Introduction
Ovulation generates oxidative stress and is
therefore comparable to an inflammatory reaction. Similar
inflammatory changes initially occur in the theca interna and
granulosa layers of follicles in response to chorionic gonadotropin
stimulation during the luteinization process. Different cytokines,
kinnins, prostaglandins, proteolytic enzymes, nitric oxide and
various steroids are produced during the final hours prior to
follicle rupture (1). These events
are believed to have effects on the blood flow in the ovaries
during the periovulatory period.
The midcycle gonadotropin surge is a major factor in
the changes of ovulation. Rapidly increasing levels of luteinizing
hormone (LH) generate numerous critical changes in oocytes and
follicular cells, which further modify the steroid and protein
micro- and macroenvironment. These physiological changes have a
significant role in oocyte normal maturation, the ovulation process
and the subsequent fertilization and implantation (2). Due to the inconsistency of the
spontaneous LH surges during controlled ovarian stimulation in any
of its forms, the increasing use of gonadotropin-releasing
hormone-agonist (GnRH-a) is used in all the successful ovarian
stimulation programs to affect the eventual triggering of oocyte
maturation and ovulation (3).
Due to the specific degree of homology between human
chorionic gonadotropin (hCG) and LH, hCG has been used as a
surrogate for the LH surge. These two hormones are complex
heterodimeric glycoproteins with molecular weights of ~30 and 40
kDa for recombinant human LH and hCG, respectively, and also have
identical α-subunits and a high cystine content. Of note, these two
hormones have the same natural function, which is to cause
luteinization and support lutein cells (4). Norjavaara et al (5) determined the redistribution of ovarian
blood flow following the injection of hCG in the adult
pseudopregnant rat and found that there was no hCG-induced change
in the luteal blood flow on days 2, 6 and 11 of pseudopregnancy.
However, in the remaining ovary the blood flow increased
>2-fold, resulting in redistribution of the blood flow.
Previously, pulsed wave Doppler has also been used
to estimate the association between the intraovarian artery blood
flow and the growth of ovarian follicles. Nargund et al
(6) investigated the association
between the ultrasound-derived indices of blood flow in individual
follicles at baseline on the day of the administration of hCG and
the following recoveries of oocytes or the production of
preimplantation embryos. There was a significant correlation
between the detection of follicular blood flow and the recovery of
an oocyte.
The quality of a follicle and an oocyte has been
estimated in terms of the incidence of apoptosis in granulosa cells
in the patients involved in in vitro fertilization and
embryo transfer (IVF-ET) treatment. Aging and endometriosis have
been known to affect the quality of follicular growth and oocytes,
which is thought to be due to an increased incidence of apoptosis
in granulosa cells (7).
In our previous study (8), which assessed 10,000 IU hCG
administration, the intraovarian artery blood flow was increased in
association with the decreased mature oocyte rate, and the
increased incidence of apoptotic granulosa cells on the day of
follicle aspiration indicated that the oocyte had overmatured.
Therefore, the present study was designed in order to compare the
same patients undergoing two protocols; the first protocol with
10,000 IU and the second with 5,000 IU hCG administration, to
investigate whether there were any significant differences between
the intraovarian artery blood flow and the development of follicles
and oocytes in an IVF-ET program between the two protocols.
Materials and methods
Patients and follicle-stimulation
protocol
The study was approved by the Harbin Medical
University Hospital Committee for Research on Human Subjects
(Heilongjiang, China). Written informed consent was obtained from
all the patients and their clinical information was concealed.
The patients in the IVF group (n=17) had the
following causes of infertility: Tubal damage (n=6), male factor
(n=6) and unknown (n=5). ‘Unknown’ means that no cause of
infertility could be determined by basal body temperature or serum
hormones [LH, follicle-stimulating hormone, prolactin, estradiol
(E2), progesterone (P), testosterone (T)] measurements,
hysterosalpingography, ultrasonography, laparoscopy or semen
analysis.
For all the patients administered a GnRH-a, and also
buserelin acetate (Suprecur nasal®; Sanofi-Aventis,
Tokyo, Japan), human menopausal gonadotropins (hMG; Humegon;
Kanebo, Tokyo, Japan) and hCG (Profasi; Serono, Geneva,
Switzerland) were also administered. The administration of GnRH-a
(600 mg/day) started during the midluteal phase. The administration
of hMG started on day 3 of the menstrual cycle. hCG injection was
administered when the dominant follicle had a mean diameter ≥16 mm
with 10,000 IU in the first protocol with 10,000 IU and 5,000 IU
hCG administration in the second protocol. As two patients were
diagnosed as pregnant in the first protocol, they were excluded
from the second protocol.
