Introduction
Mesenchymal stem cells (MSCs) possess the capacity
for self-renewal and multi-directional differentiation, and have
several characteristics, including multi-lineage differentiation
potential, hematopoietic support and stem cell implantation
promotion, immune regulation and self-renewal, which make them a
promising source for cell therapy in numerous diseases (1–3).
Since MSCs are rarely found in bone marrow or fetal tissues, the
isolation and expansion of human MSCs is crucial for their clinical
application. Human umbilical cord-derived mesenchymal stem cells
(hUCMSCs) are readily available, abundant, rich in content and able
to differentiate into a variety of types of cells. As a result,
they have been the focus of considerable attention. However,
whether the chromosomes of hUCMSCs change following culture in
vitro remains unclear and controversial (4). Therefore, in the present study
hUCMSCs were isolated and cultured in vitro, and the
alteration in karyotypes of the hUCMSCs subjected to serial passage
in vitro was determined by investigating the karyotypes of
generations of P0 and P3-P6 and analysis with the G-banding
technique.
Materials and methods
Materials
The materials used included DMEM/F12 medium
(NVF0274; Gibco-BRL, Gaithersburg, MD, USA), fetal bovine serum
(FBS; 10057; Excell Bio, Shanghai, China), collagenase II
(Gibco-BRL), trypsin (Gibco-BRL), cell culture box (Nunc; Thermo
Fisher Scientific, Waltham, MA, USA), flow cytometer (BD Accuri C6;
BD Biosciences, Franklin Lakes, NJ, USA), inverted optical
microscope (IX70-S8F; Olympus, Tokyo, Japan), mouse anti-human
CD13, CD29, CD31, CD34, CD44, CD45, CD90, CD105 and HLA-DR
antibodies (BD Biosciences), karyotyping system (CytoVision version
7.2; Leica, Mannheim, Germany) and a medium kit for chromosome
culture (RPMI; Gibco-BRL, Grand Island, NY, USA). Colchicine,
glacial acetic acid and Giemsa stain (Sigma-Aldrich, St. Louis, MO,
USA) were also used.
Isolation and culture of hUCMSCs
Umbilical cords were obtained following normal or
cesarean term deliveries from two healthy infants (one male and one
female) at Taizhou People’s Hospital (Taizhou, China) in January
2013. Written informed consent was obtained from the mothers and
the experimental procedures were approved by the Ethics Committee
of Taizhou People’s Hospital. The umbilical cords were cannulated
and washed three times with phosphate-buffered saline to remove
blood clots. Then the umbilical cords were cut into 2–3 cm pieces
and opened with a scalpel. The Wharton’s jelly was scratched out
and the vessels were removed. Thereafter, the Wharton’s jelly was
incubated in collagenase/DMEM solution for 24 h. Following
centrifugation for 10 min, the cell pellet was resuspended and
incubated in trypsin at 37°C for 30 min. Finally, the cell pellet
was resuspended in DMEM/F12 medium containing 10% FBS and cells
were seeded in a T75 culture flask. The cells were passaged at
1×104–0.4×106cells/cm2, and were
continuously cultivated for six passages. Morphological changes
were assessed by observing the cells under an inverted optical
microscope.
Detection of surface markers of hUCMSCs
using flow cytometry
Following the third passage (P3), the cells were
washed twice with PBS, and then digested with a 1:1 mixture of
trypsin (2.5 g/l) and EDTA (0.2 g/l). A suspension of
1×1010 cells/l was obtained by washing with PBS
containing bovine serum albumin (20 g/l). A total of 100 μl cell
suspension was added to each Eppendorf tube. Fluorescently labeled
anti-human-antibodies [PE-CD13, PE-CD29, APC-CD90, PE-CD105,
PE-CD31, PE-CD34, FITC-CD44, FITC-CD45 and FITC-HLA-DR] were added
in 20 μl. To the control group was added immunoglobulin G (IgG;
isotype control). The cells were incubated for 30 min at 4°C in the
dark, washed twice with PBS, then 200 μl paraformaldehyde (10 g/l)
was added to each tube. Flow cytometry was used to detect the
surface markers of hUCMSCs.
