Introduction
Periodontitis, one of the most prevalent chronic
diseases, is an infection-associated chronic inflammatory disease
of the periodontium and the major cause of tooth loss. Although
interleukin-1β (IL-1β) is critical in the host defense against
pathogens, it has been shown to be a pathogenic factor for the
destruction of periodontal tissue in periodontitis (1,2).
The processing of IL-1β is regulated by cytosolic machinery termed
as the inflammasome (3,4). The major components of the
inflammasome include the NOD-like receptor family, pyrin domain
containing protein (NLRP) and apoptosis-associated speck-like
protein containing a caspase recruitment domain (ASC), which
recruits and activates caspase-1. Activated caspase-1 in turn
cleaves pro-IL-1β to produce mature IL-1β. Therefore, the
inflammasome plays an important role in the production of
IL-1β.
Different inflammasomes, which contain different
NLRP family proteins, have been identified, such as NLRP1, NLRP3,
and NLRC4. These different inflammasomes are activated in response
to different stimuli (5). It has
been demonstrated that the NLRP3 inflammasome is the NLR subset
inflammasome that is activated in periodontitis (6). It has also been shown that
Porphyromonas gingivalis (P. gingivalis) infection activates
the NLRP3 inflammasome and increases the production of IL-1β
(7–8). Similarly, the bacterial endotoxin,
lipopolysaccharide (LPS), can also activate the NLRP3 inflammasome
and stimulate the production of IL-1β (8–10).
Gingival epithelial cells (GECs) are an important component of the
innate host response to periodontal bacteria and make a significant
contribution to the gingival health of the host (11). A recent study revealed that the
LPS-induced activation of the NLRP3 inflammasome is an important
mediator of the inflammatory response in the gingival epithelium
(8). Therefore, targeting the
inflammasome in the gingival epithelium may be a potential strategy
for the prevention and treatment of periodontitis.
Heme oxygenase-1 (HO-1) is an ubiquitous inducible
cellular stress protein and an endogenous cytoprotective enzyme
(12). HO-1 catalyzes the
rate-limiting step in heme degradation, producing equimolar
quantities of biliverdin, iron and carbon monoxide. Biliverdin is
subsequently converted to bilirubin by biliverdin reductase
(13–15). The products of HO-1 exhibit
protective biological activities, including antioxidant and
anti-inflammatory effects (12).
Reactive oxygen species have been shown to activate the
inflammasome and antioxidants inhibit the inflammasome (16,17). With a strong antioxidant function,
the HO-1 pathway may inhibit the inflammasome. Indeed, although
there are very limited reports studying the effect of the HO-1
pathway on the inflammasome, a literature search found two studies
showing that the induction of HO-1 inhibited inflammasome
activation and reduced the release of IL-1β in acute live failure
(18) and lung injury (19). Given the fact that LPS produces
oxidative stress and activates the inflammasome (8–10),
we hypothesized that the induction of the HO-1 pathway may inhibit
the inflammasome and protect against LPS-induced inflammatory
damage in human GECs. To examine this hypothesis, we first
determined the effects of hemin, a potent HO-1 inducer, on the
LPS-induced activation of the inflammasome, then measured the
production of IL-1β and the activation of nuclear factor-κB
(NF-κB), and finally, assessed the cell damage. To the best of our
knowledge, our study is the first to demonstrate that the induction
of HO-1 by hemin attenuates inflammatory damage in human GECs
through the inhibition of inflammasome activation.
Materials and methods
Cell culture
Human GECs were purchased from Yuhengfeng Biotech
(Beijing, China) and cultured according to the manufacturer’s
instructions in a defined epithelial cell medium (Yuhengfeng
Biotech) supplemented with fetal bovine serum (FBS) (2%),
epithelial cell growth supplement (1%), penicillin (100 IU/ml) and
streptomycin (100 μg/ml) at 37°C in a humidified atmosphere of 5%
CO2 in air.
Cell treatment and experimental
groups
The cells were subcultured in 10-cm dishes and
treated with the vehicle (0.2% DMSO as a control), LPS (2 μg/ml),
LPS plus hemin (20 μmol/l) or Ac-YVAD-CMK (10 μmol/l, a caspase-1
inhibitor) for 16 h when they reached 80% confluence. The cells
were then harvested for protein and RNA isolation as described
below. Some cells were cultured on glass coverslips for
immunofluorescent staining and confocal microscopy as described
below.
Preparation of cytosolic protein and
nuclear extracts, western blot analyses for the protein levels of
HO-1, NLRP3, ASC, caspase-1 and NF-κB (p65), as well as
co-immunoprecipitation (Co-IP) of ASC and NLRP3
Cytosolic and nuclear proteins were prepared as
previously described (20,21).
