Introduction
Postoperative cognitive dysfunction (POCD) is a
common complication following major surgery, particularly in
elderly individuals. POCD is defined as a decline in a variety of
neuropsychological functions, including memory, executive
functioning and speed of processing, and is often accompanied by a
decrease in social skills. POCD may last for several months or
years and affect the quality of life, or may even manifest as a
permanent cognitive decline marked by further deterioration
(1). Although the etiology and
pathophysiology of POCD are not fully understood, several risk
factors for POCD have been identified, including the type of
surgery, the extent of surgical trauma and the stress response,
while increased age has been consistently reported as a risk factor
(2–4).
Adolescent idiopathic scoliosis affects 1–3% of the
population at risk (children aged between 10 and 16 years), and is
a common disease in adolescent orthopedic hospital departments,
usually requiring surgical treatment (5). Idiopathic scoliosis surgical
treatment is often accompanied by a large trauma, a large amount of
bleeding and extensively prolonged surgery duration. In addition, a
wake-up test is often required during surgery for early detection
of spinal cord injuries. These factors induce intensive stress
responses to the patients. In addition, Welsh et al found
that verbal fluency, action sequences and complex planning skills
may not mature in adolescents until the age of 12 or later
(6). Therefore, it may be
hypothesized that these adolescents have an elevated risk for POCD,
following orthopedic surgery.
In the present study, variations in the levels of
plasma markers, including cortisol, interleukin (IL)-1β, IL-6,
IL-10, and tumor necrosis factor-α (TNF-α), were investigated to
determine whether these markers are potential predictive factors
for the development of POCD in patients with adolescent idiopathic
scoliosis following surgery.
Materials and methods
Subjects
The study was performed between July 2012 and March
2013 on 75 adolescent scoliosis patients aged between 11 and 18
years. The patients had been categorized as American Society of
Anesthesiologists (ASA) classification I or II (7), and were scheduled for orthopedic
surgery with general anesthesia induced by total intravenous
anesthesia. The patients did not suffer from severe hypotension,
hypoxemia or other serious incidents, such as cardiac arrest.
Patients with severe congenital, mental and neurological diseases,
organ dysfunction, including the liver and kidney, and hearing and
visual impairments, as well as those unwilling to comply with the
protocol or procedures, unable to understand Mandarin Chinese and
with a mini-mental state examination score of <23, were excluded
from the study. Among the 75 adolescent scoliosis patients
participating in the study, two patients obtained a score of <23
in the mini-mental state examination, one patient had severe
hearing impairment, four patients did not complete the cognitive
function test within seven days of surgery and two patients did not
complete the sample collection. Therefore, 66 patients completed
the sample collection and neurocognitive tests. All the procedures
were approved by the Ethics Committee of the Drum Tower Hospital of
Nanjing University (Nanjing, China). Informed consent was obtained
from all the patients or the patients’ families.
Anesthesia and postoperative
analgesia
All the patients followed the routine ‘nothing by
mouth’ after midnight or 6 h prior to surgery. The patients were
not administered any sedatives or other medicine. The patient’s
electrocardiogram, pulse oximetry and invasive blood pressure were
continuously monitored during anesthesia. Radial artery
catheterization for perioperative blood pressure monitoring and
internal jugular vein catheterization for central venous pressure
monitoring were required. Induction of anesthesia was achieved by
midazolam (0.1 mgkg−1), propofol (1.5
mgkg−1), vecuronium (0.15 mgkg−1) and
fentanyl (6 μgkg−1). Maintenance of anesthesia was
achieved by propofol (4–12 mgkg−1h−1),
dexmedetomidine (0.2 μgkg−1h−1), atracurium
(5 μgkg−1min−1) and remifentanil (0.2
μgkg−1min−1). All anesthetics were withheld
during the wake-up test, which was performed smoothly in all cases
with the patients cooperating well. Following the wake-up test,
propofol (4–6 mgkg−1h−1), dexmedetomidine
(0.2 μgkg−1h−1), atracurium (5
μgkg−1min−1) and fentanil (2
μgkg−1) were used to maintain anesthesia. For patients
with bradycardia, administration of 0.05–0.1 mg atropine was
required.
