|
1
|
Vallée A: Geoepidemiological perspective
on COVID-19 pandemic review, an insight into the global impact.
Front Public Health. 11(1242891)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khoury DS, Docken SS, Subbarao K, Kent SJ,
Davenport MP and Cromer D: Predicting the efficacy of
variant-modified COVID-19 vaccine boosters. Nat Med. 29:574–578.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Carabelli AM, Peacock TP, Thorne LG,
Harvey WT and Hughes J: COVID-19 Genomics UK Consortium. Peacock
SJ, Barclay WS, de Silva TI, Towers GJ and Robertson DL: SARS-CoV-2
variant biology: Immune escape, transmission and fitness. Nat Rev
Microbiol. 21:162–177. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bouhaddou M, Reuschl AK, Polacco BJ,
Thorne LG, Ummadi MR, Ye C, Rosales R, Pelin A, Batra J, Jang GM,
et al: SARS-CoV-2 variants evolve convergent strategies to remodel
the host response. Cell. 186:4597–4614.e26. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jiang S, Xia S, Ying T and Lu L: A novel
coronavirus (2019-nCoV) causing pneumonia-associated respiratory
syndrome. Cell Mol Immunol. 17(554)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Qualls N, Levitt A, Kanade N,
Wright-Jegede N, Dopson S, Biggerstaff M, Reed C and Uzicanin A:
CDC Community Mitigation Guidelines Work Group. Community
mitigation guidelines to prevent pandemic influenza-United States,
2017. MMWR Recomm Rep. 66:1–34. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Farsalinos K, Poulas K, Kouretas D,
Vantarakis A, Leotsinidis M, Kouvelas D, Docea AO, Kostoff R,
Gerotziafas GT, Antoniou MN, et al: Improved strategies to counter
the COVID-19 pandemic: Lockdowns vs. primary and community
healthcare. Toxicol Rep. 8:1–9. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li H, Burm SW, Hong SH, Ghayda RA,
Kronbichler A, Smith L, Koyanagi A, Jacob L, Lee KH and Shin JI: A
comprehensive review of coronavirus disease 2019: Epidemiology,
transmission, risk factors, and international responses. Yonsei Med
J. 62:1–11. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Perico L, Benigni A, Casiraghi F, Ng LFP,
Renia L and Remuzzi G: Immunity, endothelial injury and
complement-induced coagulopathy in COVID-19. Nat Rev Nephrol.
17:46–64. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wu VC, Hsueh PR, Lin WC, Huang JW, Tsai
HB, Chen YM and Wu KD: SARS Research Group of the National Taiwan
University College of Medicine and National University Hospital.
Acute renal failure in SARS patients: More than rhabdomyolysis.
Nephrol Dial Transplant. 19:3180–3182. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cha RH, Joh JS, Jeong I, Lee JY, Shin HS,
Kim G and Kim Y: Critical Care Team of National Medical Center.
Renal complications and their prognosis in Korean patients with
Middle East respiratory syndrome-coronavirus from the central
MERS-CoV designated hospital. J Korean Med Sci. 30:1807–1814.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chan JFW, Yuan S, Kok KH, To KK, Chu H,
Yang J, Xing F, Liu J, Yip CC, Poon RW, et al: A familial cluster
of pneumonia associated with the 2019 novel coronavirus indicating
person-to-person transmission: A study of a family cluster. Lancet.
395:514–523. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gansevoort RT and Hilbrands LB: CKD is a
key risk factor for COVID-19 mortality. Nat Rev Nephrol.
16:705–706. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pakhchanian H, Raiker R, Mukherjee A, Khan
A, Singh S and Chatterjee A: Outcomes of COVID-19 in CKD patients:
A multicenter electronic medical record cohort study. Clin J Am Soc
Nephrol. 16:785–786. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
ERA-EDTA Council; ERACODA Working Group.
Chronic kidney disease is a key risk factor for severe COVID-19: A
call to action by the ERA-EDTA. Nephrol Dial Transplant. 36:87–94.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bajwa H, Riaz Y, Ammar M, Farooq S and
Yousaf A: The dilemma of renal involvement in COVID-19: A
systematic review. Cureus. 12(e8632)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Go AS, Chertow GM, Fan D, McCulloch CE and
Hsu C: Chronic kidney disease and the risks of death,
cardiovascular events, and hospitalization. N Engl J Med.