Briefly, the cells were scraped and washed with phosphate-buffered
saline (PBS), and then homogenized in ice-cold HEPES buffer A
containing 10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM
KCl, 0.5 mM dithiothreitol (DTT), 0.5 mM phenylmethylsulfonyl
fluoride (PMSF) and 10% Nonidet P-40. Following centrifugation of
the homogenate at 1,000 × g for 5 min at 4°C, the supernatants were
collected for cytosolic protein preparation and the pellets for
nuclear protein isolation. The supernatants were centrifuged again
at 6,000 × g for 10 min and the resulting supernatants were used as
cytosolic proteins for western blot analyses of HO-1, NLRP3, ASC
and caspase-1.
For nuclear fraction isolation, the pellets from the
first centrifugation, which contains cell nuclei, were washed with
buffer A and then incubated with ice-cold HEPES buffer B containing
5 mM HEPES (pH 7.9), 1.5 mM MgCl2, 300 mM NaCl, 400 mM
KCl, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF and 26% glycerol for 30
min to release nuclear proteins. Subsequently, the reaction
mixtures were centrifuged at 23,000 rpm for 30 min, and the
supernatant was collected and stored at −80°C until use as nuclear
extracts for western blot analyses of NF-κB levels.
Western blot analysis was performed as previously
described (22). Briefly, protein
samples (20 μg) were subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto nitrocellulose membranes. The membranes were
probed with primary antibodies and then horseradish
peroxidase-labeled secondary antibodies. Following incubation with
enhanced chemiluminescence detection solution (ECL; Pierce,
Rockford, IL, USA), the membranes were exposed to Kodak X-OMAT film
(VWR International China Co., Ltd., Shanghai, China). The intensity
of the blots on the film was determined using an imaging analysis
program (ImageJ, free download from http://rsbweb.nih.gov/ij/).
The primary antibodies used in the present study
included anti-human HO-1 (1:1,000 dilution), NLRP3 (1:500), ASC
(1:1,000), caspase-1 (1:3,000) and NF-κB (p65) (1:1,000), which
were from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA,
distributor in Shanghai, China).
Co-IP of ASC and NLRP3
Co-IP was performed using a kit from Takara Bio,
Inc. (Beijing, China) following the manufacturer’s instructions. In
brief, the cell lyses were mixed with antibody against ASC followed
by the addition of protein-A beads. The beads were then collected
and subjected to western blot analysis with anti-NLRP3
antibody.
Immunofluorescence microscopy
Immunofluorescence staining was performed using the
cells cultured on glass coverslips, as previously described
(20). Following fixation with 2%
paraformaldehyde for 30 min, the cells were incubated with anti-ASC
(1:100 dilution), anti-caspase-1 (1:100 dilution), anti-E-cadherin
(1:200) and/or anti-NF-κB (1:200) antibodies at 4°C overnight.
Following washing, the slides were incubated with corresponding
Alexa Fluor 488- or Alexa Fluor 555-labeled secondary antibodies
and then subjected to examinations using a confocal laser scanning
microscope (FluoView FV1000; Olympus, Tokyo, Japan). The images
were analyzed and the co-localization co-efficients were calculated
using the computer program, Image-Pro® Plus (Media
Cybernetics, Silver Spring, MD, USA), as previously described
(23).
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) of the mRNA
expression of HO-1
Total RNA was extracted using TRIzol reagent (Life
Technologies, Inc., Rockville, MD, USA). RNA was then
reverse-transcribed and the PCR products were amplified using an
RT-PCR kit (Solarbio Science and Technology, Beijing, China) and a
thermocycler (Lab-Eye; Chuangmeng Biotechnology, Shanghai, China).
The primers used were as follows: HO-1. sense,
5′-GGGTGACAGAAGAGGCTAAGACC-3′ and antisense,
5′-AGATTCTCCCCTGCAGAGAGAAG-3′ (24). The PCR cycling conditions were as
follows: 30 cycles of 94°C for 30 sec; 55°C for 30 sec; and 72°C
for 45 sec. The amplified products were visualized by 1.5% agarose
gel electrophoresis, stained with ethidium bromide and images were
captured under ultraviolet light.
Enzyme-linked immunosorbent assay (ELISA)
of IL-1β in cell culture medium
The concentration of IL-1β in the cell culture
medium was measured using a Valukine ELISA kit (R&D Systems,
Shanghai, China) according to the manufacturer’s instructions.