Cell proliferation test
Proliferation curves were determined using the MTT
method. Each passage of hUCMSCs was respectively seeded in a
96-well plate; 200 μl cell suspension with a density of
2×104/ml was added to each well. The cells were
incubated for 72 h and 10 μl MTT reagent (Sigma-Aldrich) was then
added to each well. The cells were incubated for a further 4 h, the
medium was removed and 200 μl DMSO was added to each well to
dissolve the formazan. The plates were shaken for 15 min at 10 × g.
The number of the cells per well was counted every day for 8
successive days and used to construct a proliferation curve for the
hUCMSCs.
Karyotyping of generations
According to the result of the flow cytometric
analysis and cell proliferation test, karyotypes were analyzed in
hUCMSCs from the first passage that was isolated and cultured in
vitro using the chromosome G-banding technique. The cell
suspension (300 μl) with a density of 2×104/ml was added
to a 10-ml culture bottle, then 100 μl colchicine with a
concentration of 40 μg/ml was added to the culture bottle. The
cells were incubated for 4 h in a carbon dioxide incubator and the
adherent cells were removed and placed into a 15-ml centrifuge
tube. Following centrifugation for 8 min at 182 × g, the culture
medium containing colchicine was removed and 4 ml 0.075% KC1 was
added. Following incubation at 37°C for 5 min, 2 ml Carnoy’s
solution (3:1 v/v absolute ethanol: glacial acetic acid) was added
and the cells were maintained in culture at 37°C for 5 min. The
cells were centrifuged for a further 8 min at 182 × g, the culture
medium was removed and 4 ml Carnoy’s fixative was added. The cells
were incubated and centrifuged again, which was repeated twice. The
cell pellet was resuspended in DMEM/F12 medium containing 10% FBS
and aliquots of the suspension were dropped onto slides. After 48 h
at room temperature, the slides were placed in a slide drier 75°C
for 4 h. Giemsa staining was then performed for 15 min and