Fentanil (3 μgkg−1) was routinely
administered to each patient 15 min prior to the end of the
surgery. All patients received the same postoperative pain control
protocol, namely, patient-controlled analgesia (a constant infusion
rate of 2 ml/h with a lock time of 15 min), with administration of
fentanil (12.5 μgkg−1) and ondansetron (8 mg) for two
days.
Enzyme-linked immunosorbent assay (ELISA)
and radioimmunoassay
Blood samples were collected at 6:00 AM on the day
of admission and at day 2 following surgery. After centrifugation
at 470 × g for 10 min, the plasma samples were collected and stored
at −70°C for future use. The plasma levels of IL-1β, IL-6, IL-10
and TNF-α were measured using ELISA kits (Yunhan Biotechnology Co.,
Ltd., Shanghai, China), according to the manufacturer’s
instructions. Cortisol concentrations were determined using a
radioimmunoassay (Department of Nuclear Medicine, Drum Tower
Hospital).
Preoperative evaluation
The patient age, gender, height, body weight,
education and other pertinent information were recorded. The
subjective pain of the patients was assessed with a 10-point linear
visual analog scale, where 0 represented ‘no pain’ and 10
represented ‘severe pain’. The patients received training in the
use of the visual analog scale. Pain scores were determined on day
1 and day 7 after the surgery (8).
Cognitive function measurement
Cognitive function was assessed one day prior to and
at day 7 following surgery in a quiet room, using a cognitive
function test in Chinese (9,10).
This test battery was designed according to the International Study
for Postoperative Cognitive Dysfunction (11), as well as the situation of the
patients. A mini-mental state examination was performed first as a
screening approach, assessing the orientation, memory and ability
to follow instructions. Patients obtaining a score of <23 were
excluded from the study (12,13).
Next, a visual and verbal learning test was performed to evaluate
the word learning and memory abilities of the patients (14). The patients were required to
memorize 10 words by studying the words three times, and recall as
many words as possible after 20 min. The number of words recalled
was recorded. During the 20-min period, a two-part Stroop
color-word test was performed to evaluate the executive function of
the patients (15,16). In the first part, the patients were
required to read aloud 30 color names, while in the second part,
the patients were required to name 30 colored patches. The time
taken to complete each part of the test and the number of errors
made were recorded.
A digit span test was used to assess the short-term
memory of the patients (17). In
addition, a trail making test required the patients to cross out
digits in ascending order (range, 1–25), which were randomly
distributed in circles (18). The
time taken to complete the task was measured and the number of
errors made was counted (17,18).
Finally, a number-symbol test was performed, in which the patients
were asked to match number and symbol pairs as quickly as possible
within the required time of 90 sec (19). The amount of correctly matched
pairs were recorded for each patient.
POCD was characterized as the deterioration of one
standard deviation (SD) compared with the preoperative test
results, obtained from at least two of the aforementioned tests
(referred to as ‘the 1-SD criterion’) (2,20).
Thereafter, the patients were divided into the POCD and non-POCD
groups depending on whether POCD occurred within seven days of the
surgery.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). The data are presented as
the mean ± SD. Intergroup comparisons were conducted by
independent-sample t-tests, while intragroup comparisons were
analyzed by paired-sample t-tests. Categorical variables were
analyzed using χ2 or Fisher’s exact tests. In addition,
binary logistic analysis was used to investigate the risk factors
for the development of POCD. Intergroup comparisons of measurement
data with non-normal distribution were performed using the
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic data
Demographic data of the patients are shown in
Table I. No statistically
significant differences were observed between the POCD and non-POCD
groups with regard to the age, gender, height, body weight, years
of education, basic mini-mental state examination scores, ASA
classification and length of surgery. In addition, no statistically
significant differences were identified in the visual analog scale
scores of the two groups at day 1 and day 7 after surgery
(P>0.05). These results demonstrated that there was no
statistically significant difference in the demographic data of the
two groups.