Statistics
Data are presented as the means ± standard error
(SE). The significance of differences in mean values within and
between multiple groups was evaluated by ANOVA followed by a
Tukey’s multiple range test. The Student’s t-test was used to
evaluate the statistical significance of differences between 2
groups. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of hemin on the expression of the
inflammasome components, NLRP3, ASC and caspase-1
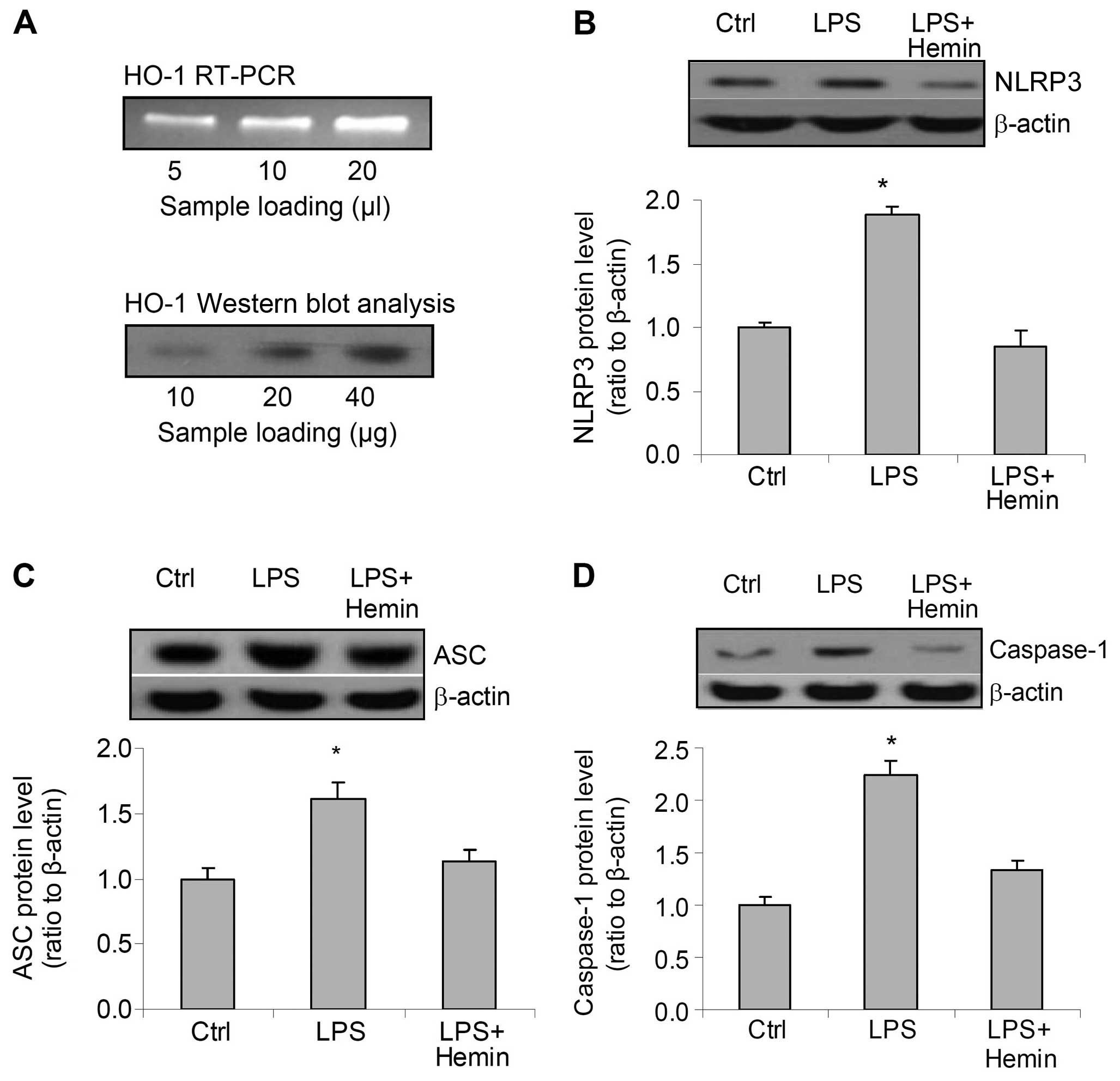
RT-PCR and western blot analysis of HO-1 mRNA and
protein revealed the predicted bands, which confirmed that HO-1 was
expressed in the cells used in the present study (Fig. 1A). Treatment of the cells with LPS
markedly increased the protein levels of all 3 components of the
inflammasome, NLRP3, ASC and caspase-1. However, hemin blocked the
increase in the levels of NLRP3, ASC and caspase-1 induced by LPS
(Fig. 1B–D). These data suggest
that LPS stimulates the expression of inflammasome components and
that hemin blocks the LPS-induced activation of the
inflammasome.