351:1296–1305. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
O'Callaghan KP, Blatz AM and Offit PA:
Developing a SARS-CoV-2 vaccine at warp speed. JAMA. 324:437–438.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Calina D, Hartung T, Docea AO, Spandidos
DA, Egorov AM, Shtilman MI, Carvalho F and Tsatsakis A: COVID-19
vaccines: Ethical framework concerning human challenge studies.
Daru. 28:807–812. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sharpe HR, Gilbride C, Allen E,
Belij-Rammerstorfer S, Bissett C, Ewer K and Lambe T: The early
landscape of coronavirus disease 2019 vaccine development in the UK
and rest of the world. Immunology. 160:223–232. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sterne JA, Hernán MA, Reeves BC, Savović
J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT,
Boutron I, et al: ROBINS-I: A tool for assessing risk of bias in
non-randomised studies of interventions. BMJ.
355(i4919)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10(ED000142)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wan X, Wang W, Liu J and Tong T:
Estimating the sample mean and standard deviation from the sample
size, median, range and/or interquartile range. BMC Med Res
Methodol. 14(135)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
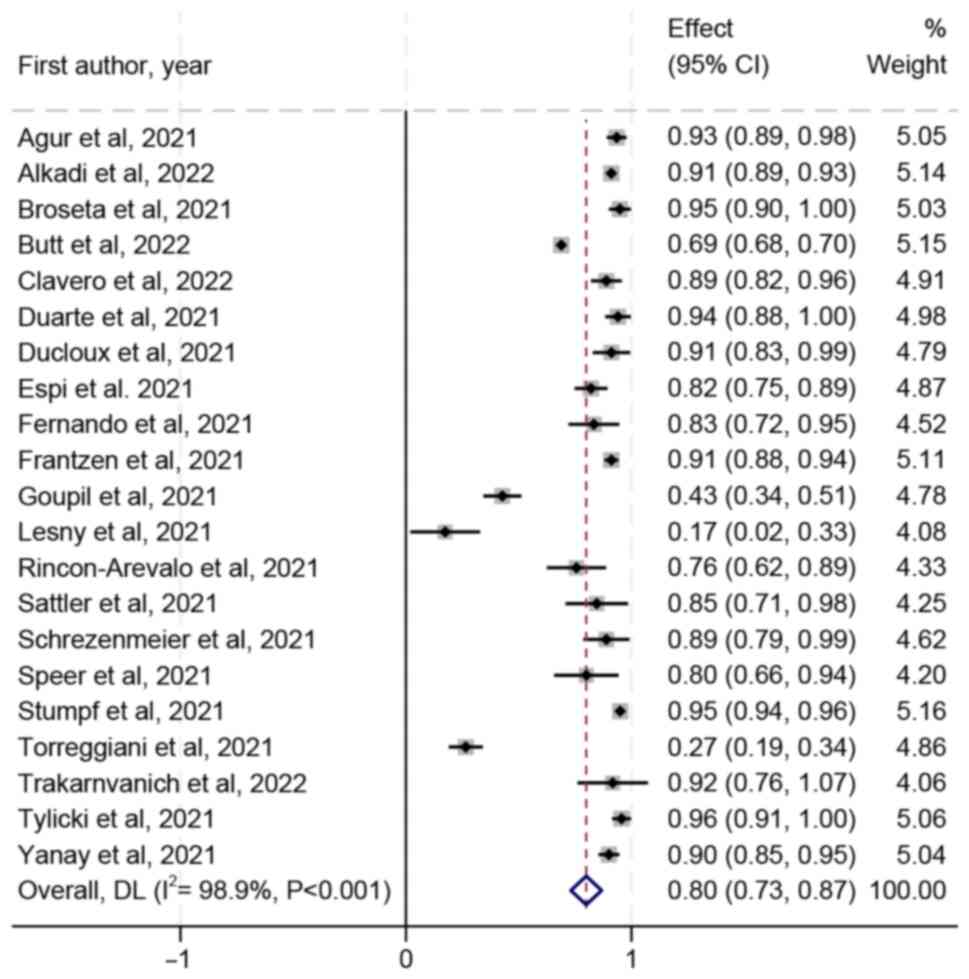
Agur T, Ben-Dor N, Goldman S, Lichtenberg
S, Herman-Edelstein M, Yahav D, Rozen-Zvi B and Zingerman B:
Antibody response to mRNA SARS-CoV-2 vaccine among dialysis
patients-a prospectivecohort study. Nephrol Dial Transplant.
