Introduction
The cause of cholesterol gallstone (CG) formation is
complicated; however, the role of small intestine motility has been
gradually noted (1,2). In our previous study, it was found
that the intestinal transit (IT) function was decreased in the
diet-induced Golden hamster CG model (3), indicating that declined IT may play
an important role in the process of CG formation.
It has been demonstrated that the interstitial cells
of Cajal (ICCs) are the pacemaker cells of the gastrointestinal
slow wave, regulating the pacing and spreading of smooth muscle
activity and participating in the conduction of nerve signal
pathways (4–6). ICCs can express the c-kit
proto-oncogene, which encodes a tyrosine kinase membrane receptor.
The signaling pathway resulting from the combination of c-kit and
its ligand stem cell factor (scf) plays an important role in the
development, differentiation and phenotypic maintenance of ICCs
(7,8). However, whether the declined IT in
the high-cholesterol diet-induced guinea pig CG model is caused by
abnormalities in the ICCs and c-kit/scf pathway remains to be
investigated. In this experiment, immunofluorescence staining and
the reverse transcription-polymerase chain reaction (RT-PCR) were
performed to detect the number of ICCs in guinea pig small
intestine tissue and to analyze the mRNA expression of c-kit and
scf, respectively. The aim of the study was to explore the cellular
and molecular mechanisms of the IT decline during the CG formation
process of the guinea pig.
Materials and methods
Animal model and grouping
The experiment was approved by the Ethics Committee
of Sheng Jing Hospital of China Medical University (Shenyang,
China). Forty healthy male guinea pigs, aged four weeks and
weighing 120–125 g, were provided by the Experimental Animal Center
of Sheng Jing Hospital of China Medical University. The animal
license number was SCXK (Liao) 2009–0016. The animals were randomly
divided into two groups: The experimental group (EG, n=20) and the
control group (CoG, n=20). The animals in the EG were fed a
high-cholesterol diet (2% cholesterol, purchased from Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China), while those in the
CoG received a normal diet. Each cage was limited to two animals.
The experiment was performed under standard laboratory conditions
(12-h light/dark cycle, 21–24°C, humidity at 50–55%). Free feeding
with cabbage every two days was maintained for eight weeks prior to
the experiment.
Bile smear observation by polarizing
microscopy and infrared spectrum analysis of the biliary
calculus.
Bile was obtained from the gallbladder with the
fine-needle penetration method. A small amount of bile was dropped
onto the slide to form the bile smear, and a DP-71 polarized light
microscope (Olympus, Tokyo, Japan) was used to observe the
cholesterol crystals. The biliary calculus was collected following
a cholecystectomy, washed with distilled water and dried naturally,
prior to a 2-mg portion being mixed with 100 mg KBr. The stone and
KBr were fully milled for 10 min and baked under an infrared lamp
for ~30 min prior to being pressed into a transparent sheet with a
3000X tablet machine (BSTD Co, Ltd., Tianjin, China). An FT-IR-55
infrared spectrometer (Bioon Co., Ltd, Bern, Switzerland) was used
to analyze the composition of the sheet.
Observation of the guinea pig small
intestine with immunofluorescence staining and laser confocal
microscopy
The guinea pigs were anesthetized, and ~2 cm
terminal ileum was reserved. The intestinal contents were then
cleaned with 0.9% sodium chloride solution. One end of the terminal
ileum was ligated, and acetone was injected through the reserved
end of the intestine. Subsequent to the segment fully expanding,
the reserved end was ligated and the intestine was retained in a
refrigerator at 4°C. The intestine was cut along the longitudinal
axis with microsurgical scissors, and then cut into pieces
measuring 3×3 mm. Under the operating microscope, the intestinal
mucosa and submucosa were peeled away with microdissection forceps,
while the muscular layer was reserved. The muscular layer was then
stretched and rinsed with 0.01 mol/l phosphate-buffered saline
(PBS), prior to being treated with 0.03 mol/l PBS-Tween 20 for 1 h
and sealed with normal goat serum for 1 h. Diluted rabbit
anti-mouse c-kit (ACK II) primary antibody (Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China) was subsequently added at
a dilution of 1:100 followed by incubation overnight. On the next
day, 1:100 cyanine 3 (CY3)-labeled goat anti-rabbit secondary
antibody (Beijing Biosynthesis Biotechnology Co., Ltd.) was added
and incubated in the dark for 1 h. DAPI was added for the
re-staining, and the slide was then mounted with glycerol mounting
medium and the coverslip. The c-kit-positive ICCs were counted and
images were captured using the Eclipse IZ laser confocal microscope
(Nikon Corp., Tokyo, Japan). The percentage ICC-positive area was
automatically calculated with the image analysis software of the
Nikon Eclipse IZ system.
