Invasive papillary carcinoma of the breast: A case report
- Authors:
- Published online on: May 2, 2024 https://doi.org/10.3892/ol.2024.14433
- Article Number: 300
-
Copyright: © Wang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Based on the latest data from the World Health Organization (WHO), breast cancer continues to be the most frequently diagnosed cancer among women, accounting for 11.6% of all cancer cases, and remains the leading cause of cancer-related deaths in women, responsible for 6.9% of all cancer-associated deaths (1). With the rise in breast cancer incidence, there is also a proportional increase in the incidence of rare histologic subtypes (2). Invasive papillary carcinoma (IPC) is a rare type of breast cancer, accounting for <1% of breast cancer cases in most case series, and is commonly seen in postmenopausal women (3–5). In 2003, the WHO classified IPC as a type of invasive mammary carcinoma in Classification of Tumors of the Breast and Female Genital Organs (6). Certain studies on IPC actually targeted variants of solid papillary carcinoma (SPC) or encapsulated papillary carcinoma (EPC) due to the lack of specificity in the description (3,7). In 2012, the WHO defined IPC as aggressive adenocarcinoma in the fourth edition of Classification of Breast Tumors, with papillary structures accounting for >90% of the invasive part (8). This definition was maintained without modification in the subsequent fifth edition of the classification (2019) (9). Currently, research on IPC is limited to case reports and small retrospective studies (2,10–12), which has resulted in a lack of understanding of this rare tumor. Diagnosing IPC can be difficult due to a lack of in-depth knowledge and understanding, and there is currently no established standard treatment in the medical community. The present article reports an exceptional case of IPC. The disease course lasted for 2 years, and the lesion involved the skin; however, no breast cancer lymph node metastasis was found. The patient received only endocrine therapy after surgery, and the prognosis is good. The present case not only highlights the favorable pathological characteristics and indolent biological behaviors inherent to this tumor type, but also offers invaluable insights for formulating effective therapeutic strategies against it.
Case report
In 2019, a 51-year-old female patient discovered a palpable lump, with a diameter of ~2 centimeters, in the right breast. The tumor progressively increased in size, and by June 2021, ulceration of the skin on the right breast became apparent, accompanied by a considerable exudation of straw-colored fluid. In September 2021, the patient was admitted to the First Affiliated Hospital of China Medical University (Shenyang, China). Throughout the period from symptom onset until hospital admission, the patient remained untreated. The patient experienced local pain; however, sleep, diet, bowel movements and body weight remained normal. The patient denied any history of chronic diseases, including diabetes mellitus, hypertension and coronary artery disease.
The physical examination of the patient revealed asymmetrical breasts, with the nipples at the different levels (Fig. 1A). The tumor involved the entire right breast, measuring ~15× 15 cm with a firm consistency, indistinct boundary and restricted movement (Fig. 1B). The skin of the breast was red and swollen with visible dehiscence (Fig. 1C). No lymph node was palpable in the bilateral axilla or in the upper and lower clavicular areas, and the skin did not show dimpling.
Laboratory tests revealed that the hepatitis B-related indicators of the patient were as follows: Hepatitis B surface antigen-positive, hepatitis B e-antibody-positive, hepatitis B core antibody-positive, hepatitis B surface antibody-negative and hepatitis B e-antigen-negative. The liver function of the patient was also assessed, with results showing alkaline phosphatase at 41 U/l, total protein at 57 g/l, and albumin at 36.6 g/l, all slightly below the reference range (13). Blood cell analyses revealed a red blood cell count of 3.38×1012/l, hemoglobin level of 83 g/l, mean corpuscular volume of 78.5 fl and mean corpuscular hemoglobin of 24.6 pg. The white blood cell count was 4.54×109/l and platelet count was 371×109/l. The urinary routine and coagulation function showed no obvious abnormalities (Table I).
