Introduction
Cardiovascular diseases are the most common
illnesses concomitant with depression. Approximately one in five
patients with cardiovascular disease suffers from major depressive
disorder (1). Depression should be
considered to be a major risk factor for cardiovascular disease,
specifically among elderly people. A previous study demonstrated
that the associated risk of heart disease in patients with
depression is ~1.6 times greater compared with individuals who do
not suffer from depression (2).
Furthermore, symptoms of depression are associated with an
increased risk of myocardial infarction and stroke (3). Treatment of these concomitant diseases
includes the prevention of heart diseases, and antidepressant
therapies.
High blood cholesterol is associated with
cardiovascular disease and is an important risk factor. The most
effective and commonly used drugs to lower the levels of
low-density lipoprotein lipids are statins;
3-hydroxy-3-methylglutaryl-CoA reductase inhibitors (4,5).
Rosuvastatin induces the most potent reduction of LDL and elevation
of high-density lipoprotein in its class of drug (6). Rosuvastatin effectively reduces the
concentration of C-reactive protein by ~37%, and diminishes the
risk of myocardial infarction, stroke and other cardiovascular
events among individuals with established coronary heart disease
(4,7). Severe adverse effects during this drug
therapy are rare and include myopathy and rhabdomyolysis (8,9).
Among the available antidepressant agents, tricyclic
antidepressants (TCAs) and selective serotonin reuptake inhibitors
(SSRIs) are commonly chosen a first-line medications (10). TCAs are effective against depression
but are associated with cardiovascular side-effects including
orthostatic hypotension and slowed cardiac conduction (10). Amitriptyline is an effective TCA
drug; however, it may cause anticholinergic side-effects (11). SSRIs are being increasingly used to
treat depressed patients, including elderly individuals (10,12).
SSRIs have comparable efficacy to TCAs against depression but are
generally better tolerated (9).
Fluoxetine is a commonly prescribed SSRI for patients with cardiac
disease suffering from depression, as it does lead to the
development of cardiovascular side-effects (13). The side-effects of fluoxetine are
temporary and usually not life threatening, including
gastrointestinal adverse effects (14). However, several clinical and animal
studies have suggested that long-term therapy of fluoxetine induces
hepatotoxicity (15,16).
Numerous studies on depression treatment in patients
with cardiovascular diseases focused on the psychiatric
consequences of medications and on the impact of antidepressant
therapies on cardiovascular outcomes (9,12,17). Few
investigations have been conducted on the influence of combined
treatment with statins and antidepressant drugs on the function of
internal organs (18,19). Both cardiovascular diseases and
depression are chronic and require treatment with agents of
different pharmacological profiles. When choosing treatment for
depression in patients with cardiovascular disease, it should be
noted that long-term simultaneous pharmacotherapy may lead to
potential drug interactions, which may in turn disrupt the function
of internal organs. All antidepressant agents are metabolized to
some extent by the cytochrome P450 system and are associated with
numerous pharmacokinetic drug interactions. Similarly, the majority
of statins are hepatically biotransformed by cytochrome P450 action
(20). Therefore, it is necessary to
conduct preliminary studies on the impact of combined therapy with
statins and antidepressants on the function of internal organs.
The present study investigated the influence of
combined treatment with rosuvastatin and amitriptyline or
fluoxetine for 14 days on the serum biochemical parameters
indicating liver and kidney function in rats. In addition, the
activity levels of aminotransferase (AST), alanine aminotransferase
(ALT), and γ-glutamyltransferase (GGT) and the concentrations of
total protein, urea, creatinine and β2-microglobulin
were assessed.
Materials and methods
Drugs and chemicals
In the present study the following reagents and
commercial test kits were used: Rosuvastatin (Polpharma S.A.
Pharmaceutical Works, Starogard Gdański, Poland), amitriptyline
hydrochloride (Sigma-Aldrich Chemie GmbH, Munich, Germany),
fluoxetine (Anpharm S.A., Warszawa, Poland), aqua pro
injectione (Polfa Warszawa S.A., Warszawa, Poland), Liquick
Cor-AST-60, Liquick Cor-ALT-60, Liquick Cor-GGT, Liquick Cor-TOTAL
PROTEIN 120, Liquick Cor-UREA 120, Liquick Cor-CREATININE 60 (all:
PZ Cormay S.A., Łomianki, Poland) and β2-microglobulin
enzyme-linked immunosorbent assay (Immundiagnostic AG, Bensheim,
Germany). The ready-made diagnostic kits from PZ Cormay S.A. were
used to determine the activity levels of AST, ALT and GGT, total
protein levels and creatinine and urea concentrations.
Animals
The present study was conducted using 48 male Wistar
rats (weight, 200–275 g) obtained from a licensed breeder (Breeding
of Laboratory Animals, Zbigniew Lipiec, Brwinów, Poland). The
animals were maintained under standard laboratory conditions with a
12-h light/dark cycle and were provided with ad libitum
access to food and water. The study design was approved by the
ethics committee at the Animal Experimentation of the Medical
University of Lublin (Lublin, Poland; ethical approval no.
2/2013).