 | Table IDemographic data of the POCD and
non-POCD patients. |
Table I
Demographic data of the POCD and
non-POCD patients.
| Admission
characteristics | POCD (n=19) | Non-POCD (n=47) |
|---|
| Age (years) | 14±2 | 14±2 |
| Height (cm) | 158±4 | 160±7 |
| Body weight (kg) | 46±9 | 48±9 |
| Gender, M/F (n) | 6/13 | 13/34 |
| Education
(years) | 7.8±1.8 | 7.6±1.6 |
| ASA classification,
I/II (n) | 3/16 | 7/40 |
| Length of surgery
(min) | 264±60 | 270±60 |
| MMSE scores | 29.2±1.1 | 29.2±1.0 |
Identification of patients with POCD
according to the International Study for Postoperative Cognitive
Dysfunction
To identify patients with POCD, cognitive function
measurements were performed with a test battery designed according
to the International Study for Postoperative Cognitive Dysfunction
and the actual situation of the patients. According to the POCD
criteria, 19 patients (28.8%) were diagnosed with POCD within seven
days of the surgery (Table II).
The patients in the POCD and non-POCD groups were subsequently
enrolled in further tests.
 | Table IINumber of patients with >1-SD
decline in the test battery at day 7 following surgery. |
Table II
Number of patients with >1-SD
decline in the test battery at day 7 following surgery.
| >1-SD decline | Patients (n=66) |
|---|
| Two tests | 13 |
| Three tests | 5 |
| Four tests | 1 |
| Five tests | 0 |
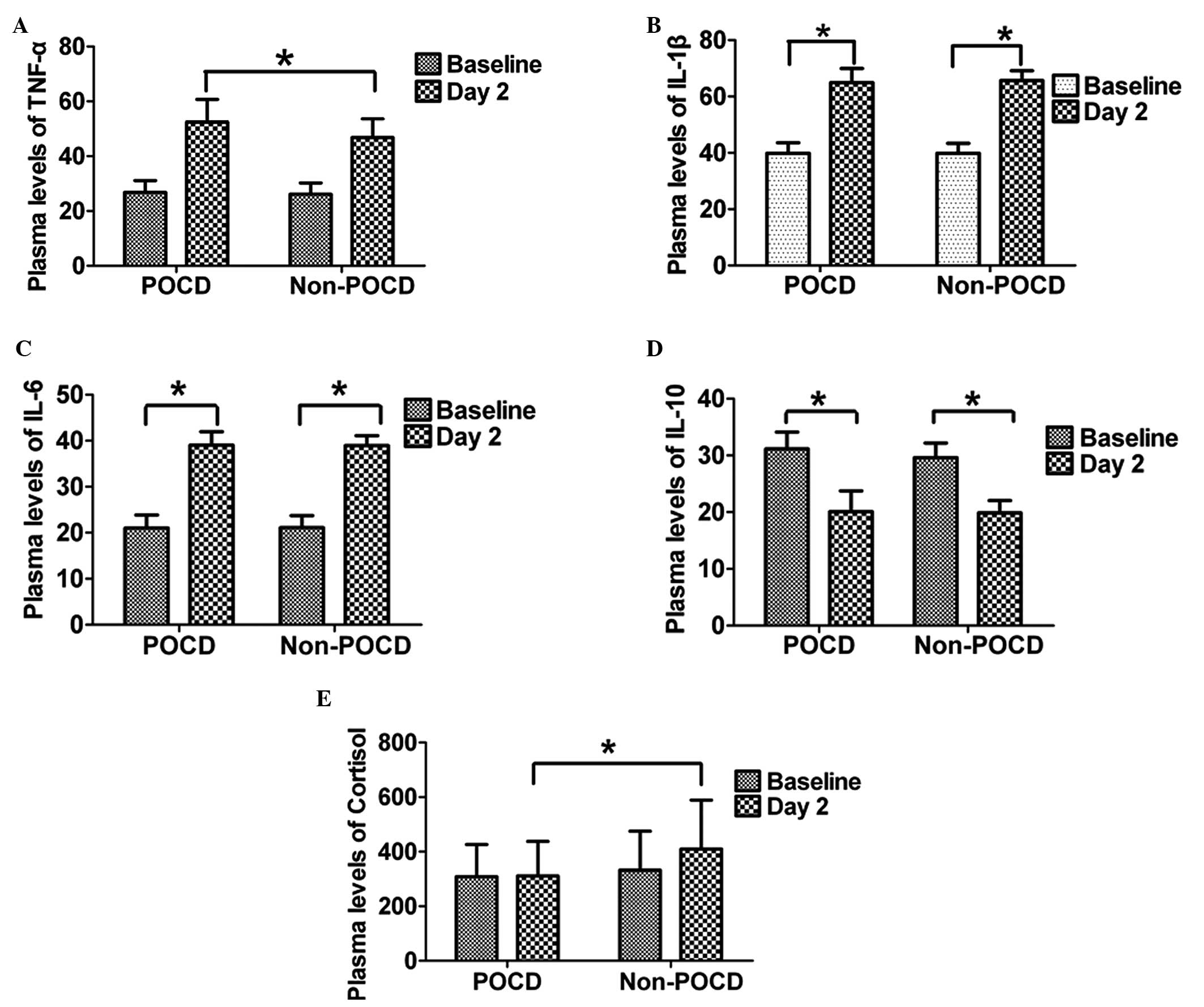
TNF-α may be a risk factor for the
indication of POCD
An ELISA was used to measure the plasma levels of
inflammatory mediators. At day 2 after surgery, the plasma levels
of IL-1β, IL-6 and TNF-α in the POCD and non-POCD groups were
higher when compared with the baseline levels (P<0.05), and no
statistically significant differences were observed in the baseline
levels between the two groups. At day 2 following surgery, the
