Association of IL‑6 and MMP‑3 gene polymorphisms with adolescent idiopathic scoliosis: A systematic review and meta‑analysis
- Authors:
- Published online on: April 26, 2024 https://doi.org/10.3892/etm.2024.12555
- Article Number: 267
-
Copyright: © Wang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Adolescent idiopathic scoliosis (AIS) is a complex three-dimensional (3D) deformity of the spine that occurs during the rapid growth and development of adolescents. The prevalence of the condition is estimated to be between 2 and 4% among the ages of 10 to 18 (1,2). It is primarily a result of the difference in growth and development between the anterior and posterior vertebrae that causes the spine to have lordosis or kyphosis. There is a lordosis in AIS because the vertebral body grows faster than the posterior elements, and the ventral vertebral body has to twist and rotate to maintain its height, as the dorsal diminished growth makes it difficult to maintain its normal height (3).
To date, the pathogenesis of AIS is not clear. Numerous scholars have studied the histomorphology, biological macromolecules and gene levels, as well as microbial and environmental cues (4,5), and have put forward a number of hypotheses, including genetic factors, an imbalance of the growth and development of the skeletal muscle system (6,7), hormone level and metabolic disorders (8-10), and genetic complexity (11-13).
At present, researchers are focusing heavily on gene polymorphism, which is considered to play an important role in the pathogenesis of AIS. The single nucleotide polymorphism (SNP) of the estrogen receptor β (ESR2) gene (10,14,15), vitamin D receptor (16-18), insulin-like growth factor-1 (IGF1) (19,20), melatonin receptor (21), ADAMTS-like protein 2 (ADAMTSL2), latent transforming growth factor β-binding protein 4 (LTBP4), Ras homolog gene family, member A (RHOA) (12,22).
In 2007, Aulisa et al (23) reported a case-control study linking interleukin-6 (IL-6) and matrix metalloproteinase-3 (MMP-3) SNPs to susceptibility to AIS development, and this study was later replicated by several other scholars, however the conclusions were quite inconsistent. Zhao et al (24) conducted a meta-analysis in 2016 which included only five related studies. Due to the low number of related articles and the small sample sizes included in each study, the conclusion was controversial in some respects. The purpose of the present study was to use an updated meta-analytic method to conduct a systematic and comprehensive analysis of all the published studies on the role of the MMP-3 rs3025058 gene polymorphism and the IL-6 rs1800795 gene polymorphism in AIS, in order to obtain more concrete and reliable results, and subsequently to scientifically evaluate the role of IL-6 and MMP-3 SNPs in the pathogenesis of AIS.
Materials and methods
Search strategy
The present study conformed to preferred reporting items for systematic review and meta-analysis guidelines. The databases searched included PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science (https://www.webofscience.com), EMBASE (https://www.embase.com), China National Knowledge Infrastructure (https://www.cnki.net/) and China Wanfang Data Knowledge Service Platform (https://www.wanfangdata.com.cn/) to analyze the relationship between MMP-3 rs3025058, IL-6 rs1800795 SNP and AIS. The search strategy included the following terms: ‘Adolescent idiopathic scoliosis’ or ‘AIS’ and ‘matrixmetalloproteinase-3’ or ‘MMP-3’ or ‘interleukin-6’ or ‘IL-6’ or ‘rs3025058’ or ‘rs1800795’ and ‘polymorphism’ or ‘single nucleotide polymorphism’ or ‘SNP’ or ‘variation’ or ‘mutation’. There was no language limit, and the publication dates were limited from January 2005 to December 2023.
Selection criteria
Studies were selected for review if they met the following inclusion criteria: i) Case-control study; ii) the case group met the diagnostic criteria of AIS, there were no other underlying diseases and the control group was healthy; and iii) the full text of the original literature was available, involvement of MMP-3 rs3025058 and IL-6 rs1800795, and specific data on sample size, genotype and gene frequency in the case group and the control group. Studies were excluded if they met the following criteria: i) Other observational study design, pedigree correlation study, case report, clinical trial, review and comment; ii) the case group did not meet the diagnosis of AIS, or it met the diagnosis of AIS but was combined with one or more underlying diseases, and the control group was not healthy; iii) MMP-3 rs3025058 and IL-6 rs1800795 were not the SNPs of interest or AIS was not the phenotype of interest.
Data extraction
The abstracts and full texts of articles matching inclusion criteria were independently reviewed by two authors. To ensure all relevant studies had been searched and indexed, data were checked thoroughly and repeatedly. Differences in information extraction results were resolved through discussion between researchers or with a third party. The following information was extracted from studies included in the reviewing: i) Last name of the first author; ii) publication year; iii) the country in which the study was conducted; iv) ethnicity; and v) sample size, genotypes and alleles of the AIS group and the control group.
Quality assessment
The modified Newcastle-Ottawa scale (NOS) was employed to evaluate the quality of the included literature on the association between MMP-3 rs3025058/IL-6 rs1800795 SNP and AIS. The modified NOS has a total of 9 stars and includes three aspects: Selection, comparability and outcome. The item of ‘selection’ can be awarded at most 4 stars, the item of ‘comparability’ can be awarded at most 2 stars and the item of ‘outcome’ can be awarded at most 3 stars. The specific scoring criteria are shown in Table I and the relative threshold may be adjusted depending on applicable technologies (25).