11(gfab155)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Broseta JJ, Rodríguez-Espinosa D, Soruco E
and Maduell F: Weekly seroconversion rate of the mRNA-1273
SARS-CoV-2 vaccine in haemodialysis patients. Nephrol Dial
Transplant. 36:1754–1755. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Labriola L, Scohy A, Van Regemorter E,
Robert A, Clerbaux G, Gillerot G, Pochet JM, Biller P, De
Schuiteneer M, Morelle J, et al: Immunogenicity of BNT162b2
SARS-CoV-2 vaccine in a multicenter cohort of nursing home
residents receiving maintenance hemodialysis. Am J Kidney Dis.
78:766–768. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lesny P, Anderson M, Cloherty G, Stec M,
Haase-Fielitz A, Haarhaus M, Santos C, Lucas C, Macario F and Haase
M: Immunogenicity of a first dose of mRNA- or vector-based
SARS-CoV-2 vaccination in dialysis patients: A multicenter
prospective observational pilot study. J Nephrol. 34:975–983.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
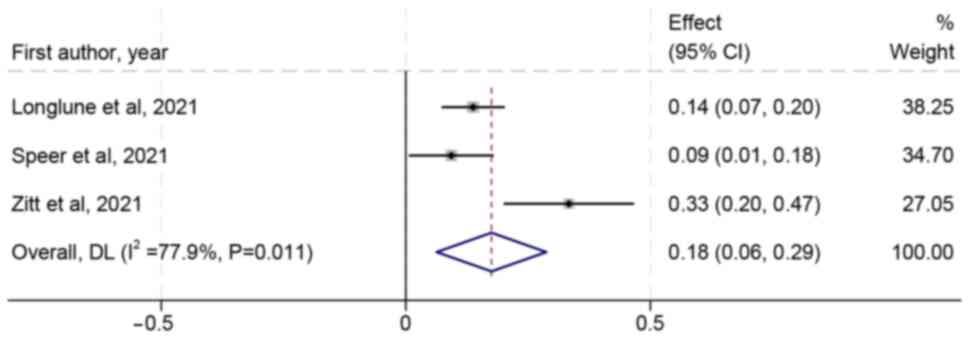
Longlune N, Nogier MB, Miedougé M, Gabilan
C, Cartou C, Seigneuric B, Del Bello A, Marion O, Faguer S, Izopet
J and Kamar N: High immunogenicity of a messenger RNA-based vaccine
against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial
Transplant. 36:1704–1709. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rincon-Arevalo H, Choi M, Stefanski AL,
Halleck F, Weber U, Szelinski F, Jahrsdörfer B, Schrezenmeier H,
Ludwig C, Sattler A, et al: Impaired humoral immunity to SARS-CoV-2
BNT162b2 vaccine in kidney transplant recipients and dialysis
patients. Sci Immunol. 6(eabj1031)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sattler A, Schrezenmeier E, Weber UA,
Potekhin A, Bachmann F, Straub-Hohenbleicher H, Budde K, Storz E,
Proß V, Bergmann Y, et al: Impaired humoral and cellular immunity
after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in
kidney transplant recipients. J Clin Invest.
131(e150175)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schrezenmeier E, Bergfeld L, Hillus D,
Lippert JD, Weber U, Tober-Lau P, Landgraf I, Schwarz T, Kappert K,
Stefanski AL, et al: Immunogenicity of COVID-19 tozinameran
vaccination in patients on chronic dialysis. Front Immunol.
12(690698)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Simon B, Rubey H, Treipl A, Gromann M,
Hemedi B, Zehetmayer S and Kirsch B: Haemodialysis patients show a
highly diminished antibody response after COVID-19 mRNA vaccination
compared with healthy controls. Nephrol Dial Transplant.