plasma level of TNF-α in the POCD group was higher compared with
the non-POCD group (P<0.05; Fig.
1A). However, no statistically significant differences were
observed in the plasma levels of IL-1β and IL-6 between the POCD
and non-POCD groups (P>0.05; Fig.
1B and C). By contrast, the plasma levels of IL-10 for patients
in the POCD and non-POCD groups at day 2 after surgery were lower
than the baseline levels; however, no statistically significant
difference was observed (P>0.05; Fig. 1D). The plasma level of TNF-α
changed significantly and binary logistic analysis demonstrated
that the elevation of TNF-α at day 2 after surgery may be a risk
factor for the occurrence of POCD (P=0.018; odds ratio, 1.108; 95%
confidence interval, 1.017–1.207).
Cortisol concentration may not be a
suitable indicator for the occurrence of POCD
A radioimmunoassay was employed to determine the
concentration of cortisol. No statistically significant difference
was identified in the baseline concentrations of cortisol between
the POCD and non-POCD groups. However, at day 2 following surgery,
the concentration of cortisol in the non-POCD group was found to be
higher compared with the POCD group (P<0.05; Fig. 1E). Using the Mann-Whitney U test,
the difference in the changing cortisol concentrations between the
two groups was investigated (changing level = preoperative cortisol
concentration - postoperative concentration). The difference in the
pre- and postoperative cortisol concentrations was found to be more
significant in the non-POCD group compared with the POCD group.
Taking these factors into account in the binary logistic analysis,
the reduced cortisol concentration in POCD patients indicated that
cortisol may not be a suitable indicator for the occurrence of
POCD.