Statistical analysis
Review Manager 5.4 software provided by The Cochrane Collaboration network was used to analyze the extracted data by meta-analysis. Notably, dichotomous variables were expressed by the odds ratio (OR) and 95% confidence interval (CI). The OR and 95% CI of the IL-6 rs1800795 and the MMP-3 rs3025058 allele model (IL-6: C vs. G; MMP-3: 5A vs. 6A), codominant model (IL-6: CC vs. GG, CG vs. GG; MMP-3: 5A5A vs. 6A6A, 5A6A vs. 6A6A), dominant model (IL-6: CC + CG vs. GG; MMP-3: 5A5A + 5A6A vs. 6A6A) and recessive model (IL-6: CC vs. CG + GG; MMP-3: 5A5A vs. 5A6A + 6A6A), and it was considered statistically significant when the P-value was <0.05. A Hardy-Weinberg equilibrium (HWE) calculation was performed on the gene frequency of the control group included in the literature. P>0.05 indicated that the gene frequency distribution of the control group conformed to HWE, while P<0.05 indicated that it did not. According to ethnicity, the included population was divided into Asian and Caucasian subgroups for analysis. Heterogeneity was evaluated by I2: I2<50%, the heterogeneity is small and a fixed effect model was used; I2≥50%, heterogeneity is large and a random effect model was adopted. Trim funnel plots were used to analyze the publication bias of the article, and Egger's test and Begg's test were used to test the publication bias; if P>0.05, the publication bias was not significant.
Results
Description of included studies
According to the aforementioned retrieval strategy, a total of 154 related articles were retrieved. By reading the titles and abstracts, a total of 95 non-case-control studies, repeated publications and articles not related to the purpose of the study were excluded. Subsequently, 59 related articles were screened out, and the full text was further read and screened strictly