Extraction of total RNA
Fresh intestinal tissue (200 mg) of the guinea pig
was placed in liquid nitrogen, then repeatedly crushed prior to the
addition of 1 ml TRIzol® solution (Invitrogen Life
Technologies, Carlsbad, CA, USA). The total RNA was extracted
according to the TRIzol reagent kit instructions (Invitrogen Life
Technologies).
c-kit and scf gene cloning with
RT-PCR
The purified RNA was used to synthesize cDNA through
the RT reaction. According to the RT kit (Takara Corporation,
Dalian, China), the first chain of cDNA was initially synthesized
and used as the template for the PCR. The gene primer sequences
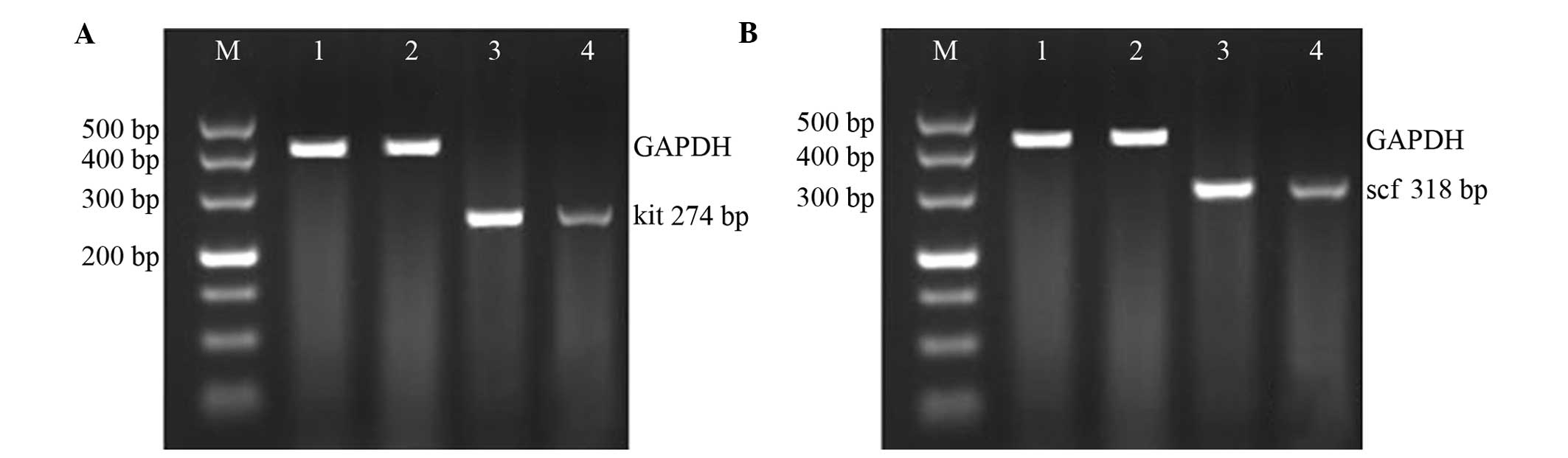
were as follows: c-kit, upstream 5′-CACAGAGGCTTAGCGG-3′, and
downstream 5′-CGTGAAGGCAACATACC-3′; scf, upstream
5′-GCAGCATAATACCACG-3′, and downstream 5′-AATACCATCATCCGTTC-3′;
internal reference GAPDH, upstream 5′-ACCACAGTCCATGCCATCAC-3′, and
downstream 5′-TCCACCACCCTGTTGGGTA-3′. The PCR products were then
separated by electrophoresis, with reference to the blank control
and molecular size of the DNA Ladder Marker. The positive
electrophoretic bands were confirmed and the desired strips were
obtained for the recovery of the c-kit and scf DNA fragments
according to the instructions of the gel extraction kit (Takara
Corporation). Following the 1.5% agarose gel electrophoresis, the
PCR products were stained with ethidium bromide, observed and
photographed under ultraviolet light, with ΦX174/HaeIII and
DL2000 DNA Marker as the molecular weight standards. A GIS-2020
digital gel-scanning imaging and analysis system (Dobio Co., Ltd.,
Shanghai, China) was used to perform the electrophoretic band
analysis, and the mRNA expression levels of c-kit and scf were
calculated according to the relative expression levels of the
internal reference mRNA GAPDH.
