Introduction
Depression is a state of low mood and the avoidance
of activity that affects a sense of well-being, thoughts, behavior
and feelings (1). Depression can
result in numerous feelings, including anxiety, sadness, worry,
helplessness and worthlessness. Patients with depression may lose
interest in activities that were previously enjoyable, have a
reduction of appetite or may begin overeating, experience problems
concentrating, remembering details or making decisions, and may
contemplate, attempt or commit suicide. Other symptoms include
insomnia, fatigue, excessive sleeping, loss of energy, or aches,
pains or digestive problems (2).
Numerous studies have indicated that adiponectin is
associated with depression (3).
Adiponectin is secreted into the bloodstream from adipose tissue
only and compared to a number of hormones, it is extremely abundant
in the plasma (4). Adiponectin has
also been shown to regulate glucose levels and fatty acid breakdown
(5). However, there has been no
meta-analysis to clarify the precise associations between
adiponectin levels and depression. The present study investigated
whether adiponectin is associated with depression by performing a
meta-analysis of the available data from previous studies.
Materials and methods
Selection of studies
Information was carefully extracted from all the
eligible studies by two investigators (Yaozhi Hu and Xiaomeng Dong)
independently, using a standardized data extraction form. Any
disagreements were resolved by discussion during a consensus
meeting with a third investigator (Jinbo Chen). A search through
the electronic databases, including PubMed, Cochrane Central
Register of Controlled Trials and Embase, and the following Chinese
databases: The China National Knowledge Infrastructure, China
Biology Medicine disc, VIP Database for Chinese Technical
Periodicals and Wan Fang Data, were conducted for studies using the
key words ‘adiponectin’ and ‘depression’ for the relevant
citations. The reference lists of retrieved reviews and studies
were manually screened. The publication language was restricted to
English or Chinese. The literature search was updated on June 20,
2014.
Selection criteria
The selection criteria used to determine the
eligible studies included: i) Study design should be a randomized
controlled trial; ii) depression was diagnosed by the Diagnostic
and Statistical Manual of Mental Disorders IV (DSM-IV) (6), Center for Epidemiologic Studies
Depression Scale or Mini-International Neuropsychiatric Interview;
iii) the control group must be healthy subjects whose age and
gender matched the patients with depression; and iv) overnight
fasting antecubital vein blood samples were collected from each
consenting subject.
Data extraction
The following information was extracted from the
eligible studies: i) Name of the first author, ii) year of
publication, iii) region, iv) sample size of the subjects with and
without depression, v) diagnostic instrument, vi) age, vii) the
type of blood sample and viii) the technique that was used for
detecting adiponectin levels.
Statistical analysis
In order to estimate the association between the
adiponectin levels and depression, the standardized mean difference
(SMD) and 95% confidence interval (CI) from each study were
calculated using Cochrane Collaboration's Review Manager 5.2
software (The Nordic Cochrane Centre, Copenhagen, Denmark). The
heterogeneity of SMDs was assessed by using the Cochran's Q test
and I2. When heterogeneity was present, SMDs were pooled
using the random-effects model (the DerSimonian and Laird method).
Otherwise, the fixed-effects model (the Mantel-Haenszel method) was
used. The statistical significance of the pooled SMDs was analyzed
using the Z test. For the studies with insufficient information,
the investigators contacted the primary researchers to acquire and
verify data whenever possible.