Experimental procedures
The rats were treated with rosuvastatin (10 mg/kg),
amitriptyline (10 mg/kg) or fluoxetine (10 mg/kg) once a day for 14
days. Previous studies (16,17) demonstrated that the above-mentioned
doses of statin and antidepressant drugs were effective. The drugs
were suspended in aqua pro injectione with one drop of Tween
80 (Sigma-Aldrich Chemie GmbH) and injected intraperitoneally in
volumes of 0.5 ml/100 g. Six groups of rats (I–VI; n=8 per group),
were administered the following: i) Control rats (treated with
aqua pro injectione), ii) rosuvastatin-treated rats, iii)
amitriptyline-treated rats, iv) fluoxetine-treated rats, v) rats
treated first with rosuvastatin and after 15 min amitriptyline, and
vi) rats treated first with rosuvastatin and after 15 min
fluoxetine. The rats were sacrificed by decapitation at 24 h
following the final injection. The blood from each animal was
obtained, allowed to clot, and centrifuged at 1,360 × g for 10 min
at room temperature. The serum fraction was separated and divided
as follows: One part was used to estimate enzymes activity levels,
the other part was frozen at −20°C until use for further
biochemical experimentation. Designation have been conducted for
two weeks.
Statistical analysis
The data were presented as means ± standard error of
the mean. Statistical significance among groups was determined
using analysis of variance. P<0.05 was considered to indicate a
statistically significant result.
Results
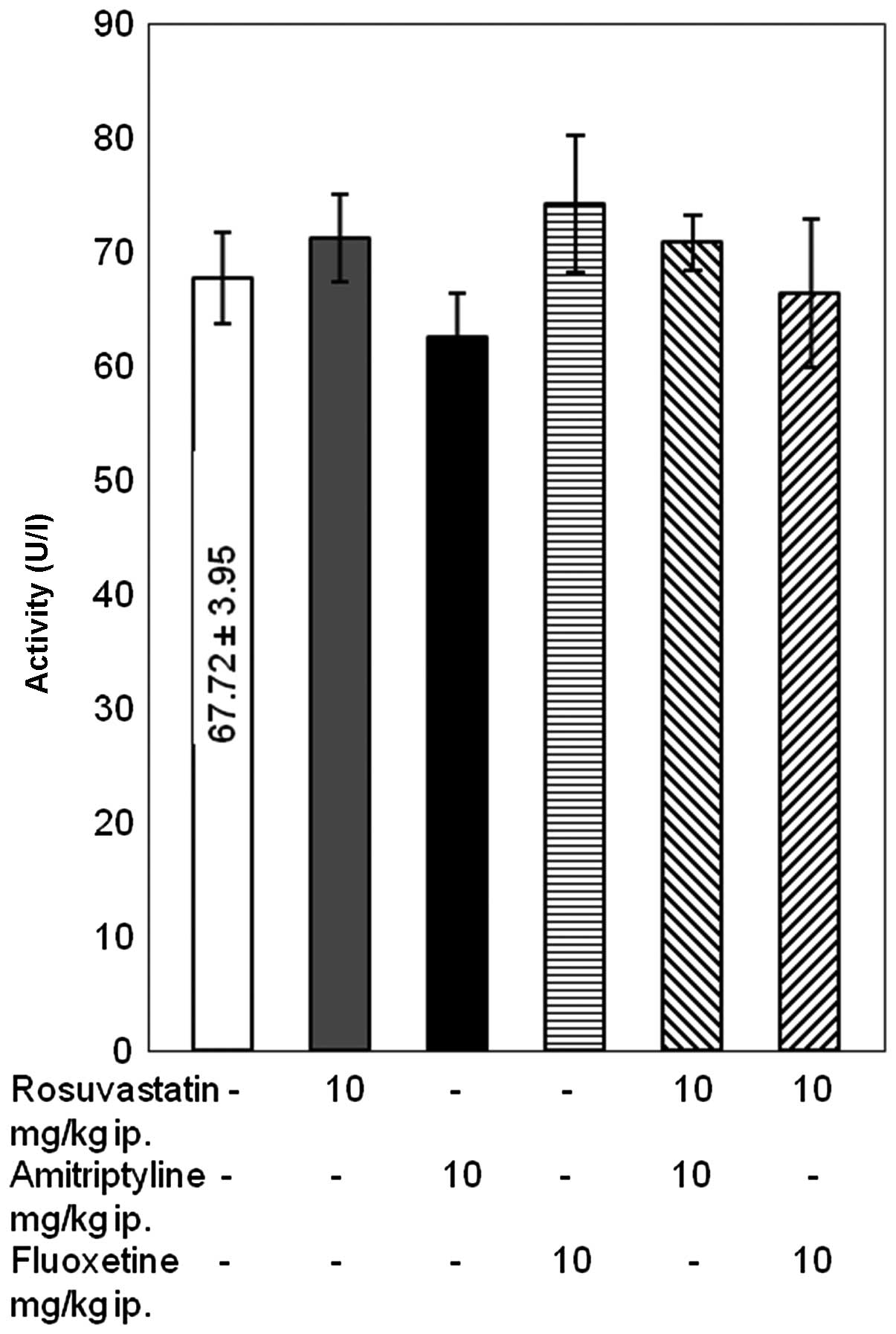
Differences in the levels of total
protein, AST, β2-microglobulin and ALT
The results of the present study indicated that the
activity levels of AST and the concentrations of total protein and
β2-microglobulin remained unaffected following combined
treatment with rosuvastatin and amitriptyline or fluoxetine for 14
days (Fig. 1 and Table I). The administration of rosuvastatin
with amitriptyline did not influence the activity of ALT either.
