Introduction
Lung cancer is a leading cause of cancer-associated
mortality worldwide, with >1 million mortalities annually
(1). Small-cell lung cancer (SCLC)
accounts for 10–15% of lung cancer cases (2). It is estimated that 70% of patients
with SCLC have extensive-stage SCLC (ES-SCLC) at the time of
diagnosis (3).
Currently, the first-line therapy for ES-SCLC
remains dependent on platinum-based chemotherapy with
cisplatin-etoposide (EP) (4). The
addition of thoracic radiotherapy (TRT) is suggested in certain
patients due to its confirmed value in the improvement of the
overall survival (OS) rate (5,6).
Although SCLC is sensitive to chemotherapy and radiotherapy, with a
60–80% response rate, the median survival rate of patients with
ES-SCLC is estimated to be only 7–12 months following 4–6 cycles of
standard chemotherapy (4).
Systemic chemotherapy in combination with concurrent
TRT have been demonstrated to be effective in the treatment of
limited-stage SCLC (LS-SCLC) by improving the progression-free
survival (PFS) and OS rates (7–9). The
National Comprehensive Cancer Network (NCCN) guidelines recommend
that TRT should be initiated during the first or second cycles of
chemotherapy in cases of LS-SCLC at 45 Gy administered twice per
day in 1.5 Gy fractions, or 60–70 Gy in 2 Gy daily fractions
(10–12). However, the effect of TRT on ES-SCLC
remains unknown. The present retrospective study evaluated the
effect of 3-dimensional (3D) conformal TRT on the clinical outcome
of patients with ES-SCLC.
Materials and methods
Subjects
A total of 165 patients with histologically or
cytologically confirmed ES-SCLC, enrolled at the Shandong Cancer
Hospital (Jinan, China) from January 2005 to December 2008, were
involved in the present study. The patients included 140 males and
25 females, with a median age of 60 years (range, 26–87 years). The
subjects were assigned to two groups. Subjects in the ChT group
(n=83) underwent chemotherapy only, while those in the ChT/TRT
group (n=82) were given chemotherapy concurrently with TRT.
Therapy
The chemotherapy schedule consisted of the
administration of etoposide 100 mg/m2 on days 1–5,
combined with cisplatin 30 mg/m2 on days 1–3 or
carboplatin 300 mg/m2 on day 1. 3D-conformal TRT was
performed following 1–6 cycles of chemotherapy at a dose of 1.5
Gy/fraction twice per day or 2 Gy/fraction daily, with a total dose
of 40–62 Gy.
The target volume was defined according to the
preoperative radiotherapy response assessment. If a stable disease
(SD) was achieved, the gross target volume (GTV) included the
primary tumor and the positive lymph node. If a complete response
(CR) was achieved, the GTV included the primary tumor bed and the
locations of the positive lymph node, which were diagnosed by
computed tomography (CT) scanning prior to chemotherapy. If a
progressive disease (PD) occurred, the GTV included the primary
tumor, the positive lymph node and the new lesions. The clinical
target volume (CTV) was defined as the GTV plus a 7 mm margin,
while the planning target volume (PTV) was defined as the CTV plus
a 10 mm margin. Prophylactic cranial irradiation (PCI) was
administered to the 5 patients who achieved CR.
Assessment of response to therapy
All patients underwent a physical examination,
electrocardiography, blood chemistry analyses, brain magnetic
resonance imaging (MRI), thoracic and abdominal CT scans, and
radionuclide bone imaging prior to treatment. All examinations,
with the exception of radionuclide bone imaging, were repeated
every 2 cycles of chemotherapy or prior to TRT, and then every 3
months until 2 years, followed by every 3–6 months.
The response to the therapy was assessed using the
Response Evaluation Criteria in Solid Tumors (13). A CR was defined as the complete
disappearance of all objective evidence of disease for ≥4 weeks; a
partial response (PR) was defined as ≥50% reduction in the sum of
the longest diameter (LD) of target lesions lasting ≥4 weeks,
taking as reference the baseline sum LD and without the appearance
of any new lesions. PD was defined as the development of new
lesions or a ≥20% increase in the sum of the LD of the target
lesions; SD was defined as neither sufficient reduction to qualify
for PR nor sufficient increase to qualify for PD lasting ≥6 weeks,
taking as reference the smallest sum LD (13).
Evaluation of therapy-associated
toxicity
Toxic effects associated with the therapy, including
leukopenia, thrombocytopenia, anemia, nausea and vomiting, were
assessed according to the National Cancer Institute Common Toxicity
Criteria (NCI-CTC) version 3.0. Radiation-induced pneumonitis and
esophagitis were evaluated in the ChT/TRT group only.
Ethical consideration
The present study was approved by the Ethics Review
Committee of Shandong Cancer Hospital. Informed consent was
obtained from the patients or their relatives prior to their
participation, with a detailed description of the potential
benefits of the study.
Statistical analysis
Differences in proportions were tested for
statistical significance using the χ2 test. The
Kaplan-Meier method was used to estimate OS and PFS. Factors used
in multivariate survival analysis included gender, age, Karnofsky
Performance Status (KPS) score, smoking status, TRT and the number
of chemotherapy cycles. A Cox proportional hazards model was
employed to identify significant variables. The associations
between clinical factors and OS rate were evaluated using
univariate survival analysis with the Kaplan-Meier method. All
statistical analyses were performed using SPSS software, version
18.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically statistical significant difference.
Results
Patient characteristics
All patients underwent a follow-up subsequent to
therapy, with a median period of 43.2 months (range, 27–73 months).
In the ChT/TRT group, 64 cases had metastasis in one organ, 17
cases had metastasis in two organs, and 1 case had metastasis in
three organs; metastasis to bone was detected in 31 patients, brain
metastasis in 17 patients, lung metastasis in 16 patients, liver
metastasis in 13 patients, adrenal gland metastasis in 17 patients
and metastasis to other organs in 7 patients. In the ChT group, 47
patients had metastasis in one organ, 33 patients had metastasis in
two organs, and 3 patients had metastasis in three organs; bone
metastasis was detected in 33 cases, brain metastasis in 21 cases,
lung metastasis in 18 cases, liver metastasis in 18 cases, adrenal
gland metastasis in 19 cases and there was metastasis to other
organs in 13 cases. There were no significant differences in
gender, age, KPS score, smoking status and the number of
chemotherapy cycles between the ChT and ChT/TRT groups (P>0.05).