Results
Characteristics of the studies
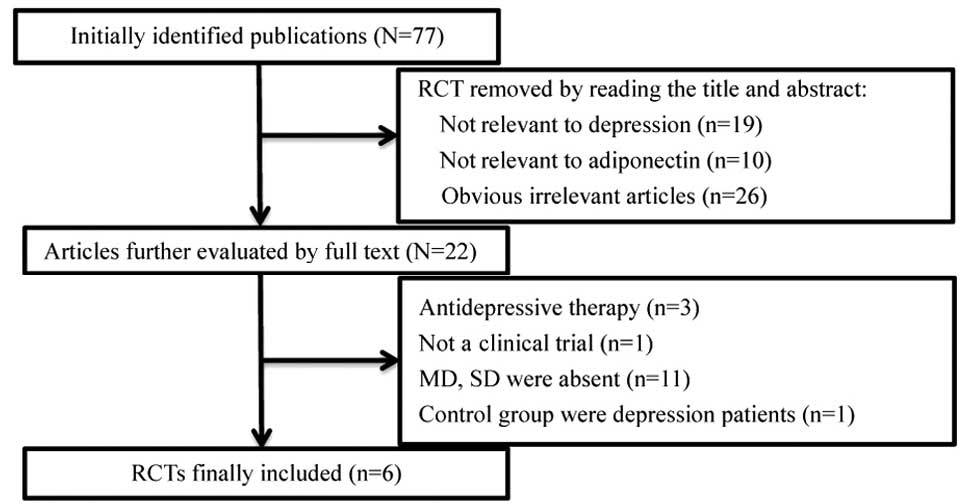
There were 77 studies relevant to the search words.
Through screening the title and reading the entire study, six
eligible studies were selected for additional analysis. The flow
diagram shows the detailed process of selection (Fig. 1). The total data of the six studies
(7–12) were gathered from 506 patients with
depression and 3,714 controls worldwide. Of the six studies, DSM-IV
was used for diagnosing depression in four studies (Table I).
 | Table ISummary of the clinical studies
regarding adiponectin levels and patients with depression. |
Table I
Summary of the clinical studies
regarding adiponectin levels and patients with depression.
| Study (year) | Area | Age, years | Subjects, n | Diagnostic
instrument | Blood sample | (Refs.) |
|---|
| Pan et al
(2008) | China | A: 58.26±6.17 | A: 312 | CES-D | Plasma | (12) |
| B: 58.64±5.99 | B: 2977 | Luminex kit | | |
| Diniz et al
(2012) | Brazil | A: 70.2±4.7 | A: 47 | DSM-IV | Serum | (10) |
| B: 68.7±5.6 | B: 51 | ELISA kit | | |
| Barbosa et al
(2012) | Brazil | A: 49.03±10.87 | A: 30 | M.I.N.I.-Plus | Plasma | (7) |
| B: 47.13±7.36 | B: 30 | ELISA kit | | |
| Lehto et al
(2010) | Finland | A: 54.33±9.32 | A: 70 | DSM-IV | Serum | (11) |
| B: 54.20±9.20 | B: 70 | Linkoplex kit | | |
| Leo et al
(2006) | Italy | A: 34.85±5.88 | A: 32 | DSM-IV | Plasma | (8) |
| B: 35.11±5.22 | B: 32 | ELISA kit | | |
| Hung et al
(2007) | Taiwan | A: 23.8±0.7 | A: 15 | DSM-IV | Serum | (9) |
| B: 23.8±0.6 | B: 14 | RIA | | |
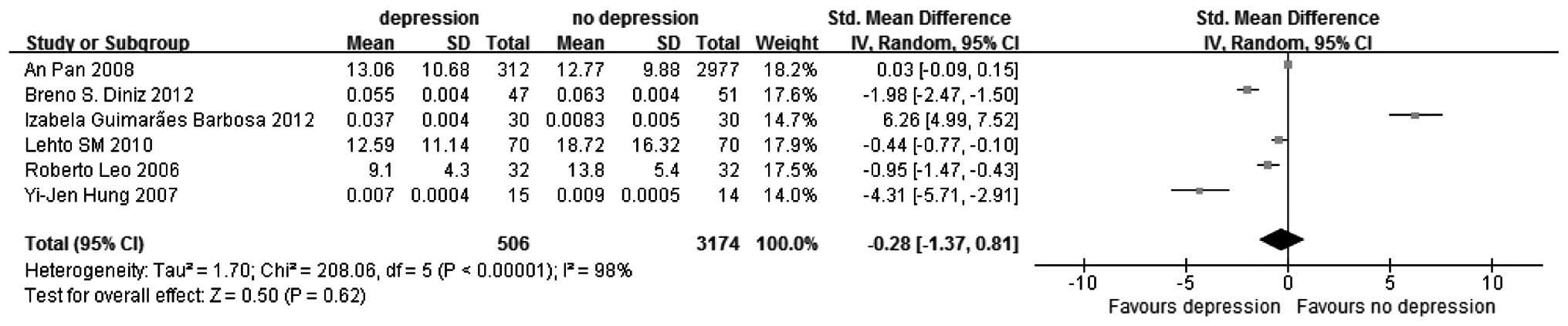
Results of the meta-analysis. In the present
continuous variable meta-analysis, tests for heterogeneity were
first performed (I2=98%, P<0.00001) and subsequently
the random-effect model (SMD, −0.28; 95% CI, −1.37 to 0.81), but
not the fixed-effect model (Fig.