Conversely, combined treatment with rosuvastatin and fluoxetine
resulted in an increase in ALT activity levels in the rat serum
compared with fluoxetine alone (P<0.05). However, in rats
treated with the SSRI fluoxetine only exhibited a significant
reduction in serum ALT activity compared with the control group
(P<0.05) (Fig. 2).
 | Table I.Assessment of the concentrations of
total protein and β2-microglobulin in the serum of
rats. |
Table I.
Assessment of the concentrations of
total protein and β2-microglobulin in the serum of
rats.
| Treatment
(mg/kg) | Total protein
(g/dl) |
β2-microglobulin (mg/l) |
|---|
| Control | 3.7±0.09 | 0.39±0.03 |
| Rosuvastatin | 3.6±0.07 | 0.41±0.07 |
| Amitriptyline | 3.5±0.08 | 0.43±0.06 |
| Fluoxetine | 3.4±0.01 | 0.46±0.04 |
| Rosuvastatin +
amitriptyline | 3.7±0.02 | 0.42±0.02 |
| Rosuvastatin +
fluoxetine | 3.5±0.01 | 0.45±0.03 |
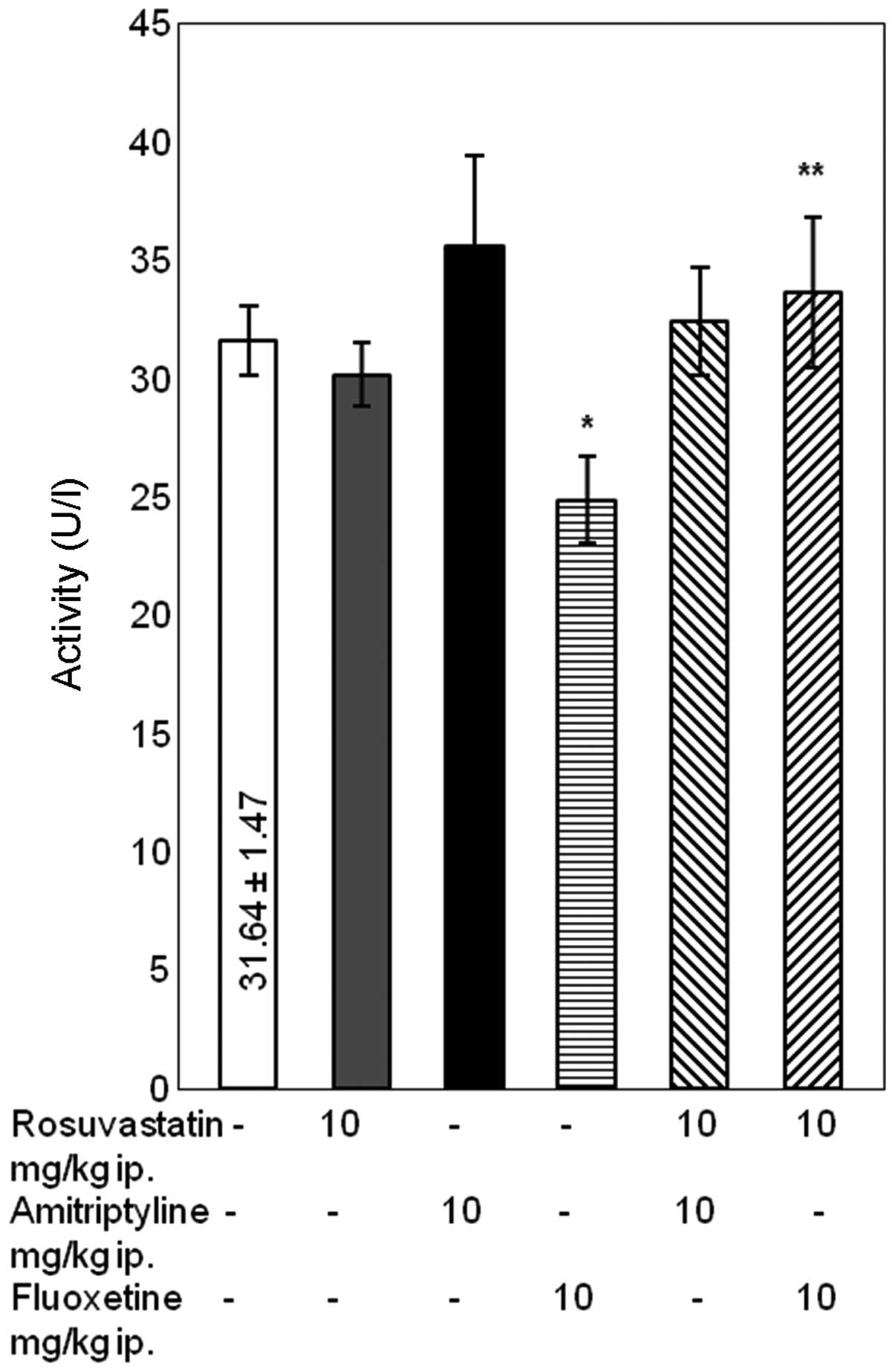
Differences in the levels of GGT
Simultaneous treatment with rosuvastatin and
amitriptyline caused a significant increase in the activity levels
of GGT compared with treatment with rosuvastatin alone (P<0.05).