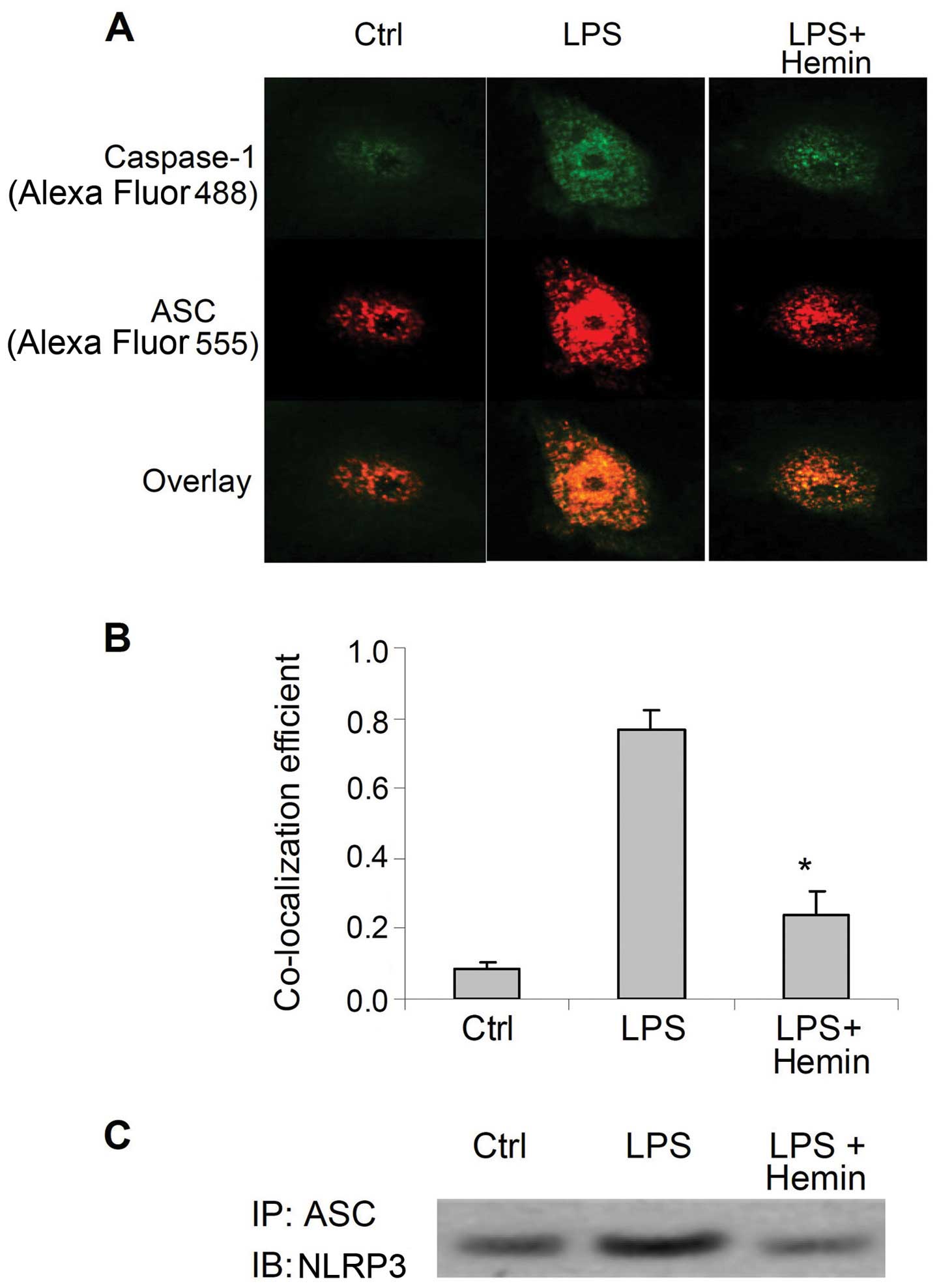
Effect of hemin on the formation of the
inflammasome
LPS markedly increased the immunostaining of
caspase-1 and ASC (Fig. 2A), as
well as the co-localization of these 2 proteins (Fig. 2B). (Co-IP) assay demonstrated that
LPS markedly increased the binding of ASC with NLRP3 (Fig. 2C). The increased co-localization
of caspase-1 and ASC, as well as the binding of NLRP3 and ASC were
markedly inhibited by hemin (Fig.
2). These results indicated that LPS increased the assembly of
inflammasome components and the recruitment of caspase-1, further
suggesting that LPS activated the inflammasome and that hemin
blocked the LPS-induced activation of the inflammasome.