Discussion
In the present study, a degree of inflammation was
found in all the patients following idiopathic scoliosis surgery.
At day 2 after surgery, the plasma level of TNF-α in the POCD
patients was found to be significantly higher when compared with
the non-POCD patients. In addition, non-POCD patients exhibited
significantly higher postoperative concentrations of cortisol when
compared with the POCD patients.
In previous studies, the incidence of POCD at day 7
after surgery varied widely (11,21).
The conflicting results may be attributed to the different criteria
for the diagnosis of POCD, as well as the different study
populations and types of surgery (22). To the best of our knowledge, no
studies exist on POCD in adolescents.
POCD is increasingly recognized as a common
complication following major surgery; however, the exact
pathophysiology remains unclear. Previous studies have primarily
focused on the risk factors associated with early POCD (23–25).
In terms of baseline factors associated with the demographic data
of the patients, an increased age and lower level of education have
been identified as the main risk factors for POCD, according to the
International Study for Postoperative Cognitive Dysfunction
(1,11). However, the patients enrolled in
the present study were not of increased age or lower education
levels, and a number of patients developed POCD, which may be due
to a variety of reasons. Adolescent scoliosis orthopedic surgery
often leads to a large trauma and a large amount of bleeding during
the surgery. Cibelli et al found that surgery may result in
complex systemic responses, including neuroinflammation (26). Systemic and neural inflammation, as
a result of surgery, may directly affect the cognitive outcomes of
patients (1,27,28).
To a certain extent, this observation concurs with the results of
present study, which demonstrated that the plasma level of TNF-α in
POCD patients was significantly higher compared with non-POCD
patients at day 2 following surgery. However, the present study
only measured the extent of peripheral inflammation. Terrando et
al demonstrated in preclinical experiments that peripheral
surgery disrupts the blood-brain barrier through the release of
TNF-α, which facilitates the migration of macrophages into the
hippocampus and impairs cognitive function (29). The results of the present study
revealed that inflammatory responses were more severe in POCD
patients compared with non-POCD patients. In addition, blood loss
and tissue injury in orthopedic procedures may stimulate the immune
system to produce more cytokines, increasing inflammatory
responses. Data from preclinical studies have supported the
hypothesis that inflammation is a possible pathogenic mechanism for
POCD (30–32).
The stress response is a physiological reaction for
survival, occurring in response to perceived harmful events,
attacks or threats (33), and is
recognized as the first stage of a general adaptation syndrome.
Moderate stress is beneficial to the body, helping to maintain
homeostasis and increasing the ability to adapt to the environment.
However, an extensive or intense stress response is potentially
harmful (34). A large number of
studies have hypothesized that excessive stress may be a possible
pathogenic mechanism for the development of POCD (35). Bisschop et al indicated that
high levels of cortisol were closely associated with cognitive
decline (4). In addition, Ji et
al revealed that the plasma cortisol concentration of elderly
POCD patients was higher compared with elderly non-POCD patients at
day 7 after hip fracture surgery, performed with spinal anesthesia
(36). In the present study, the
baseline cortisol concentrations were found to be similar in POCD
and non-POCD patients. However, at day 2 following surgery, the
level of cortisol in the non-POCD group was higher compared with
the POCD group. The self-regulation ability of the immune system in
POCD patients was hypothesized to be abnormal; thus, adaptive
changes were unable to be made to avoid the risks of intense
stress. As the pathophysiology of adolescent idiopathic scoliosis
is unknown, the present study indicates that the adolescent
population may be susceptible to POCD.
In conclusion, the observations of the present study
indicated that high plasma levels of TNF-α in patients with
adolescent idiopathic scoliosis at day 2 after surgery can predict
the incidence of early POCD. However, a large number of clinical
studies are required to clarify the role of TNF-α as a predictive
factor for POCD.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (nos. 81371207, 81171047,
81070892 and 81171048), the Natural Science Foundation of Jiangsu
Province (no. BK2010105) and the Department of Health, Jiangsu,
China (nos. XK201140 and RC2011006).