2), and subgroup analysis were carried out according to their
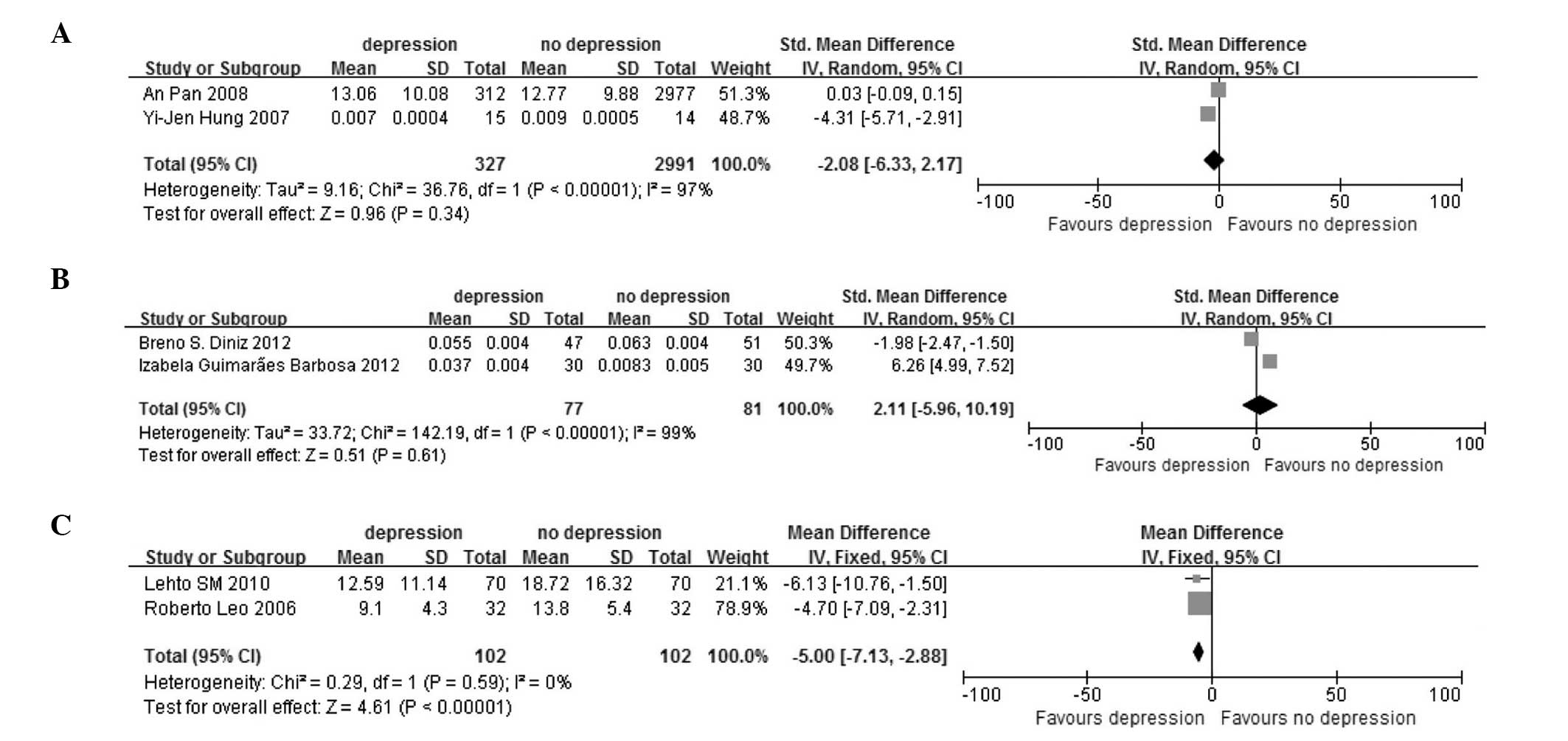
region, age, blood sample and detection method. In the region group
were the Chinese (SMD, −2.08; 95% CI, −6.33 to 2.17; P<0.00001
for heterogeneity, by random-effect model; Fig. 3A), Brazilian (SMD, 2.11; 95% CI,
−5.96 to 10.19; P<0.00001 for heterogeneity, by random-effect
model; Fig. 3B) and European
subgroups (SMD, −5.00; 95% CI, −7.13 to −2.88; P=0.59 for
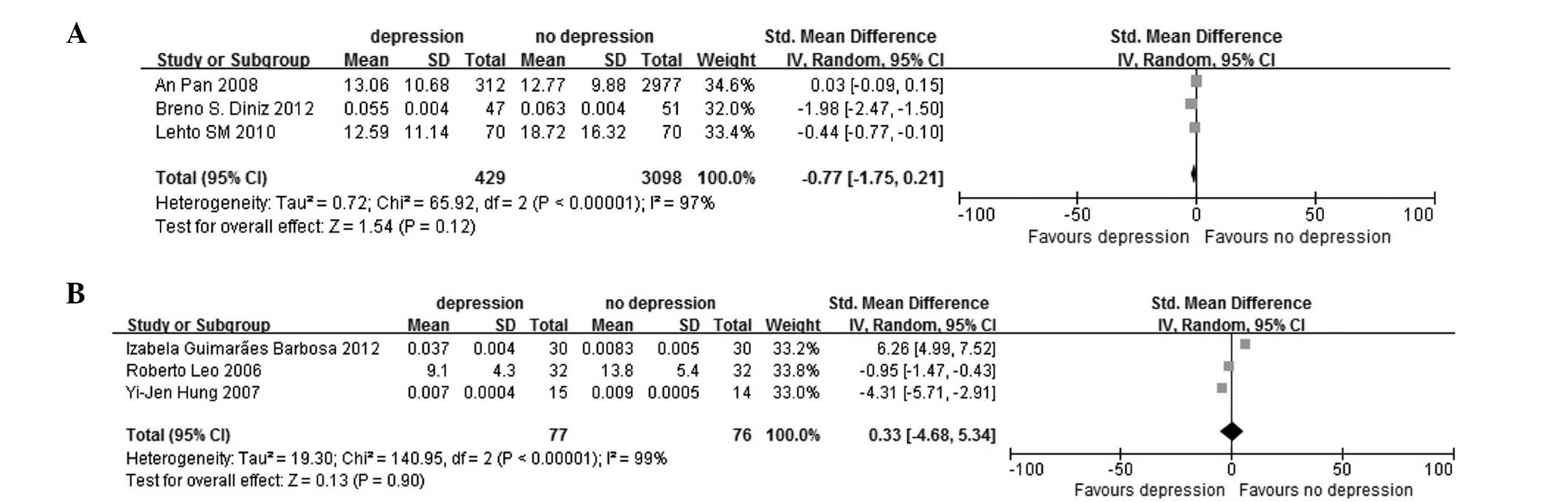
heterogeneity, by fixed-effect model; Fig. 3C). In the age group were the older
(mean age, >50 years) (SMD, −0.77; 95% CI, −1.75 to 0.21;