Imaging revealed a space-occupying lesion with bleeding in the right breast parenchyma, leveled Breast Imaging-Reporting and Data System 5 (14). High-resolution (HR) computed tomography (CT) revealed a large irregular low-density mass in the right breast (Fig. 2A). A Doppler ultrasound indicated the loss of the normal glandular architecture of the right breast, with mixed cystic (>90%) and solid echoes (Fig. 2B). Additionally, a lymph node echo was detected in the right axilla, measuring 1.09×0.61 cm with slight cortical thickening (Fig. 2C). Magnetic resonance imaging (MRI) indicated tortuous vessels within the solid portion and a fluid-fluid plane within the lesion capsule (Fig. 2D). Mammography with splint compression was not performed due to the size, cystic nature and lesions over the skin surface of the mass.
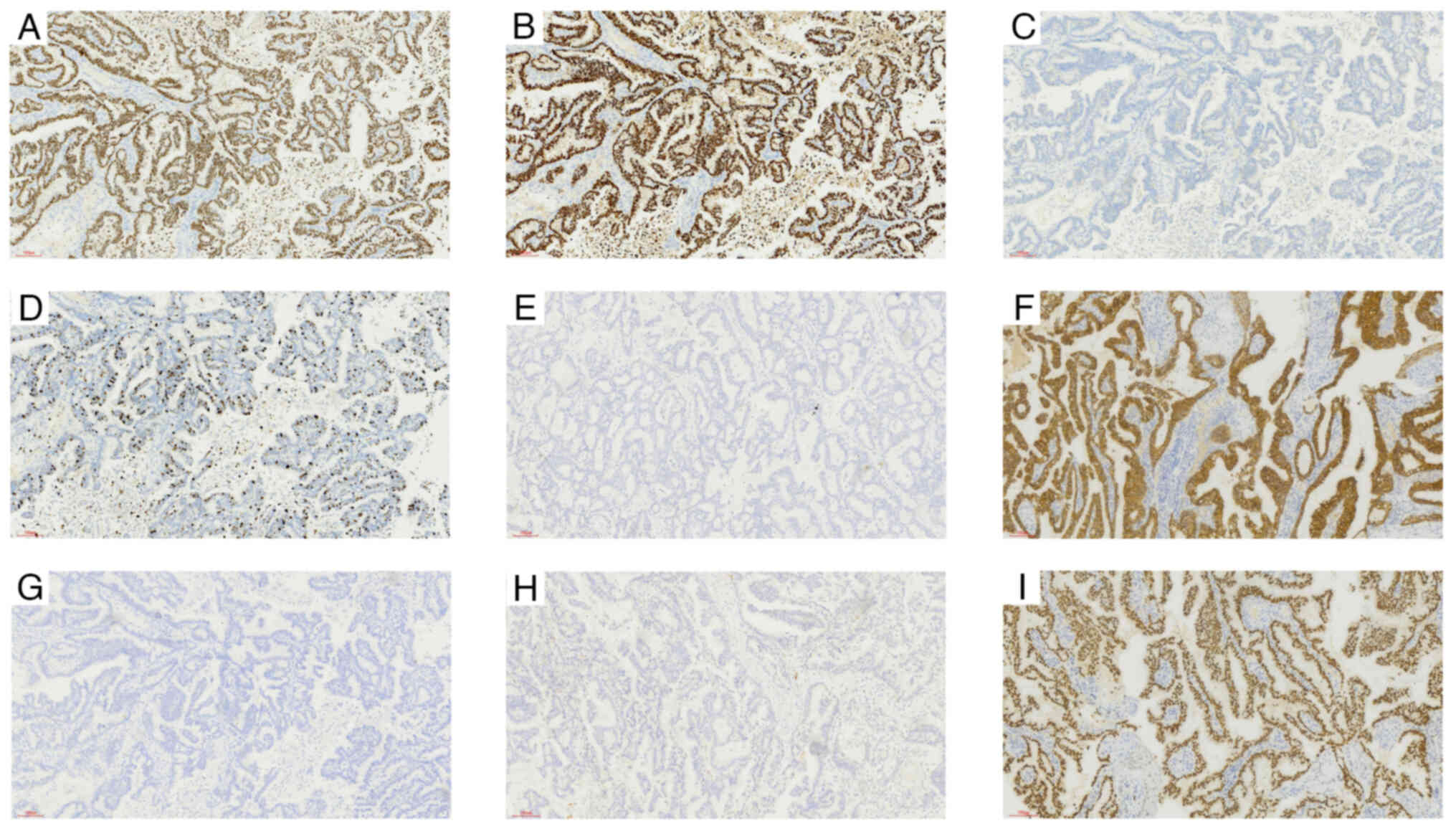
Tissue from the lesion was obtained using core needle biopsy (CNB), which was followed by hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC). Microscopically, the dilated ducts exhibited broad papillary structures with a monolayer-multilayer epithelial covering on their surface, revealing densely packed tumor cells with weak eosinophilic cytoplasm and moderately atypical nuclei, thus pointing towards a preliminary diagnosis of papillary neoplasms (Fig. 3). Papillary neoplasms include several subtypes such as benign papilloma, intraductal papillary carcinoma, EPC, SPC and IPC (15). IHC revealed a luminal-type breast cancer, which was characterized by estrogen receptor (ER) positivity (90%), progesterone receptor (PR) positivity (90%), human epidermal growth factor receptor-2 (HER-2) negativity (−), a Ki-67 index of 20%, cytokeratin 5/6 (CK5/6)(−) and E-cadherin positivity (+) (Fig. 4A-F). The assessment of myoepithelial cells was performed through the utilization of specific immune markers, namely p63 and calponin (Fig. 4G,H); however, both markers returned negative outcomes, a characteristic that rules out benign papilloma and intraductal papillary carcinoma (16). Encapsulated papillary carcinoma is typically characterized by a distinct fibrous cystic capsule, whilst solid papillary carcinoma demonstrates expansive growth with minimal fibrous-vascular cores (17,18). In the present case, the absence of a fibrous cystic capsule, along with fused papillary networks and abundant fibrous-vascular cores, allowed the exclusion of both encapsulated and solid papillary carcinomas. Consequently, the diagnosis was narrowed down to IPC (8,9). Furthermore, the positivity of ER, PR and GATA binding protein 3 (GATA3; Fig. 4I), combined with the medical history of the patient, strongly suggested that the lesion originated from the breast. Therefore, a preoperative diagnosis of primary IPC of the breast was made via CNB.
The patient had locally advanced breast cancer but declined preoperative adjuvant therapy, including neoadjuvant chemotherapy and endocrine therapy, due to economic reasons. Therefore, surgical treatment was considered, accounting for the request of the patient and the lack of standardized treatment guidelines and evidence-based medical evidence for neoadjuvant treatment of IPC. In September 2021, the patient underwent a modified radical mastectomy to remove the lesion and drain lymph nodes in the axillary region, reducing the risk of metastasis and recurrence. In addition, as the tumor occupied the entire breast, the surgery resulted in significant loss of local skin and tissue. To address this, a pedicled transverse rectus abdominis myocutaneous flap was used for immediate reconstruction upon completion of the modified radical mastectomy (Fig. 5) (19,20). Notably, there is presently no standardized surgical protocol for IPC, and the aforementioned surgical procedures were primarily based on the prevalent surgical techniques used for non-specific types of breast cancer (2).
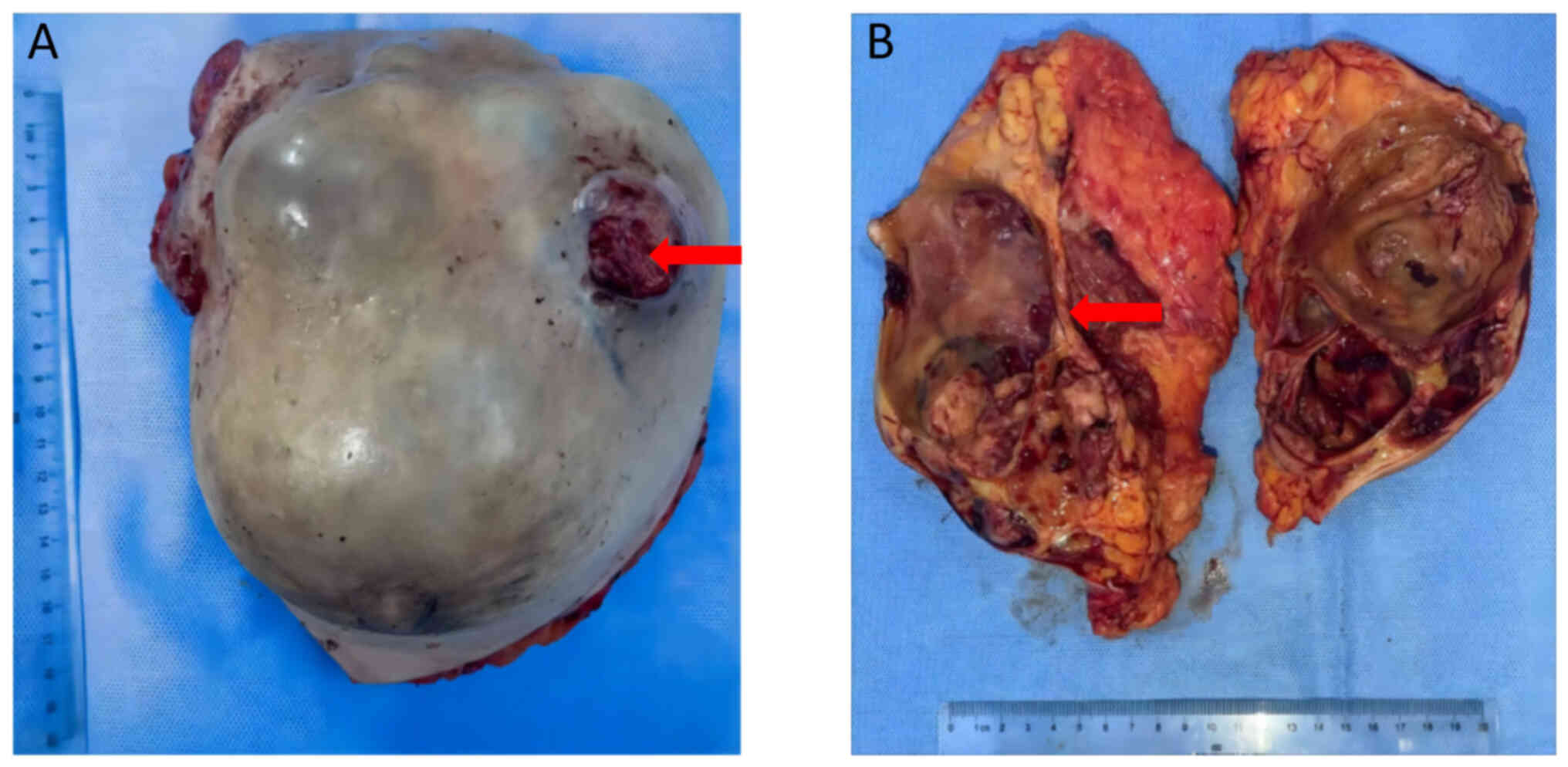
The specimen appeared wrinkled on the surface and had two fissures, measuring ~6×6 and 3×3 cm, respectively (Fig. 6A). Multiple fine papillary masses were observed immediately adjacent to the skin. The cut surface exhibited a grayish-white texture and was brittle, more delicate and felt tough in certain areas. Dark-brown blood clots were observed within the capsule and the luminal wall, with a substantial amount of necrosis in certain areas (Fig. 6B). To definitively ascertain the type and nature of the tumor, tissue samples were collected from several regions and H&E staining and IHC were performed. The cancerous tissue primarily exhibited papillary structures, with papillae fusing to form complex papillae and a reticulated papillary structure. The papillae surface was covered by atypical epithelium (Nottingham score of 7) (21), with a central fibrovascular core (Fig. 7A). The lesion had extended to involve the skin, where histological examination revealed an incomplete basal cell layer with visible tumor cells infiltrating the epidermis, whilst the dermis had completely vanished (Fig. 7B). However, lymph node examination did not detect the presence of any tumor cells (Fig. 7C). The IHC results aligned with the preoperative CNB results, affirming the diagnosis of IPC (Fig. 8A-I).