However, a significant difference was detected in the number of
metastatic organs between the two groups (P=0.005; Table I).
 | Table I.Comparison of the demographic and
clinical characteristics of patients with extensive-stage
small-cell lung cancer in the ChT/TRT and ChT groups. |
Table I.
Comparison of the demographic and
clinical characteristics of patients with extensive-stage
small-cell lung cancer in the ChT/TRT and ChT groups.
| Characteristic | Total | ChT/TRT group
(%) | ChT group (%) | P-value |
|---|
| Gender, n |
|
|
| 0.836 |
| Male | 138 | 68 (82.9) | 70 (84.3) |
|
|
Female | 27 | 14 (17.1) | 13 (15.7) |
|
| Age |
|
|
| 0.339 |
| Range,
years | 26–87 | 26–83 | 36–87 |
|
| Median,
years | 59 | 55 | 64 |
|
| <60
years, n | 79 | 54 (65.9) | 25 (30.1) |
|
| ≥60
years, n | 86 | 28 (34.1) | 58 (69.9) |
|
| KPS score, n |
|
|
| 0.704 |
|
≥80 | 131 | 64 (78) | 67 (80.7) |
|
|
<80 | 34 | 18 (22) | 16 (19.3) |
|
| Smoking status,
n |
|
|
| 0.522 |
|
Yes | 102 | 53 (64.6) | 49 (59) |
|
| No | 63 | 29 (35.4) | 34 (41) |
|
| No. of ChT
cycles |
|
|
| 0.355 |
|
<4 | 20 | 8 (9.8) | 12 (14.5) |
|
| ≥4 | 145 | 74 (90.2) | 71 (85.5) |
|
| No. of metastatic
organs |
|
|
| 0.005 |
| 1 | 111 | 64 | 47 |
|
| ≥2 | 54 | 18 | 36 |
|
Response to therapy
Table II lists the
therapies administered to the 165 patients, including the 82
individuals undergoing chemotherapy and TRT, and the 83 individuals
undergoing chemotherapy alone. A total of 124 cases (75.2%)
responded to the therapy. There were 69 cases (84.1%) responsive to
therapy in the ChT/TRT group, including 12 cases achieving a CR.
There were 55 cases (66.2%) responsive in the ChT group, including
9 cases achieving a CR. The initial chemotherapy prior to TRT
resulted in CR in 2 cases, PR in 68 patients, SD in 11 cases and PD
in 1 patient in the ChT/TRT group. The timing of TRT with relation
to the course of chemotherapy is also presented in Table II.
 | Table II.Initial treatment of patients with
extensive-stage small-cell lung cancer in the ChT/TRT and ChT
groups. |
Table II.
Initial treatment of patients with
extensive-stage small-cell lung cancer in the ChT/TRT and ChT
groups.
| Treatment | ChT/TRT group
(n) | ChT group (n) |
|---|
| No. of ChT cycles
prior to TRT |
|
|
| 1 | 4 | - |
| 2 | 32 | - |
| 3 | 15 | - |
| 4 | 13 | - |
| 5 | 5 | - |
| 6 | 13 | - |
| No. of initial ChT
cycles |
| - |
| 3 | 1 | 2 |
| 4 | 16 | 21 |
| 5 | 11 | 7 |
| 6 | 29 | 33 |
| 7 | 9 | 6 |
| 8 | 16 | 14 |
| ChT regimen |
| - |
| EP | 67 | 62 |
| CE | 15 | 21 |
| Response to ChT
prior to TRT |
| - |
| CR | 2 | - |
| PR | 68 | - |
| SD | 11 | - |
| PD | 1 | - |
| TRT dose (Gy) |
| - |
| 40 | 5 | - |
| 42 | 1 | - |
| 44 | 4 | - |
| 45 | 22 | - |
| 46 | 1 | - |
| 48 | 4 | - |
| 50 | 12 | - |
| 52 | 3 | - |
| 54 | 3 | - |
| 56 | 5 | - |
| 58 | 1 | - |
| 60 | 20 | - |
| 62 | 1 | - |
| Radiation
dosing |
| - |
| 1.5
Gy/fraction BID | 22 | - |
| 2
Gy/fraction QD | 20 | - |
| PCI 30
Gy | 3 | 2 |
|
WBRT | 38 | 26 |
| Bone
RT | 31 | 33 |
Whole-brain radiotherapy was administered to 38
patients in the ChT/TRT group and 26 patients in the ChT group. The
64 cases with bone metastasis received bisphosphonate treatment at
the beginning of therapy. PCI was administered to the 5 patients
with CR. Additional chemotherapy or palliative radiotherapy was
given to 67 patients in the ChT/TRT group and 62 patients in the
ChT group with PD, while 6 patients in the ChT/TRT group and 9
patients in the ChT group accepted supportive care, which included
nutritional support and symptomatic treatment.
