles. Clin Pharmacokinet. 38:305–314. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Okusanya BO, Oladapo OT, Long Q, Lumbiganon P, Carroli G, Qureshi Z, Duley L, Souza JP and Gülmezoglu AM: Clinical pharmacokinetic properties of magnesium sulphate in women with pre-eclampsia and eclampsia. BJOG. 123:356–366. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Wu Y, Wang DJ, Zhang Y, Zhang YX and Zhang R: Regulation of magnesium sulfate combined with nifedipine and labetalol on disease-related molecules in serum and placenta in the treatment of preeclampsia. Eur Rev Med Pharmacol Sci. 24:5062–5070. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Podymow T and August P: Antihypertensive drugs in pregnancy. Semin Nephrol. 31:70–85. 2011.PubMed/NCBI View Article : Google Scholar | |
|
DeSisto CL, Robbins CL, Ritchey MD, Ewing AC, Ko JY and Kuklina EV: Hypertension at delivery hospitalization-United States, 2016-2017. Pregnancy Hypertens. 26:65–68. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Nzelu D, Dumitrascu-Biris D, Hunt KF, Cordina M and Kametas NA: Pregnancy outcomes in women with previous gestational hypertension: A cohort study to guide counselling and management. Pregnancy Hypertens. 12:194–200. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Mhiri R, Mvogo A, Kamga A, Yassinguezo S, Fagla H, Dotou D and Kallel H: Epidemiology and maternal prognosis of hypertension disorders of pregnancy in French Guiana. Pregnancy Hypertens. 20:96–101. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Rodrigues Â, Barata C, Marques I and Almeida MC: Diagnosis of White Coat Hypertension and pregnancy outcomes. Pregnancy Hypertens. 14:121–124. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Akbar MIA, Adibrata MA, Aditiawarman Aryananda RA, Angsar MD and Dekker G: Maternal and perinatal outcome related to severity of chronic hypertension in pregnancy. Pregnancy Hypertens. 16:154–160. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Bowen L, Pealing L, Tucker K, McManus RJ and Chappell LC: Adherence with blood pressure self-monitoring in women with pregnancy hypertension, and comparisons to clinic readings: A secondary analysis of OPTIMUM-BP. Pregnancy Hypertens. 25:68–74. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Nguyen NTT, Kurtovic K, Mitchell C, Evangelista M, Del Valle R, McWay Boling S and Hughes BL: Improving treatment of severe hypertension in pregnancy and postpartum using a hypertensive pathway. Pregnancy Hypertens. 30:1–6. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Easterling T, Mundle S, Bracken H, Parvekar S, Mool S, Magee LA, von Dadelszen P, Shochet T and Winikoff B: Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: An open-label, randomised controlled trial. Lancet. 394:1011–1021. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Gupta A, Nayak D, Sharma J and Keepanasseril A: Comparing the efficacy of oral labetalol with oral amlodipine in achieving blood pressure control in women with postpartum hypertension: Randomized controlled trial (HIPPO study-Hypertension In Pregnancy & Postpartum Oral-antihypertensive therapy). J Hum Hypertens. 37:1056–1062. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Muhammad S, Usman H, Dawha YM, Yahya A, Yekeen A and Bako B: Comparison of intravenous labetalol and hydralazine for severe hypertension in pregnancy in Northeastern Nigeria: A randomized controlled trial. Pregnancy Hypertens. 29:1–6. 2022.PubMed/NCBI View Article : Google Scholar | |
|
He Y, Tian J, Blizzard L, Oddy WH, Dwyer T and Venn AJ: Associations of childhood adiposity and changes in adiposity status from childhood to adulthood with pregnancy hypertension. Pregnancy Hypertens. 19:218–225. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Wang Y, Zhang H, Chen Q, Jiao F, Shi C, Pei M, Lv J, Zhang H, Wang L and Gong Z: TNF-α/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif. 53(e12829)2020.PubMed/NCBI View Article : Google Scholar | |
|
Xu X, Piao HN, Aosai F, Zeng XY, Cheng JH, Cui YX, Li J, Ma J, Piao HR, Jin X and Piao LX: Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br J Pharmacol. 177:5224–5245. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Webster LM, Reed K, Myers JE, Burns A, Gupta P, Patel P, Wiesender C, Seed PT and Nelson-Piercy C and Nelson-Piercy C: Quantifying adherence to antihypertensive medication for chronic hypertension during pregnancy. Pregnancy Hypertens. 17:12–14. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Gressner AM, Lahme B, Meurer SK, Gressner O and Weiskirchen R: Variable expression of cystatin C in cultured trans-differentiating rat hepatic stellate cells. World J Gastroenterol. 12:731–738. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Xu CC, Fu GX, Liu QQ and Zhong Y: Association between cystatin C and heart failure with preserved ejection fraction in elderly Chinese patients. Z Gerontol Geriatr. 51:92–97. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Dikovskaya MA, Russkikh GS, Loktev KV, Johnston TP, Gevorgyan MM, Voronina NP, Chernykh VV, Trunov AN and Korolenko TA: Cystatin C and cystatin SN as possible soluble tumor markers in malignant uveal melanoma. Radiol Oncol. 56:83–91. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Xu B, Charlton F, Makris A and Hennessy A: PP042. Anti-hypertensive drugs hydralazine, clonidine and labetalol improve trophoblast integration into endothelial cellular networks in vitro. Pregnancy Hypertens. 2(264)2012.PubMed/NCBI View Article : Google Scholar | |
|
Xu B, Bobek G, Makris A and Hennessy A: Antihypertensive methyldopa, labetalol, hydralazine, and clonidine reversed tumour necrosis factor-α inhibited endothelial nitric oxide synthase expression in endothelial-trophoblast cellular networks. Clin Exp Pharmacol Physiol. 44:421–427. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Tooher J, Thornton C, Makris A, Korda A, Ogle R, Horvath J and Hennessy A: PP102. Hypertension in pregnancy and long term cardiovascular mortality outcomes. Pregnancy Hypertens. 2(295)2012.PubMed/NCBI View Article : Google Scholar | |
|
MacCarthy EP and Bloomfield SS: Labetalol: A review of its pharmacology, pharmacokinetics, clinical uses and adverse effects. Pharmacotherapy. 3:193–219. 1983.PubMed/NCBI View Article : Google Scholar | |
|
Visser VS, Hermes W, Twisk J, Franx A, van Pampus MG, Koopmans C, Mol BWJ and de Groot CJM: Prognostic model for chronic hypertension in women with a history of hypertensive pregnancy disorders at term. Pregnancy Hypertens. 10:118–123. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Magee LA, Rey E, Asztalos E, Hutton E, Singer J, Helewa M, Lee T, Logan AG, Ganzevoort W, Welch R, et al: Management of non-severe pregnancy hypertension-A summary of the CHIPS Trial (Control of Hypertension in Pregnancy Study) research publications. Pregnancy Hypertens. 18:156–162. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Oogaki Y, Ozawa R, Seshima K, Shinoda R, Torii Y, Takahashi H, Iwata H, Kuwayama T and Shirasuna K: Uncaria tomentosa extract (AC-11) improves pregnancy hypertension together with suppression of sFlt-1 and sEng. Pregnancy Hypertens. 26:127–132. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Hauspurg A, Countouris ME, Jeyabalan A, Hubel CA, Roberts JM, Schwarz EB and Catov JM: Risk of hypertension and abnormal biomarkers in the first year postpartum associated with hypertensive disorders of pregnancy among overweight and obese women. Pregnancy Hypertens. 15:1–6. 2019.PubMed/NCBI View Article : Google Scholar |