Postoperatively, the patient reported slight incision pain but denied upper extremity numbness, swelling, chills, fever or shivering. Pressure dressing and daily dressing changes were administered. On postoperative day 21, during the dressing change, the incision appeared well healed without signs of redness, swelling or infection. The patient requested discharge, and upon a comprehensive assessment of the physical condition and recovery progress of the patient, they were discharged in October 2021. Due to financial constraints, the patient received only anastrozole endocrine therapy at a dosage of 1 mg/day for 5 years (22). Additionally, the patient did not return for hospital follow-ups or undergo any imaging examinations such as CT, MRI or ultrasound. Postoperative telephone follow-ups were performed every 3 months. From October 2021 to April 2024, the patient reported no discomfort, and self-examination revealed no lumps in the surgical area, axilla or contralateral breast. However, it should be noted that the postoperative follow-up has limitations, necessitating a longer and more comprehensive evaluation.
Imaging instrumentation and parameters
HRCT
CT scanning was performed using a Siemens 64-Row 128-Slice Spiral CT Machine and a tube voltage of 100 keV and automatic milliampere-second tube current modulation. The scanning process was performed in a spiral fashion, progressing from the apex to the base of the lungs, with a pitch of 0.8 and a slice thickness of 1 mm. A matrix of 512×512 was implemented. For image interpretation, the lung window was adjusted to a window width ranging from 1,200-1,500 HU, with a window level between −600 and −700 HU. Similarly, the mediastinal window was set to a width of 400 to 500 HU, and a level of 40–50 HU.
Ultrasonography
Ultrasonography was performed using a Philips ATL HDI 5000 Ultrasound Machine. The patient was positioned supine, with their upper limbs extended laterally and elevated. The breasts and axillae were exposed to facilitate bilateral scanning using a probe operating within a frequency range of 8–12 MHz. Lesion scope, characteristics and the distribution of blood flow were observed using color Doppler flow imaging.
MRI
MRI was performed using a GE HealthCare Signa HDxt 3.0T MRI Scanner. The patient entered the examination room, lay flat on the examination bed in a prone position, and allowed both breasts to naturally hang due to gravity and fit into the concave cavity of the coil. The anterior chest wall was positioned tightly against the coil. During the examination, the team endeavored to scan the axilla and anterior chest wall regions of the patient. The routinely performed MRI scans included horizontal T1 weighted image (WI) plain scans, T2WI fat-suppressed scans and diffusion-weighted imaging (DWI) scans. Dynamic contrast-enhanced scanning was performed using dynamic-enhanced T1 high-resolution isotropic volume excitation (dyn-eTHRIVE) technology. The scanning parameters were set as follows: i) T1WI/turbo spin echo: repetition time (TR), 639 msec; echo time (TE), 6.8 msec; slice thickness, 5 mm; slice gap, 1 mm; field-of-view (FOV), 340×340 mm; ii) T2WI/Spectral Attenuated Inversion Recovery: TR, 5,620 msec; TE, 110 msec; slice thickness, 4.5 mm; slice gap, 1 mm; FOV, 340×340 mm; iii) DWI (b=1,000): TR, 3,894 msec; TE, 69 msec; slice thickness, 4.5 mm; slice gap, 1 mm; FOV, 350×350 mm; iv) dyn-eTHRIVE dynamic contrast-enhanced scanning: TR, 4.2 msec; TE, 2.0 msec; slice thickness, 2.0 mm; FOV, 200×200 mm. Following the plain scans, Gadolinium-diethylenetriaminepentaacetic acid, a paramagnetic contrast agent, was intravenously injected through the median cubital vein for contrast-enhanced scanning. The standard dosage administered was ~0.1 mmol/kg at a flow rate of ~2 m/sec. Immediately after the contrast agent injection, 20 ml normal saline was flushed through at the same rate of 2 ml/sec. A mask acquisition was taken prior to the intravenous bolus injection of the contrast agent, and subsequently, eight consecutive image acquisitions were performed over a total duration of 7–9 min.