Survival rate
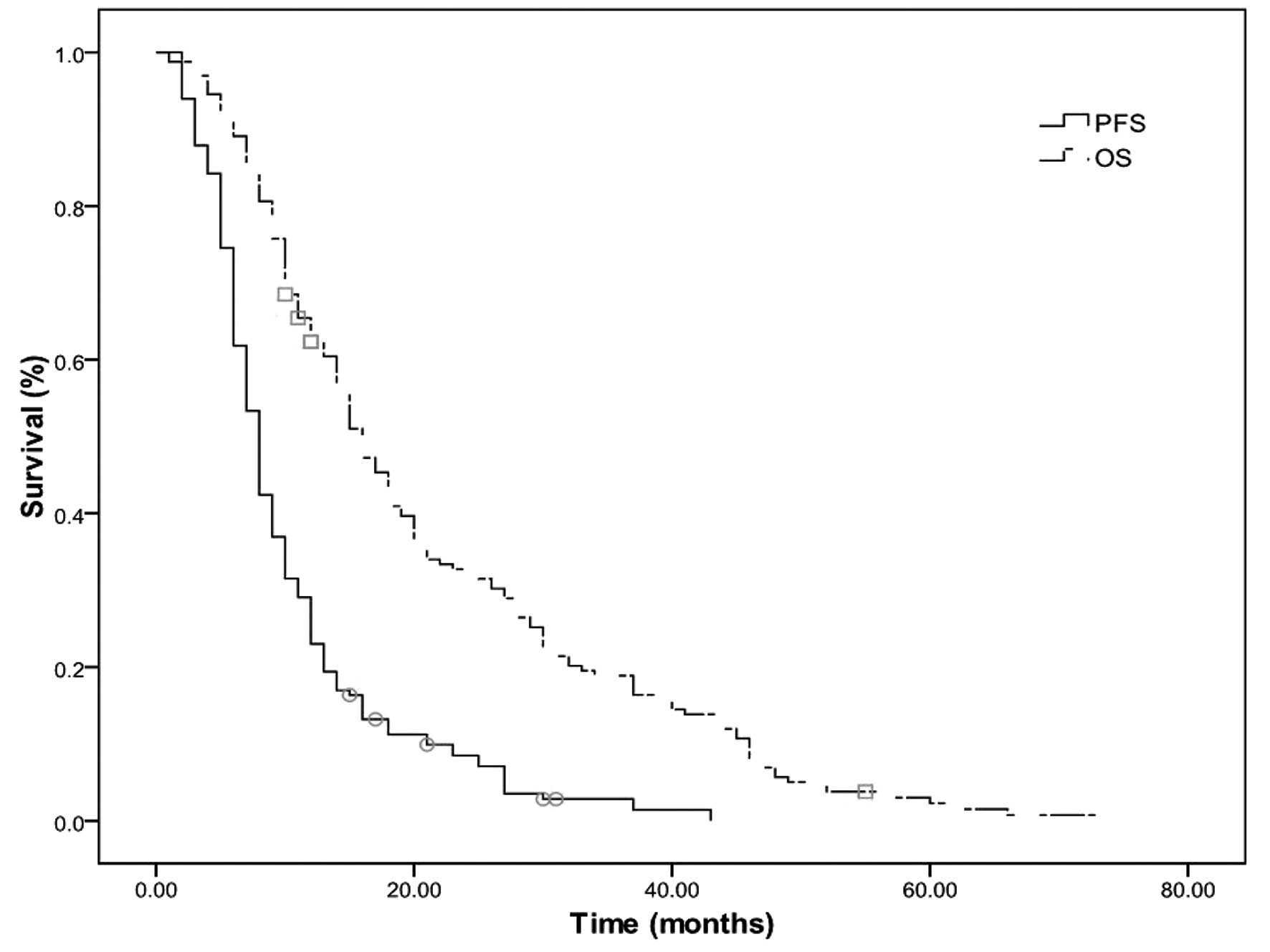
The median OS and PFS times were 15 and 8 months,
respectively, and the median 2- and 5-year OS rates were 31.5 and
2.4%, respectively (Fig. 1). The
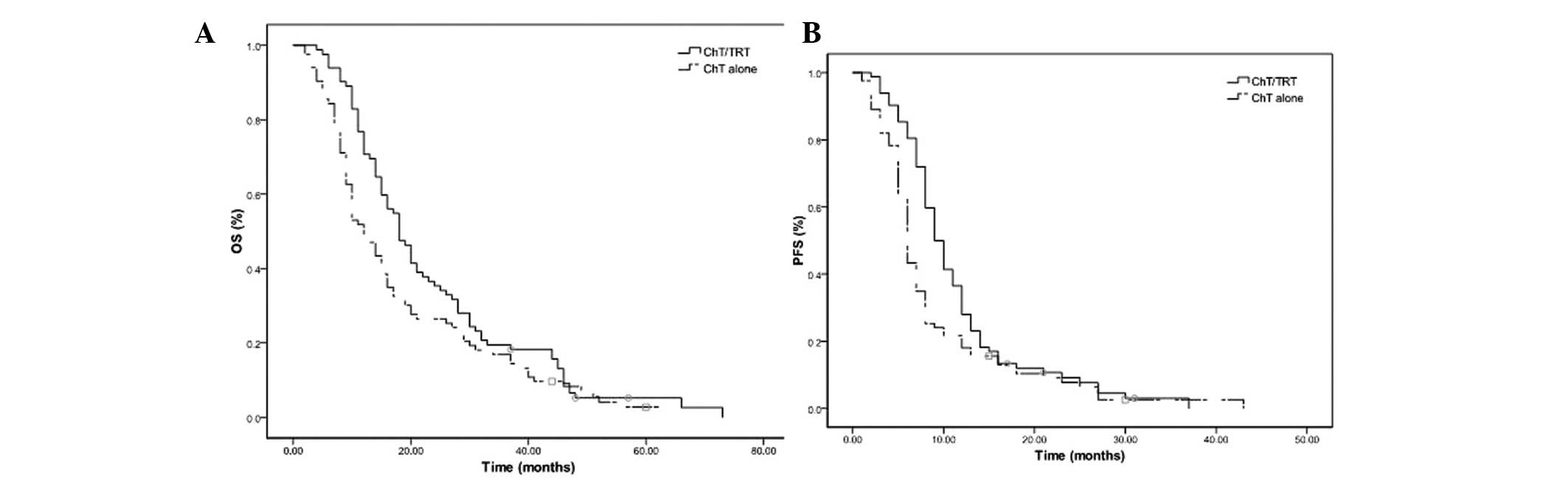
median OS and PFS times were 18 and 9 months in the ChT/TRT group,
with median 2- and 5-year OS rates of 35.3 and 2.4%, and 1- and
2-year PFS rates of 35.4 and 6.0%, respectively. In the ChT group,
the median OS and PFS times were 12 and 6 months, with median 2-
and 5-year OS rates of 14.5 and 2.4% (P=0.033; Fig. 2A) and 1- and 2-year PFS rates of 20.5
and 6.0%, respectively (P=0.011; Fig.