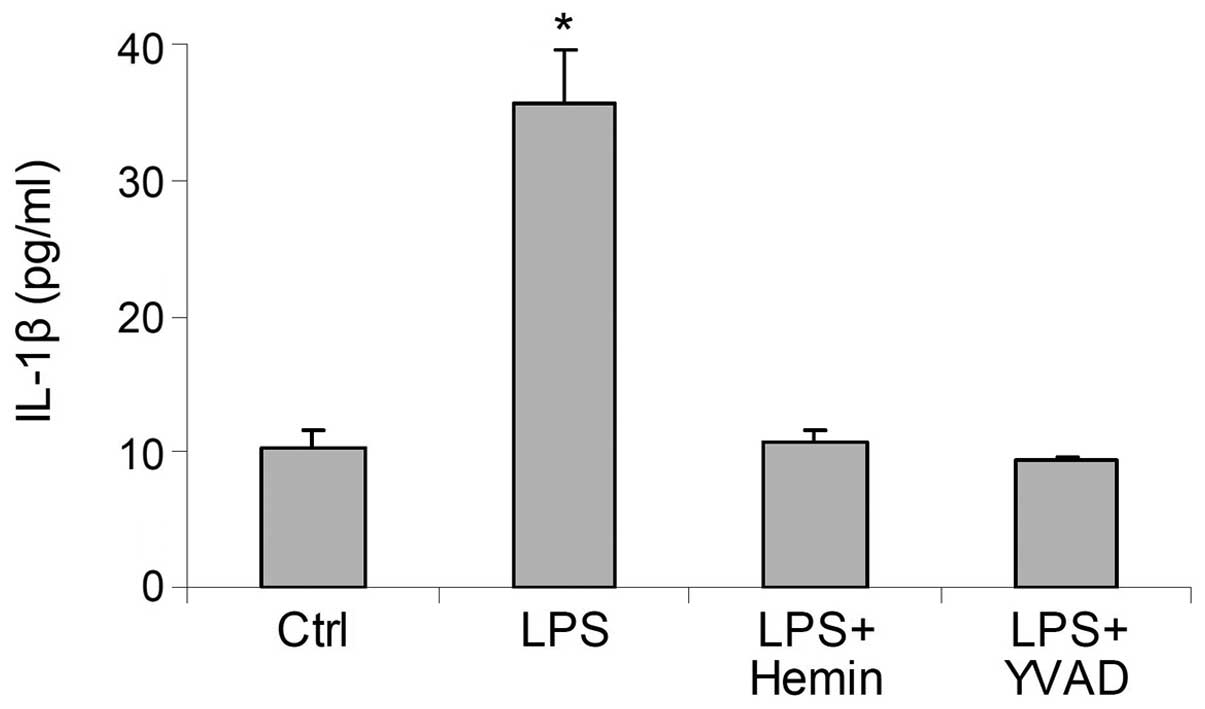
Effects of hemin on the production of
IL-1β
In the LPS-treated cells, the levels of IL-1β were
markedly increased; this effect was blocked by hemin (Fig. 3). These results suggested that
hemin inhibited the LPS-induced activation of the inflammasome and
reduced the production of IL-1β. The inhibitory effects of hemin on
the production of IL-1β were similar to those of YVAD, a caspase-1
inhibitor (Fig. 3), further
indicating that treatment with hemin resulted in an inhibition of
caspase-1 activity through the deactivation of the
inflammasome.
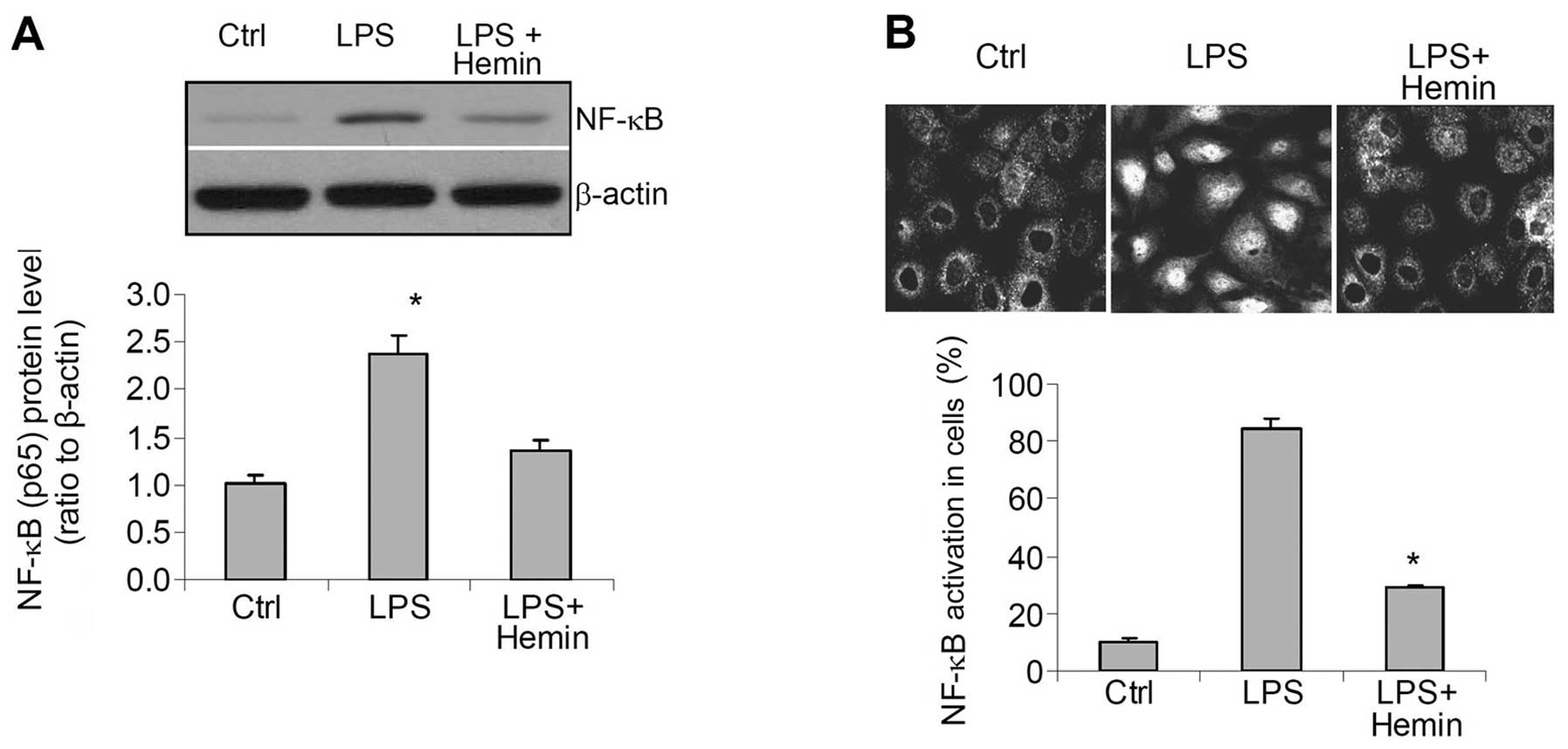
Effects of hemin on the activity of the
inflammatory factor, NF-κB
Western blot analysis revealed a significant
increase in the levels of NF-κB in the nuclear extracts (Fig. 4A). Confocal imaging indicated that
NF-κB was mainly located in the cytoplasm of the control cells
(Fig. 4B), whereas in the
LPS-treated cells, NF-κB was mainly observed in the nucleus,
indicating the activation and enhanced nuclear translocation of
NF-κB. These increases in the translocation of NF-κB into the
nuclei were also blocked in the hemin-treated cells (Fig. 4).
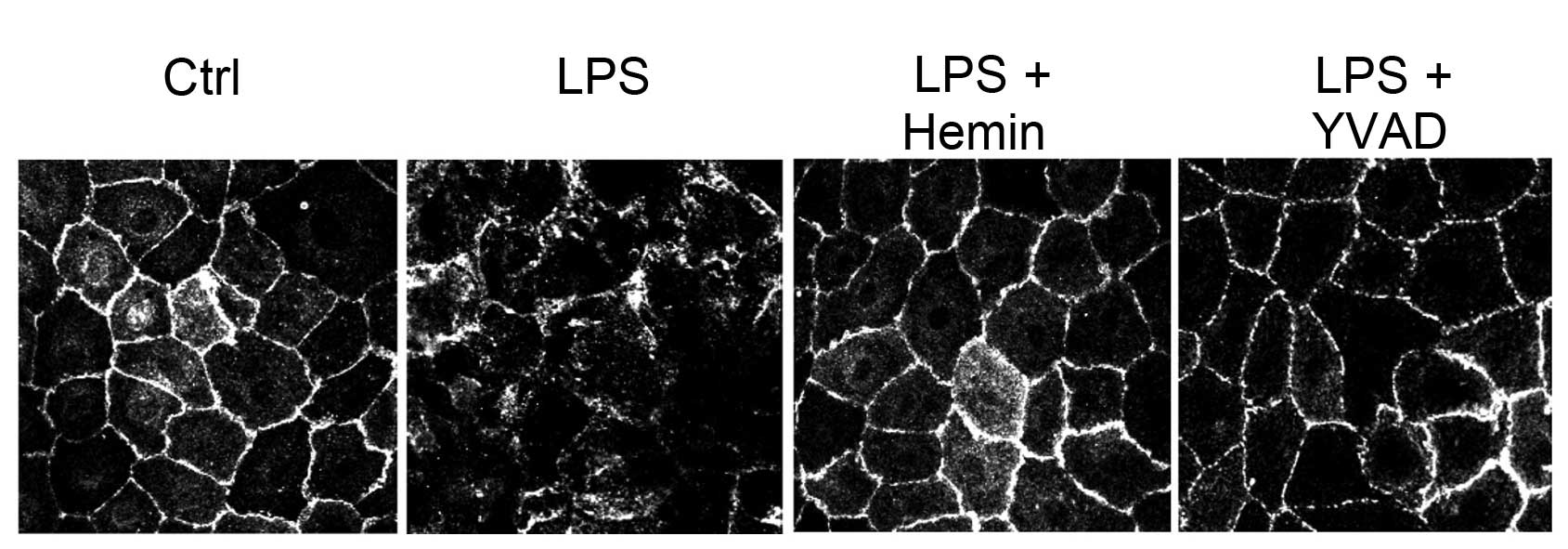
Effects of hemin on cell damage
Confocal imaging revealed a derangement of the
immunostaining pattern of the cell junction protein, E-cadherin, in
the LPS-treated cells, which was blocked in the hemin-treated cells
and in the cells treated with the caspase-1 inhibitor, YVAD,
(Fig. 5), suggesting that the
inhibition of the activation of the inflammasome and its downstream
pro-inflammatory effectors by hemin protected the cells from
LPS-induced damage.
Discussion
The present study demonstrated that the induction of
HO-1 by hemin suppressed the LPS-induced increase in the expression
of inflammasome components (NLRP3, ASC and caspase-1), inhibited