P<0.00001 for heterogeneity, by random-effect model; Fig. 4A) and younger subgroups (mean age,
<50 years) (SMD, 0.33; 95% CI, −4.68 to 5.34; P<0.00001 for
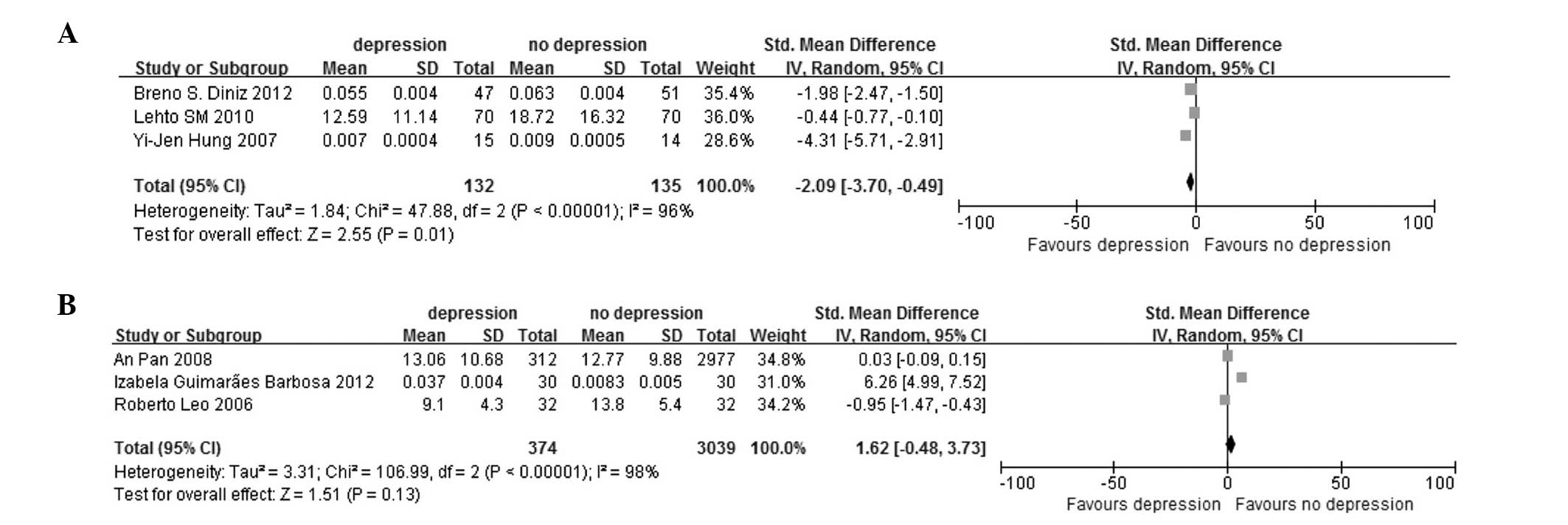
heterogeneity, by random-effect model; Fig. 4B). The serum (SMD, −2.09; 95% CI,
−3.70 to −0.49; P<0.00001 for heterogeneity, by random-effect
model; Fig. 5A) and plasma
subgroups (SMD, 1.62; 95% CI, −0.48 to 3.73; P<0.00001 for
heterogeneity, by random-effect model; Fig. 5B) were present in the blood sample
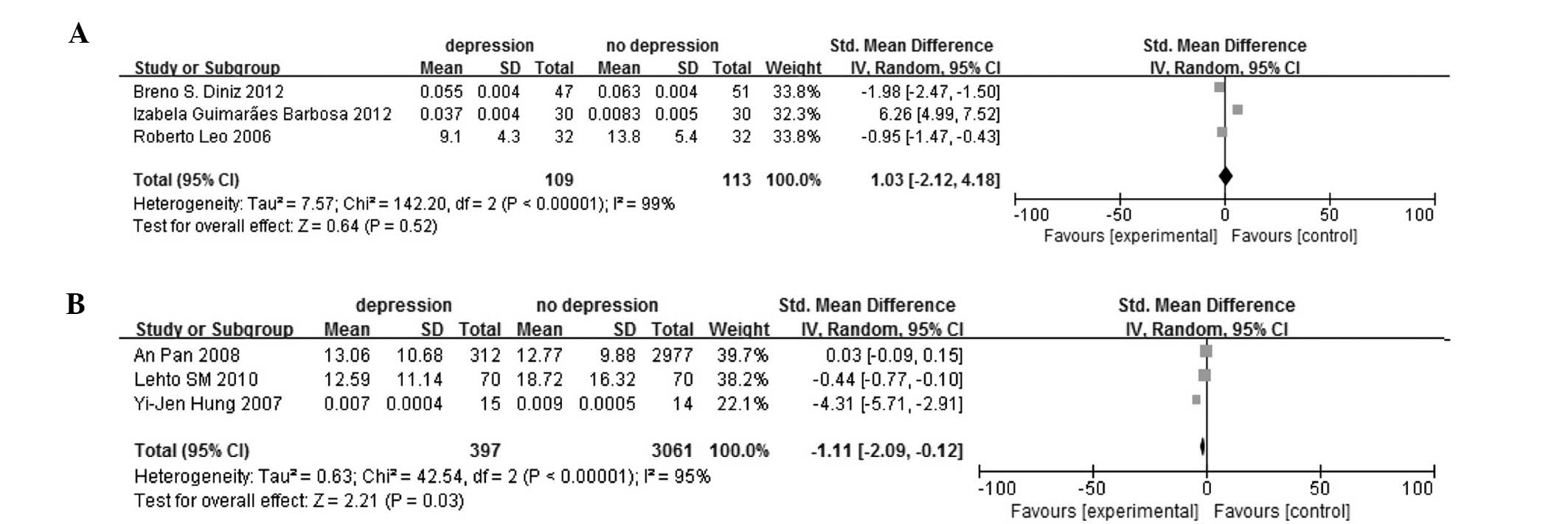
group. In the method group, the ELISA, which was used for detecting
adiponectin levels, (SMD, 1.03; 95% CI, −2.12 to 4.18; P<0.00001