Statistical analysis
Statistical analyses were performed using SPSS 11.5
statistical software (SPSS, Inc., Chicago, IL, USA), and all
experimental data are expressed as the mean ± standard deviation.
Inter-group comparisons were conducted with the Student’s t-test,
and the rate comparisons were performed with the χ2
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Guinea pig growth and stone
formation
Three animals in the EG died, with the mortality
rate at 15%. In the later stage of the feeding time, the animals
moved slowly, exhibited dull responses, ate less and shed hair. The
autopsies revealed that the causes of death were pneumonia (n=1),
splenic abscess (n=1) and cervical lymphadenitis (n=1). There were
no cases of mortality in the CoG, and the guinea pigs grew in good
condition.
At the end of the lithogenic period, the dissection
revealed that the gallbladders of the EG guinea pigs were swollen,
with evident granular, yellow, single or multiple stones. By
contrast, the gallbladders of the CoG were normal with clear and
bright bile. There was no stone formation in the CoG guinea pigs,
and the stone formation rate was 0% (0/20); in the EG, stones
formed in the remaining 17 guinea pigs, and the stone formation
rate was 100% (17/17). Polarized light microscopy revealed no
cholesterol crystals in the gallbladder bile of the CoG guinea
pigs, while the typical needle-like crystals of cholesterol could
be observed in the EG. Infrared spectroscopy of the stone-KBr
sheets showed that at the wavelengths of 1,418 cm−1 and