2B).
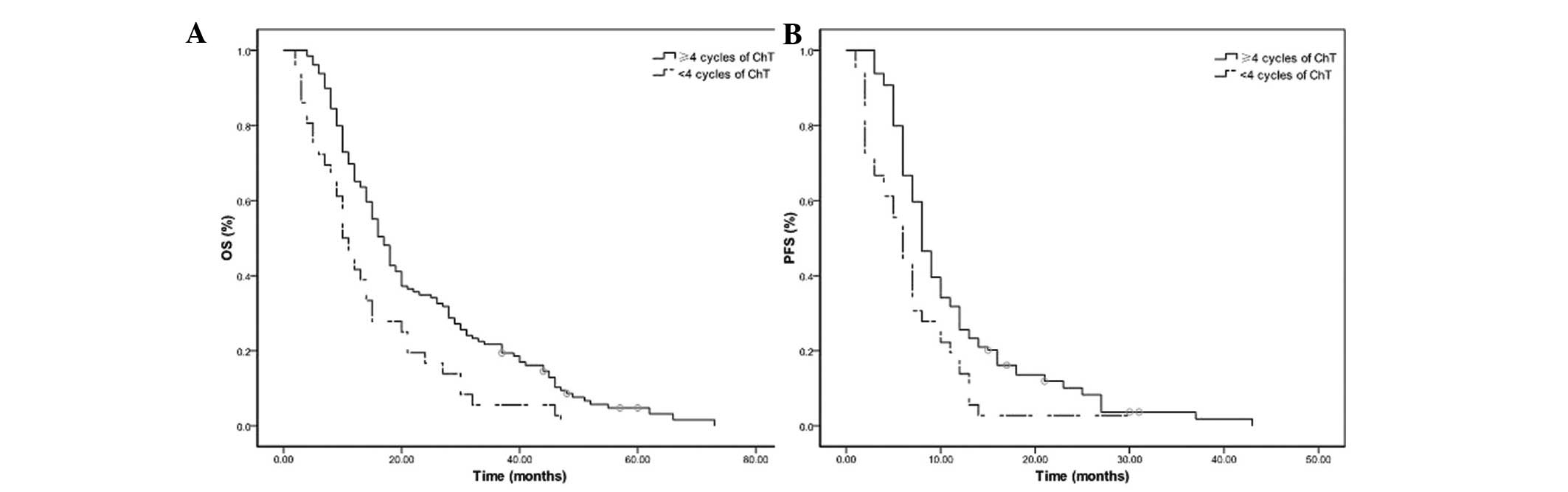
The median OS and PFS times were 17 and 8 months in
the 129 patients receiving ≥4 cycles of chemotherapy, and 10 and 6
months in the 36 patients receiving <4 cycles of chemotherapy
(all P=0.001; Fig. 3). The median
PFS was 11 months in the 20 patients undergoing 45 Gy radiotherapy
at 1.5 Gy/fraction twice per day, and 9 months in the 22 patients
undergoing 60 Gy radiotherapy at 2 Gy/fraction daily (P=0.043). No
significant differences were observed in the OS and PFS rates
between the patients receiving ≥6 and <6 cycles of chemotherapy
(P>0.05). Furthermore, there were no significant differences in
the OS and PFS rates among the patients receiving 2, 4 or 6 cycles
of chemotherapy prior to TRT in the ChT/TRT group (all P>0.05).
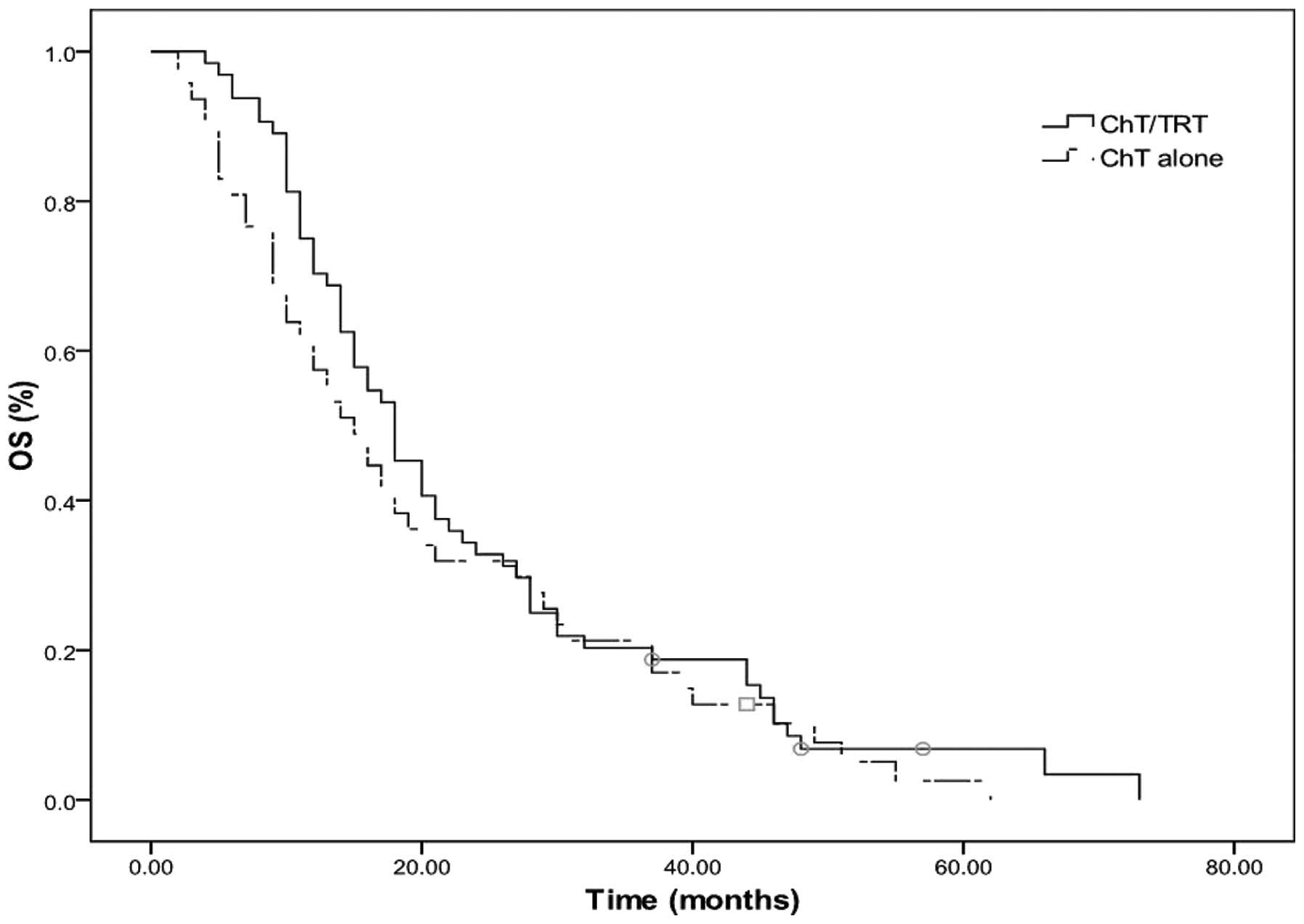
In patients with metastasis in 1 organ, the median OS time, 2- and
5-year OS rates were 18 months, 32.8 and 3.1% in the ChT/TRT group,
respectively, whereas they were 15 months, 48.9 and 2.1% in the ChT
group, respectively (Fig. 4).
Prognostic factors
Univariate survival analysis revealed that KPS
score, age at diagnosis, gender, smoking status, and number of
metastatic organs had no association with OS rate; however, TRT and
≥4 cycles of chemotherapy were found to correlate with survival
(Table III). Multivariate analysis
revealed that receiving ≥4 cycles of chemotherapy (P=0.001) and TRT
(P=0.008) were favorable prognostic factors for OS (Table IV).
 | Table III.Univariate analysis of the prognostic
factors for survival in patients with extensive-stage small-cell
lung cancer. |
Table III.
Univariate analysis of the prognostic
factors for survival in patients with extensive-stage small-cell
lung cancer.
| Characteristic | Mean survival
(months) | 2-year OS, % | 5-year OS, % | χ2 | P-value |
|---|
| Gender |
|
|
| 0.052 | 0.820 |
|
Male | 15 | 31.3 | 2.2 |
|
|
|
Female | 14 | 30.8 | 3.8 |
|
|
| Age, years |
|
|
| 2.590 | 0.108 |
|
<60 | 18 | 34.2 | 3.8 |
|
|
|