karyotypes of generations were analyzed using the G-banding
technique.
Results
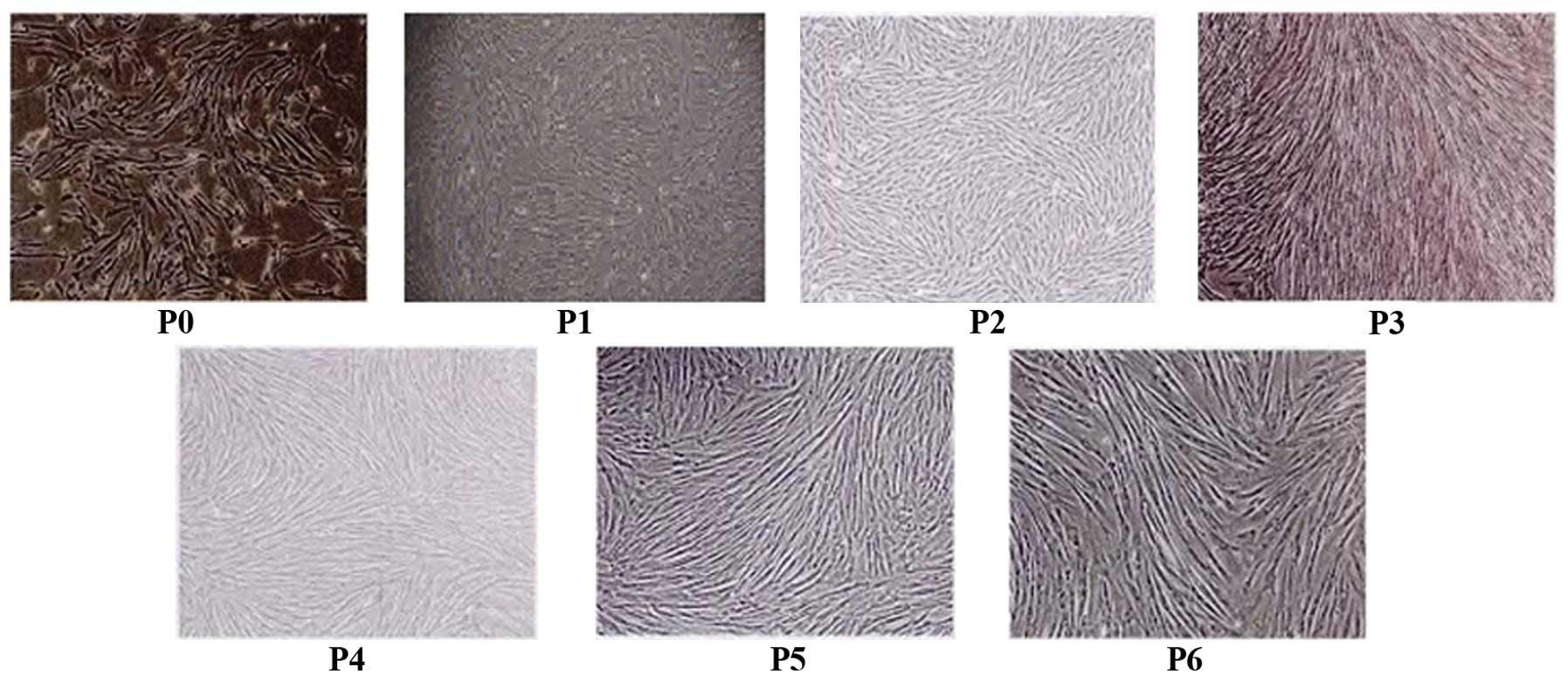
Morphology of hUCMSCs
After 7 days of adherent culture, hUCMSCs with
fibroblastic morphology were presented from the human umbilical
cord tissue. Following serial passage, the cells grew and
proliferated quickly. When cultured for 6 passages in vitro,
hUCMSCs maintained a stable spindle-shaped morphology (Fig. 1).
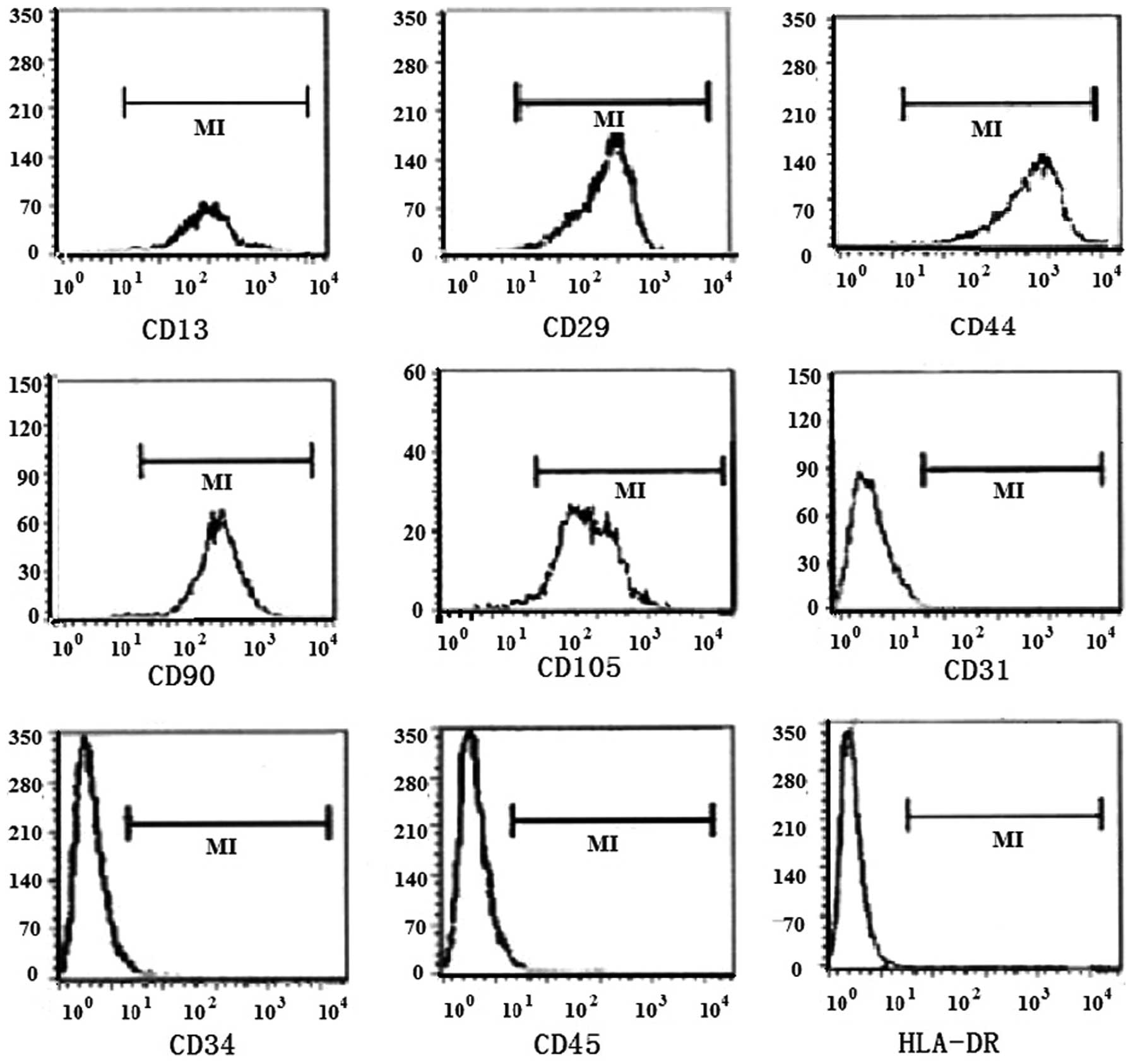
Immunophenotype
The results from the flow cytometric analysis
revealed that standardized culture of hUCMSCs in vitro
resulted in the stable expression of surface markers following
serial passage. CD13, CD29, CD44, CD90 and CD105 were highly
expressed at a level of >95% on the surface of hUCMSCs, but the
expression of CD31, CD34, CD45 and HLA-DR was negative and <2%
(Table I, Fig. 2).
 | Table IImmunophenotype of human umbilical
cord-derived mesenchymal stem cells detected using flow cytometry
(%). |
Table I
Immunophenotype of human umbilical