1,640 cm−1, clear cholesterol absorption peaks could be
observed.
Small intestine c-kit and scf mRNA
expression results
Compared with the CoG, the small intestinal mRNA
expression levels of c-kit (0.316±0.056 vs. 0.912±0.103; t=6.582,
P<0.01) and scf (0.499±0.012 vs. 0.899±0.124; t=6.163,
P<0.01) in the EG guinea pigs were significantly decreased
(Fig. 1A and B).
Immunofluorescence staining results of
the guinea pig small intestine
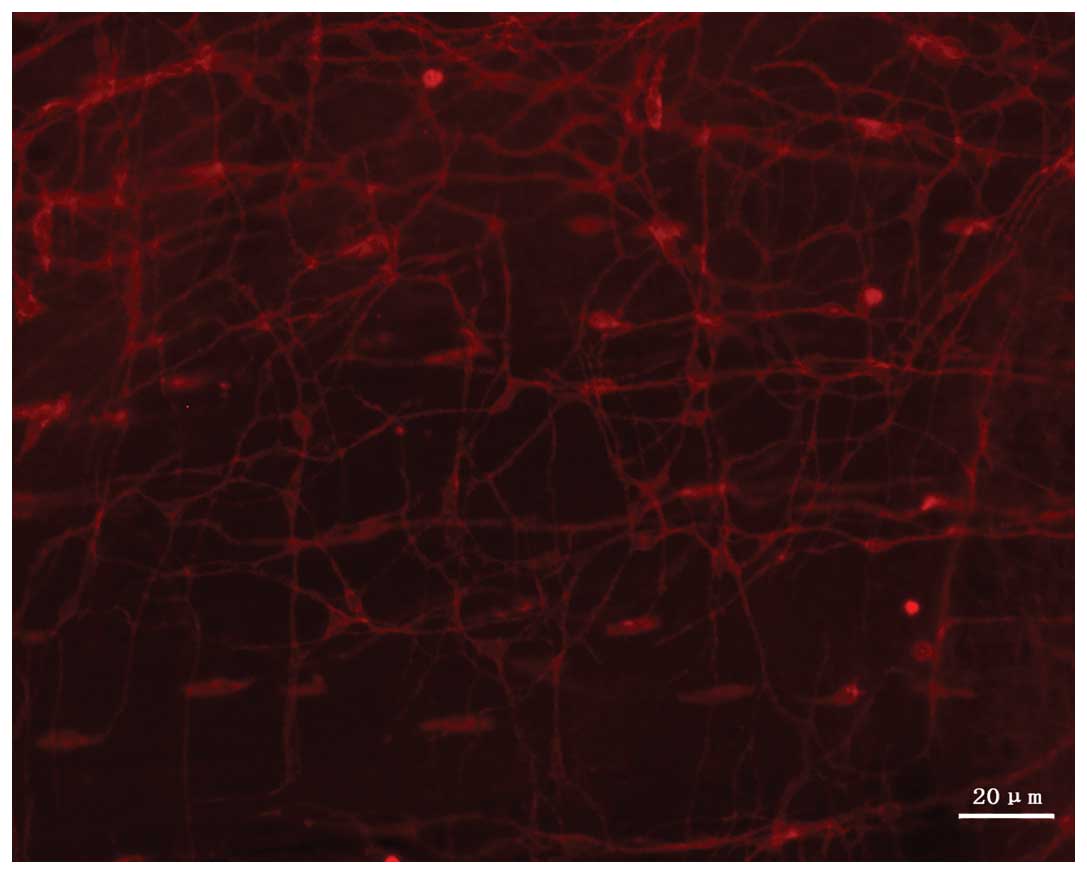
The c-kit-positive ICCs were present in the muscular
layer of the small intestine in the guinea pigs. When stained by
CY3-conjugated secondary antibody, the cells exhibited red
fluorescence with confocal laser microscopy, and this was
significantly highlighted against the black background. Under the
confocal microscope, ICCs were observed to exhibit a fusiform or
satellite shape, with large oval DAPI-stained (blue) nuclei, little
perinuclear cytoplasm and between two and five long, branched cell
processes. The cells thus appeared to be spindle- or satellite-like
cells. The ICCs were connected to each other, forming a
network-like structure (Fig. 2).
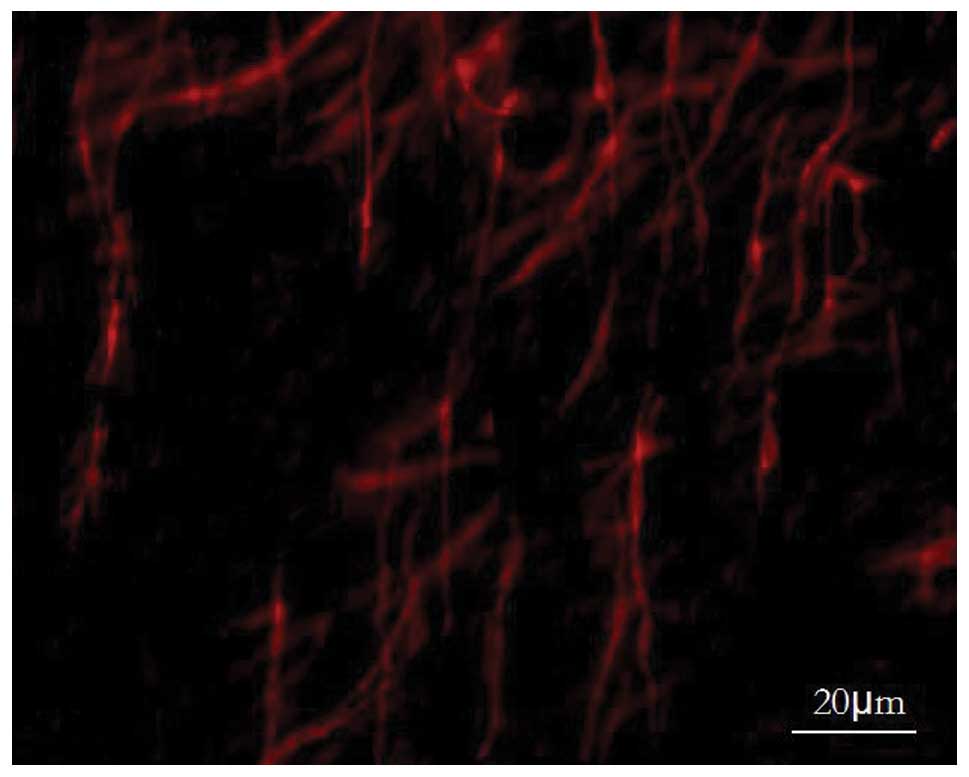
The small intestinal slides of the EG also exhibited c-kit-positive
ICCs, showing no significant difference in single cell morphology
with the CoG under the light microscope, while the number of ICCs
observed within the vision field was significantly reduced in the
EG, and the network structure was found to have disappeared
(Fig. 3).