≥60 | 14 | 26.7 | 1 |
|
|
| KPS score |
|
|
| 0.056 | 0.813 |
|
≥80 | 16 | 32.3 | 1.6 |
|
|
|
<80 | 12 | 27.3 |
|
|
|
| Smoking status |
|
|
| 2.127 | 0.145 |
|
Yes | 15 | 26.5 | 0 |
|
|
| No | 15 | 34.9 | 4.8 |
|
|
| No. of ChT
cycles |
|
|
| 10.527 | 0.001 |
|
<4 | 10 | 16.7 | 0 |
|
|
| ≥4 | 17 | 34.1 | 3.1 |
|
|
| Treatment |
|
|
| 4.534 | 0.033 |
|
ChT/TRT | 18 | 35.3 | 2.4 |
|
|
| ChT
alone | 12 | 14.5 | 2.4 |
|
|
| No. of metastatic
organs |
|
|
| 2.435 | 0.119 |
| 1 | 17 | 32.4 | 2.7 |
|
|
| ≥2 | 12 | 25.9 | 1.9 |
|
|
 | Table IV.Multivariate analysis of the
prognostic factors for survival in patients with extensive-stage
small-cell lung cancer. |
Table IV.
Multivariate analysis of the
prognostic factors for survival in patients with extensive-stage
small-cell lung cancer.
| Characteristic | HR | 95% CI | P-value |
|---|
| Gender (female vs.
male) | 1.096 | 0.693–1.732 | 0.696 |
| Age, years (<60
vs. ≥60) | 1.023 | 0.719–1.454 | 0.901 |
| KPS score (<80
vs. ≥80) | 0.999 | 0.655–1.493 | 0.958 |
| Smoking status (yes
vs. no) | 0.836 | 0.608–1.203 | 0.368 |
| No. of ChT cycles
(<4 vs. ≥4) | 0.440 | 0.279–0.712 | 0.001 |
| Treatment (ChT
alone vs. ChT/TRT) | 0.607 | 0.418–0.880 | 0.008 |
| No. of ChT cycles
(<6 vs. ≥6) | 1.006 | 0.673–1.468 | 0.975 |
| No. of metastasis
organs (>2 vs. 1) | 0.791 | 0.563–1.108 | 0.172 |
Treatment failure
SCLC progression and distant metastasis were
observed in 23 and 39 patients, respectively, in the ChT/TRT group,
and 41 and 35 patients, respectively, in the ChT group. A higher
local control of SCLC was observed in the ChT/TRT compared with the
ChT group (48.8 vs. 28%; P=0.006), while no significant difference
in the distant control was found between the two groups
(P>0.05).
Therapy-associated toxicity
Therapy-induced toxicity was evaluated in the 165
eligible patients. Leukopenia occurred more frequently in the
ChT/TRT group than in the ChT group, while no significant
differences were detected in the occurrence of other toxicities.