Measurement of ovarian artery blood
flow and follicle aspiration
On the hCG administration day and the follicle
aspiration day, both sides of intraovarian artery flow for each
patient were measured by transvaginal color ultrasonographic pulsed
wave Doppler. The values of pulsatility indices (PI) were printed
on a wave-form image. The PI of each patient was defined as the
mean value of the two measured sides.
Oocyte retrieval was performed 35 h after the hCG
administration by follicle aspiration under transvaginal
ultrasonographic guidance. All the follicles with a mean diameter
>12 mm were aspirated, using an 18-gauge needle connected to a
connecting tube and 20-ml syringe for suction.
Granulosa cell treatment
The oocyte-cumulus complex was removed from the
follicular fluid, washed twice in culture medium (human tubal
fluid) and placed in fresh culture medium. The cumulus granulosa
cells (ApoC) were mechanically separated from the oocyte using
26-gauge needles under the microscope and placed on glass slides.
The mural cell mass was collected from the follicular fluid and
washed twice in the culture medium. The ApoC and mural granulosa
cells (ApoM) were dispersed separately by hyaluronidase (0.1% w/v)
and fixed with 4% neutral formalin on glass slides. The granulosa
cells were fixed <15 min after the oocyte recovery. Subsequent
to drying the glass slides, they were rinsed twice by
phosphate-buffered saline. The fixed granulosa cells were stained
with Hoechst 33258 fluorescent dye solution (0.5 mg/ml in distilled
water; Wako, Osaka, Japan) that contained
1,4-diazabicyclo-2,2,2-octane (Sigma, St. Louis, MO, USA) to
prevent fading, and were then mounted with a coverglass.
A total of 1,000 granulosa cells were observed under
a fluorescent microscope and the number of apoptotic cells was
counted. Apoptotic cells were defined as those with fragmented and
uniformly dense nuclei or fragmented cytoplasm containing a
uniformly dense fragmented nucleus.
Follicular fluids collection and
steroids assay
Each follicular fluid sample was centrifuged at 300
x g for 10 min and the cleared fluid was stored at −20˚C until the
assay for E2, P and T. The quantification of the
hormonal concentration was performed using commercially available
immunoassays: E2 by time-resolved fluoroimmunoassay
(DELFIA Estradiol; Pharmacia, Tokyo, Japan), P by radioimmunoassay
(RIA) (DPC progesterone; DPC, Inc., Tokyo, Japan) and free T by RIA
(DPC progesterone kit and DPC free testosterone kit; DPC, Inc.).
Reliability criterions for all the assays were established. The
hormones were assayed in duplicate within the same assay (8).
Assessment of oocyte Oocyte
quality
Oocyte quality was scored by the incidence of
apoptotic granulosa cells with condensed and fragmented nuclei.
Oocyte maturity
Oocyte maturity was judged by the condition of the
corona radiata and the ApoC, as described previously.
Oocyte-corona-cumulus complexes with a large and loose cumulus and
distinct corona radiata were defined as mature, and complexes with
a small and dense cumulus and opaque corona radiata were defined as
immature (9).