cord-derived mesenchymal stem cells detected using flow cytometry
(%).
| Passage | CD13 | CD29 | CD44 | CD90 | CD105 | CD31 | CD34 | CD45 | HLA-DR |
|---|
| P1 | 96.70 | 98.30 | 95.60 | 99.20 | 97.10 | 0.69 | 1.24 | 0.63 | 0.18 |
| P2 | 97.30 | 96.40 | 96.10 | 99.30 | 95.90 | 0.18 | 0.79 | 0.51 | 0.24 |
| P3 | 99.20 | 96.90 | 95.40 | 99.80 | 95.10 | 0.71 | 1.00 | 0.24 | 0.56 |
| P4 | 95.40 | 97.20 | 97.20 | 99.60 | 96.40 | 0.62 | 0.68 | 0.72 | 0.05 |
| P5 | 98.70 | 98.60 | 96.30 | 98.70 | 96.70 | 0.34 | 0.92 | 0.29 | 0.25 |
| P6 | 96.50 | 95.10 | 95.80 | 99.10 | 98.10 | 0.59 | 0.75 | 0.46 | 0.36 |
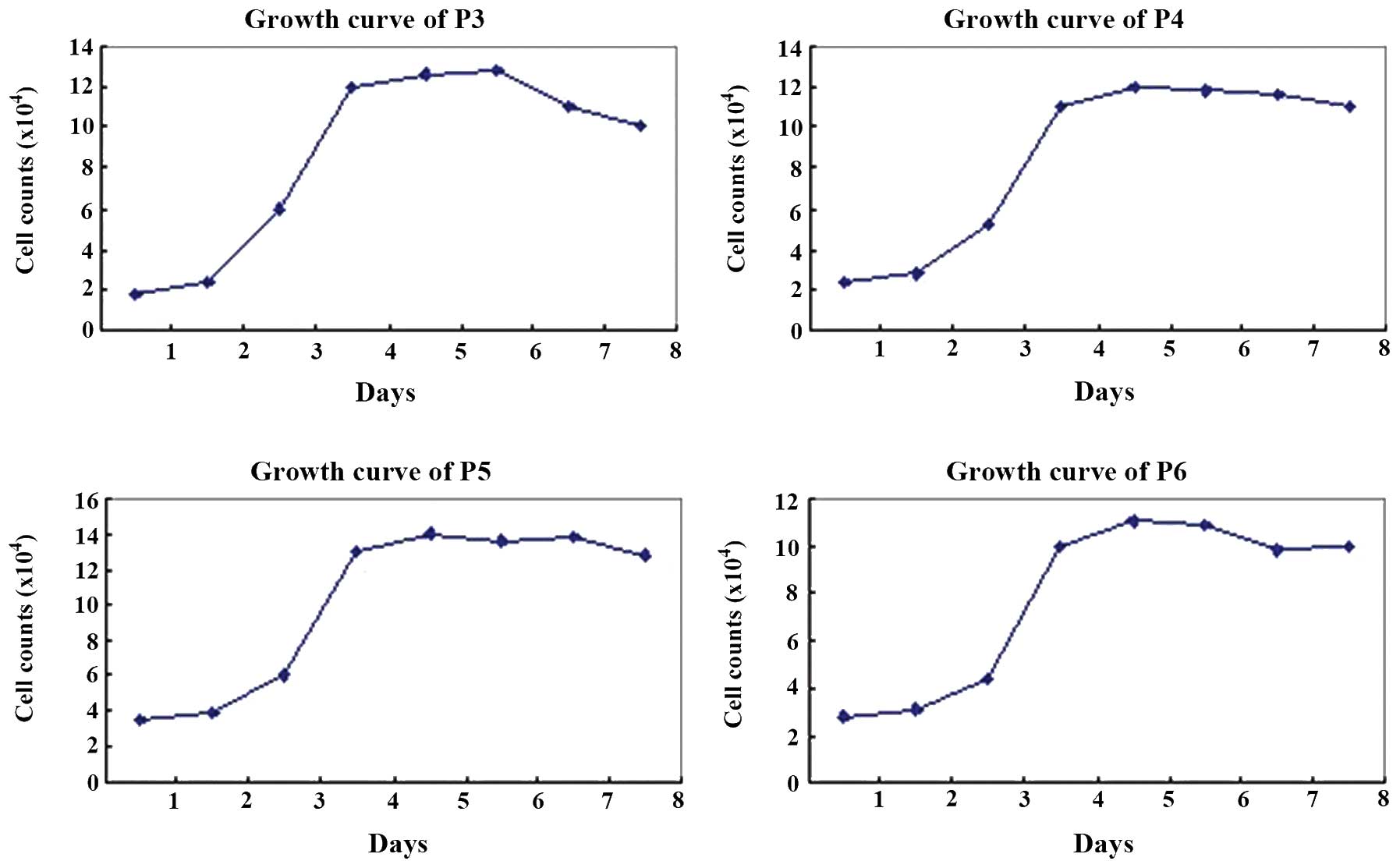
Proliferation of hUCMSCs
Each passage of hUCMSCs presented a typical S-like
proliferation curve. Cells were in a slow growth period at 1–2 days
of culture, but reached a logarithmic growth phase at 3–4 days and
then a plateau phase at 5–7 days. After 8 days of culture, the
cells were observed to have diminished proliferation potency
(Fig. 3).