References
|
1
|
Tsai TL, Sands LP and Leung JM: An update
on postoperative cognitive dysfunction. Adv Anesth. 28:269–284.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meybohm P, Renner J, Broch O, et al:
Postoperative neurocognitive dysfunction in patients undergoing
cardiac surgery after remote ischemic preconditioning: a
double-blind randomized controlled pilot study. PloS One.
8:e647432013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vacas S, Degos V, Feng X and Maze M: The
neuroinflammatory response of postoperative cognitive decline. Br
Med Bull. 106:161–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bisschop PH, de Rooij SE, Zwinderman AH,
van Oosten HE and van Munster BC: Cortisol, insulin, and glucose
and the risk of delirium in older adults with hip fracture. J Am
Geriatr Soc. 59:1692–1696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weinstein SL, Dolan LA, Cheng JC,
Danielsson A and Morcuende JA: Adolescent idiopathic scoliosis.
Lancet. 371:1527–1537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Welsh MC, Labbé EE and Delayney D:
Cognitive strategies and personality variables in adherence to
exercise. Psychol Rep. 68:1327–1335. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fitz-Henry J: The ASA classification and
peri-operative risk. Ann R Coll Surg Engl. 93:185–187.
2011.PubMed/NCBI
|
|
8
|
Otunctemur A, Dursun M, Besiroglu H, et
al: The effectivity of periprostatic nerve blockade for the pain
control during transrectal ultrasound guided prostate biopsy. Arch
Ital Urol Androl. 85:69–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ballard C, Jones E, Gauge N, et al:
Optimised anaesthesia to reduce post operative cognitive decline
(POCD) in older patients undergoing elective surgery, a randomised
controlled trial. PloS One. 7:e374102012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lili X, Zhiyong H and Jianjun S: A
preliminary study of the effects of ulinastatin on early
postoperative cognition function in patients undergoing abdominal
surgery. Neurosci Lett. 541:15–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moller JT, Cluitmans P, Rasmussen LS, et
al: Long-term postoperative cognitive dysfunction in the elderly
ISPOCD1 study. ISPOCD investigators International Study of
Post-Operative Cognitive Dysfunction. Lancet. 351:857–861. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong GK, Lam SW, Wong A, Ngai K, Poon WS
and Mok V: Comparison of montreal cognitive assessment and
mini-mental state examination in evaluating cognitive domain
deficit following aneurysmal subarachnoid haemorrhage. PloS One.
8:e599462013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kasten M, Bruggemann N, Schmidt A and
Klein C: Validity of the MoCA and MMSE in the detection of MCI and
dementia in Parkinson disease. Neurology. 75:478–479. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kudiaki C and Aslan A: Executive functions
in a Turkish sample: associations with demographic variables and
normative data. Appl Neuropsychol. 15:194–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong RH, Howe PR, Bryan J, Coates AM,
Buckley JD and Berry NM: Chronic effects of a wild green oat
extract supplementation on cognitive performance in older adults: a
randomised, double-blind, placebo-controlled, crossover trial.
Nutrients. 4:331–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aslanyan G, Amroyan E, Gabrielyan E,
Nylander M, Wikman G and Panossian A: Double-blind,
placebo-controlled, randomised study of single dose effects of
ADAPT-232 on cognitive functions. Phytomedicine. 17:494–499. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Attia A, Rapp SR, Case LD, et al: Phase II
study of Ginkgo biloba in irradiated brain tumor patients: effect
on cognitive function, quality of life, and mood. J Neurooncol.