Statistical analysis
The significance of the differences in the data was
analyzed by the Student's t-test (two group t-test, paired) and
Wilcoxon signed-ranks test in computer-assisted statistical
analysis. Statistical power was determined by analysis based on
sample size and was sufficient to detect the mean differences of
approximately one standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
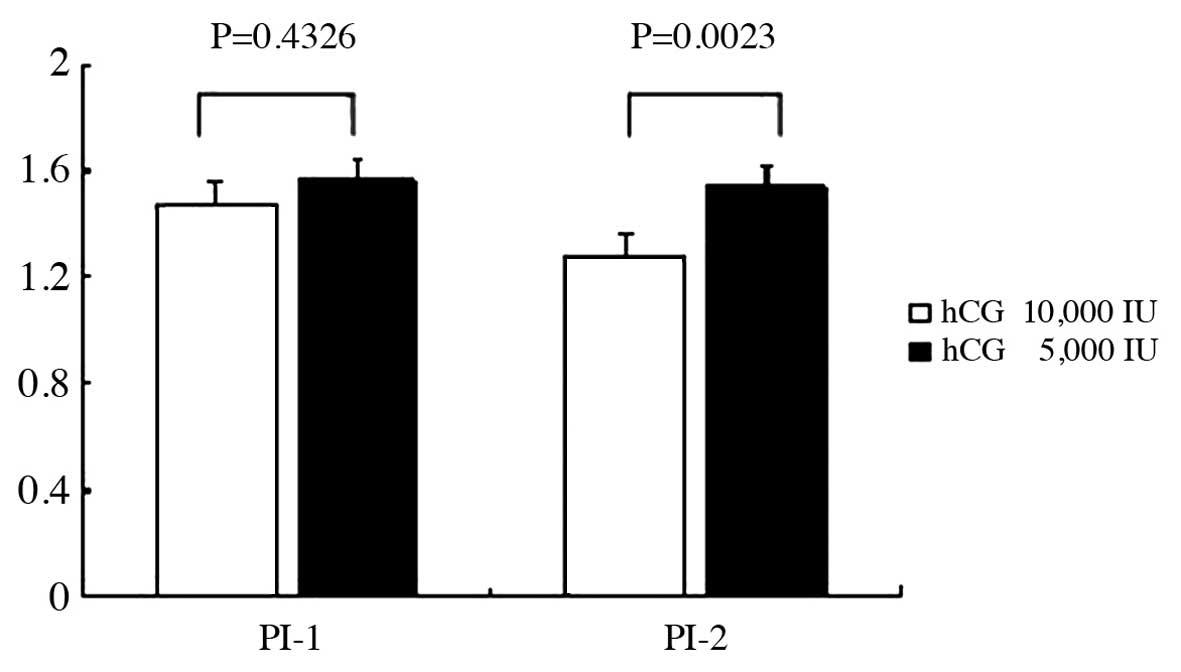
Intraovarian artery blood flow
The difference of intraovarian artery blood flow
between the first protocol with 10,000 IU and the second protocol
with 5,000 IU hCG administration is shown in Fig. 1. There were no statistically
significant differences between the two protocols prior to hCG
administration (P=0.4326). However, there were statistically
significant differences between the two protocols for 10,000 and
5,000 IU hCG administration in the follocle aspiration day
(P=0.0023), which indicated that the nitraovarian artery blood flow
significantly decreased due to 5,000 compared to 10,000 IU hCG
administration.
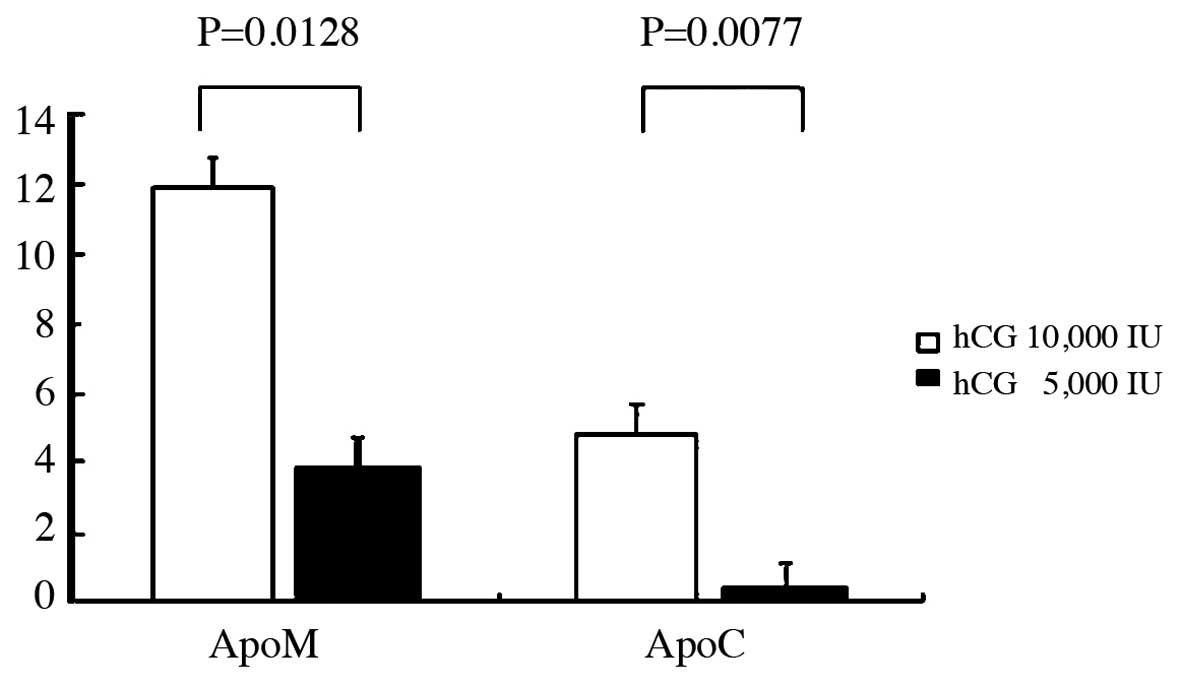
Incidence of apoptosis
The incidence difference of apoptotic granulosa cell
between the two protocols is shown in Fig. 2. There were statistically
significant differences between the two protocols for 10,000 and
5,000 IU hCG administration in the incidence of ApoC (P=0.0077) and
ApoM (P=0.0128). This indicated that there was an increased
incidence of ApoC and ApoM in 10,000 compared to in 5,000 IU
administration.
Differences in hCG administration
The differences between the two protocols in the
follicle fluid progesterone and estradiol concentration are shown
in Table I. There was a
statistically significant difference between the two protocols for
10,000 and 5,000 IU hCG administration in the follicle fluid
progesterone concentration (P=0.0044), as 10,000 IU significantly
increased the follicle fluid progesterone concentration compared to
5,000 IU administration. There was no statistically significant