for heterogeneity, by random-effect model; Table II and Fig. 6A) and not ELISA subgroups (SMD,
−1.11; 95% CI, −2.09 to −0.12; P<0.00001 for heterogeneity, by
random-effect model; Fig. 6B) were
assessed.
 | Table IIStratified meta-analysis of the
circulating adiponectin levels and depression. |
Table II
Stratified meta-analysis of the
circulating adiponectin levels and depression.
| Heterogeneity |
|---|
|
|
|---|
| Characteristic | Studies, n | Random-effects SMD
(95% CI) | P-value | I2, % | P-value |
|---|
| All studies | 6 | −0.28 (-1.37 to
0.81) | 0.62 | 91 | <0.0001 |
| Region |
|
China | 2 | −2.08 (-6.33 to
2.17) | 0.34 | 97 | <0.0001 |
|
Brazil | 2 | 2.11 (-5.96 to
10.19) | 0.61 | 99 | <0.0001 |
|
European | 2 | −5.00 (−7.13 to
−2.88) | 0.00 | 0 | 0.590 |
| Age, year (mean) |
|
>50 | 3 | −0.77 (-1.75 to
0.21) | 0.12 | 97 | <0.0001 |
|
<50 | 3 | 0.33 (-4.68 to
5.34) | 0.90 | 99 | <0.0001 |
| Blood sample |
|
Serum | 3 | −2.09 (−3.70 to
−0.49) | 0.01 | 96 | <0.0001 |
|
Plasma | 3 | 1.62 (-0.48 to
3.73) | 0.13 | 98 | <0.0001 |
| Method |
|
ELISA | 3 | 1.03 (-2.12 to
4.18) | 0.52 | 99 | <0.0001 |
| Not
ELISA | 3 | −1.11 (−2.09 to
−0.12) | 0.03 | 95 | <0.0001 |
The investigators did not draw a funnel plot based
on the comparison of the adiponectin levels in plasma of two groups
(depression verses no depression) due to the small sample size.