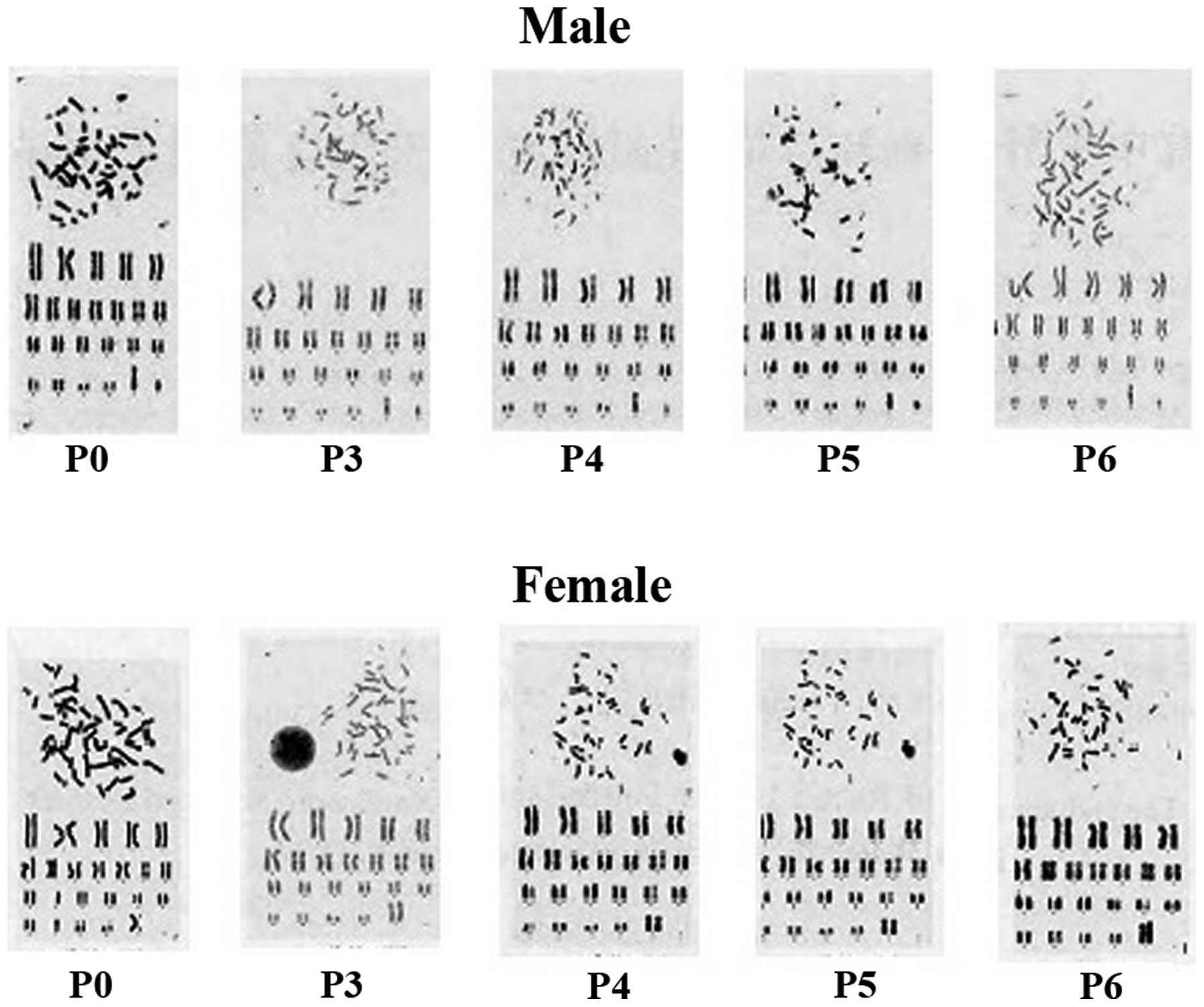
Karyotype analysis of hUCMSCs
Following treatment with colchicine, P3-P6 of
hUCMSCs were arrested in the metaphase stage. A normal diploid
karyotype with 46 chromosomes and no abnormal changes in chromosome
structure was observed by the analysis of 30 metaphase cells
(Fig. 4). However, abnormal
morphology was observed in the seventh and eighth passages, and the
ratio of non-hUCMSCs also increased in these passages.