the assembly of the NLRP3 inflammasome, and blocked the production
of IL-1β, as well as the activation of the pro-inflammatory factor,
NF-κB. As a result, hemin attenuated the LPS-induced cell damage in
the cultured human GECs. To the best of our knowledge, the results
of the present study are the first to suggest that the induction of
HO-1 inhibits the activation of the inflammasome and protects human
GECs against LPS-induced inflammatory damage.
Epithelial cells are the first line cells in contact
with pathogens and danger signals/factors, including bacterial
toxins. These cells also act as a physical barrier, protecting
other cells, such as fibroblasts and osteoblasts, from exposure to
pathogens. We thus selected to use GECs in the present study. We
first confirmed the presence of HO-1 in the cells by detecting the
mRNA and protein expression of HO-1, which is consistent with
previous studies showing that HO-1 is expressed in oral epithelial
cells (25,26). Similar to the results of a recent
study (8), our results revealed
an increased expression of NLRP3 inflammasome components in the
GECs following treatment with LPS. Using confocal and Co-IP
analyses, we further demonstrated that LPS not only increased the
expression of NLRP3 inflammasome components, but also stimulated
the assembly/formation of this machinery, further demonstrtating
the activation of the NLRP3 inflammasome by LPS. Consequently, the
production of IL-1β was increased in the LPS-treated cells.
However, the LPS-induced activation of the NLRP3 inflammasome and
the production of IL-1β were abolished by hemin, suggesting that
the acvitation of HO-1 inhibits NLRP3 inflammasome activation, as
well as IL-1β production in GECs.
Although it is well recognized that the induction of
HO-1 exerts anti-inflammatory effects, the mechanisms involved have