according to the inclusion criteria and exclusion criteria. Finally, a total of seven English articles and two Chinese articles were included. The nine articles included 1,601 patients with AIS (case group) and 1,899 controls (control group). The literature screening process and results are presented in Fig. 1, and the basic characteristics included in the literature research are presented in Table II.
Quality evaluation of included literature
In the present study, the quality of literature was evaluated using the modified NOS, and nine articles received research quality scores >5 stars (Table III), indicating higher overall quality. All the inclusion studies were case-control studies, and all met the aforementioned inclusion and exclusion criteria.
Meta-analysis results
The nine articles (23,26-33) included in this meta-analysis examined the association between IL-6 rs1800795 SNP and AIS, involving 1,214 patients with AIS (case group) and 1,505 controls (control group). A total of six (23,27-29,31,32) out of the nine articles investigated the relationship between MMP-3 rs3025058 SNP and AIS, including 1,143 patients with AIS (case group) and 1,517 controls (control group).
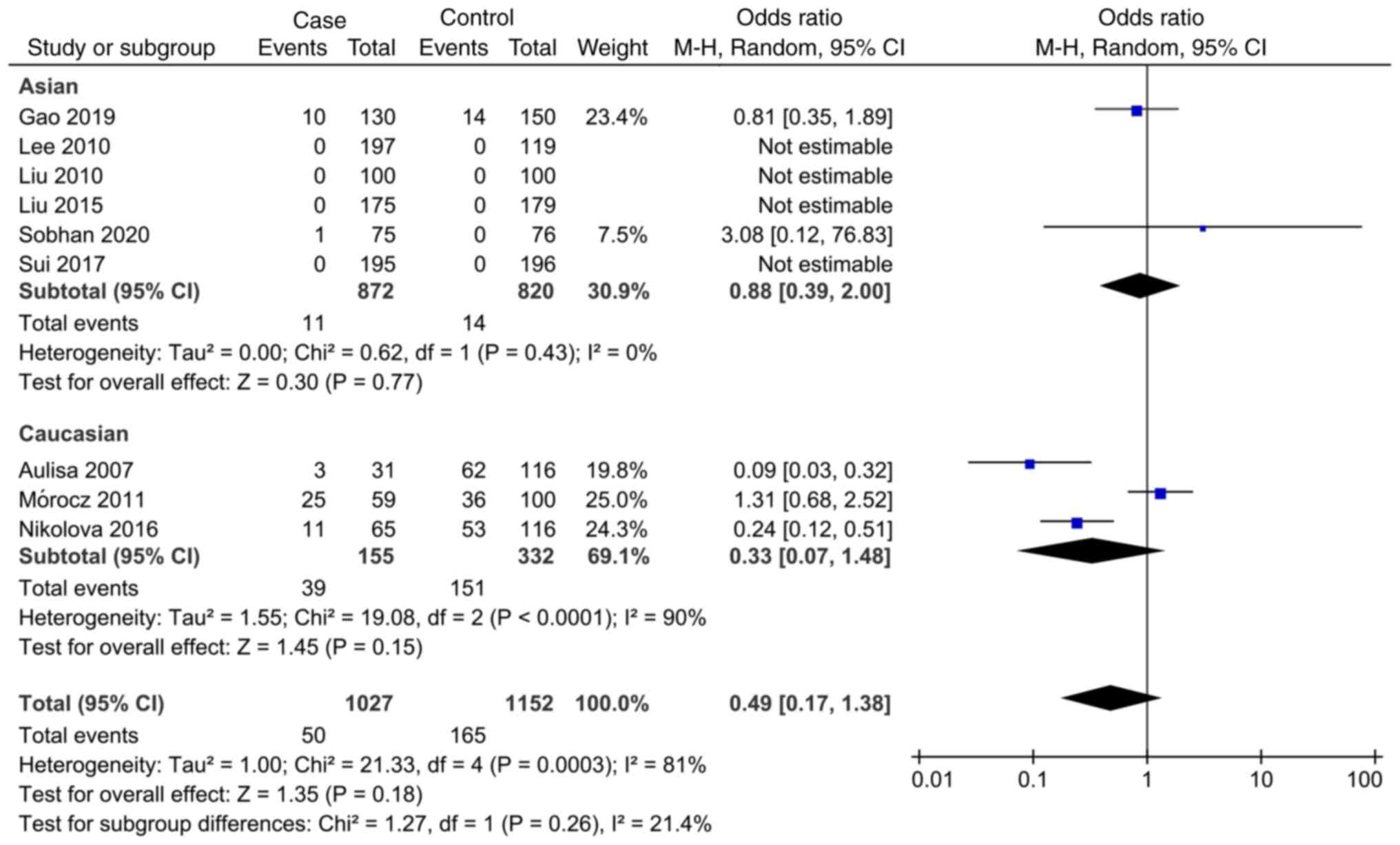
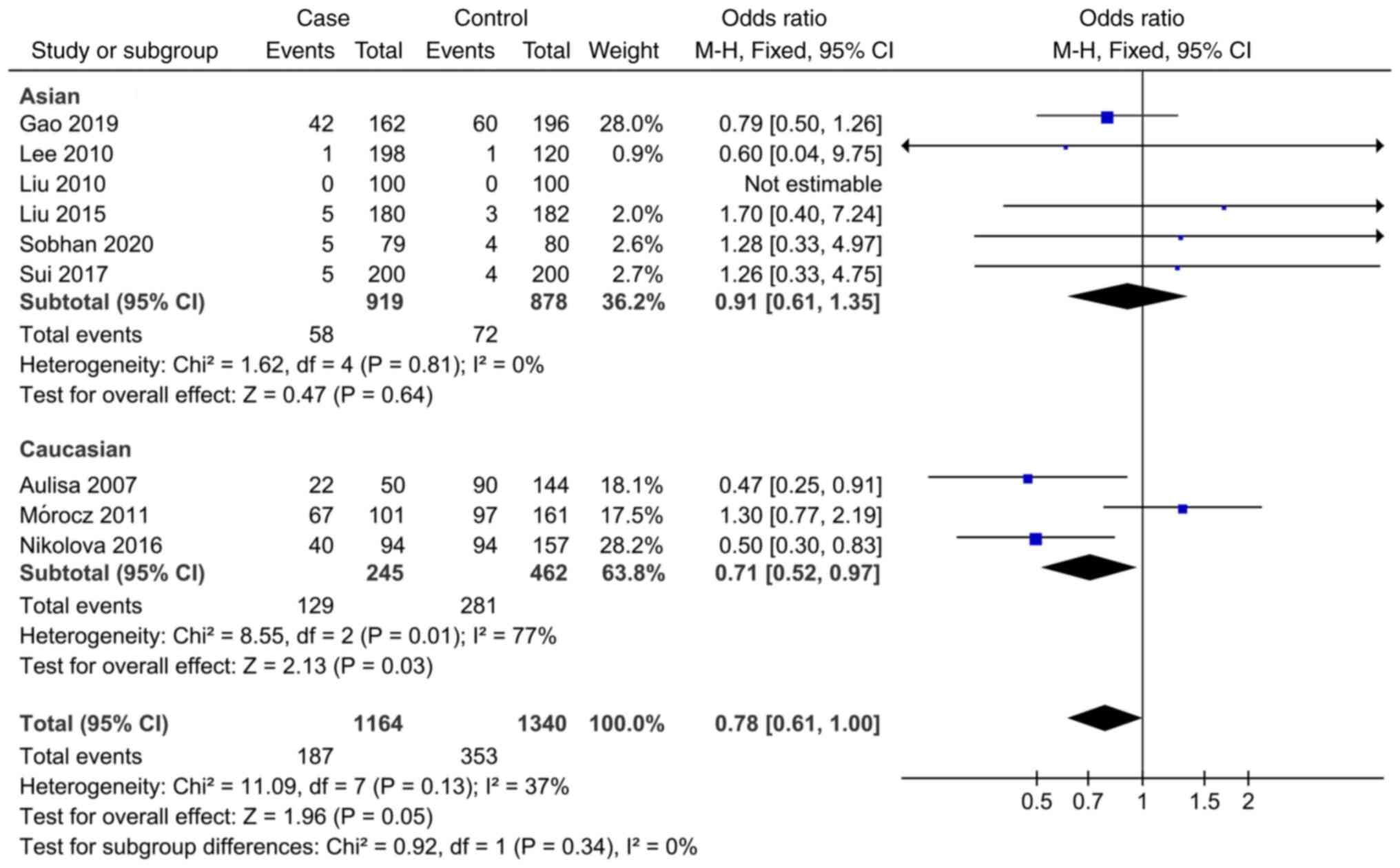
In the study of the association between IL-6 rs1800795 SNP and AIS, the meta-analysis showed that the allele model (C vs. G), codominant model (CC vs. CG and CG vs. GG), dominant model (CC + CG vs. GG) and recessive model (CC vs. CG + GG) were not associated with AIS susceptibility (Fig. 2, Fig. 3, Fig. 4, Fig. 5 and Fig. 6). Subgroup analysis showed that there was an association between the dominant model (CG vs. GG, OR=0.71; 95% CI: 0.52-0.97) and AIS susceptibility in the Caucasian population (Fig. 4). The specific results are shown in Table IV.