Discussion
MSCs offer a lot of promise for the development of
novel alternative cell-based therapies. These unique cells possess
two major features: their ability for self-renewal and
differentiation potential. At present, MSCs are primarily obtained
from bone marrow. However, bone marrow-derived MSCs are easily
contaminated by viruses and have a significantly decreased capacity
for proliferation and differentiation with the aging of the donor.
In addition, as there are a variety of ethical and legal issues,
their application is restrained (5,6).
Umbilical cord blood (UCB) is a source of additional stem cells for
experimental and potentially clinical uses. However, the presence
of MSCs in UCB is controversial (7). Human umbilical cord, a connecting
tissue of extraembryonic origin lying between the mother and fetus,
consists of two arteries, one vein, inter-vessel connective tissue
and umbilical epithelium. The connective tissue, also referred as
Wharton’s jelly, is composed of a sponge-like structure woven by
collagen fibers, proteoglycan and embedded stromal cells. The
umbilical cord is a promising source of MSCs due to its wide
availability, differentiation potential and lack of ethical
concerns. Compared with bone marrow MSCs, hUCMSCs are easier to
isolate and expand, and so they would be an advantageous, novel
source of adult MSCs. A number of studies have demonstrated that
hUCMSCs may have an important role in the application and
experimental research of adult human MSCs (8,9).
Due to differences between culture systems in
vitro and conditions in vivo, whether MSCs maintain a
stable chromosome structure following serial passage and
proliferation is an important index of clinical safety. Numerous
studies have found that an abnormal chromosome structure is
positively correlated with the incidence of tumors (10,11).
The results of investigations of the karyotype stability of MSCs
during a long-term culture in vitro remain inconsistent. It
has been demonstrated that MSCs passaged >10 times have a
chromosome analysis that is normal (4,12);
however, other studies have found that karyotype variation in MSCs
may occur following long-term culture in vitro and have
tumorigenicity in nude mice (13–15).
Although hUMSCs are a desirable source of cells for use in tissue
repair and regeneration engineering, few studies have addressed the
safety of hUMSCs that have been subjected to long-term culture
in vitro.
In the present study, by digestion with collagenase
and trypsin, a large number of adherent hUMSCs were harvested
within a short time period. The results showed that following
resuscitation, these cells maintain high cell viability, and could
be cultured for a long time, enabling large numbers of cells to be
harvested. The results from the flow cytometric analysis
demonstrated that the hUCMSCs harvested in the present study
maintained their phenotypes following long-term in vitro
proliferation and serial passage. CD13, CD29, CD44, CD90 and CD105
were highly expressed on >95% of the surface of hUCMSCs;
however, the expression of CD31,CD34, CD45 and HLA-DR was negative
and <2%, which is consistent with a previous study (16). The results from the proliferation
test demonstrated that each passage of hUCMSCs presented a typical
S-like proliferation curve. Cells were in a logarithmic growth
phase after 3–4 days of culture. According to the results of
proliferation test, the hUCMSCs of P3-P6 were selected, which were
then cultured for 3–4 days for karyotyping. The karyotype analysis
showed a normal diploid karyotype with 46 chromosomes and no
abnormal changes were observed in chromosome structure in the
hUCMSCs of P3-P6 following treatment with colchicine. In the
present study, it was found that there was abnormal morphology in
the seventh and eighth passages and the ratio of non-hUCMSCs was
also increased in these passages.
In conclusion, the present study demonstrated that
hUCMSCs maintain a stable immunophenotype and chromosome structure
when cultured for 6 passages in vitro, which provides an
experimental basis for the safety of hUCMSC cytotherapy.
There were a number of limitations in the present
study. The number of the subjects in this study was small, which
may have affected the reliability of the results. The karyotype
stability of hUCMSCs was not evaluated following long-term culture.
It remains to be determined whether the chromosomes of hUCMSCs
change following in vitro culture for larger numbers of
generations and over a longer period of time in future studies.