In addition, the combined administration of rosuvastatin with
fluoxetine caused a significant increase in the activity levels of
GGT, compared with treatment with rosuvastatin or fluoxetine alone
(P<0.05) (Fig. 3).
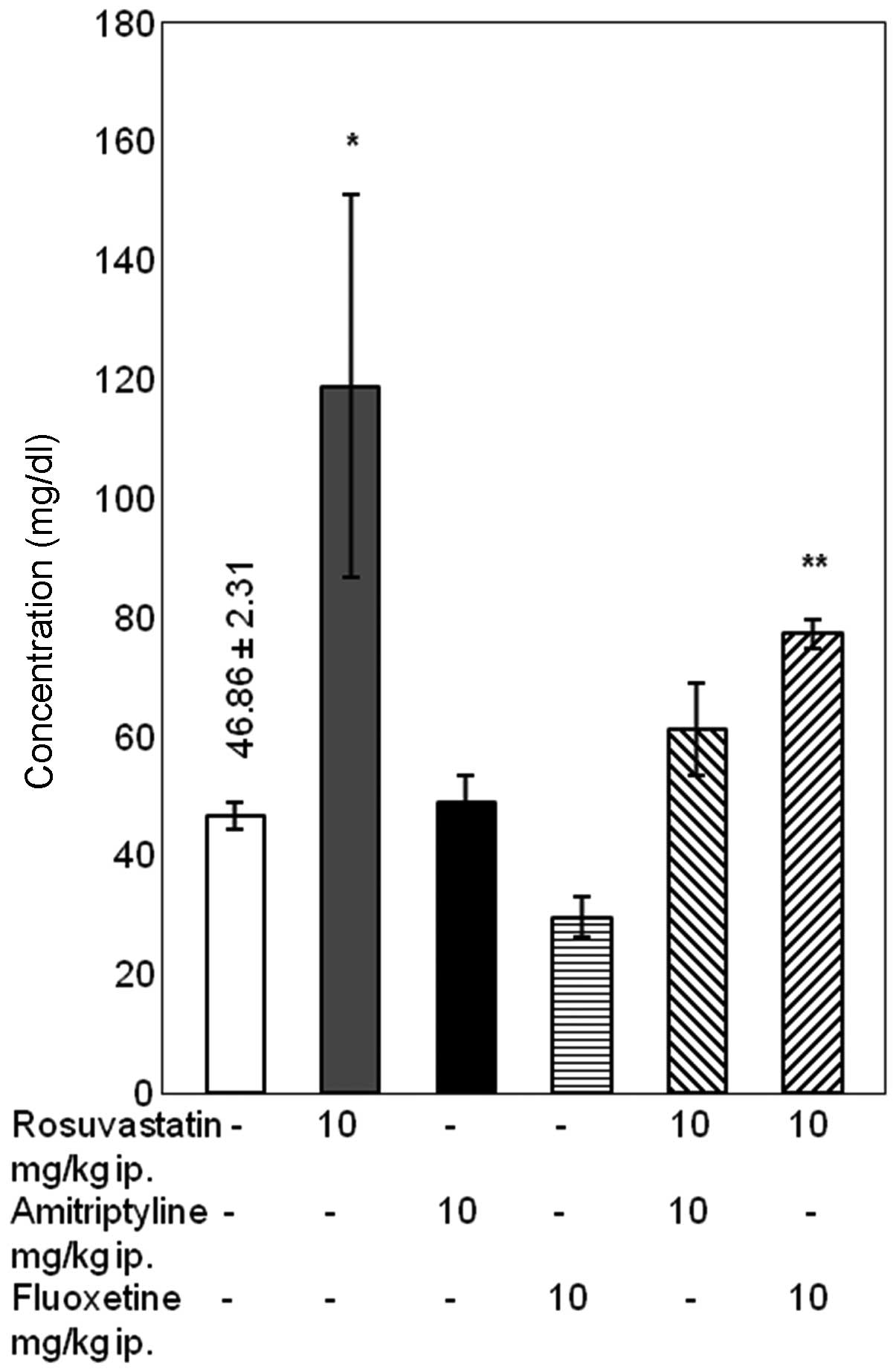
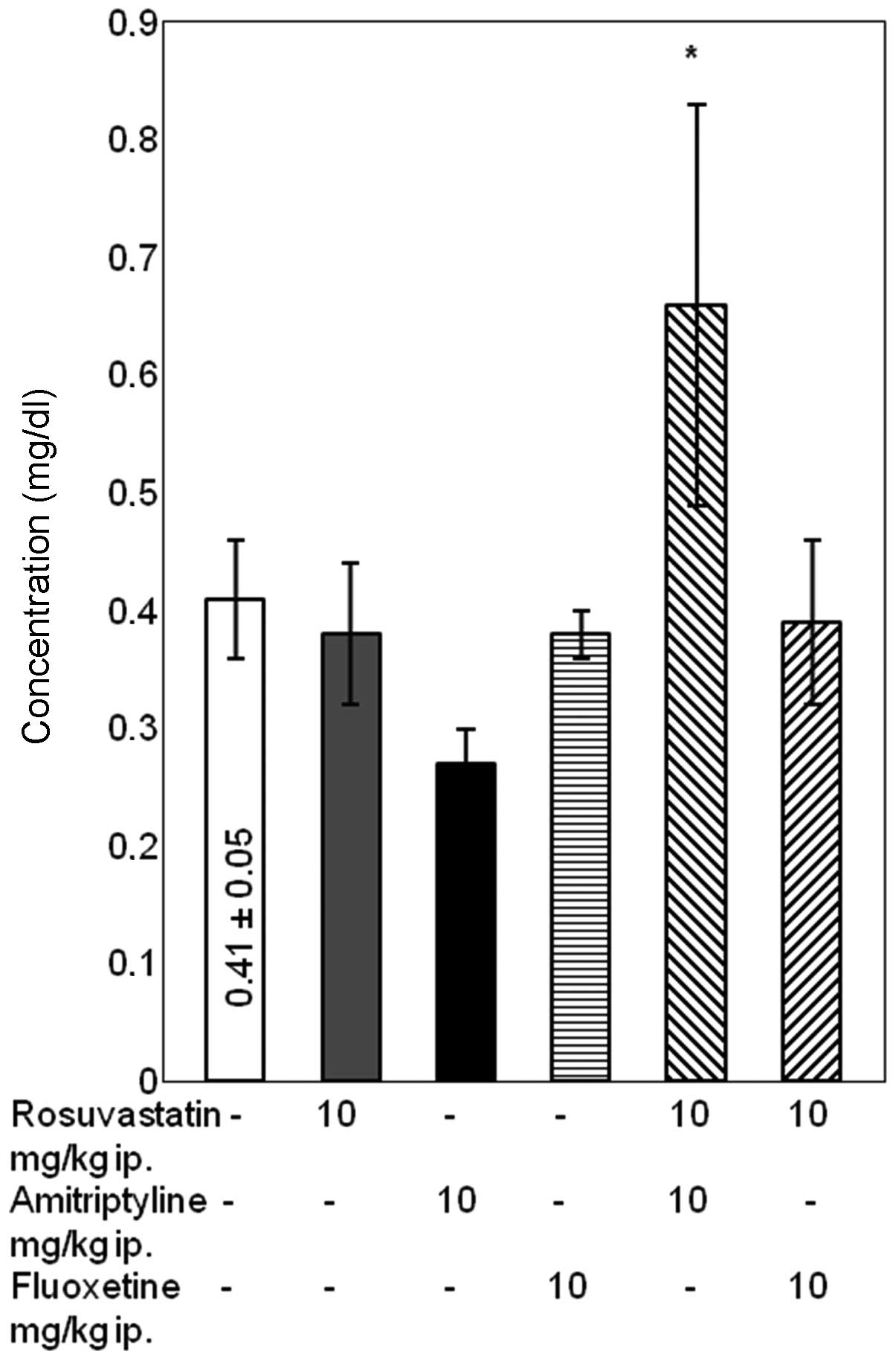
Differences in urea concentration
Combined treatment with rosuvastatin and
amitriptyline for 14 days had no effect on the concentration of
urea. Conversely, in rats treated with rosuvastatin and fluoxetine
a significant in urea concentration was noted compared with the
rats treated with fluoxetine alone (P<0.05). In rats treated
with rosuvastatin alone, a significant increase in urea
concentration was observed compared with the control group
(P<0.05), although it is worth noting that the standard error of
the mean was high (Fig. 4).