not yet been fully elucidated. The inhibition of inflammasome
activation and IL-1β production may present one of the mechanisms
through which HO-1 executes anti-inflammatory functions. In the
present study, we did not attempt to explore the mechanisms through
which the HO-1 pathway inhibits the LPS-induced activation of the
NLRP3 inflammasome. In this regard, the antioxidant activities of
HO-1 may be accountable for this effect. Oxidative stress has been
shown to activate the inflammassome (16,17), and at the same time, the products
of HO-1 exhibit antioxidant functions (27). Thus, the mechanisms through which
the HO-1 pathway inhibits the inflammasome may possibly be mediated
through the antioxidant activities, as LPS has been shown to
activate the inflammasome through oxidative stress (9,10).
Since IL-1β has been shown to activate NF-κB
(28,29) and IL-1β mediates the LPS-induced
activation of NF-κB (10), we
also evaluated the effects of hemin on the activation of NF-κB
(p65). Our results demonstrated that LPS stimulated the
translocation of NF-κB into the nucleus, as shown by the increased
staining of NF-κB in the nucleus in the confocal images and the
elevated levels of NF-κB in the nuclear extracts by western blot
analysis. Hemin blocked the LPS-induced translocation of NF-κB into
the nucleus. These data indicate that the activation of the HO-1
pathway blocks the activation of downstream inflammatory factors
associated with the inflammasome. Since NF-κB, as a transcription
factor, also stimulates IL-1β production (4), we cannot rule out the possibility
that hemin directly inhibited the LPS-induced NF-κB activation in
addition to its effect on the inflammasome and IL-1β. Nevertheless,
our data provide compelling evidence that the activation of the
HO-1 pathway inhibits inflammasome activation and thereby blocks
the downstream inflammatory response induced by LPS.