Table IVAnalysis of the genetic models on the association of MMP-3 rs3025058 and IL-6 rs1800795 polymorphism with adolescent idiopathic scoliosis. |
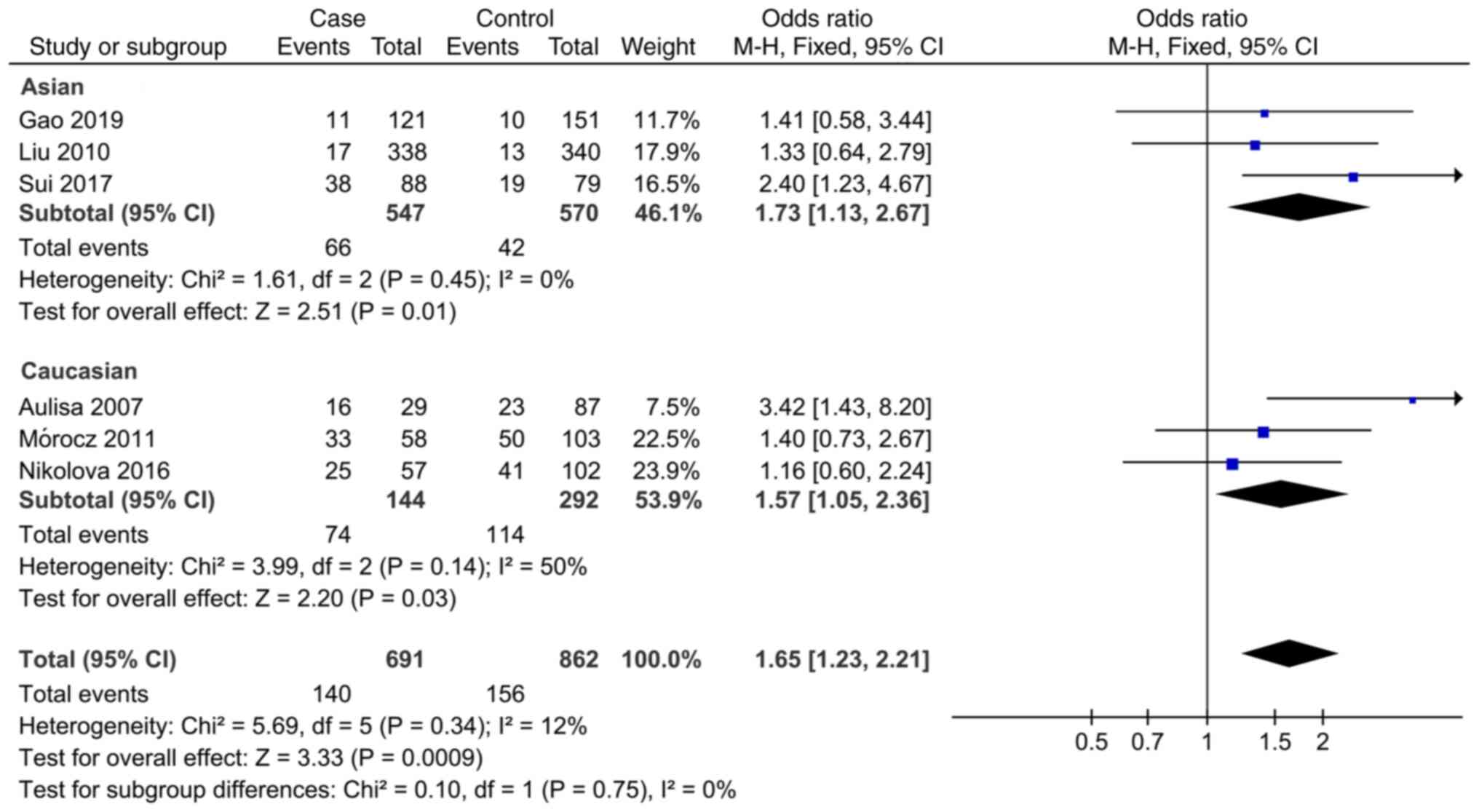
In the study of the association between the MMP-3 rs3025058 SNP and AIS, the meta-analysis showed that the allele model (5A vs. 6A, OR=1.18; 95% CI: 1.04-1.33), codominant model (5A5A vs. 6A6A, OR=1.65; 95% CI: 1.23-2.21) and recessive model (5A5A vs. 5A6A + 6A6A, OR=1.54; 95% CI: 1.19-1.99) were associated with the susceptibility to AIS. There was no significant association between the codominance model (5A6A vs. 6A6A) and dominance model (5A5A + 5A6A vs. 6A6A) and susceptibility to AIS. The results of subgroup analysis revealed that the codominant model (5A5A vs. 6A6A, OR=1.73; 95% CI: 1.13-2.67) and implicit model (5A5A vs. 5A6A + 6A6A, OR=1.73; 95% CI: 1.15-2.59) were associated with the susceptibility to AIS in the Asian population, while the allele model (5A vs. 6A, OR=1.22; 95% CI: 1.00-1.49), codominant model (5A5A vs. 6A6A, OR=1.57; 95% CI: 1.05-2.36) and recessive model (5A5A vs. 5A6A + 6A6A, OR=1.41; 95% CI: 1.01-1.98) were associated with the susceptibility to AIS in the Caucasian population (Fig. 7, Fig. 8 and Fig. 9). The specific results are presented in Table IV.