H&E staining
The tissue samples were preserved in a 10% neutral formalin solution at room temperature for 22–24 h, subsequently placed in molds filled with liquid paraffin, and allowed to cool and solidify. After fixation, the samples were sliced into 4-µm sections using a microtome. Following the slicing process, the tissue sections were dewaxed procedure at 45°C for ~6 min to remove any residual paraffin from the tissue, preparing it for subsequent staining. The Roche Ventana HE 600 automated staining system (Roche Diagnostics) was used for staining, in which the tissue sections were stained with H&E, two commonly used histological dyes, for 3 min at room temperature. After staining, the tissue sections were dehydrated and clarified by sequential immersion in 95% alcohol I for 5 min, 95% alcohol II for another 5 min, absolute ethanol I for 5 min, absolute ethanol II for 5 min, xylene I for 5 min and xylene II for 5 min. The sections were then removed from the xylene, allowed to air dry briefly and sealed with neutral gum. The staining quality and tissue morphology were then evaluated under a light microscope at a magnification of ×200.
IHC
The reagents and steps used for paraffin sectioning are consistent with those described for the aforementioned H&E staining. Tissue sections were placed in a 60°C incubator for 30 min to melt the paraffin, followed by thorough dewaxing using xylene (xylene I, 30 min and xylene II, 30 min) and gradual rehydration in a graded ethanol series (100% ethanol, 10 min; 95% ethanol, 10 min; 80% ethanol, 10 min; and 70% ethanol, 10 min). The sections were then rinsed with tap water for 10 min. Subsequently, they were immersed in 0.01 M citrate buffer (pH 6.0), microwaved to boiling for 5 min, and allowed to cool naturally to room temperature. To block endogenous peroxidase/phosphatase activity, the tissue sections were incubated in a 3% hydrogen peroxide solution for 10 min. Subsequently, at room temperature, antibody treatment commenced with the blocking of non-specific binding sites on the sections using 5% bovine serum albumin (Fuzhou Maixin Biotechnology Development Co., Ltd.) for 30 min to reduce background staining. Diluted primary monoclonal antibodies, purchased from Fuzhou Maixin Biotechnology Development Co., Ltd., were applied and incubated overnight at 4°C to ensure sufficient antigen-antibody binding. These antibodies, along with their respective dilution ratios, were as follows: Rabbit anti-human ER (clone SP1; cat. no. Kit-0012) at 1:800, rabbit anti-human PR (clone SP2; cat. no. Kit-0013) at 1:800, rabbit anti-human HER-2 (clone MXR001; cat. no. Kit-0043) at 1:400, rabbit anti-human Ki-67 (clone SP6; cat. no. RMA-0542) at 1:400, mouse anti-human CK5/6 (clone MX040; cat. no. MAB-0744) at 1:800, mouse anti-human E-cadherin (clone MX020; cat. no. MAB-0738) at 1:400, mouse anti-human P63 (clone MX013; cat. no. MAB-0694) at 1:400, mouse anti-human Calponin (clone MX023; cat. no. MAB-0712) at 1:400 and mouse anti-human GATA3 (clone L50-823; cat. no. MAB-0695) at 1:400. The following day, the sections were thoroughly washed with PBS (cat. no. PBS-0060; Fuzhou Maixin Biotechnology Development Co., Ltd.) to remove unbound primary antibodies. Biotinylated secondary antibodies, specifically Goat anti-Mouse IgG (H+L) Secondary Antibody, Biotin (cat. no. A-11008) and Goat anti-Rabbit IgG (H+L) Secondary Antibody, Biotin (cat. no. A-11021), both purchased from Fuzhou Maixin Biotechnology Development Co., Ltd., were then applied at a dilution ratio of 1:400 and incubated for 30 min at room temperature, followed by washing with PBS. Streptavidin-peroxidase conjugate was added and incubated for another 30 min at room temperature before another PBS wash. Finally, DAB chromogen (cat. no. DAB-1031; Fuzhou Maixin Biotechnology Development Co., Ltd.) was used for color development. Positive control slides (cat. no. P-0023) from Fuzhou Maixin Biotechnology Development Co., Ltd. were used, and a blocking serum (cat. no. BS-012-015) served as the negative control. The color development process was closely monitored under a light microscope (Leica DM2000), and once the desired staining intensity was achieved, the reaction was stopped by rinsing with tap water. The sections were then counterstained with hematoxylin (cat. no. CTS-1090; Fuzhou Maixin Biotechnology Development Co., Ltd.) at room temperature for 30 sec to enhance tissue structure visualization. Following this, the sections were dehydrated through a graded ethanol series, washed with xylene and mounted with neutral balsam. Evaluation was performed under an optical microscope at ×200 magnification. All immunohistochemical sections were interpreted according to standard criteria (23).
Discussion
Based on the 5th edition of the WHO Classification of Breast Tumors, papillary neoplasms of the breast comprise a diverse group of diseases, encompassing benign papilloma, intraductal papillary carcinoma, as well as EPC, SPC and IPC (15). The rarest subtype of papillary neoplasms is IPC, characterized by a predominantly (>90%) papillary infiltrating component (24–26). Compared with other papillary neoplasms, IPC possesses distinct clinical and histological characteristics. However, the lack of large-scale epidemiological investigations poses significant challenges for clinical management. This challenge is reflected not only in the difficulty of histological diagnosis but also in the clinical treatment process (27). To the best of our knowledge, the present study is the first instance of three characteristics being included together: Firstly, the present study is the largest IPC case reported so far, with a diameter >15 cm; secondly, despite the patient having a long history of disease, a large tumor burden and skin lesions, there was no occurrence of axillary lymph node metastasis. This reflects, to a certain extent, the favorable pathological characteristics and biological behaviors of IPC; and finally, the patient only received endocrine therapy after surgery, and no recurrence or metastasis was found during the 2.5-year follow-up. The present case has never been reported before, to the best of our knowledge, and provides an important reference for the treatment of IPC. It is crucial to increase awareness of this rare tumor in the medical community and aid doctors to recognize and properly handle similar cases in the future.
IPC typically occurs in postmenopausal women of non-Caucasian descent, usually between the ages of 60–80 (3,28). In 2013, Liu et al (29) reviewed 284 IPC cases and 300 invasive ductal carcinoma (IDC) cases, and reported that most patients with IPC (79.23%) were >50 years at diagnosis, which was more than patients with IDC (39.00%). Additionally, most patients with IPC (74.30%) were postmenopausal, which was higher than patients with IDC (35.00%) (29). A large retrospective study recently demonstrated that IPC was more prevalent in older postmenopausal women, African-Americans and individuals with government insurance (30). Similarly, Chen et al (31) reported that 85.9% of patients with IPC were >50 years old at diagnosis, compared with 73.4% of patients with IDC.