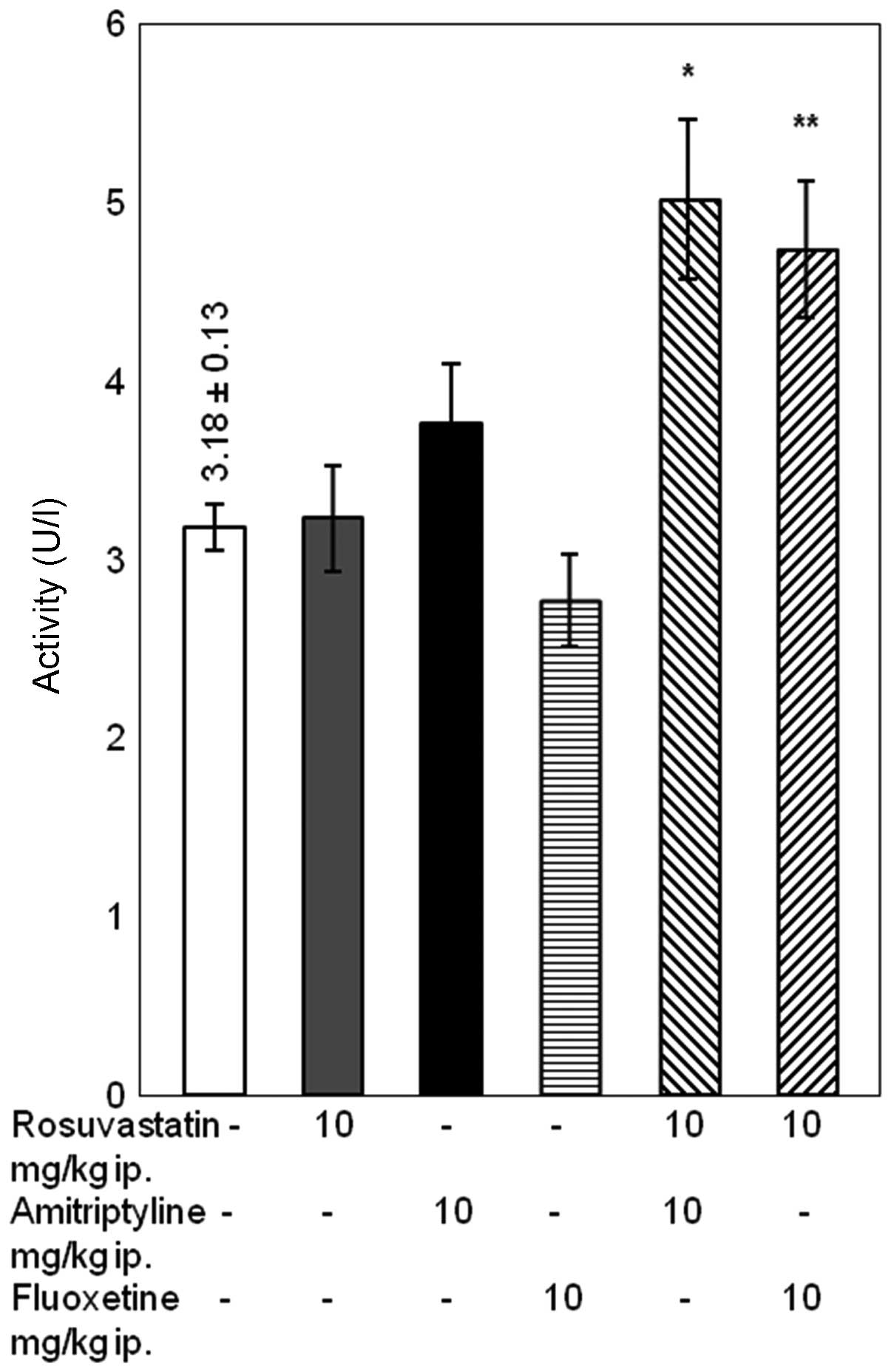
Differences in creatinine
concentration
Rosuvastatin administered simultaneously with
amitriptyline caused a significant increase in the concentration of
creatinine compared with amitriptyline alone (P<0.05).
Conversely, the administration of rosuvastatin and fluoxetine did
not significantly affect the concentration of creatinine in the
blood serum of the rats (Fig.
5).
Discussion
The results of the present study demonstrated that
treatment with rosuvastatin alone increased the urea concentration
compared with the control group. Although a high standard error of
the mean value was observed, there were no effects on the other
parameters to indicate renal function. Previous studies reported
that rosuvastatin induces low levels of transient proteinuria
(6,21). In short-term placebo-controlled
trials, transient proteinuria was detected in 0.1% patients
receiving rosuvastatin at a dose of 40 mg. Patient elevated
proteinuria levels during rosuvastatin therapy were generally
resolved spontaneously later during the trial (4,6,9). In addition, long-term data indicates
that rosuvastatin appears to marginally improve renal function
(6). Few studies have been conducted
on kidney dysfunction during therapy with amitriptyline. The
available data suggests that amitriptyline drug clearance is
reduced in elderly people (12).
Several studies have demonstrated the safety of fluoxetine in
depressed patients with renal impairment (22,23). The
results of the present study indicated that neither amitriptyline
nor fluoxetine caused significant changes in the markers of renal
function, including concentrations of total protein, urea,
creatinine and β2-microglobulin, as compared with the
control group.
The data obtained from this study suggested that the
administration of either rosuvastatin and amitriptyline or
rosuvastatin and fluoxetine for 14 days did not significantly
influence AST activity levels in the serum of rats. In addition,
combined treatment with rosuvastatin and amitriptyline did not
induce any significant changes in the activity levels of ALT, as
compared with the rosuvastatin and amitriptyline alone groups.
However, combined treatment with rosuvastatin and fluoxetine
resulted in an increase in ALT activity levels in rat serum, as
compared with rats treated with fluoxetine alone. Serum AST and ALT
levels are the most useful indicators of liver cell injury,
although AST is less liver-specific than ALT (24,25). It
should be noted that an increase in ALT activity levels was
associated with the group of rats receiving fluoxetine alone,
whereas in the serum of the rats pretreated with fluoxetine a
significant reduction in ALT activity was observed, as compared
with the control group. Finally, the activity levels of this ALT in
the rats treated with both rosuvastatin and fluoxetine was not
significantly different compared with the control group. Our
previous studies (18,19) demonstrated that combined treatment
with simvastatin (10 mg/kg) and amitriptyline (10 mg/kg) for 14
days increased the activity levels of AST, whereas combined
treatment with simvastatin (20 mg/kg) and doxepin (20 mg/kg)
significantly increased the activity levels of both AST and ALT.