For the comparative observation of the slides of the
CoG and EG, three fields of each slide were randomly scanned, and
the immunofluorescence image analysis software was used to count
the percentage ICC-positive area. The results showed that the
average ICC-positive area in the EG was significantly reduced when
compared with that in the CoG (22.26±1.14 vs. 56.24±2.68%;
t=15.256, P<0.01).
Discussion
The incidence of CGs increases with age, and
the condition exhibits a complex pathogenesis. In the past two
decades, studies on the motility function of the small
intestine-gallbladder have increased, showing that patients with
CGs experience a decline in IT function, leading to a prolongation
of the transit time of bile in the small intestine and increased
levels of bile deoxycholic acid (DCA) (9,10).
DCA is positively correlated with the cholesterol saturation index
and cholesterol crystallization rate (11); furthermore, the gallbladder
contraction and IT function during the digestion period are
associated with the serum motilin level. The decline in IT induces
disordered gallbladder emptying, and increases in bile
concentration (12). Extension of
the IT period also prolongs the enterohepatic circulation of bile
acids, thus slowing the bile salt reabsorption rate. As a result,
the bile salt content falls, which promotes cholesterol
supersaturation and crystallization (13). However, the mechanism underlying
the decline in IT has not been reported, to date.
In 1893, Cajal, the Spanish neuroanatomist, first
reported the existence of a class of mesenchymal cells that were
present in the gastrointestinal tract and exhibited a network
structure, namely the ICCs (14).
Recent studies have confirmed that ICCs play an important role in
the pacing and dissemination of the smooth muscle activity in the
gastrointestinal tract, and also in the nerve signal transduction
pathway (15–18). ICCs can specifically express c-kit,
a type III tyrosine kinase membrane receptor. In the present study,
c-kit immunofluorescence staining was successfully performed on the
whole layer slice of the small intestine of the guinea pig. The
results showed that, during the CG formation process of the
high-cholesterol diet-induced guinea pig, the number of intestinal
ICCs was significantly decreased when compared with that of the
CoG, and the network-like structure between the cells disappeared.
This indicated that the changes in the number and function of ICCs
and pacemaker cells in IT played an important role in the declined
IT function during the process of stone formation.
It has also been indicated that the c-kit receptor
has an important role in the development, growth and phenotypic
maintenance of ICCs. Animals with a spontaneous c-kit mutation do
not exhibit a normal occurrence and development of ICCs (19). Furthermore, when the c-kit
neutralizing antibody ACK II was intraperitoneally injected into a
new-born mouse, it was revealed that the normal contraction phase
of the mouse intestine was disordered, and the electric slow wave
activity and ICCs disappeared (20). Numerous studies have found a
missing or damaged ICCs network in a variety of human diseases,
including intestinal pseudo-obstruction, Hischsprung’s disease,
Crohn’s disease, extensive bowel resection, hereditary
transthyretin amyloidosis, diabetic gastroparesis and slow transit
constipation (21–23). The experimental data from the
present study showed that, in the small intestine of the
high-cholesterol diet-induced CG guinea pig, the expression of
c-kit mRNA was significantly decreased. This indicated that
cholesterol could inhibit the expression of c-kit mRNA in
intestinal tissues, which may explain the phenomenon of the reduced
number of ICCs in the small intestine during the process of CG
formation.