IPC can occur at any location within the ductal system, spanning from the nipple to the terminal duct lobular unit (32). According to Zheng et al (2), among 524 reported cases of IPC, 50% occurred beneath the areola, whilst the remaining cases were found outside the areola. In addition, the presentation of IPC is typically characterized by bloody nipple discharge, an abnormal mass or radiographic abnormalities (5,29). In medical imaging, there are no distinct radiological features that differentiate between IPC and IDC. Both malignancies commonly manifest as irregular masses, occasionally accompanied by calcifications. Ultrasonography may reveal hypoechoic or mixed echogenic masses, whilst MRI may demonstrate heterogeneous enhancement in both tumor types. In the series of 18 IPC cases presented by Mitnick et al (33), the majority showcased a distinct multinodular pattern, featuring a notable increase in density within a segmental distribution. Additionally, IPC typically presents as a solid or cystic-solid mass, with the solid component being smaller than that of solid papillary carcinoma, and larger than that of encapsulated papillary carcinoma of the same size (18). In the present case, the patient was a 53-year-old premenopausal female patient with a mass located in the upper outer quadrant of the right breast that occasionally caused pain. Preoperative imaging and postoperative analysis of the gross specimen revealed multiple cystic-solid lesions in the breast that had fused together. Evaluating a tumor with a cystic component can be challenging due to its large volume, which may lead to overdetermination. Therefore, it is important to consider any solid components present. In breast pathology, the presence and distribution of myoepithelium are crucial for the identification and classification of papillary neoplasms: i) Benign papilloma: the lesion is characterized by uneven proliferation of epithelial cells around the fibrovascular core. There are myoepithelial cells present around the affected ducts and within the lesion (Fig. 9A); ii) Intraductal papillary carcinoma: the lesion is typically characterized by papillary hyperplasia of homogeneous tumor cells. There are no myoepithelial cells present within the lesion, however, there are clearly visible myoepithelial cells surrounding the affected ducts (Fig. 9B) (4,34); iii) EPC: the lesion is surrounded by a thick fibrous capsule-like structure. The tumor cells are homogeneous and exhibit papillary hyperplasia in the capsule. Myoepithelial cells do not surround the lesion and the vast majority of affected ducts (Fig. 9C) (35,36); iv) SPC: the lesion is characterized by expansive nodules and solid growth patterns, with an indistinct fibrovascular core. It is often accompanied by neuroendocrine and mucus secretion characteristics. Neuroendocrine markers, neuron-specific enolase, synaptophysin and chromogranin A, are positively expressed (17,37,38). The lesion lacks myoepithelial cells and, in most cases, the involved ducts are also devoid of myoepithelial cells (Fig. 9D) (39); however, in a few cases, it can be observed around the affected ducts (Fig. 9E) (40); and v) IPC: the lesion primarily forms delicate papillary structures, and the papillae fuse with each other to form larger, complex papillae and reticulated papillary structures (4). The papillary structures and the affected ducts lack myoepithelial cells (41) (Fig. 9F). Furthermore, in a study by Fisher et al (42). mucin secretion was noted in 2/3 of IPC cases. Notably, metastatic papillary carcinoma from other organs, such as the thyroid, ovary and lung, should not be misdiagnosed as IPC (43–45). Immunohistochemical markers such as paired-box gene 8, Wilms' tumor 1, thyroid transcription factor 1, napsin-A and thyroglobulin, combined with relevant clinical history, can help identify the origin of the tumor outside the breast. Moreover, it is important to distinguish between invasive micropapillary carcinoma and papillary neoplasms. The former is a distinct tumor type featuring clusters or nests of neoplastic cells without a true fibrovascular core and surrounded by empty spaces (46,47).
In the present case, the patient had a disease duration of >2 years with skin involvement but no axillary lymph node metastases. No recurrent metastases were detected in the 2.5 years of postoperative follow-up. This may reflect the relatively indolent biological behaviors of this type of tumor to a certain extent. The biological behaviors of tumors refers to the characteristics and abilities exhibited by tumor cells during proliferation, development and metastasis (48,49). In most cases, IPC progresses slowly and poses a low risk of local metastasis. According to Suh et al (50), disease-free progression can occur for >10 years under the natural history of the disease. In 2012, Liu et al performed a review of 284 cases of IPC and 300 cases of IDC (29). The results indicated that the rate of axillary lymph node metastasis in IPC (17.25%) was markedly lower than that in IDC (49.00%). These characteristics were associated with the pathological characteristics and gene expression of the tumors. In many studies, IPC has been reported to be smaller in size, of lower histological grade, and have higher positivity rates for ER and PR, as well as a lower Ki-67 proliferation index compared with IDC (12,51). A retrospective study in 2016 reported that patients with IPC, in comparison with patients with IDC, presented with a higher proportion of tumors that were <20 mm (67.4 vs. 63.9%) and a greater incidence of grade 1 disease (32.6 vs. 18.6%) (2). Moreover, a retrospective study conducted by Hashmi et al (52) reported that IPC cases exhibited a more favorable pathological profile in terms of prognostic features, including a lower Ki-67 index, tumor stage and histological grade, compared with IDC. Similarly, a higher expression of PR and a lower expression of HER2 was associated with a superior biomarker profile in IPC. Additionally, the occurrence rates of lymphovascular invasion and axillary metastasis were also lower in IPC (52). However, Terzi and Uner (53) documented a unique instance of IPC exhibiting high-grade nuclei, pronounced karyorrhexis and absence of ER or PR expression, which implied a high-grade malignancy, albeit without axillary lymph node metastasis. Furthermore, papillary neoplasms represent entities with varying biological behaviors and differential responses to treatment, suggesting that they may be driven by a few specific genomic events. It is important to note that papillary carcinoma, including EPC, SPC and IPC, may be part of the ER(+) breast cancer lineage due to their highly similar gene expression patterns (41). They are considered low-aggressive, exhibiting low levels of genes associated with cell adhesion, migration and movement (54). Moreover, research has indicated that IPC exhibits lower p53 expression, fewer gene copy number aberrations and a higher mutation rate of phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α compared with IDC (55,56). A more in-depth investigation into the histopathology and genomics of IPC is necessary to gain a deeper understanding of its biological behaviors.