109:357–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sarrar L, Ehrlich S, Merle JV, Pfeiffer E,
Lehmkuhl U and Schneider N: Cognitive flexibility and
Agouti-related protein in adolescent patients with anorexia
nervosa. Psychoneuroendocrinology. 36:1396–1406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hunt LA and Bassi CJ: Near-vision acuity
levels and performance on neuropsychological assessments used in
occupational therapy. Am J Occup Ther. 64:105–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rudolph JL, Schreiber KA, Culley DJ, et
al: Measurement of post-operative cognitive dysfunction after
cardiac surgery: a systematic review. Acta Anaesthesiol Scand.
54:663–677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Voigt Hansen M, Rasmussen LS, Jespersgaard
C, Rosenberg J and Gogenur I: There is no association between the
circadian clock gene HPER3 and cognitive dysfunction after
noncardiac surgery. Anesth Analg. 115:379–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salazar F, Doñate M, Boget T, et al:
Intraoperative warming and post-operative cognitive dysfunction
after total knee replacement. Acta Anaesthesiol Scand. 55:216–222.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan MT, Cheng BC, Lee TM and Gin T; CODA
Trial Group. BIS-guided anesthesia decreases postoperative delirium
and cognitive decline. J Neurosurg Anesthesiol. 25:33–42. 2013.
View Article : Google Scholar
|
|
24
|
Wang Y, Sands LP, Vaurio L, Mullen EA and
Leung JM: The effects of postoperative pain and its management on
postoperative cognitive dysfunction. Am J Geriatr Psychiatry.
15:50–59. 2007. View Article : Google Scholar
|
|
25
|
Block RI, Thomas JJ, Bayman EO, Choi JY,
Kimble KK and Todd MM: Are anesthesia and surgery during infancy
associated with altered academic performance during childhood?
Anesthesiology. 117:494–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cibelli M, Fidalgo AR, Terrando N, et al:
Role of interleukin-1beta in postoperative cognitive dysfunction.
Ann Neurol. 68:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobbe P, Vodovotz Y, Kaczorowski DJ,
Mollen KP, Billiar TR and Pape HC: Patterns of cytokine release and
evolution of remote organ dysfunction after bilateral femur
fracture. Shock. 30:43–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie G, Zhang W, Chang Y and Chu Q:
Relationship between perioperative inflammatory response and
postoperative cognitive dysfunction in the elderly. Med Hypotheses.
73:402–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Terrando N, Eriksson LI, Ryu JK, et al:
Resolving postoperative neuroinflammation and cognitive decline.
Ann Neurol. 70:986–995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Belarbi K, Jopson T, Tweedie D, et al:
TNF-α protein synthesis inhibitor restores neuronal function and
reverses cognitive deficits induced by chronic neuroinflammation. J
Neuroinflammation. 9:232012. View Article : Google Scholar
|
|
31
|
Steinman L: Modulation of postoperative
cognitive decline via blockade of inflammatory cytokines outside
the brain. Proc Natl Acad Sci USA. 107:20595–20596. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perez-Asensio FJ, Perpiñá U, Planas AM and
Pozas E: Interleukin-10 regulates progenitor differentiation and
modulates neurogenesis in adult brain. J Cell Sci. 126:4208–4219.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kazmierski J, Banys A, Latek J, Bourke J
and Jaszewski R: Cortisol levels and neuropsychiatric diagnosis as
markers of postoperative delirium: a prospective cohort study. Crit
Care. 17:R382013. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chrousos GP and Kino T: Glucocorticoid
action networks and complex psychiatric and/or somatic disorders.
Stress. 10:213–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rasmussen LS, O’Brien JT, Silverstein JH,
et al; ISPOCD2 Investigators. Is peri-operative cortisol secretion
related to post-operative cognitive dysfunction? Acta Anaesthesiol
Scand. 49:1225–1231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji MH, Shen JC, Gao R, et al: Early
postoperative cognitive dysfunction is associated with higher
cortisol levels in aged patients following hip fracture surgery. J
Anesth. 27:942–944. 2013. View Article : Google Scholar : PubMed/NCBI
|