Heterogeneity and sensitivity analyses
As a result of the meta-analysis, heterogeneity was observed. There are a number of factors that can explain heterogeneity, including HWE, studies conducted in different countries and sample size limitations. There was no significant change in genotypes studied when the studies selected were limited to high quality and HWE studies. In addition, when analyzing the relationship between IL-6 rs1800795SNP and AIS, evident heterogeneity was detected, and the heterogeneity mainly emerged from the study of Mórocz et al (31), and it is hypothesized that this heterogeneity may be caused by ethnic differences and the weight of the number of cases. Following further careful reading and analysis of the literature, it was found that the study was carried out in the Hungarian population, and the gene frequency of risk gene (C) reported by the authors was much higher than that of other studies, suggesting that the occurrence of this phenomenon may be related to race specificity. Concurrently in this study, there were 126 cases in the case group and 197 cases in the control group. The weight of the number of cases in this study was larger, and the conclusion of this study was opposite to that of the other two (27,28), thus it greatly increased the heterogeneity of meta-analysis. However, considering the large sample size and high quality of the study conducted by Mórocz et al (31) it was not excluded from the present study. In the subgroup analysis, no significant heterogeneity was detected in several studies of the Asian subgroup, indicating a good consistency. At the same time, a sensitivity analysis was conducted, and when any study was removed, there was no significant difference in the results of meta-analysis, therefore it was finally concluded that the results were stable and the meta-analysis results were accurate. The detailed results are shown in Table IV.
Publication bias
All comparisons were included and publication bias was estimated using trim funnel plots test (Fig. 10). For statistical evidence, Egger's and Begg's tests (P>0.05) were conducted, and indicated that publication bias was not apparent. Although funnel plots are symmetrical, publication bias may be present because of the few related studies on the whole.
The comparisons were the following: i) The allelic comparison (C vs. G); ii) the codominance model comparison (CC vs. GG); iii) the codominance model comparison (CG vs. GG); iv) the dominant model comparison (CC + CG vs. GG); v) the recessive model comparison (CC vs. CG + GG); vi) the allelic model comparison (5A vs. 6A); vii) the codominance model comparison (5A5A vs. 6A6A); and viii) the recessive model comparison (5A5A vs. 5A6A + 6A6A).
Discussion
Scoliosis is a 3D deformity of the spine with a complex etiology, which often occurs in adolescence. As the initial symptoms are not obvious, the occurrence and development of the disease are often ignored. If adolescent patients with idiopathic scoliosis do not receive effective prevention and timely treatment, a serious condition may develop, affecting the appearance of the spine, cardiopulmonary function and even threatening the patient's life. As adolescence is a stage of maturation with physical and psychological development, the occurrence of scoliosis may seriously harm the physical and mental health of adolescents. Thus, active measures should be taken to prevent the occurrence of AIS (34,35). Specific pathogenic genes of AIS remain inconclusive, and greater attention has been paid to its genetic factors, especially the relationship between candidate genes and AIS (36,37). In recent years, some studies on the correlation between IL-6 rs1800795, MMP-3 rs3025058 and AIS have emerged, but the conclusions are not consistent. At present, the total amount of research literature on the relationship between IL-6 rs1800795, MMP-3 rs3025058 and AIS remains limited.
IL-6 is a multifunctional cytokine from a wide range of sources. Current studies have shown that IL-6 has both pro-inflammatory and anti-inflammatory effects, and its main function is to mediate inflammatory response (38,39). A number of studies have revealed that the expression of IL-6 in herniation intervertebral disc cells was significantly increased, suggesting that IL-6 is involved in the pathogenesis of intervertebral disc degeneration to a certain extent (40-42). The 5A allele in the MMP-3 promoter sequence is a risk factor for lumbar degenerative diseases and is highly expressed in human degenerative intervertebral disc samples, suggesting that MMP-3 plays an important role in intervertebral disc degeneration (43,44). The upregulated expression of IL-6 and MMP-3 can promote intervertebral disc degeneration, and numerous studies have found that scoliosis areas of patients with AIS are more prone to intervertebral disc degeneration than normal areas, suggesting that MMP-3 and IL-6 may interact to induce intervertebral disc degeneration and participate in the occurrence of scoliosis (38,42-44).
Research in several countries has identified a relationship between IL-6 rs1800795 and MMP-3 rs3025058 SNPs and AIS incidence, gradually expanding evidence. However, the conclusions remain uncertain, and controversial points continue to exist. According to the results of the present meta-analysis, the IL-6 rs1800795 gene polymorphism does not appear to be associated with AIS incidence. Based on a meta-analysis published in 2020, Sobhan et al (26) found that the IL-6 rs1800795 SNP and AIS risk were statistically significant. In the subgroup analysis, the IL-6 rs1800795 SNP was significantly associated with the risk of AIS in the Caucasian population, which runs counter to the results of the present study. After studying the article carefully, it was observed that the author did not exclude the repeated published literature and there may be the phenomenon of repeated statistical data. For example, related articles published by Nikolova et al (29,45) in 2015 and 2016 involve the phenomenon of repetition of statistical data. The author also included data from degenerative lumbar scoliosis that was not relevant to this study, which led to discrepancies in the data. Based on this, it is assumed that the data and conclusions are not reliable.
After screening, newly published articles were selected for inclusion in the present study. Notably, significant heterogeneity was also detected and it mainly emerged from the study conducted by Mórocz et al (31); is hypothesized that this heterogeneity may be caused by ethnic differences and the weight of the number of cases. This leads to differences in the analytical results, and thus the data need to be confirmed by further study. Statistical analysis in the present study showed that there was no significant correlation between IL-6 rs1800795 locus gene polymorphism and the pathogenesis of AIS.