scf is a multifunctional growth factor involved in
the growth regulation of a variety of cells in the body. scf can
combine with its natural ligand, c-kit, forming the c-kit/scf
system, which is associated with the differentiation, development,
proliferation and phenotypic maintenance of ICCs (24). A previous study demonstrated that
when a non-lethal mutation of scf occurred, heterozygous mice
(Sl/Sld) exhibited severely disordered scf synthesis, and were only
capable of synthesizing and secreting a small amount of soluble
scf. Ten days after the birth of the Sl/Sld mice, the intestinal
myenteric ICCs (ICCs-MY) showed poor development, forming a loose
and atypical network structure compared with normal mice, with
fewer cytoplasmic mitochondria and without membrane foveola. The
ICCs-MY were difficult to identify 20 days after birth (25). In the ICC cell culture environment,
scf should be added to aid the growth, development and phenotypic
maintenance of ICCs (26). The
aforementioned evidence shows that scf is essential for the
occurrence, development and phenotypic maintenance of ICCs. In
addition, the present experimental data show that, in the small
intestine of the high-cholesterol diet-induced CG guinea pig, the
expression of scf mRNA is significantly decreased, and cholesterol
can inhibit the expression of scf mRNA in intestinal tissues,
affecting the regulation of the number and function of ICCs.
In conclusion, the results of the present study
indicated that the high-cholesterol diet was the causative factor
that induced CG formation in the guinea pig. This occurred through
the inhibition of the c-kit/scf signaling pathway, which resulted
in a reduced number of ICCs and dysfunction in the guinea pig small
intestine, including dysfunctional spontaneous rhythmic
contraction, as well as the decline in IT function. This ultimately
led to CG formation.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81000183), Shenyang Scientific and
Technological Funding (no. F11-264-1-24) and the Natural Science
Foundation of Liaoning (no. 2013021060).
References
|
1
|
Hussaini SH, Pereira SP, Dowling RH and
Wass JA: Slow intestinal transit and gallstone formation. Lancet.
341:6381993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spathis A, Heaton KW, Emmett PM, Norboo T
and Hunt L: Gallstones in a community free of obesity but prone to
slow intestinal transit. Eur J Gastroenterol Hepatol. 9:201–206.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan Y, Wu SD and Fu BB: Effect of
intestinal transit on the formation of cholesterol gallstones in
hamsters. Hepatobiliary Pancreat Dis Int. 6:513–515.
2007.PubMed/NCBI
|
|
4
|
Jun JY: The important roles of
interstitial cells of cajal and cholinergic receptors on diabetes
related dysfunction of colon. J Neurogastroenterol Motil.
17:333–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iino S, Horiguchi S and Horiguchi K:
Interstitial cells of Cajal in the gastrointestinal musculature of
W(jic) c-kit mutant mice. J Smooth Muscle Res. 47:111–121. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cole WC: ANO1-ther brick in the wall -
role of Ca2+-activated Cl- channels of
interstitial cells of Cajal in cholinergic motor control of
gastrointestinal smooth muscle. J Physiol. 589:4641–4642.
2011.PubMed/NCBI
|
|
7
|
Tanaka C, Kaji H, He J, et al: Rab27b
regulates c-kit expression by controlling the secretion of stem
cell factor. Biochem Biophys Res Commun. 419:368–373. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He X, Yang WC, Wen XY, et al: Late
embryonic and postnatal development of interstitial cells of cajal
in mouse esophagus: distribution, proliferation and kit dependence.
Cells Tissues Organs. 196:175–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Portincasa P, Di Ciaula A, Wang HH, et al:
Coordinate regulation of gallbladder motor function in the
gut-liver axis. Hepatology. 47:2112–2116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu QW, Scott RB, Tan DT and Shaffer EA:
Slow intestinal transit: a motor disorder contributing to
cholesterol gallstone formation in the ground squirrel. Hepatology.