36:1709–1716. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Speer C, Benning L, Töllner M, Nusshag C,
Kälble F, Reichel P, Schaier M, Bartenschlager M, Schnitzler P,
Zeier M, et al: Neutralizing antibody response against variants of
concern after vaccination of dialysis patients with BNT162b2.
Kidney Int. 100:700–702. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Speer C, Morath C, Töllner M, Buylaert M,
Göth D, Nusshag C, Kälble F, Schaier M, Grenz J, Kreysing M, et al:
Humoral responses to single-dose BNT162b2 mRNA vaccination in
dialysis patients previously infected with SARS-CoV-2. Front Med
(Lausanne). 8(721286)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Strengert M, Becker M, Ramos GM, Dulovic
A, Gruber J, Juengling J, Lürken K, Beigel A, Wrenger E, Lonnemann
G, et al: Cellular and humoral immunogenicity of a SARS-CoV-2 mRNA
vaccine in patients on haemodialysis. EBioMedicine.
70(103524)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cserep G, Morrow D, Latchford K, Jesset R,
Dosa A and Kirmizis D: The effect of a single dose of BNT162b2
vaccine on the incidence of severe COVID-19 infection in patients
on chronic hemodialysis: A single-centre study. Clin Exp Nephrol.
26:54–58. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Stumpf J, Siepmann T, Lindner T, Karger C,
Schwöbel J, Anders L, Faulhaber-Walter R, Schewe J, Martin H,
Schirutschke H, et al: Humoral and cellular immunity to SARS-CoV-2
vaccination in renal transplant versus dialysis patients: A
prospective, multicenter observational study using mRNA-1273 or
BNT162b2 mRNA vaccine. Lancet Reg Heal Eur.
9(100178)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Torreggiani M, Blanchi S, Fois A, Fessi H
and Piccoli GB: Neutralizing SARS-CoV-2 antibody response in
dialysis patients after the first dose of the BNT162b2 mRNA
COVID-19 vaccine: The war is far from being won. Kidney Int.
99:1494–1496. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
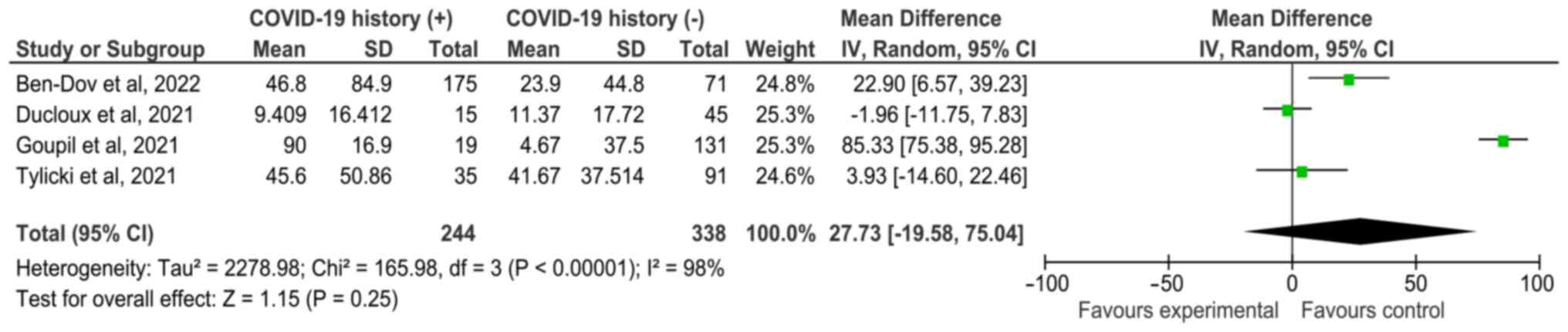
Tylicki L, Biedunkiewicz B, Dąbrowska M,
Ślizień W, Tylicki P, Polewska K, Rosenberg I, Rodak S and
Dębska-Ślizień A: Humoral response to SARS-CoV-2 vaccination
promises to improve the catastrophic prognosis of hemodialysis
patients as a result of COVID-19: The COViNEPH project. Polish Arch
Intern Med. 131:797–801. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Weigert A, Bergman ML, Gonçalves LA,
Godinho I, Duarte N, Abrantes R, Borges P, Brennand A, Malheiro V,
Matoso P, et al: Longitudinal analysis of antibody responses to the
mRNA BNT162b2 vaccine in patients undergoing maintenance
hemodialysis: A 6-month follow-up. Front Med (Lausanne).