Discussion
Depression is a common psychiatric disorder with a
10–20% lifetime prevalence rate (13). Depressive disorder is a complex and
multifactorial disorder with biological heterogeneity. Increasing
evidence indicates that the mood disorder is associated with
insulin resistance and inflammation (14,15).
As an anti-inflammatory cytokine, the adiponectin level will
decrease in patients with depression (16). There has been particular
inconsistency, with certain studies in support of this finding and
others not. In the present meta-analysis, data was extracted from
six eligible studies, including a total of 506 patients with
depression and 3,714 controls. Furthermore, all the relevant
studies published in English or Chinese were included for the
meta-analysis, which reduced language biases. The results indicate
that there was no significant association between the adiponectin
level and depression in all the included populations, but patients
with depression had a lower adiponectin level in the European
subgroup when compared to healthy subjects. This analogous
situation did not occur in the Chinese or Brazilian subgroups.
Although subgroup analysis may produce false positive result due to
small sample size, it also demonstrates a tendency in certain ways.
Different region and ethnic lines may cause heterogeneities.
Intracerebroventricular administration of
adiponectin produces an antidepressant-like effect in mice
(3). The same study also described
that a chronic social defeat (depression model) reduces circulating
adiponectin concentrations. Adiponectin stimulated proliferation of
adult hippocampal neural stem cells through activation of p38
mitogen-activated protein kinase/glycogen synthase kinase
3β/β-catenin signaling cascade (17). These studies demonstrated that mice
with depression had a lower adiponectin level and it also described
the mechanism of antidepressant-like activity regarding
adiponectin. These results may consistent with European subgroup in
humans being in our meta-analysis.
In one clinical trial, premenopausal females with
major depression exhibited lower circadian plasma adiponectin
concentrations compared to the closely matched control subjects
(18), and it detected adiponectin
level once an hour, which lead to the exclusion of the study from
the present meta-analysis. High molecular weight adiponectin/total
adiponectin was also negatively associated with the depression
score (19). In addition, numerous
studies had other views. Mamalakis et al found that serum
adiponectin was not associated with depression in adolescents
(20). Jeong et al (21) also described that the plasma
adiponectin concentration was elevated in subsyndromal depression
patients. A significantly positive correlation between plasma
adiponectin levels and the BDI-II cognitive-affective factor
measure was also found in another study (22). Approximately six studies included in
the present study found that adiponectin levels were present as
pg/ml or µg/ml, and these were subsequently calculated into µg/ml
simultaneously. An enormous difference appeared in these values,
which may be associated with age, region and ethnicity. The
heterogeneity of the European subgroup was appropriate and patients
with depression had a lower adiponectin level compared to healthy
controls. A number of animal experiments and a section of clinical
trials were in accord with this conclusion, but a consistent
conclusion could not be reached due to limited clinical data.
However, there were limitations of the
meta-analysis, which included the small number of studies and
subjects in the studies that were in the specific subgroups.
Therefore, larger samples sizes are required in more studies.
In conclusion, the results of the present study
indicate that the association between adiponectin levels and
depression was not clarified in all the different populations, but
the adiponectin levels of patients with depression were lower in
the European subgroup. Due to the limitations mentioned, additional
investigations should be performed to investigate these
associations.
References
|
1
|
Silva MT, Galvao TF, Martins SS and
Pereira MG: Prevalence of depression morbidity among Brazilian
adults: a systematic review and meta-analysis. Rev Bras Psiquiatr.
36:262–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson J, Weissman MM and Klerman GL:
Service utilization and social morbidity associated with depressive
symptoms in the community. JAMA. 267:1478–1483. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu J, Guo M, Zhang D, et al: Adiponectin
is critical in determining susceptibility to depressive behaviors
and has antidepressant-like activity. Proc Natl Acad Sci USA.