This may be due to the fact that rosuvastatin is a poor substrate
for metabolism by cytochrome P450, and it is less likely to cause
metabolic drug-drug interactions. Furthermore, a study in which an
increase in the activity levels of both AST and ALT were observed,
drug concentrations twice as high were used (19). In the present study, combined
treatment with rosuvastatin and amitriptyline in rats caused a
significant increase in the activity levels of GGT compared with
those treated with rosuvastatin alone. In addition, the combined
administration of rosuvastatin and fluoxetine caused a significant
increase in the activity levels of GGT compared with rosuvastatin
or fluoxetine alone. GGT activity has been observed in numerous
types of tissue, although it is considered as a serum marker
primarily for the diagnosis of liver disease. Elevated serum GGT
activity levels have been widely used as an early and sensitive
marker of liver dysfunction (8).
Therefore, the increased activity levels of the marked enzymes that
were observed in the present study may suggest the possibility of
liver function impairment during combined therapy with rosuvastatin
and amitriptyline or fluoxetine. However, the administration of
rosuvastatin for 14 days did not significantly influence the serum
activity levels of the enzymes compared with the control group.
Previous studies demonstrated that rosuvastatin may occasionally
induce alterations in hepatic enzyme activity levels; however,
these changes infrequently caused liver failure (4,6). In the
controlled clinical trials of rosuvastatin, the incidence of
clinically significant ALT increases was low, and similar across
all doses (0.1–0.5%), and these elevations were dose-dependent
(24,25). The results of the present study
demonstrated that amitriptyline did not significantly affect the
activity levels of the marker enzymes, compared with the control
group. However, according to previous studies amitriptyline is an
antidepressant drug associated with numerous adverse effects,
including infrequent toxic liver injury (26). Furthermore, amitriptyline therapy may
result in idiosyncratic hepatotoxicity, although this rarely occurs
(27). Numerous studies have
reported that fluoxetine induces liver damage, as demonstrated by
elevated levels of aminotransferases, glutathione
S-transferases, and acute hepatitis in humans and animals
(15,16,28).
However, in present study fluoxetine administered to the rats for
14 days lowered the activity levels of ALT compared with the
control group.
The concentrations of total protein and
β2-microglobulin remained unaffected in the serum of the
rats treated with rosuvastatin and amitriptyline or fluoxetine.
Similarly, combined treatment with rosuvastatin and amitriptyline
did not significantly influence urea concentration. However,
rosuvastatin administered simultaneously with fluoxetine
significantly increased the concentration of urea, as compared with
the rats treated with fluoxetine alone. It should be noted that any
significant changes observed in the serum of rats treated with
rosuvastatin and fluoxetine were as compared with the control
group. Additionally, any adverse effects of the simultaneous
administration of rosuvastatin and fluoxetine on creatinine
concentration were indicated. The results of the present
investigation also demonstrated that combined treatment with
rosuvastatin and amitriptyline for 14 days caused a significant
increase in the concentration of creatinine, compared with rats
treated with TCA alone. However, in the group of rats treated with
rosuvastatin and amitriptyline, the high standard error of the mean
was recorded and the concentration of creatinine was estimated to
be within the limits of the control group. The alterations in serum
markers observed in the present study do not clearly indicate an
impaired renal function. The differences between combined treatment
with rosuvastatin and amitriptyline or fluoxetine and the
experimental marker activity levels and concentrations may be a
result of the metabolism of these drugs. Rosuvastatin and
fluoxetine are biotransformed in the liver by cytochrome P450
izoenzyme CYP2C9, although this izoenzyme is not involved in the
metabolism of amitriptyline (4,29).
In conclusion, combined treatment with rosuvastatin
and either antidepressant for 14 days increased the activity levels
of GGT in the serum of rats compared with the control group, which
suggested the impairment of liver function. Therefore, further
long-term (28- and/or 90-day) animal model studies and prospective
clinical analyses that specifically focus on the mechanisms
underlying the adverse effects of combined treatment with statins
and antidepressants on the liver and kidney functions, are
required. However, according to the estimation of the marked
biochemical parameters of renal function, it appears that the
above-mentioned therapy does not adversely affect renal function in
rats.
Acknowledgements
The present study was supported by a grant from the
Medical University of Lublin (grant no. DS38/2013-2014).
References
|
1
|
Elderon L and Whooley MA: Depression and
Cardiovascular Disease. Prog Cardiovasc Dis. 55:511–523. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rugulies R: Depression as a predictor for
coronary heart disease. A review and meta-analysis. Am J Prev Med.
23:51–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramasubbu R and Patten SB: Effect of
depression on stroke morbidity and mortality. Can J Psychiatry.
48:250–257. 2003.PubMed/NCBI
|
|
4
|
Luvai A, Mbagaya W, Hall AS and Barth JH:
Rosuvastatin: A review of the pharmacology and clinical
effectiveness in cardiovascular disease. Clin Med Insights Cardiol.