8(796676)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yanay NB, Freiman S, Shapira M, Wishahi S,
Hamze M, Elhaj M, Zaher M and Armaly Z: Experience with SARS-CoV-2
BNT162b2 mRNA vaccine in dialysis patients. Kidney Int.
99:1496–1498. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zitt E, Davidovic T, Schimpf J,
Abbassi-Nik A, Mutschlechner B, Ulmer H, Benda MA, Sprenger-Mähr H,
Winder T and Lhotta K: The safety and immunogenicity of the
mRNA-BNT162b2 SARS-CoV-2 vaccine in Hemodialysis patients. Front
Immunol. 12(704773)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Alkadi MM, Hamad A, Ghazouani H,
Elshirbeny M, Ali MY, Ghonimi T, Ibrahim R, Abuhelaiqa E,
Abou-Samra AB, Al-Malki H and Butt AA: Effectiveness of messenger
RNA vaccines against SARS-CoV-2 infection in hemodialysis patients:
A case-control study. Vaccines (Basel). 11(49)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ben-Dov IZ, Oster Y, Tzukert K, Alster T,
Bader R, Israeli R, Asayag H, Aharon M, Burstein I, Pri-Chen H, et
al: Impact of tozinameran (BNT162b2) mRNA vaccine on kidney
transplant and chronic dialysis patients: 3-5 months follow-up. J
Nephrol. 35:153–164. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bielopolski D, Libresco G, Barda N, Dagan
N, Steinmetz T, Yahav D, Charytan DM, Balicer RD and Rozen-Zvi B:
BNT162b2 vaccine effectiveness in chronic kidney disease
patients-an observational study. Clin Kidney J. 15:1838–1846.
2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Butt AA, Talisa VB, Yan P, Shaikh OS, Omer
SB and Mayr FB: Real-World effectiveness of the severe acute
respiratory syndrome Coronavirus 2 (SARS-CoV-2) mRNA vaccines in
preventing confirmed infection in patients on chronic hemodialysis.
Clin Infect Dis. 75:e617–e622. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Duarte R, Roldão M, Figueiredo C, Luz I,
Ferrer F, Gonçalves H, Sofia F and Lopes K: Humoral response to
BNT162b2 mRNA COVID-19 vaccine in peritoneal and hemodialysis
patients: A comparative study. Ther Apher Dial. 26:790–796.
2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Davidovic T, Schimpf J, Abbassi-Nik A,
Stockinger R, Sprenger-Mähr H, Lhotta K and Zitt E: Humoral and
cellular immune response after a 3-dose heterologous SARS-CoV-2
vaccination using the mRNA-BNT162b2 and viral vector Ad26COVS1
vaccine in hemodialysis patients. Front Immunol.
13(907615)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Quiroga B, Soler MJ, Ortiz A, Vaquera SM,
Mantecón CJ, Useche G, Márquez MG, Carnerero M, Rodríguez MT, Ramos
PM, et al: Safety and immediate humoral response of COVID-19
vaccines in chronic kidney disease patients: The SENCOVAC study.
Nephrol Dial Transplant. 37:1868–1878. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Trakarnvanich T, Ngamvichchukorn T,
Phumisantiphong U, Pholtawornkulchai K, Phochanasomboon K and
Manomaipiboon A: Immune response after COVID-19 vaccination among
patients with chronic kidney disease and kidney transplant.
Vaccine. 40:6499–6511. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ducloux D, Colladant M, Chabannes M,
Yannaraki M and Courivaud C: Humoral response after 3 doses of the
BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney
Int. 100:702–704. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Espi M, Charmetant X, Barba T, Koppe L,
Pelletier C, Kalbacher E, Chalencon E, Mathias V, Ovize A,
Cart-Tanneur E, et al: The ROMANOV study found impaired humoral and
cellular immune responses to SARS-CoV-2 mRNA vaccine in
virus-unexposed patients receiving maintenance hemodialysis. Kidney
Int. 100:928–936. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fernando E and Govindan S: Neutralizing
SARS-CoV-2 antibody response and protective effect of 2 Doses of
ChAdOx1 nCoV-19 and BBV152 vaccines in hemodialysis patients: A
preliminary report. Kidney Int Rep. 6:2521–2522. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Frantzen L, Cavaille G, Thibeaut S and
El-Haik Y: Efficacy of the BNT162b2 mRNA Covid-19 vaccine in a
hemodialysis cohort. Nephrol Dial Transplant. 36:1756–1757.