Subgroup analyses revealed that there was a higher occurrence of
leukopenia in the patients receiving 45 Gy radiotherapy at 1.5
Gy/fraction twice per day compared with those receiving 60 Gy
radiotherapy at 2 Gy/fraction daily. Grade 2 esophagitis was
observed in 2 patients and pneumonitis was reported in 3 patients
(grade 2 pneumonitis in 2 patients; grade 3 pneumonitis in 1
patient) in the ChT/TRT group (Table
V).
 | Table V.Incidence of toxic effects in the
ChT/TRT and ChT groups. |
Table V.
Incidence of toxic effects in the
ChT/TRT and ChT groups.
|
| Group (n) |
|
|---|
|
|
|
|
|---|
| Toxic
effect/grade | ChT/TRT | ChT | P-value |
|---|
| Hematologic
toxicity grade ≥3 |
|
|
|
|
Leukopenia | 47 | 29 | 0.004 |
|
Thrombocytopenia | 6 | 9 | 0.431 |
|
Anemia | 7 | 6 | 0.755 |
| Nonhematologic
toxicity |
|
|
|
|
Nausea/vomiting grade ≥3 | 5 | 4 | 0.718 |
| TRT-induced
toxicity |
|
|
|
|
Esophagitis |
|
|
|
| Grade
2 | 2 | - | - |
| Grade
3 | 0 | - | - |
| Pneumonitis |
|
|
|
| Grade
2 | 2 | - | - |
| Grade
3 | 1 | - | - |
| Grade
4 | 0 | - | - |
| Grade
5 | 0 | - | - |
Discussion
Although much progress has been achieved in the
treatment of lung cancer, it remains the leading cause of
cancer-associated mortality throughout the world, and is
characterized by an extremely high mortality rate (14). There are two types of lung cancer:
SCLC accounts for 10–15% of all lung cancers, while non-small-cell
lung cancer (NSCLC) accounts for ∼85% of all lung cancers (15). Untreated SCLC follows an aggressive
course, with a median survival time of 2–4 months (16). Despite its generally late
presentation and high risk of dissemination, SCLC is extremely
sensitive to chemotherapy and radiotherapy (17). Chemotherapy is the mainstay of
treatment for SCLC, while radiation therapy that involves the use
of high energy X-rays focused on the specific disease site to
directly destroy cancer cells, is usually recommended during
chemotherapy to relieve pain and prevent and cure brain metastases
(17–21).
Combined chemotherapy has been the standard
treatment for ES-SCLC for almost 30 years and the administration of
carboplatin or cisplatin with etoposide remains the standard of
care for patients with ES-SCLC (4).
In the American College of Chest Physicians (ACCP), NCCN and the
European Society for Medical Oncology (ESMO) guidelines, patients
with ES-SCLC are advised to receive 4–6 cycles of cisplatin- or
carboplatin-based combination chemotherapy (such as cisplatin plus
etoposide or irinotecan) (16,22,23).
Although EP remains the most widely used combination, a randomized
trial that compared the combination of cisplatin with either
etoposide or irinotecan in ES-SCLC demonstrated that the
irinotecan-cisplatin combination (IP) was superior to EP. The
median survival time was 12.8 months in patients treated with IP
vs. 9.4 months in patients treated with the EP combination; the
2-year survival rate was also superior at 19.5% for IP vs. 5.2% for
EP (24). However, a confirmatory
study in the United States failed to show the superiority of either
regimen (25). Although the EP and
IP combinations have shown comparable OS rates and survival
outcomes, the IP combination has been found to cause increased
gastrointestinal toxicity (26).
Generally, radiotherapy is only used in patients with ES-SCLC to
palliate symptoms if required (such as for painful bone
metastases). Response rates are high; however, patients invariably
relapse (27). The ACCP guidelines
suggest that consolidative TRT to the chest is a treatment option
for patients who achieve a CR outside the chest and CR or PR in the
chest (16). However, to the best of
our knowledge, the present study is the first study to examine the
efficacy of TRT for the treatment of ES-SCLC.
In the current study, 75.2% of the total individuals
studied responded to therapy; of these, 84.1% of the subjects in
the ChT/TRT group responded and 66.2% of the subjects in the ChT
group responded. The ES-SCLC patients receiving chemotherapy and 3D
conformal TRT had a median OS time of 18 months, and 2- and 5-year
OS rates of 35.3 and 2.4%, respectively. The patients receiving
chemotherapy alone had a median OS time of 12 months, and 2- and
5-year OS rates of 14.5 and 2.4%, respectively (all P<0.05). The
median PFS times were 9 and 6 months in the ChT/TRT and ChT groups,
respectively, while the 1- and 2-year PFS rates were 35.4 and 6.0%
in the ChT/TRT group, and 20.5 and 6.0% in the ChT group,
respectively. Furthermore, multivariate analysis showed that
receiving ≥4 cycles of chemotherapy and TRT were independent,
favorable prognostic factors for OS.
A previous study investigated the efficacy and
toxicity of EP chemotherapy with or without accelerated
hyperfractionated radiotherapy and combined daily
carboplatin/etoposide (CE) in patients with EC-SCLC. In patients
with a CR or PR following 3 cycles of standard EP, it was found
that patients who subsequently received thoracic radiotherapy with
54 Gy in 36 fractions over 18 treatment days in combination with CE
followed by two cycles of EP had a significantly improved survival
rate compared with that of patients given an additional 4 cycles of
EP. Local control was improved in the radiotherapy group, although
not significantly. No difference in distant metastasis-free
survival was observed between the groups, and acute high-grade
toxicity was higher in the patients that received chemotherapy.
Thus, the addition of accelerated hyperfractionated radiotherapy to
the treatment of the most favorable subset of patients led to
improved survival over that obtained with chemotherapy alone
(28). The present results also
suggest that TRT may be a beneficial treatment for patients with
ES-SCLC.