Breast cancer is a diverse group of diseases with varying histological and clinical characteristics. Treatment options are primarily based on the more prevalent types, such as non-specific IDC. However, for the rarer types of breast cancer, treatment guidelines are not always clear, and options are often inferred from comparisons with the more common types (57). There is no clear consensus on the treatment of IPC, a rare type of breast cancer (52). Due to its prevalence in postmenopausal elderly women and the favorable pathological characteristics and biological behaviors of tumor, it is generally recommended to avoid overtreatment (4). Local surgical intervention can not only prevent breast cancer progression but also improve the quality of life of patients with locally advanced disease (58,59). Arora et al (60) reported a case of a 62-year-old male patient with IPC who underwent a simple mastectomy. The patient remained disease-free at the 1-year follow-up. For comprehensive evaluation and treatment, it is necessary to completely remove IPC tumors and perform a sentinel lymph node biopsy (61,62). When cystic structures are present, neoadjuvant therapy should be considered for tumor reduction and skin graft or flap metastasis, as IPC tend to be larger in size. Additionally, due to the low malignancy of IPC and the low risk of metastasis recurrence, breast-conserving surgery combined with radiation therapy may be appropriate (63). Furthermore, IPC is an invasive carcinoma, and a systematic adjuvant treatment plan needs to be determined based on characteristics such as recurrence risk and tumor molecular classification. Patients with IPC with low recurrence risk may be more suitable for endocrine therapy compared with chemotherapy. The toxic and side effects of chemotherapy may affect its use, especially in postmenopausal elderly female patients who are commonly affected by IPC (64). Combination therapy or sequential therapy with multiple chemical drugs may cause liver function damage, digestive tract damage and leukocyte reduction (65). The integration of gene expression profiling, chemotherapy benefit and toxicity prediction tools is expected to better inform chemotherapy decisions in this population (66). Conversely, whilst IPC often expresses HR, HER-2 expression is often negative, and the Ki-67 index is low, which may result in poor efficacy of chemotherapy (67,68). However, endocrine therapy has lower toxicity and side effects, improved patient adherence, and is particularly effective for patients who are HR(+). Furthermore, the use of CDK4/6 inhibitors in combination with endocrine therapy has brought new hope to patients with HR(+)/HER-2(−) breast cancer (69). In 2016, a case of HR(+)/HER-2(−) invasive papillary carcinoma in an 83-year-old postmenopausal female patient was reported in Japan. The patient achieved a pathological complete response after undergoing neoadjuvant endocrine therapy with letrozole at a dosage of 2.5 mg/day for 12 months (70). In the present case, due to financial constraints, the patient underwent aromatase inhibitor therapy solely with anastrozole, administered at a daily dosage of 1 mg for 5 years post-surgery, whilst eschewing adjunctive therapies like radiotherapy and chemotherapy. Notably, anastrozole, letrozole and exemestane all belong to the class of aromatase inhibitors, and their efficacy in treating hormone-sensitive breast cancer is equivalent (71). Liu et al (29) demonstrated that IPC was associated with a higher 5-year overall survival rate (92.77%) and disease-free survival rate (87.95%) compared with IDC (87.95 and 80.72%, respectively). IPC typically exhibits favorable pathological features and biological behaviors; however, further evidence-based medical research is required to determine its true prognosis. A recent large retrospective study compared IPC and IDC and reported similar 5-year overall survival rates for both (86.8 vs. 88.7%). The study further reported that age (ranging from 80 to 90 years), locally advanced disease and the lack of radiation therapy are independent risk factors for poor prognosis in patients with IPC (30). Zheng et al (2) reported that, after adjusting for confounders, patients treated with IPC did not have a significant survival advantage over those treated with IDC. In the present case, the patient received telephone follow-ups every 3 months post-surgery, primarily to communicate about the post-surgical recovery progress, discuss medication side effects and assess for any signs of metastasis or recurrence of breast cancer. To date, the patient has reported no discomfort during this period, and self-examination has not revealed any significant masses in the affected chest wall, axilla, contralateral breast or axilla. Further observation, along with comprehensive assessments using imaging and other auxiliary examinations, is necessary to determine the long-term prognosis of the patient.
The present study reports a rare case of giant IPC and not only highlights the benign pathological characteristics and indolent growth behavior typically associated with this tumor, but also provides valuable data for the study of IPC treatment and prognosis. Due to the economic limitations of the patient, the follow-up was unable to include comprehensive auxiliary examinations and laboratory tests. Therefore, the absence of recurrence or metastasis in the patient cannot be fully confirmed. However, despite the 2-year duration from the self-discovery of the mass by the patient to treatment, and the large size of the tumor with extensive skin involvement, no axillary lymph node or distant metastasis was detected after surgery. Furthermore, the patient has maintained a good quality of life for 2.5 years following the operation. Based on previous research, it is believed that the current status of the patient is positive, although the true prognosis still requires longer-term and more comprehensive follow-up observations.
In conclusion, IPC is a rare type of breast cancer and its favorable prognosis is attributed to its pathological features and biological behaviors. Accurate diagnosis and avoidance of overtreatment are crucial in clinical management. Due to the limited clinical data and absence of clear treatment guidelines, doctors must exercise caution and individualize treatment plans. Endocrine therapy may be an effective treatment modality, but further prospective clinical studies are necessary.
Acknowledgements
Not applicable.
Funding
The present work was supported by the National Nature Science Foundation of China (grant no. 12374413).
Availability of data and materials
The datasets generated in the present study may be requested from the corresponding author.
Authors' contributions
SW, XM, QZ and TZ contributed to the study design, writing and revisions of this manuscript. SW and XM confirm the authenticity of all the raw data. All authors reviewed the results and have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the patient for publication of the present case report and the accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
|
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. Apr 4–2024.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI | |
|
Zheng YZ, Hu X and Shao ZM: Clinicopathological characteristics and survival outcomes in invasive papillary carcinoma of the breast: A SEER population-based study. Sci Rep. 6:240372016. View Article : Google Scholar : PubMed/NCBI | |
|