23:1664–1672. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang DQ, Cohen DE and Carey MC: Biliary
lipids and cholesterol gallstone disease. J Lipid Res. 50(Suppl):
S406–S411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang ZH, Wu SD, Su Y, et al: Differences
and significance of motilin, vasoactive intestinal peptide and
gastrin in blood and gallbladder tissues of patients with
gallstones. Hepatobiliary Pancreat Dis Int. 7:58–64.
2008.PubMed/NCBI
|
|
13
|
Xie M, Kotecha VR, Andrade JD, Fox JG and
Carey MC: Augmented cholesterol absorption and sarcolemmal sterol
enrichment slow small intestinal transit in mice, contributing to
cholesterol cholelithogenesis. J Physiol. 590:1811–1824. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torihashi S, Horisawa M and Watanabe Y:
c-Kit immunoreactive interstitial cells in the human
gastrointestinal tract. J Auton Nerv Syst. 75:38–50. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCann CJ, Hwang SJ, Bayguinov Y, Colletti
EJ, Sanders KM and Ward SM: Establishment of pacemaker activity in
tissues allotransplanted with interstitial cells of Cajal.
Neurogastroenterol Motil. 25:e418–e428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neuhann TM, Mansmann V, Merkelbach-Bruse
S, et al: A novel germline KIT mutation (p. L576P) in a family
presenting with juvenile onset of multiple gastrointestinal stromal
tumors, skin hyperpigmentations, and esophageal stenosis. Am J Surg
Pathol. 37:898–905. 2013. View Article : Google Scholar
|
|
17
|
Hebert MD: Signals controlling Cajal body
assembly and function. Int J Biochem Cell Biol. 45:1314–1317. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JH, Kim SY, Kwon YK, Kim BJ and So I:
Characteristics of the cholecystokinin-induced depolarization of
pacemaking activity in cultured interstitial cells of Cajal from
murine small intestine. Cell Physiol Biochem. 31:542–554. 2013.
View Article : Google Scholar
|
|
19
|
Albertí E, Mikkelsen HB, Wang XY, et al:
Pacemaker activity and inhibitory neurotrans- mission in the colon
of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol.
292:G1499–G1510. 2007.
|
|
20
|
Torihashi S, Ward SM, Nishikawa S, et al:
c-kit-dependent development of interstitial cells and electrical
activity in the murine gastrointestinal tract. Cell Tissue Res.
280:97–111. 1995.PubMed/NCBI
|
|
21
|
Chen J, Du L, Xiao YT and Cai W:
Disruption of interstitial cells of Cajal networks after massive
small bowel resection. World J Gastroenterol. 19:3415–3422. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wixner J, Obayashi K, Ando Y, Karling P
and Anan I: Loss of gastric interstitial cells of Cajal in patients
with hereditary transthyretin amyloidosis. Amyloid. 20:99–106.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mogami S, Suzuki H, Tsugawa H, Fukuhara S
and Hibi T: Impaired heme oxygenase-1 induction in the gastric
antrum induces disruption of the interstitial cells of Cajal
network in a rat model of streptozotocin-induced diabetes.
Neurogastroenterol Motil. 25:609–e465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li F, Zhang L, Li C, et al:
Adenovirus-mediated stem cell leukemia gene transfer induces rescue
of interstitial cells of Cajal in ICC-loss mice. Int J Colorectal
Dis. 25:557–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mikkelsen HB, Malysz J, Huizinga JD and
Thuneberg L: Action potential generation, Kit receptor
immunohistochemistry and morphology of steel-Dickie (Sl/Sld) mutant
mouse small intestine. Neurogastroenterol Motil. 10:11–26. 1998.
View Article : Google Scholar
|
|
26
|
Rich A, Miller SM, Gibbons SJ, Malysz J,
Szurszewski JH and Farrugia G: Local presentation of Steel factor
increases expression of c-kit immunoreactive interstitial cells of
Cajal in culture. Am J Physiol Gastrointest Liver Physiol.
284:G313–G320. 2003.PubMed/NCBI
|