2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Goupil R, Benlarbi M, Beaubien-Souligny W,
Nadeau-Fredette AC, Chatterjee D, Goyette G, Gunaratnam L, Lamarche
C, Tom A, Finzi A, et al: Short-term antibody response after 1 dose
of BNT162b2 vaccine in patients receiving hemodialysis. CMAJ.
193:E793–E800. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Clavero R, Parra-Lucares A, Méndez-Valdés
G, Villa E, Bravo K, Mondaca E, Aranda J, Brignardello R, Gajardo
C, Ordenes A, et al: Humoral immune response of BNT162b2 and
coronavac vaccinations in hemodialysis patients: A multicenter
prospective cohort. Vaccines (Basel). 10(1542)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liu W, Zhou L, Yin W, Wang J and Zuo X:
Global, regional, and national burden of chronic kidney disease
attributable to high sodium intake from 1990 to 2019. Front Nutr.
10(1078371)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kovesdy CP: Epidemiology of chronic kidney
disease: An update 2022. Kidney Int Suppl (2011). 12:7–11.
2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Locatelli F, Nissenson AR, Barrett BJ,
Walker RG, Wheeler DC, Eckardt KU, Lameire NH and Eknoyan G:
Clinical practice guidelines for Anemia in chronic kidney disease:
Problems and solutions. A position statement from kidney disease:
Improving Global Outcomes (KDIGO). Kidney Int. 74:1237–1240.
2008.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zou X, Chen K, Zou J, Han P, Hao J and Han
Z: Single-cell RNA-seq data analysis on the receptor ACE2
expression reveals the potential risk of different human organs
vulnerable to 2019-nCoV infection. Front Med. 14:185–192.
2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Diao B, Wang C, Wang R, Feng Z, Zhang J,
Yang H, Tan Y, Wang H, Wang C, Liu L, et al: Human kidney is a
target for novel severe acute respiratory syndrome coronavirus 2
infection. Nat Commun. 12(2506)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Martinez-Rojas MA, Vega-Vega O and
Bobadilla NA: Is the kidney a target of SARS-CoV-2? Am J Physiol
Renal Physiol. 318:F1454–F1462. 2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Sanhueza ME, Martín PS, Brantes L, Caro S,
Carrasco G and Machuca E: Efficacy of vaccination against the
SARS-CoV-2 virus in patients with chronic kidney disease on
hemodialysis. Hum Vaccin Immunother. 19(2173904)2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Hou YC, Lu KC and Kuo KL: The efficacy of
COVID-19 vaccines in chronic kidney disease and kidney
transplantation patients: A narrative review. Vaccines (Basel).
9(885)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Rossi M, Pessolano G and Gambaro G: What
has vaccination against COVID-19 in CKD patients taught us? J
Nephrol. 36:1257–1266. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Martins MP and de Oliveira RB: COVID-19
and chronic kidney disease: A narrative review. COVID. 3:1092–1105.
2023.
|
|
67
|
Babel N, Hugo C and Westhoff TH:
Vaccination in patients with kidney failure: Lessons from COVID-19.
Nat Rev Nephrol. 18:708–723. 2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Jiang Z, Liu J, Geng L, Zhong Z, Tan J,
Wen D, Zhou L, Tang Y and Qin W: The influences of COVID-19 on
patients with chronic kidney disease: A multicenter cross-sectional
study. Front Psychiatry. 12(754310)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Rozen-Zvi B, Yahav D, Agur T, Zingerman B,
Ben-Zvi H, Atamna A, Tau N, Mashraki T, Nesher E and Rahamimov R:
Antibody response to SARS-CoV-2 mRNA vaccine among kidney
transplant recipients: A prospective cohort study. Clin Microbiol
Infect. 27:1173.e1–1173.e4. 2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Kunutsor SK and Laukkanen JA: Markers of
liver injury and clinical outcomes in COVID-19 patients: A
systematic review and meta-analysis. J Infect. 82:159–198.