The selection of radiotherapy dose is a critical
factor that determines its efficacy. Currently, TRT delivered at 60
Gy/30 fractions daily is more widely used for ES-SCLC treatment
compared with 45 Gy/30 fractions twice per day due to convenience,
a higher effective dose and the shorter duration of the treatment
course (29). The two radiotherapy
regimens have been recommended for the treatment of LS-SCLC;
however, significant differences have been reported in the
toxicity, disease control and survival rates between the two
regimens (30). A phase III trial
comparing daily with twice-daily radiotherapies for LS-SCLC showed
that radiotherapy at 50.4 Gy in 28 fractions daily resulted in
survival rates comparable with those of split-course twice-daily
radiotherapy at 24 Gy in 16 fractions, a 2.5-week break and 24 Gy
in 16 fractions (31). In the
current study, the median PFS rate was 11 months in the 20 patients
receiving TRT at 45 Gy/30 fractions twice per day, and 9 months in
the 22 patients receiving radiotherapy at 60 Gy/30 fractions daily
(P=0.043); however, no significant difference was detected in the
OS rate between the two radiotherapy regimens. Thus, TRT delivered
at 45 Gy/30 fractions twice daily appears feasible for the
treatment of patients with ES-SCLC.
Consolidative TRT at 40 Gy/15 fractions, 45 Gy/30
fractions delivered twice daily and 36 Gy/12 fractions has been
found to result in 1- and 2-year locoregional failure rates of 26
and 39%, 1- and 2-year distant failure rates of 58 and 74%, a
median OS time of 14 months, and 1- and 2-year OS rates of 58 and
14% in patients with ES-SCLC with minimal metastatic disease,
respectively. Consolidative TRT is therefore shown to control the
locoregional disease in the majority of patients with minimal acute
toxicity, though distant failure remains a significant problem
(32). In the present study, SCLC
progression and distant metastasis were observed in 23 and 39
patients in the ChT/TRT group, and 41 and 35 patients in the ChT
group, respectively. A higher local control of SCLC was observed in
the ChT/TRT group compared with the ChT group (48.8 vs. 28%;
P=0.006), while no significant difference in the distant control
was found between the two groups (P>0.05).
The results of the present study showed that
receiving ≥4 cycles of chemotherapy improved OS and PFS, while no
further benefit was achieved from chemotherapy for >6 cycles.
These results support the hypothesis that 4–6 cycles of
chemotherapy, administered either alone or concurrently with
radiotherapy, may be an appropriate choice for the treatment of
patients with ES-SCLC. This hypothesis has been validated by a
previous study (33).
Concurrent radiochemotherapy administered at an
early stage has been shown to improve local control and survival
rates in patients with LS-SCLC (9);
however, the optimum timing of TRT administration for patients with
ES-SCLC remains unclear. In the Shandong Cancer Hospital, TRT is
usually administered to patients with ES-SCLC who respond to
therapy following >2 cycles of chemotherapy. In the current
study, TRT was initiated following 2 cycles of chemotherapy in 32
subjects, 4 cycles in 13 subjects and 6 cycles in 13 subjects. Such
therapies resulted in comparable OS and PFS rates with those
observed in the entire ChT/TRT group, which indicated that the
timing of TRT addition has no effect on treatment efficacy in
ES-SCLC. However, it is suggested that TRT should be administered
at a late stage of integrated therapy to ensure that more patients
complete the initial 4–6 cycles of chemotherapy.
In general, ES-SCLC patients with metastasis in a
single organ are given radiotherapy following chemotherapy, while
those with metastasis in >1 organ are recommended to receive
chemotherapy with radiotherapy used only as a palliative treatment
(28). In the present retrospective
study, 64 patients in the ChT/TRT group and 47 patients in the ChT
group had metastasis in a single organ. A significant difference
was detected in the number of metastatic organs between the two
groups (P=0.005). Further prospective, multicenter and randomized
trials are required to evaluate the value of TRT in the treatment
of ES-SCLC patients with metastasis in one organ.
PCI, which has been found to reduce the incidence
rate of symptomatic brain metastases and prolong disease-free
survival and OS times, is now recommended for patients with ES-SCLC
with CR to chemotherapy (34). In
the present retrospective study, PCI was only administered to 5
patients with a CR to chemotherapy. Thus, the association between
PCI and survival rate was not evaluated.
During SCLC therapy, treatment-induced toxicity was
carefully monitored. Hematologic toxicity was the most common
adverse reaction observed, with leukopenia at grades 3 or 4 in 47
patients (57.3%) in the ChT/TRT group and 29 patients (34.9%) in
the ChT group (P=0.004). No significant differences were detected
in the occurrence of other toxicities between the two groups,
including thrombocytopenia, anemia, nausea and vomiting. Three
patients had pneumonitis (grade 2 in 2 patients and grade 3 in 1
patient) in the ChT/TRT group, with a lower occurrence rate of
radiation pneumonitis observed as compared with that found in a
previous study (35).
In conclusion, the present study found that the
addition of TRT improved the OS and PFS of patients with ES-SCLC.
It is thus cautiously recommended that 4–6 cycles of standard
chemotherapy followed by TRT at 45 Gy/30 fractions twice daily may
be an appropriate treatment protocol for patients with ES-SCLC.
References
|
1
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simon GR and Wagner HAmerican College of
Chest Physicians: Small cell lung cancer. Chest. 123
(Suppl):259S–271S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jett JR, Schild SE, Kesler KA and
Kalemkerian GP: Treatment of small cell lung cancer: Diagnosis and
management of lung cancer, 3rd ed: American College of Chest
Physicians evidence-based clinical practice guidelines. Chest. 143
(5 Suppl):e400S–e419S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu H, Zhou Z, Wang Y, et al: Thoracic
radiation therapy improves the overall survival of patients with
extensive-stage small cell lung cancer with distant metastasis.
Cancer. 117:5423–5431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yee D, Butts C, Reiman A, et al: Clinical
trial of post-chemotherapy consolidation thoracic radiotherapy for
extensive-stage small cell lung cancer. Radiother Oncol.
102:234–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pignon JP, Arriagada R, Ihde DC, Johnson
DH, Perry MC, Souhami RL, et al: A meta-analysis of thoracic
radiotherapy for small-cell lung cancer. N Engl J Med.