difference between the two protocols in the follicle fluid
estradiol concentration (P=0.8939). There were also no differences
between the two protocols in serum progesterone and estradiol
concentration (P, P>0.9999; E2, P=0.8589).
 | Table I.Difference between the two protocols
for the concentration of steroid hormone. |
Table I.
Difference between the two protocols
for the concentration of steroid hormone.
| hCG | |
|---|
|
| |
|---|
| Steroid hormone | 10,000 IU | 5,000 IU | P-value |
|---|
| Follicular
estradiol | 460.6±152.2 | 529.6±454.4 |
0.8393 |
| Follicular progesterone | 5.9±1.9 | 2.3±3.3 |
0.0044a |
| Serum estradiol
aspiration day | 865.0±573.9 | 815.6±535.2 |
0.8589 |
| Serum progesterone aspiration
day | 5.0±3.9 | 4.8±2.5 |
0.9999 |
| Serum estradiol hCG applied
day | 1175.2±951.0 | 839.3±516.8 |
0.3078 |
| Serum progesterone hCG applied
day | 0.7±0.4 | 1.6±1.2 |
0.0546 |
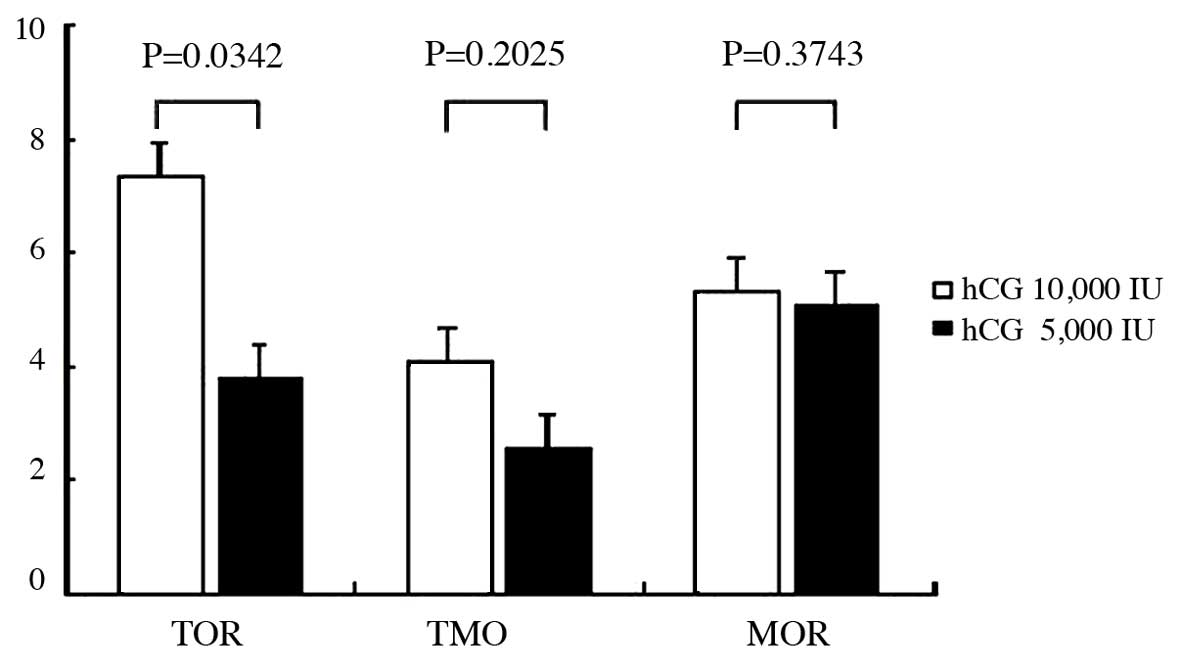
The differences between the two protocols in total
oocytes retrieved, total mature oocytes retrieved and mature oocyte
rate are shown in Fig. 3. There
were statistically significant differences between the two
protocols in total oocytes retrieved (P=0.0342), as 10,000 IU
significantly increased the total oocytes retrieved compared to
5,000 IU administration. There were no statistically significant
differences between the two protocols in total mature oocytes
retrieved (P=0.2026) and mature oocyte rate (P=0.3743).
Discussion
Currently in the IVF-ET program, hCG is used in all
successful ovarian stimulation programs to affect the eventual
triggering of oocyte maturation and ovulation and it has been used
as a surrogate LH surge due to the degree of homology between the
two hormones. hCG has a slower plasma metabolic clearance,
consisting of a rapid phase in the initial 5–9 h and a slower phase
in the 1–1.3 days after hCG administration. After 36 h, the
calculated half-life of hCG was 2–3.2 days (10).
The study by Bjercke et al (11) determined whether there was any