2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Bohn MK, Loh TP, Wang CB, Mueller R, Koch
D, Sethi S, Rawlinson WD, Clementi M, Erasmus R, Leportier M, et
al: IFCC interim guidelines on serological testing of antibodies
against SARS-CoV-2. Clin Chem Lab Med. 58:2001–2008.
2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Ma BM, Tam AR, Chan KW, Ma MKM, Hung IFN,
Yap DYH and Chan TM: Immunogenicity and safety of COVID-19 vaccines
in patients receiving renal replacement therapy: A systematic
review and meta-analysis. Front Med (Lausanne).
9(827859)2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Javadinia SA, Alizadeh K, Mojadadi MS,
Nikbakht F, Dashti F, Joudi M, Harati H, Welsh JS, Farahmand SA and
Attarian F: COVID-19 vaccination in patients with malignancy; a
systematic review and meta-analysis of the efficacy and safety.
Front Endocrinol (Lausanne). 13(860238)2022.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Joudi M, Binabaj MM, Porouhan P,
PeyroShabany B, Tabasi M, Fazilat-Panah D, Khajeh M, Mehrabian A,
Dehghani M, Welsh JS, et al: A cohort study on the immunogenicity
and safety of the inactivated SARS-CoV-2 vaccine (BBIBP-CorV) in
patients with breast cancer; does trastuzumab interfere with the
outcome? Front Endocrinol (Lausanne). 13(798975)2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Ciarambino T, Para O and Giordano M:
Immune system and COVID-19 by sex differences and age. Womens
Health (Lond). 17(17455065211022262)2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ho JQ, Sepand MR, Bigdelou B, Shekarian T,
Esfandyarpour R, Chauhan P, Serpooshan V, Beura LK, Hutter G and
Zanganeh S: The immune response to COVID-19: Does sex matter?
Immunology. 166:429–443. 2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Collier DA, Ferreira IATM, Kotagiri P,
Datir RP, Lim EY, Touizer E, Meng B and Abdullahi A: CITIID-NIHR
BioResource COVID-19 Collaboration. Elmer A, et al: Age-related
immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2.
Nature. 596:417–422. 2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Chen Q, Zhao H, Yao X, Lin Z, Li J, Lin B,
Wang R, Huang Y, Su Y, Wu T, et al: Comparing immunogenicity of the
Escherichia coli-produced bivalent human papillomavirus vaccine in
females of different ages. Vaccine. 38:6096–6102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Fernandes M da CR, Vasconcelos GS, de Melo
ACL, Matsui TC, Caetano LF, de Carvalho Araújo FM and Fonseca MHG:
Influence of age, gender, previous SARS-CoV-2 infection, and
pre-existing diseases in antibody response after COVID-19
vaccination: A review. Mol Immunol. 156:148–155. 2023.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zheng YY, Ma YT, Zhang JY and Xie X:
COVID-19 and the cardiovascular system. Nat Rev Cardiol.
17:259–260. 2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Barnes E, Goodyear CS, Willicombe M,
Gaskell C, Siebert S, de Silva TI, Murray SM, Rea D, Snowden JA,
Carroll M, et al: SARS-CoV-2-specific immune responses and clinical
outcomes after COVID-19 vaccination in patients with
immune-suppressive disease. Nat Med. 29:1760–1774. 2023.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Letizia AG, Ge Y, Vangeti S, Goforth C,
Weir DL, Kuzmina NA, Balinsky CA, Chen HW, Ewing D,
Soares-Schanoski A, et al: SARS-CoV-2 seropositivity and subsequent
infection risk in healthy young adults: A prospective cohort study.
Lancet Respir Med. 9:712–720. 2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Brüssow H: COVID-19: Vaccination problems.