In the current study, the association between MMP-3 rs3025058SNP and AIS was analyzed by meta-analysis. The results showed that allele model (5A vs. 6A), the codominant model (5A5A vs. 6A6A) and the recessive model (5A5A vs. 5A6A + 6A6A) were related to the susceptibility to AIS. Results of the subgroup analysis demonstrated that the codominant model (5A5A vs. 6A6A) and the recessive model (5A5A vs. 5A6A + 6A6A) were associated with the susceptibility to AIS in Asian population. In Caucasian population, the allele model (5A vs. 6A), codominant model (5A5A vs. 6A6A) and recessive model (5A5A vs. 5A6A + 6A6A) are associated with AIS susceptibility, and the combined OR value of the codominant model (5A5A vs. 6A6A) in the Caucasian population was statistically significant, which is consistent with the overall population, suggesting that this association is more common in Caucasian population. Further study is required to clarify the correlation of other populations. On the whole, there is an association between allele 5A at this locus and susceptibility to AIS. It is considered that although the sample size has increased, it is still less overall, and the reliability of the conclusion still needs more research for verification. In this case, a false negative may be obtained. In the analysis of the association between MMP-3 rs3025058SNP and AIS, the overall allele model (5A vs. 6A) OR=1.18; 95% CI: 1.04-1.33, the codominant model (5A5A vs. 6A6A) OR=1.65, 95% CI: 1.23-2.21 and the recessive model (5A5A vs 5A6A + 6A6A) OR=1.54; 95% CI: 1.19-1.99, indicated an association between the two. In addition, in the overall and subgroup analyses of the relationship between MMP-3 rs3025058SNP and AIS, the heterogeneity was small, suggesting that the results are more reliable. At the same time, the results of subgroup analysis showed that the proportion of risk gene (5A) was similar in Asian and Caucasian population. It is possible to detect the MMP-3 rs3025058 gene early in children and provide long-term prevention guidance for children who may have AIS, by including the following methods: i) Bone nutrition strengthening (high-protein, high-carbohydrate, high-vitamin, high-fiber food consumption); ii) correct sitting and standing posture, which may delay the deterioration of symptoms; and iii) health education, strengthening physical exercise, reasonable nutrition, reasonable regulation of body weight and psychological adjustment. Concurrently, it is recommended to use traditional methods such as Baduanjin, Tai Chi and schroth exercises as interventions (46-48). In addition, regular follow-up and review of children and adolescents' body posture test index, low back pain score, spinal positive position and lateral X-ray Cobb angle is important.
The main advantages of the present meta-analysis include the following: i) Most of the studies included in the present meta-analysis are high quality case-control studies; and ii) most were based on relatively large samples, and specific gene results were extracted and analyzed. However, the current meta-analysis also has numerous limitations: i) Although the meta-analysis contains a relatively large sample size (1,601 patients with AIS and 1,899 controls) so the conclusion should be relatively accurate, the results could still be an overestimation that could be overturned with the addition of more data; ii) the subjects only included East Asian and Caucasian races, and thus the study does not reflect the overall population. In the subgroup analysis, the sample size of each subgroup was smaller, which may also cause the results of the analysis to deviate from the actual situation; and iii) when informed patient consent is required in clinical research, when it comes to medical ethical issues, it may also lead to the loss of a significant number of reluctant patients, which will affect the reliability of meta-analysis conclusions. Therefore, the conclusion needs to be further verified by larger sample randomized controlled trials.
In conclusion, the present meta-analysis revealed that there was no significant association between the gene polymorphism of IL-6 rs1800795 locus and the pathogenesis of AIS, but the 5A allele of the MMP-3 rs3025058 locus was associated with the susceptibility to AIS, especially in the Caucasian population. The 5A5A genotype of the MMP-3 rs3025058 locus is associated with susceptibility to AIS, both in Asians and Caucasians. Based on the synthesis of the relevant case-control studies regarding the association between IL-6 rs1800795/MMP-3 rs3025058 and AIS, the present study obtained more reliable and credible results, although, further research is still required to better understand its specific pathogenesis and confirm the conclusions of the previous studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
YPW and JPH confirm the authenticity of all the raw data. YPW and SLQ designed and performed the research, collected and analyzed the data, wrote the article and performed the statistical analysis. KDH and JPH conceived the present study. SY, PFH and AHL collected the data. YFX, KDH and JPH reviewed the articles and interpreted the data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Kuznia AL, Hernandez AK and Lee LU: Adolescent idiopathic scoliosis: Common questions and answers. Am Fam Physician. 101:19–23. 2020.PubMed/NCBI | |
|
Addai D, Zarkos J and Bowey AJ: Current concepts in the diagnosis and management of adolescent idiopathic scoliosis. Childs Nerv Syst. 36:1111–1119. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Hefti F: Pathogenesis and biomechanics of adolescent idiopathic scoliosis (AIS). J Child Orthop. 7:17–24. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Smit TH: Adolescent idiopathic scoliosis: The mechanobiology of differential growth. JOR Spine. 3(e1115)2020.PubMed/NCBI View Article : Google Scholar | |
|