difference in the outcome of IVF when retrieval of oocytes was
performed 34 (group A) and 38 h (group B) after hCG injection in a
total 170 patients with tubal failure. The study found no
significant difference for any of the parameters tested for in
groups A and B. Nargund et al (12) investigated whether the hCG-oocyte
collection interval had an influence on the oocyte recovery rate,
fertilization rate and outcome of IVF-ET cycles with 533
consecutive patients undergoing their first IVF-ET cycle. There was
no significant difference found among the hCG-oocyte collection
intervals examined (33–41 h). None of the females studied had
ovulated prior to oocyte collection.
Thus, we hypothesized that the oocyte collection
intervals within 33–41 h cannot be significantly affected after the
same dose of hCG administration. Furthermore, in the present study,
follicle aspiration was attempted at 36 h after hCG administration.
Therefore, the effect of oocyte collection intervals following hCG
administration can be accepted.
In the study, the parameters between the two
protocols with the same group of patients following 10,000 and
5,000 IU hCG administration were analyzed by Student's t-test (two
group t-test, paired) and Wilcoxon signed-ranks test in
computer-assisted statistical analysis. There were statistically
significant differences between the two protocols in the
intraovarian artery blood flow, the incidence of ApoC and ApoM,
follicle fluid progesterone concentration and total oocytes
retrieved.
There were numerous types of regional hormones,
enzymes and cytokines that are correlated with a similar
inflammatory reaction of the intravessels in the ovulation process.
Certain substances can promote the increase of dilated vessels and
extravasation of resin from weakened vessels, promote the
production of progesterone following a hCG surge, promote the
oocyte maturation, regulate the activity of collagenase and
prolylhydroxylase for follicle rupture and prevent the premature
degeneration of intrafollicular oocytes (1, 13).