Pal SK, Lau SK, Kruper L, Nwoye U, Garberoglio C, Gupta RK, Paz B, Vora L, Guzman E, Artinyan A and Somlo G: Papillary carcinoma of the breast: An overview. Breast Cancer Res Treat. 122:637–645. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Tay TKY and Tan PH: Papillary neoplasms of the breast-reviewing the spectrum. Mod Pathol. 34:1044–1061. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Louwman MWJ, Vriezen M, van Beek MWPM, Nolthenius-Puylaert MCBJET, van der Sangen MJC, Roumen RM, Kiemeney LALM and Coebergh JWW: Uncommon breast tumors in perspective: Incidence, treatment and survival in the Netherlands. Int J Cancer. 121:127–135. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Soe AM, Joseph G, Guevara E and Xiao P: Primary neuroendocrine carcinoma of the breast metastatic to the bones, which chemotherapy? Breast J. 23:589–593. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Blaumeiser B, Tjalma WA, Verslegers I, De Schepper AM and Buytaert P: Invasive papillary carcinoma of the male breast. Eur Radiol. 12:2207–2210. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Sinn HP and Kreipe H: A brief overview of the WHO classification of breast tumors, 4th edition, focusing on issues and updates from the 3rd edition. Breast Care (Basel). 8:149–154. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al: The 2019 World Health Organization classification of tumours of the breast. Histopathology. 77:181–185. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Gogoi G, Dowerah S, Kashyap AS and Terangpi M: Papillary carcinoma of breast: An institutional overview of a rare histopathologic type. J Cancer Res Ther. 19:511–515. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Pant I and Joshi SC: Invasive papillary carcinoma of the male breast: Report of a rare case and review of the literature. J Cancer Res Ther. 5:216–218. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Vani D, Geetanjali S, Punja GM and Bharathi M: A case of invasive papillary breast carcinoma: Fierce façade with favorable prognosis. J Cancer Res Ther. 11:10292015. View Article : Google Scholar : PubMed/NCBI | |
|
Bajaj JS, O'Leary JG, Lai JC, Wong F, Long MD, Wong RJ and Kamath PS: Acute-on-chronic liver failure clinical guidelines. Am J Gastroenterol. 117:225–252. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Spak DA, Plaxco JS, Santiago L, Dryden MJ and Dogan BE: BI-RADS® fifth edition: A summary of changes. Diagn Interv Imaging. 98:179–190. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Rehman B, Mumtaz A, Sajjad B, Urooj N, Khan SM, Zahid MT, Mannan H, Chaudhary MZ, Khan A and Parvaiz MA: Papillary carcinoma of breast: Clinicopathological characteristics, management, and survival. Int J Breast Cancer. 2022:54278372022. View Article : Google Scholar : PubMed/NCBI | |
|
Kulka J, Madaras L, Floris G and Lax SF: Papillary lesions of the breast. Virchows Arch. 480:65–84. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Morgan S, Dodington D, Wu JM and Turashvili G: Solid papillary carcinoma and encapsulated papillary carcinoma of the breast: Clinical-pathologic features and basement membrane studies of 50 cases. Pathobiology. 88:359–373. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Bai J and Wang G: Encapsulated papillary carcinoma of the breast. Radiology. 308:e2310382023. View Article : Google Scholar : PubMed/NCBI | |
|
Hartrampf CR, Scheflan M and Black PW: Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 69:216–225. 1982. View Article : Google Scholar : PubMed/NCBI | |
|
Ter Louw RP and Nahabedian MY: Prepectoral breast reconstruction. Plast Reconstr Surg. 140:51S–59S. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Rakha EA, El-Sayed ME, Lee AHS, Elston CW, Grainge MJ, Hodi Z, Blamey RW and Ellis IO: Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 26:3153–3158. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Gnant M, Fitzal F, Rinnerthaler G, Steger GG, Greil-Ressler S, Balic M, Heck D, Jakesz R, Thaler J, Egle D, et al: Duration of adjuvant aromatase-inhibitor therapy in postmenopausal breast cancer. N Engl J Med. 385:395–405. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wolff AC, Somerfield MR, Dowsett M, Hammond MEH, Hayes DF, McShane LM, Saphner TJ, Spears PA and Allison KH: Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-college of american pathologists guideline update. J Clin Oncol. 41:3867–3872. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Elghobashy M, Jenkins S, Shulman Z, O'Neil A, Kouneli S and Shaaban AM: Tall cell carcinoma with reversed polarity: Case report of a rare special type of breast cancer and review of the literature. Biomedicines. 11:23762023. View Article : Google Scholar : PubMed/NCBI | |
|
Rakha EA and Ellis IO: Diagnostic challenges in papillary lesions of the breast. Pathology. 50:100–110. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Eiada R, Chong J, Kulkarni S, Goldberg F and Muradali D: Papillary lesions of the breast: MRI, ultrasound, and mammographic appearances. AJR Am J Roentgenol. 198:264–271. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Dugandzija T, Sekerija M, Hinic N, Rajcevic S and Kusturica MP: Trend analyses of breast cancer incidence and mortality in Vojvodina. J BUON. 25:655–661. 2020.PubMed/NCBI | |
|
Anderson WF, Chu KC, Chang S and Sherman ME: Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 13:1128–1135. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Liu ZY, Liu N, Wang YH, Yang CC, Zhang J, Lv SH and Niu Y: Clinicopathologic characteristics and molecular subtypes of invasive papillary carcinoma of the breast: A large case study. J Cancer Res Clin Oncol. 139:77–84. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Huang K, Appiah L, Mishra A, Bagaria SP, Gabriel ME and Misra S: Clinicopathologic characteristics and prognosis of invasive papillary carcinoma of the breast. J Surg Res. 261:105–112. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen S, Wang J, Yang L, Ji M and Chen S: Comparative analysis of clinicopathologic characteristics and molecular subtypes of invasive papillary carcinoma of the breast and invasive ductal carcinoma: Results from SEER database. J BUON. 26:1991–2002. 2021.PubMed/NCBI | |
|
Catanzariti F, Avendano D, Cicero G, Garza-Montemayor M, Sofia C, Venanzi Rullo E, Ascenti G, Pinker-Domenig K and Marino MA: High-risk lesions of the breast: Concurrent diagnostic tools and management recommendations. Insights Imaging. 12:632021. View Article : Google Scholar : PubMed/NCBI | |
|
Mitnick JS, Vazquez MF, Harris MN, Schechter S and Roses DF: Invasive papillary carcinoma of the breast: Mammographic appearance. Radiology. 177:803–806. 1990. View Article : Google Scholar : PubMed/NCBI | |
|
Vdovenko AA: Pathology of breast papillary neoplasms: Community hospital experience. Ann Diagn Pathol. 49:1516052020. View Article : Google Scholar : PubMed/NCBI | |
|
Ghannam SF, Rutland CS, Allegrucci C, Mongan NP and Rakha E: Encapsulated papillary carcinoma of the breast: Does it have a native basement membrane? Histopathology. 83:376–393. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Rakha EA, Gandhi N, Climent F, van Deurzen CHM, Haider SA, Dunk L, Lee AHS, Macmillan D and Ellis IO: Encapsulated papillary carcinoma of the breast: An invasive tumor with excellent prognosis. Am J Surg Pathol. 35:1093–1103. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao H and Lou S: Clinicopathological characteristics and treatment of solid papillary carcinoma of the breast. Asian J Surg. 46:3389–3390. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Otsuki Y, Suwa K, Ohtsuka S, Mori N, Yoshida M, Serizawa A, Shimizu SI and Kobayashi H: A large-scale clinicopathological and long-term follow-up study of solid papillary carcinoma of the breast. Virchows Arch. 482:687–695. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Saremian J and Rosa M: Solid papillary carcinoma of the breast: A pathologically and clinically distinct breast tumor. Arch Pathol Lab Med. 136:1308–1311. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Huang B, Wu K and Fu S: Infiltrative solid papillary carcinoma of the breast with axillary lymph node metastasis: A case report. BMC Womens Health. 23:4532023. View Article : Google Scholar : PubMed/NCBI | |