Lin J, Wong CKH, Cheung JPY, Cheung PWH and Luo N: Psychometric performance of proxy-reported EQ-5D youth version 5-level (EQ-5D-Y-5L) in comparison with three-level (EQ-5D-Y-3L) in children and adolescents with scoliosis. Eur J Health Econ. 23:1383–1395. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Kaviani R, Londono I, Parent S, Moldovan F and Villemure I: Growth plate cartilage shows different strain patterns in response to static versus dynamic mechanical modulation. Biomech Model Mechanobiol. 15:933–946. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Swany LM, Larson AN, Milbrandt TA, Sanders JO, Neal KM, Blakemore LC, Newton PO, Pahys JM, Cahill PJ and Alanay A: Inter- and intra-rater reliability and accuracy of sanders skeletal maturity staging system when used by surgeons performing vertebral body tethering. Spine Deform. 10:97–106. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Gargano G, Oliva F, Migliorini F and Maffulli N: Melatonin and adolescent idiopathic scoliosis: The present evidence. Surgeon. 20:e315–e321. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Liang ZT, Guo CF, Li J and Zhang HQ: The role of endocrine hormones in the pathogenesis of adolescent idiopathic scoliosis. FASEB J. 35(e21839)2021.PubMed/NCBI View Article : Google Scholar | |
|
Chmielewska M, Janusz P, Andrusiewicz M, Kotwicki T and Kotwicka M: Methylation of estrogen receptor 2 (ESR2) in deep paravertebral muscles and its association with idiopathic scoliosis. Sci Rep. 10(22331)2020.PubMed/NCBI View Article : Google Scholar | |
|
Wise CA: What causes AIS? Ask the genome! Stud Health Technol Inform. 280:3–8. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Liu B, Zhao S, Liu L, Du H, Zhao H, Wang S, Niu Y, Li X and Qiu G: Deciphering disorders Involving Scoliosis COmorbidities (DISCO) study group et al. Aberrant interaction between mutated ADAMTSL2 and LTBP4 is associated with adolescent idiopathic scoliosis. Gene. 814(146126)2022.PubMed/NCBI View Article : Google Scholar | |
|
Sharma S, Gao X, Londono D, Devroy SE, Mauldin KN, Frankel JT, Brandon JM, Zhang D, Li QZ, Dobbs MB, et al: Genome-wide association studies of adolescent idiopathic scoliosis suggest candidate susceptibility genes. Hum Mol Genet. 20:1456–1466. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Zhao L, Roffey DM and Chen S: association between the estrogen receptor beta (ESR2) Rs1256120 single nucleotide polymorphism and adolescent idiopathic scoliosis: A systematic review and meta-analysis. Spine (Phila Pa 1976). 42:871–878. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Kotwicki T, Janusz P, Andrusiewicz M, Chmielewska M and Kotwicka M: Estrogen receptor 2 gene polymorphism in idiopathic scoliosis. Spine (Phila Pa 1976). 39:E1599–E1607. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Dai J, Lv ZT, Huang JM, Cheng P, Fang H and Chen AM: Association between polymorphisms in vitamin D receptor gene and adolescent idiopathic scoliosis: A meta-analysis. Eur Spine J. 27:2175–2183. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Goździalska A, Jaśkiewicz J, Knapik-Czajka M, Drąg J, Gawlik M, Cieśla M, Kulis A, Zarzycki D and Lipik E: Association of calcium and phosphate balance, vitamin D, PTH, and calcitonin in patients with adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 41:693–697. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Suh KT, Eun IS and Lee JS: Polymorphism in vitamin D receptor is associated with bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J. 19:1545–1550. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Guan M, Wang H, Fang H, Zhang C, Gao S and Zou Y: Association between IGF1 gene single nucleotide polymorphism (rs5742612) and adolescent idiopathic scoliosis: A meta-analysis. Eur Spine J. 26:1624–1630. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Yang Y, Wu Z, Zhao T, Wang H, Zhao D, Zhang J, Wang Y, Ding Y and Qiu G: Adolescent idiopathic scoliosis and the single-nucleotide polymorphism of the growth hormone receptor and IGF-1 genes. Orthopedics. 32(411)2009.PubMed/NCBI View Article : Google Scholar | |
|
Nelson LM, Ward K and Ogilvie JW: Genetic variants in melatonin synthesis and signaling pathway are not associated with adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 36:37–40. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Nowak R, Kwiecien M, Tkacz M and Mazurek U: Transforming growth factor-beta (TGF-β) signaling in paravertebral muscles in juvenile and adolescent idiopathic scoliosis. Biomed Res Int. 2014(594287)2014.PubMed/NCBI View Article : Google Scholar | |
|
Aulisa L, Papaleo P, Pola E, Angelini F, Aulisa AG, Tamburrelli FC, Pola P and Logroscino CA: Association between IL-6 and MMP-3 gene polymorphisms and adolescent idiopathic scoliosis: A case-control study. Spine (Phila Pa 1976). 32:2700–2702. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Zhao J, Yang M and Li M: Association of IL-6 and MMP-3 gene polymorphisms with susceptibility to adolescent idiopathic scoliosis: A meta-analysis. J Genet. 95:573–579. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Norris JM, Simpson BS, Ball R, Freeman A, Kirkham A, Parry MA, Moore CM, Whitaker HC and Emberton M: A modified newcastle-ottawa scale for assessment of study quality in genetic urological research. Eur Urol. 79:325–326. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Sobhan MR, Mahdinezhad-Yazdi M, Dastgheib SA, Ahrar H, Aghili K and Neamatzadeh H: Association of the IL-6-174G > C (rs1800795) polymorphism with adolescent idiopathic scoliosis: Evidence from a case-control study and meta-analysis. Rev Bras Ortop (Sao Paulo). 55:17–26. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Gao S: Study on the relationship between Interleukin-6, Matrix metalloproteinase-3 Gene Polymorphism and Adolescent Idiopathic Scoliosis. Doctoral thesis, Zhengzhou University, 2019. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFDLAST2019&filename=1019115140.nh. | |
|