Expecting the effect of hCG-oocyte retrieval intervals following
hCG administration, it can be concluded that the differences
between the two protocols with 10,000 and 5,000 IU hCG
administration was due to the dose of hCG administration and not
intervals. The 5,000 IU hCG administration protocol significantly
increased PI and decreased follicle fluid P and total oocytes
retrieved compared to the 10,000 IU hCG administration protocol,
due to the lower hCG dose. However, the decreased ApoM and ApoC can
be explained by the presence of more oocytes in the premature step
in the 5,000 IU compared to the 10,000 IU hCG protocol. Therefore,
it was more difficult for oocyte retrieval by follicle aspiration
and as a result the total oocytes retrieved were significantly
decreased in comparison. The serum was recovered 35–36 h after hCG
administration, not 5–9 h, so there were no siginificant deference
between the two protocols in serum progesterone and estradiol
concentration.
In conclusion, the present study demonstrates that
there were statistically significant differences between the two
protocol with 10,000 and 5,000 IU hCG administration in the
intraovarian artery blood flow, incidence of apoptotic granulosa
cells, follicle fluid progesterone concentration and total oocyte
retrieved in the IVF-ET program. The dose of hCG administration can
affect the oocyte maturation or the outcome of the IVF-ET
program.
Acknowledgements
The present study was supported in part by the
Education Overseas Scholars (grant no. 1151hq041) of Heilongjiang
Province Education Administration and the Provincial Health Issues
(major project, grant no. 2006-098) of Heilongjiang Province Health
Bureau.
References
|
1
|
Espey LL: Current status of the hypothesis
that mammalian ovulation is comparable to an inflammatory reaction.
Biol Reprod. 50:233–238. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shoham Z, Schacter M, Loumaye E, Weissman
A, MacNamee M and Insler V: The luteinizing hormone surge - the
final stage in ovulation induction: modern aspects of ovulation
triggering. Fertil Steril. 64:237–251. 1995.PubMed/NCBI
|
|
3
|
European Recombinant LH Study Group, .
Human recombinant luteinizing hormone is as effective as, but safer
than, urinary human chorionic gonadotropin in inducing final
follicular maturation and ovulation in in vitro fertilization
procedures: results of a multicenter double-blind study. J Clin
Endocrinol Metab. 86:2607–2618. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weissman A, Lurie S, Zalel Y, Goldchmit R
and Shoham Z: Human chorionic gonadotropin: pharmacokinetics of
subcutaneous administration. Gynecol Endocrinol. 10:273–276. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Norjavaara E, Olofsson J, Gåfvels M and
Selstam G: Redistribution of ovarian blood flow after injection of
human chorionic gonadotropin and luteinizing hormone in the adult
pseudopregnant rat. Endocrinology. 120:107–114. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nargund G, Doyle PE, Bourne TH, Parsons
JH, Cheng WC, Campbell S and Collins WP: Ultrasound derived indices
of follicular blood flow before HCG administration and the
prediction of oocyte recovery and preimplantation embryo quality.
Hum Reprod. 11:2512–2517. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakahara K, Saito H, Saito T, Ito M, Ohta
N, Takahashi T and Hiroi M: The incidence of apoptotic bodies in
membrana granulosa can predict prognosis of ova from patients
participating in in vitro fertilization programs. Fertil Steril.
68:312–317. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du B, Takahashi K, Ishida GM, Nakahara K,
Saito H and Kurachi H: Usefulness of intraovarian artery
pulsatility and resistance indices measurement on the day of
follicle aspiration for the assessment of oocyte quality. Fertil
Steril. 85:366–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saito H, Saito T, Kaneko T, Sasagawa I,
Kuramoto T and Hiroi M: Relatively poor oocyte quality is an
indication for intracytoplasmic sperm injection. Fertil Steril.
73:465–469. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Damewood MD, Shen W, Zacur HA, Schlaff WD,
Rock JA and Wallach EE: Disappearance of exogenously administered
human chorionic gonadotropin. Fertil Steril. 52:398–400.
1989.PubMed/NCBI
|
|
11
|
Bjercke S, Tanbo T, Dale PO and Abyholm T:
Comparison between two hCG-to-oocyte aspiration intervals on the
outcome of in vitro fertilization. J Assist Reprod Genet.
17:319–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nargund G, Reid F and Parsons J: Human
chorionic gonadotropin-to-oocyte collection interval in a
superovulation IVF program. A prospective study. J Assit Reprod
Genet. 18:87–90. 2001. View Article : Google Scholar
|
|
13
|
Wang LJ and Norman RJ: Concentrations of
immunoreactive interleukin-1 and interleukin-2 in human
preovulatory follicular fluid. Hum Reprod. 7:147–150.
1992.PubMed/NCBI
|