References
|
1
|
Tong CK, Vellasamy S, Tan BC, Abdullah M,
Vidyadaran S, Seow HF and Ramasamy R: Generation of mesenchymal
stem cell from human umbilical cord tissue using a combination and
mechanical disassociation method. Cell Biol Int. 35:221–226. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arufe MC, De la Fuente A, Fuentes I, Toro
FJ and Blanco FJ: Umbilical cord as a mesenchymal stem cell source
for treating joint pathologies. World J Orthop. 2:43–50.
2011.PubMed/NCBI
|
|
3
|
Méndez-Ferrer S, Michurina TV, Ferraro F,
Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A,
Enikolopov GN and Frenette PS: Mesenchymal and haematopoietic stem
cells form a unique bone marrow niche. Nature. 466:829–834.
2010.PubMed/NCBI
|
|
4
|
Montanucci P, Basta G, Pescara T, Pennoni
I, Di Giovanni F and Calafiore R: New simple and rapid method for
purification of mesenchymal stem cells from the human umbilical
cord Wharton jelly. Tissue Eng Part A. 17:2651–2661. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stewart MC and Stewart AA: Mesenchymal
stem cells: characteristics, sources, and mechanisms of action. Vet
Clin N Am Equine Pract. 27:243–261. 201l.PubMed/NCBI
|
|
6
|
Ringden O and Le Blanc K: Mesenchymal stem
cells for treatment of acute and chronic graft-versus-host disease,
tissue toxicity and hemorrhages. Best Pract Res Clin Haematol.
24:65–72. 201l. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kern S, Eichler H, Stoeve J, Klüter H and
Bieback K: Comparative analysis of mesenchymal stem cells from bone
marrow, umbilical cord blood, or adipose tissue. Stem Cells.
24:1294–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho H, Seo YK, Jeon S, Yoon HH, Choi YK
and Park JK: Neural differentiation of umbilical cord mesenchymal
stem cells by sub-sonic vibration. Life Sci. 90:591–599. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuzuka T, Rachakatla RS, Doi C, Maurya
DK, Ohta N, Kawabata A, Pyle MM, Pickel L, Reischman J, Marini F,
Troyer D and Tamura M: Human umbilical cord matrix-derived stem
cells expressing interferon-beta gene significantly attenuate
bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer.
70:28–36. 2010. View Article : Google Scholar
|
|
10
|
Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis
Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder
TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L,
Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE and
Blazar BR: Sarcoma derived from cultured mesenchymal stem cells.
Stem Cells. 25:371–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bagley RG, Weber W, Rouleau C, Yao M,
Honma N, Kataoka S, Ishida I, Roberts BL and Teicher BA: Human
mesenchymal stem cells from bone marrow express tumor endothelial
and stromal markers. Int J Oncol. 34:619–627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fotino C, Ricordi C, Lauriola V, Alejandro
R and Pileggi A: Bone marrow-derived stem cell transplantation for
the treatment of insulin dependent diabetes. Rev Diabet Stud.
7:144–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis
Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder
TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L,
Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE and
Blazar BR: Sarcoma derived from cultured mesenchymal stem cells.
Stem Cells. 25:371–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tasso R, Augello A, Carida’ M, Postiglione
F, Tibiletti MG, Bernasconi B, Astigiano S, Fais F, Truini M,
Cancedda R and Pennesi G: Development of sarcomas in mice implanted
with mesenchymal stemcells seeded onto bioscaffolds.
Carcinogenesis. 30:150–157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Hisha H, Takaki T, Adachi Y, Li M,
Song CH, Feng W, Okazaki S, Mizokami T, Kato J, Inaba M, Hosaka N,
Maki M and Ikehara S: Trabsformation potential of bone marrow
stromal cells into undifferentiated high-grade pleomorphic sarcoma.
J Canc Res Clin Oncol. 136:829–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chabannes D, Hill M, Merieau E, Rossignol
J, Brion R, Soulillou JP, Anegon I and Cuturi CM: A role for heme
oxygenase-1 in the immunosuppressive effect of adult rat and human
mesenchymal stem cells. Blood. 110:3691–3694. 2007. View Article : Google Scholar : PubMed/NCBI
|