109:12248–12253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adam T, Schamarek I, Springer EA, Havel PJ
and Epel EE: Adiponectin and negative mood in healthy premenopausal
and postmenopausal women. Horm Behav. 58:699–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jaziri R, Aubert R, Roussel R, et al
DIABHYCAR SURDIAGENE Study Groups: Association of ADIPOQ genetic
variants and plasma adiponectin isoforms with the risk of incident
renal events in type 2 diabetes. Nephrol Dial Transplant.
25:2231–2237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kessler RC, Birnbaum HG, Shahly V, et al:
Age differences in the prevalence and co-morbidity of DSM-IV major
depressive episodes: results from the WHO World Mental Health
Survey Initiative. Depress Anxiety. 27:351–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barbosa IG, Rocha NP, de Miranda AS, et
al: Increased levels of adipokines in bipolar disorder. J Psychiatr
Res. 46:389–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leo R, Di Lorenzo G, Tesauro M, et al:
Decreased plasma adiponectin concentration in major depression.
Neurosci Lett. 407:211–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hung YJ, Hsieh CH, Chen YJ, et al: Insulin
sensitivity, proinflammatory markers and adiponectin in young males
with different subtypes of depressive disorder. Clin Endocrinol
(Oxf). 67:784–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Diniz BS, Teixeira AL, Campos AC, et al:
Reduced serum levels of adiponectin in elderly patients with major
depression. J Psychiatr Res. 46:1081–1085. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lehto SM, Huotari A, Niskanen L, et al:
Serum adiponectin and resistin levels in major depressive disorder.
Acta Psychiatr Scand. 121:209–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan A, Ye X, Franco OH, et al: The
association of depressive symptoms with inflammatory factors and
adipokines in middle-aged and older Chinese. PLoS One. 3:e13922008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hasin DS, Goodwin RD, Stinson FS and Grant
BF: Epidemiology of major depressive disorder: results from the
National Epidemiologic Survey on Alcoholism and Related Conditions.
Arch Gen Psychiatry. 62:1097–1106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miller AH, Maletic V and Raison CL:
Inflammation and its discontents: the role of cytokines in the
pathophysiology of major depression. Biol Psychiatry. 65:732–741.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steinman L: Nuanced roles of cytokines in
three major human brain disorders. J Clin Invest. 118:3557–3563.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ouchi N, Kihara S, Funahashi T, Matsuzawa
Y and Walsh K: Obesity, adiponectin and vascular inflammatory
disease. Curr Opin Lipidol. 14:561–566. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang D, Guo M, Zhang W and Lu XY:
Adiponectin stimulates proliferation of adult hippocampal neural
stem/progenitor cells through activation of p38 mitogen-activated
protein kinase (p38MAPK)/glycogen synthase kinase 3β
(GSK-3β)/β-catenin signaling cascade. J Biol Chem. 286:44913–44920.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cizza G, Nguyen VT, Eskandari F, et al
POWER Study Group: Low 24-hour adiponectin and high nocturnal
leptin concentrations in a case-control study of community-dwelling
premenopausal women with major depressive disorder: the
Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate,
Depression (POWER) study. J Clin Psychiatry. 71:1079–1087. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Narita K, Murata T, Hamada T, et al:
Adiponectin multimer distribution, not absolute amount of plasma,
correlates with depression severity in healthy elderly subjects.
Prog Neuropsychopharmacol Biol Psychiatry. 32:124–127. 2008.
View Article : Google Scholar
|
|
20
|
Mamalakis G, Kiriakakis M, Tsibinos G, et
al: Depression and serum adiponectin and adipose omega-3 and
omega-6 fatty acids in adolescents. Pharmacol Biochem Behav.
85:474–479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeong HG, Min BJ, Lim S, et al: Plasma
adiponectin elevation in elderly individuals with subsyndromal
depression. Psychoneuroendocrinology. 37:948–955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilhelm CJ, Choi D, Huckans M, Manthe L
and Loftis JM: Adipocytokine signaling is altered in Flinders
sensitive line rats, and adiponectin correlates in humans with some
symptoms of depression. Pharmacol Biochem Behav. 103:643–651. 2013.
View Article : Google Scholar : PubMed/NCBI
|