|
Duprez R, Wilkerson PM, Lacroix-Triki M, Lambros MB, MacKay A, A'Hern R, Gauthier A, Pawar V, Colombo PE, Daley F, et al: Immunophenotypic and genomic characterization of papillary carcinomas of the breast. J Pathol. 226:427–441. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Fisher ER, Palekar AS, Redmond C, Barton B and Fisher B: Pathologic findings from the national surgical adjuvant breast project (protocol no. 4). VI. Invasive papillary cancer. Am J Clin Pathol. 73:313–322. 1980. View Article : Google Scholar : PubMed/NCBI | |
|
Coca-Pelaz A, Shah JP, Hernandez-Prera JC, Ghossein RA, Rodrigo JP, Hartl DM, Olsen KD, Shaha AR, Zafereo M, Suarez C, et al: Papillary thyroid cancer-aggressive variants and impact on management: A narrative review. Adv Ther. 37:3112–3128. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kim HJ, Kim JK and Cho KS: CT features of serous surface papillary carcinoma of the ovary. AJR Am J Roentgenol. 183:1721–1724. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Silver SA and Askin FB: True papillary carcinoma of the lung: A distinct clinicopathologic entity. Am J Surg Pathol. 21:43–51. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Ye F, Yu P, Li N, Yang A, Xie X, Tang H and Liu P: Prognosis of invasive micropapillary carcinoma compared with invasive ductal carcinoma in breast: A meta-analysis of PSM studies. Breast. 51:11–20. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yang YL, Liu BB, Zhang X and Fu L: Invasive micropapillary carcinoma of the breast: An update. Arch Pathol Lab Med. 140:799–805. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Liu C, Zhou J, Chang C and Zhi W: Feasibility of shear wave elastography imaging for evaluating the biological behavior of breast cancer. Front Oncol. 11:8201022021. View Article : Google Scholar : PubMed/NCBI | |
|
Peck DR: Biologic behavior of breast cancers. JAMA. 241:27841979. View Article : Google Scholar : PubMed/NCBI | |
|
Suh YJ, Shin H and Kwon TJ: Natural history of invasive papillary breast carcinoma followed for 10 years: A case report and literature review. Case Rep Med. 2017:37253912017. View Article : Google Scholar : PubMed/NCBI | |
|
Rakha EA, Ahmed MA and Ellis IO: Papillary carcinoma of the breast: diagnostic agreement and management implications. Histopathology. 69:862–870. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Hashmi AA, Munawar S, Rehman N, Ahmed O, Islam S, Asghar IA, Afzal A, Irfan M, Shamail F and Ali SJ: Invasive papillary carcinoma of the breast: Clinicopathological features and hormone receptor profile. Cureus. 13:e134802021.PubMed/NCBI | |
|
Terzi A and Uner AH: An unusual case of invasive papillary carcinoma of the breast. Indian J Pathol Microbiol. 55:543–545. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Piscuoglio S, Ng CKY, Martelotto LG, Eberle CA, Cowell CF, Natrajan R, Bidard FC, De Mattos-Arruda L, Wilkerson PM, Mariani O, et al: Integrative genomic and transcriptomic characterization of papillary carcinomas of the breast. Mol Oncol. 8:1588–1602. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Di Cosimo S and Baselga J: Phosphoinositide 3-kinase mutations in breast cancer: A ‘good’ activating mutation? Clin Cancer Res. 15:5017–5019. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Natrajan R, Lambros MB, Rodríguez-Pinilla SM, Moreno-Bueno G, Tan DSP, Marchió C, Vatcheva R, Rayter S, Mahler-Araujo B, Fulford LG, et al: Tiling path genomic profiling of grade 3 invasive ductal breast cancers. Clin Cancer Res. 15:2711–2722. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Jenkins S, Kachur ME, Rechache K, Wells JM and Lipkowitz S: Rare breast cancer subtypes. Curr Oncol Rep. 23:542021. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Huang R, Ma L, Liu S and Zong X: Locoregional surgical treatment improves the prognosis in primary metastatic breast cancer patients with a single distant metastasis except for brain metastasis. Breast. 45:104–112. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lee CM, Zheng H, Tan VKM, Tan TJY, Kanesvaran R, Wong FY, Sim YR, Yong WS, Madhukumar P, Ong KW and Tan BKT: Surgery for early breast cancer in the extremely elderly leads to improved outcomes-an Asian population study. Breast. 36:44–48. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Arora R, Gupta R, Sharma A and Dinda AK: Invasive papillary carcinoma of male breast. Indian J Pathol Microbiol. 53:135–137. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Hong R and Xu B: Breast cancer: An up-to-date review and future perspectives. Cancer Commun (Lond). 42:913–936. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Waks AG and Winer EP: Breast cancer treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Fakhreddine MH, Haque W, Ahmed A, Schwartz MR, Farach AM, Paulino AC, Bonefas E, Miltenburg D, Niravath P, Butler EB and Teh BS: Prognostic factors, treatment, and outcomes in early stage, invasive papillary breast cancer: A SEER investigation of less aggressive treatment in a favorable histology. Am J Clin Oncol. 41:532–537. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Lewis L, Thompson B, Stellmaker R and Koelmeyer L: Body composition and chemotherapy toxicities in breast cancer: A systematic review of the literature. J Cancer Surviv. Jan 11–2024.(Epub ahead of print). View Article : Google Scholar | |
|
Licaj I, Coquan E, Dabakuyo-Yonli TS, Dauchy S, Vaz Luis I, Charles C, Lemogne C, Tredan O, Vanlemmens L, Jouannaud C, et al: Baseline quality of life and chemotherapy toxicities in patients with early breast cancer. Cancer. 129:1085–1095. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Battisti NML, Joshi K, Nasser MS and Ring A: Systemic therapy for older patients with early breast cancer. Cancer Treat Rev. 100:1022922021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Liu Y, Liu X, Liu Y and Zhang J: Prognoses of patients with hormone receptor-positive and human epidermal growth factor receptor 2-negative breast cancer receiving neoadjuvant chemotherapy before surgery: A retrospective analysis. Cancers (Basel). 15:11572023. View Article : Google Scholar : PubMed/NCBI | |
|
Coates AS, Colleoni M and Goldhirsch A: Is adjuvant chemotherapy useful for women with luminal a breast cancer? J Clin Oncol. 30:1260–1263. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Goel S, Bergholz JS and Zhao JJ: Targeting CDK4 and CDK6 in cancer. Nat Rev Cancer. 22:356–372. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Saita C, Goto R, Aruga T, Idera N, Honda Y, Horiguchi K, Miyamoto H, Horiguchi S, Yamashita T and Kuroi K: Invasive papillary carcinoma treated with neoadjuvant endocrine therapy in which pathological complete response was achieved. BMC Res Notes. 9:462016. View Article : Google Scholar : PubMed/NCBI | |
|
De Placido S, Gallo C, De Laurentiis M, Bisagni G, Arpino G, Sarobba MG, Riccardi F, Russo A, Del Mastro L, Cogoni AA, et al: Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): A randomised, phase 3 trial. Lancet Oncol. 19:474–485. 2018. View Article : Google Scholar : PubMed/NCBI |