Sui W, Yang J, Huang Z, Wang Q, Fan H and Deng Y: Polymorphisms in promoter regions of MMP-3 and IL-6 genes are not associated to adolescent idiopathic scoliosis (AIS) gender bias. J Back Musculoskelet Rehabil. 30:559–563. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Nikolova ST, Yablanski VT, Vlaev EN, Stokov LD, Savov AS, Kremensky IM and Loukanov AR: Association between IL-6 and MMP3 common genetic polymorphisms and idiopathic scoliosis in Bulgarian patients: A case-control study. Spine (Phila Pa 1976). 41:785–791. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Liu S: Association between Gene Polymorphism and Adolescent Idiopathic Scoliosis in Northern Chinese Han population. Doctoral thesis, Peking Union Medical College, 2015. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFDLAST2015&filename=1015353939.nh. | |
|
Mórocz M, Czibula A, Grózer ZB, Szécsényi A, Almos PZ, Raskó I and Illés T: Association study of BMP4, IL6, Leptin, MMP3, and MTNR1B gene promoter polymorphisms and adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 36:E123–E130. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Liu Z, Tang NLS, Cao XB, Liu WJ, Qiu XS, Cheng JCY and Qiu Y: Lack of association between the promoter polymorphisms of MMP-3 and IL-6 genes and adolescent idiopathic scoliosis: A case-control study in a Chinese Han population. Spine (Phila Pa 1976). 35:1701–1705. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Lee JS, Suh KT and Eun IS: Polymorphism in interleukin-6 gene is associated with bone mineral density in patients with adolescent idiopathic scoliosis. J Bone Joint Surg Br. 92:1118–1122. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Labrom FR, Izatt MT, Askin GN, Labrom RD, Claus AP and Little JP: Quantifying typical progression of adolescent idiopathic Scoliosis: Longitudinal three-dimensional MRI measures of disc and vertebral deformities. Spine (Phila Pa 1976). 48:1642–1651. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Tang NLS, Dobbs MB, Gurnett CA, Qiu Y, Lam TP, Cheng JCY and Hadley-Miller N: A decade in review after idiopathic scoliosis was first called a complex trait-a tribute to the late Dr. Yves Cotrel for his support in studies of etiology of scoliosis. Genes (Basel). 12(1033)2021.PubMed/NCBI View Article : Google Scholar | |
|
Yang Y, Yang M, Shi D, Chen K, Zhao J, He S, Bai Y, Shen P and Ni H: Single-cell RNA Seq reveals cellular landscape-specific characteristics and potential etiologies for adolescent idiopathic scoliosis. JOR Spine. 4(e1184)2021.PubMed/NCBI View Article : Google Scholar | |
|
Khanshour AM, Kou I, Fan Y, Einarsdottir E, Makki N, Kidane YH, Kere J, Grauers A, Johnson TA, Paria N, et al: Genome-wide meta-analysis and replication studies in multiple ethnicities identify novel adolescent idiopathic scoliosis susceptibility loci. Hum Mol Genet. 27:3986–3998. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Kreiner FF, Kraaijenhof JM, von Herrath M, Hovingh GKK and von Scholten BJ: Interleukin 6 in diabetes, chronic kidney disease, and cardiovascular disease: mechanisms and therapeutic perspectives. Expert Rev Clin Immunol. 18:377–389. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Albrakati A, Alsharif KF, Al Omairi NE, Alsanie WF, Almalki ASA, Elmageed ZY, Elshopakey GE, Lokman MS, Bauomy AA, Moneim AE and Kassab RB: Neuroprotective efficiency of prodigiosins conjugated with selenium nanoparticles in rats exposed to chronic unpredictable mild stress is mediated through antioxidative, anti-inflammatory, anti-apoptotic, and neuromodulatory activities. Int J Nanomedicine. 16:8447–8464. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Yamamoto Y, Kokubo Y, Nakajima H, Honjoh K, Watanabe S and Matsumine A: Distribution and polarization of hematogenous macrophages associated with the progression of intervertebral disc degeneration. Spine (Phila Pa 1976). 47:E149–E158. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Schmidli MR, Sadowska A, Cvitas I, Gantenbein B, Lischer HEL, Forterre S, Hitzl W, Forterre F and Wuertz-Kozak K: Fibronectin fragments and inflammation during canine intervertebral disc disease. Front Vet Sci. 7(547644)2020.PubMed/NCBI View Article : Google Scholar | |
|
Chen J, Mei Z, Huang B, Zhang X, Liu J, Shan Z, Wang J, Wang X and Zhao F: IL-6/YAP1/β-catenin signaling is involved in intervertebral disc degeneration. J Cell Physiol. 234:5964–5971. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Zawilla NH, Darweesh H, Mansour N, Helal S, Taha FM, Awadallah M and El Shazly R: Matrix metalloproteinase-3, vitamin D receptor gene polymorphisms, and occupational risk factors in lumbar disc degeneration. J Occup Rehabil. 24:370–381. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Takahashi M, Haro H, Wakabayashi Y, Kawa-uchi T, Komori H and Shinomiya K: The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br. 83:491–495. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Nikolova S, Dikova M, Dikov D, Djerov A, Dzhebir G, Atanasov V, Savov A and Kremensky I: Role of the IL-6 gene in the etiopathogenesis of idiopathic scoliosis. Anal Cell Pathol (Amst). 2015(621893)2015.PubMed/NCBI View Article : Google Scholar | |
|
Ng PTT, Tucker K, Zahir SF, Izatt MT, Straker L and Claus A: Comparison of physiological and behavioral nutrition-related factors in people with and without adolescent idiopathic scoliosis, from cohort data at 8 to 20 years. JBMR Plus. 8(ziad013)2024.PubMed/NCBI View Article : Google Scholar | |
|
Qin X, Sun K, Xu W, Gao J, Jiang H, Chen W, Zhang L, Li Z, Li W, Yuan P, et al: An evidence-based guideline on treating lumbar disc herniation with traditional Chinese medicine. J Evid Based Med. 17:187–206. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Burger M, Coetzee W, du Plessis LZ, Geldenhuys L, Joubert F, Myburgh E, van Rooyen C and Vermeulen N: The effectiveness of Schroth exercises in adolescents with idiopathic scoliosis: A systematic review and meta-analysis. S Afr J Physiother. 75(904)2019.PubMed/NCBI View Article : Google Scholar |