327:1618–1624. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Warde P and Payne D: Does thoracic
irradiation improve survival and local control in limited-stage
small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol.
10:890–895. 1992.PubMed/NCBI
|
|
9
|
Takada M, Fukuoka M, Kawahara M, et al:
Phase III study of concurrent versus sequential thoracic
radiotherapy in combination with cisplatin and etoposide for
limited-stage small-cell lung cancer: Results of the Japan Clinical
Oncology Group Study 9104. J Clin Oncol. 20:3054–3060. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simon GR and Turrisi AAmerican College of
Chest Physicians: Management of small cell lung cancer: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132 (3 Suppl):324–339. 2007. View Article : Google Scholar
|
|
11
|
National Comprehensive Cancer Network, .
Prostate cancer. NCCN Clinical practice guidelines in oncology. J
Natl Compr Canc Netw. 2:224–248. 2004.PubMed/NCBI
|
|
12
|
Faivre-Finn C, Lorigan P, West C and
Thatcher N: Thoracic radiation therapy for limited-stage small-cell
lung cancer: Unanswered questions. Clin Lung Cancer. 7:23–29. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: Revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoffman PC, Mauer AM and Vokes EE: Lung
cancer. Lancet. 355:479–485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samson DJ, Seidenfeld J, Simon GR, et al:
Evidence for management of small cell lung cancer: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132 (3 Suppl):314S–323S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cooper S and Spiro SG: Small cell lung
cancer: treatment review. Respirology. 11:241–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kallianos A, Rapti A, Zarogoulidis P,
Tsakiridis K, Mpakas A, Katsikogiannis N, et al: Therapeutic
procedure in small cell lung cancer. J Thorac Dis. 5 (Suppl
4):S420–S424. 2013.PubMed/NCBI
|
|
19
|
Ganti AK, West WW and Zhen W: Current
concepts in the management of small cell lung cancer. Indian J Med
Res. 137:1043–1051. 2013.PubMed/NCBI
|
|
20
|
Califano R, Abidin AZ, Peck R, Faivre-Finn
C and Lorigan P: Management of small cell lung cancer: recent
developments for optimal care. Drugs. 72:471–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simon M, Argiris A and Murren JR: Progress
in the therapy of small cell lung cancer. Crit Rev Oncol Hematol.
49:119–133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology. Small Cell Lung
Cancer. v.2:2011 https://www.nccn.org/Accessed. May
21–2011
|
|
23
|
Früh M, De Ruysscher D, Popat S, Crinò L,
Peters S and Felip EESMO Guidelines Working Group: Small-cell lung
cancer (SCLC): ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 24 (Suppl 6):vi99–vi105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noda K, Nishiwaki Y, Kawahara M, et al:
Irinotecan plus cisplatin compared with etoposide plus cisplatin
for extensive small-cell lung cancer. N Engl J Med. 346:85–91.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanna N, Bunn PA Jr, Langer C, et al:
Randomized phase III trial comparing irinotecan/cisplatin with
etoposide/cisplatin in patients with previously untreated
extensive-stage disease small-cell lung cancer. J Clin Oncol.
24:2038–2043. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Natale RB, Lara PN, Chansky K, Crowley JJ,
Jett JR, Carleton JE, et al: S0124: A randomized phase III trial
comparing irinotecan/cisplatin (IP) with etoposide/cisplatin (EP)
in patients (pts) with previously untreated extensive stage small
cell lung cancer (E-SCLC). J Clin Oncol. 26 (Suppl):7512–2008
|
|
27
|
Toy E, Macbeth F, Coles B, Melville and
Eastwood A: Palliative thoracic radiotherapy for non-small-cell
lung cancer: A systematic review. Am J Clin Oncol. 26:112–120.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeremic B, Shibamoto Y, Nikolic N, et al:
Role of radiation therapy in the combined-modality treatment of
patients with extensive disease small-cell lung cancer: A
randomized study. J Clin Oncol. 17:2092–2099. 1999.PubMed/NCBI
|
|
29
|
Turrisi AT III, Kim K, Blum R, Sause WT,
Livingston RB, Komaki R, et al: Twice-daily compared with
once-daily thoracic radiotherapy in limited small-cell lung cancer
treated concurrently with cisplatin and etoposide. N Engl J Med.
340:265–271. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watkins JM, Fortney JA, Wahlquist AE,
Shirai K, Garrett-Mayer E, Aguero EG, et al: Once-daily
radiotherapy to > or =59.4 Gy versus twice-daily radiotherapy to
> or =45.0 Gy with concurrent chemotherapy for limited-stage
small-cell lung cancer: a comparative analysis of toxicities and
outcomes. Jpn J Radiol. 28:340–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schild SE, Bonner JA, Shanahan TG, et al:
Long-term results of a phase III trial comparing once-daily
radiotherapy with twice-daily radiotherapy in limited-stage
small-cell lung cancer. Int J Radiat Oncol Biol Phys. 59:943–951.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giuliani ME, Atallah S, Sun A, et al:
Clinical outcomes of extensive stage small cell lung carcinoma
patients treated with consolidative thoracic radiotherapy. Clin
Lung Cancer. 12:375–379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schiller JH, Adak S, Cella D, et al:
Topotecan versus observation after cisplatin plus etoposide in
extensive-stage small-cell lung cancer: E7593 - a phase III trial
of the Eastern Cooperative Oncology Group. J Clin Oncol.
19:2114–2122. 2001.PubMed/NCBI
|
|
34
|
Slotman B, Faivre-Finn C, Kramer G, Rankin
E, Snee M, Hatton M, et al: Prophylactic cranial irradiation in
extensive small-cell lung cancer. N Engl J Med. 357:664–672. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roeder F, Friedrich J, Timke C, Kappes J,
Huber P, Krempien R, Debus J and Bischof M: Correlation of
patient-related factors and dose-volume histogram parameters with
the onset of radiation pneumonitis in patients with small cell lung
cancer. Strahlenther Onkol. 186:149–156. 2010. View Article : Google Scholar : PubMed/NCBI
|