Environ Microbiol. 23:2878–2890. 2021.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Sáez-Peñataro J, Torres F, Bartra J,
Bascuas J, Vilella A, Tortajada M, Quesada S, González E,
López-Suñé E, Castells A, et al: Tolerability and reactogenicity
profile of mRNA SARS-Cov-2 vaccines from a mass vaccination
campaign in a tertiary hospital: Between-vaccine and
between-population prospective observational study (VigilVacCOVID
Study). BioDrugs. 36:509–520. 2022.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Al-Sadeq DW, Shurrab FM, Ismail A,
Amanullah FH, Thomas S, Aldewik N, Yassine HM, Rahim HF, Abu-Raddad
L and Nasralla GK: Comparison of antibody immune responses between
BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in naïve and previously
infected individuals. J Travel Med. 28(taab190)2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Kitagawa H, Kaiki Y, Sugiyama A, Nagashima
S, Kurisu A, Nomura T, Omori K, Akita T, Shigemoto N, Tanaka J and
Ohge H: Adverse reactions to the BNT162b2 and mRNA-1273 mRNA
COVID-19 vaccines in Japan. J Infect Chemother. 28:576–581.
2022.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Hulme WJ, Horne EMF, Parker EPK, Keogh RH,
Williamson EJ, Walker V, Palmer TM, Curtis HJ, Walker AJ, Andrews
CD, et al: Comparative effectiveness of BNT162b2 versus mRNA-1273
covid-19 vaccine boosting in England: Matched cohort study in
OpenSAFELY-TPP. BMJ. 380(e072808)2023.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Ono S, Michihata N, Yamana H, Uemura K,
Ono Y, Jo T and Yasunaga H: Comparative effectiveness of BNT162b2
and mRNA-1273 booster dose after BNT162b2 primary vaccination
against the omicron variants: A retrospective cohort study using
large-scale population-based registries in Japan. Clin Infect Dis.
76:18–24. 2023.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Puspitasari M, Sattwika PD, Rahari DS,
Wijaya W, Hidayat ARP, Kertia N, Purwanto B and Thobari JA:
Immunogenicity and safety of inactivated SARS-CoV-2 vaccine in
haemodialysis patients: A prospective cohort study. Sci Rep.
13(11557)2023.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Mehta N, Shah S, Paudel K, Chamlagain R
and Chhetri S: Safety and efficacy of coronavirus disease-19
vaccines in chronic kidney disease patients under maintenance
hemodialysis: A systematic review. Heal Sci Rep.
5(e700)2022.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Wang Y, Yang L and Xu G: New-onset acute
interstitial nephritis Post-SARS-CoV-2 infection and COVID-19
vaccination: A panoramic review. J Epidemiol Glob Health.
13:615–636. 2023.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zhang S, He J, Tang B, Zhou Q, Hu Y, Yu Y,
Chen J, Liu Y, Li C, Ren H and Liao X: Cellular and humoral
responses to recombinant and inactivated SARS-CoV-2 vaccines in CKD
Patients: An observational study. J Clin Med.
12(1225)2023.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Qaderi K, Golezar MH, Mardani A, Mallah
MA, Moradi B, Kavoussi H, Shamsabadi A and Golezar S: Cutaneous
adverse reactions of COVID-19 vaccines: A systematic review.
Dermatol Ther. 35(e15391)2022.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Tan SW, Tam YC and Pang SM: Cutaneous
reactions to COVID-19 vaccines: A review. JAAD Int. 7:178–186.
2022.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Sharif N, Alzahrani KJ, Ahmed SN and Dey
SK: Efficacy, immunogenicity and safety of COVID-19 vaccines: A
systematic review and meta-analysis. Front Immunol.
12(714170)2021.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Xia S, Duan K, Zhang Y, Zhao D, Zhang H,
Xie Z, Li X, Peng C, Zhang Y, Zhang W, et al: Effect of an
inactivated vaccine Against SARS-CoV-2 on safety and immunogenicity
outcomes: Interim analysis of 2 randomized clinical trials. JAMA.
324:951–960. 2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J,
Feng F, Zou H and Sun C: Safety of SARS-CoV-2 vaccines: A
systematic review and meta-analysis of randomized controlled
trials. Infect Dis Poverty. 10(94)2021.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wu Q, Dudley MZ, Chen X, Bai X, Dong K,
Zhuang T, Salmon D and Yu H: Evaluation of the safety profile of
COVID-19 vaccines: A rapid review. BMC Med. 19(173)2021.PubMed/NCBI View Article : Google Scholar
|