6:17–33. 2012.PubMed/NCBI
|
|
5
|
You H, Lu W, Zhao S, Hu Z and Zhang J: The
relationship between statins and depression: A review of the
literature. Expert Opin Pharmacother. 14:1467–1476. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guthrie RM and Martin DR: The safety of
rosuvastatin: Effect of renal and hepatic function. Expert Opin
Drug Saf. 6:573–581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan KL, Dumesnil JG, Tam J, Ni A and Teo
K: Effect of rosuvastatin on C-reactive protein and progression of
aortic stenosis. Am Heart J. 161:1133–1139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cohen DE, Anania FA and Chalasani N:
National Lipid Association Statin Safety Task Force Liver Expert
Panel: An assessment of statin safety by hepatologists. Am J
Cardiol. 97:77C–81C. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soran H and Durrington P: Rosuvastatin:
Efficacy, safety and clinical effectiveness. Expert Opin
Pharmacother. 9:2145–2160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jian W and Davidson JR: Antidepressant
therapy in patients with ischemic heart disease. Am Heart J.
150:871–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leucht C, Huhn M and Leucht S:
Amitriptyline versus placebo for major depressive disorder.
Cochrane Database Syst Rev. 12:CD0091382012.PubMed/NCBI
|
|
12
|
von Moltke LL, Greenblatt DJ and Shader
RI: Clinical pharmacokinetics of antidepressants in the elderly.
Therapeutic implications. Clin Pharmacokinet. 24:141–160. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roose SP, Glassman AH, Attia E, Woodring
S, Giardina EG and Bigger JT Jr: Cardiovascular effects of
fluoxetine in depressed patients with heart disease. Am J
Psychiatry. 155:660–665. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brambilla P, Cipriani A, Hotopf M and
Barbui C: Side-effect profile of fluoxetine in comparison with
other SSRIs, tricyclic and newer antidepressants: A meta-analysis
of clinical trial data. Pharmacopsychiatry. 38:69–77. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Castiella A and Arenas J: Fluoxetine
hepatotoxicity. Am J Gastroenterol. 89:458–459. 1994.PubMed/NCBI
|
|
16
|
Inkielewicz-Stępniak I: Impact of
fluoxetine on liver damage in rats. Pharmacol Rep. 63:441–447.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mavrides N and Nemeroff C: Treatment of
depression in cardiovascular disease. Depress Anxiety. 30:328–341.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herbet M, Gawrońska-Grzywacz M and
Jagiełło-Wójtowicz E: The influence of combined treatment of
simvastatin and amitriptyline on some biochemical parameters in rat
serum. Ann UMCS Sect DDD. 23:121–127. 2010.
|
|
19
|
Herbet M, Gawrońska-Grzywacz M, Kwiatek K
and Jagiełło-Wójtowicz E: Evaluation of selected biochemical
parameters in the serum of rats pretreated with simvastatin,
doxepin or their combination. Ann UMCS Sect DDD. 24:107–113.
2011.
|
|
20
|
Karnik NS and Maldonaldo JR:
Antidepressant and statin interactions: A review and case report of
simvastatin and nefazodone-induced rhabdomyolysis and
transaminitis. Psychosomatics. 46:565–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kasiske BL, Wanner C and O'Neill WC: An
Assessment of statin safety by nephrologists. Am J Cardiol.
97:82C–85C. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levy NB, Blumenfield M, Beasley CM Jr,
Dubey AK, Solomon RJ, Todd R, Goodman A and Bergstrom RR:
Fluoxetine in depressed patients with renal failure and in
depressed patients with normal kidney function. Gen Hosp
Psychiatry. 18:8–13. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blumenfield M, Levy NB, Spinowitz B,
Charytan C, Beasley CM Jr, Dubey AK, Solomon RJ, Todd R, Goodman A
and Bergstrom RF: Fluoxetine in depressed patients on dialysis. Int
J Psychiatry Med. 27:71–80. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vasudevan AR, Hamirani YS and Jones PH:
Safety of statins: Effects on muscle and the liver. Cleve Clin J
Med. 72:990–993. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang LS, Liu ZX, Lü W and Hu XY: Effects
of statins on the liver: Clinical analysis of patients with
ischemic stroke. Chin Med J (Engl). 124:897–900. 2011.PubMed/NCBI
|
|
26
|
Wille SM, Cooreman SG, Neels HM and
Lambert WE: Relevant issues in the monitoring and the toxicology of
antidepressants. Crit Rev Clin Lab Sci. 45:25–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wen B, Ma L and Zhu M: Bioactivation of
the tricyclic antidepressant amitriptyline and its metabolite
nortriptyline to arene oxide intermediates in human liver
microsomes and recombinant P450s. Chem Biol Interact. 173:59–67.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Apella D, Bruguera M, Figueras A and
Laporte J: Fluoxetine-induced hepatitis: Why is postmarketing
surveillance needed? Eur J Clin Pharmacol. 55:545–546. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mandrioli R, Forti GC and Raggi MA:
Fluoxetine metabolism and pharmacological interactions: The role of
cytochrome P450. Curr Drug Metab. 7:127–133. 2006. View Article : Google Scholar : PubMed/NCBI
|