The inhibition of LPS-induced pro-inflammatory
factors IL-1β and NF-κB by hemin is expected to protect the cell
damage. We then observed the immunostaining patter of a cell
junction protein E-cadherin, which determines the epithelial
integrity. In consistent with previous study (30), our result demonstrated that LPS
disrupted cell junction as shown by the derangement of E-cadherin
staining pattern. However, hemin blocked LPS-induced disturbance in
E-cadherin staining pattern, suggesting that induction of HO-1
protected against LPS-induced inflammatory damage in gingival
cells, probably through inhibition of LPS-induced activation of
inflammasome.
In conclusion, the present study demonstrates that
induction of HO-1 activity by hemin inhibits the LPS-induced
activation of the NLRP3 inflammasome, reduces the resulting
production of pro-inflammatory factors, and consequently attenuates
cell damage. Thus, it can be concluded that the activation of HO-1
protects LPS-induced inflammatory damage in GECs, which may be used
as a strategy for the prevention and treatment of chronic
periodontitis.
References
|
1
|
Bascones A, Noronha S, Gómez M, Mota P,
Gonzalez Moles MA and Villarroel Dorrego M: Tissue destruction in
periodontitis: bacteria or cytokines fault? Quintessence Int.
36:299–306. 2005.PubMed/NCBI
|
|
2
|
Orozco A, Gemmell E, Bickel M and Seymour
GJ: Interleukin-1beta, interleukin-12 and interleukin-18 levels in
gingival fluid and serum of patients with gingivitis and
periodontitis. Oral Microbiol Immunol. 21:256–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pedra JH, Cassel SL and Sutterwala FS:
Sensing pathogens and danger signals by the inflammasome. Curr Opin
Immunol. 21:10–16. 2009. View Article : Google Scholar
|
|
4
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Broz P and Monack DM: Molecular mechanisms
of inflammasome activation during microbial infections. Immunol
Rev. 243:174–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bostanci N, Emingil G, Saygan B, et al:
Expression and regulation of the NALP3 inflammasome complex in
periodontal diseases. Clin Exp Immunol. 157:415–422. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park E, Na HS, Song YR, Shin SY, Kim YM
and Chung J: Activation of NLRP3 and AIM2 inflammasomes by
Porphyromonas gingivalis infection. Infect Immun. 82:112–123. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yilmaz O, Sater AA, Yao L, Koutouzis T,
Pettengill M and Ojcius DM: ATP-dependent activation of an
inflammasome in primary gingival epithelial cells infected by
Porphyromonas gingivalis. Cell Microbiol. 12:188–198. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hua KF, Chou JC, Lam Y, et al:
Polyenylpyrrole derivatives inhibit NLRP3 inflammasome activation
and inflammatory mediator expression by reducing reactive oxygen
species production and mitogen-activated protein kinase activation.
PLoS One. 8:e767542013. View Article : Google Scholar
|
|
10
|
Kamo N, Ke B, Ghaffari AA, et al:
ASC/caspase-1/IL-1β signaling triggers inflammatory responses by
promoting HMGB1 induction in liver ischemia/reperfusion injury.
Hepatology. 58:351–362. 2013.
|
|
11
|
Yilmaz O: The chronicles of
Porphyromonas gingivalis: the microbium, the human oral
epithelium and their interplay. Microbiology. 154:2897–2903.
2008.
|
|
12
|
Pae HO, Kim EC and Chung HT: Integrative
survival response evoked by heme oxygenase-1 and heme metabolites.
J Clin Biochem Nutr. 42:197–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maines MD: The heme oxygenase system: a
regulator of second messenger gases. Annu Rev Pharmacol Toxicol.
37:517–554. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ndisang JF, Tabien HE and Wang R: Carbon
monoxide and hypertension. J Hypertens. 22:1057–1074. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abraham NG and Kappas A: Heme oxygenase
and the cardiovascular-renal system. Free Radic Biol Med. 39:1–25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Villegas LR, Kluck D, Field C, et al:
Superoxide dismutase mimetic, MnTE-2-PyP, attenuates chronic
hypoxia-induced pulmonary hypertension, pulmonary vascular
remodeling, and activation of the NALP3 inflammasome. Antioxid
Redox Signal. 18:1753–1764. 2013. View Article : Google Scholar
|
|
17
|
Komada T, Usui F, Shirasuna K, et al: ASC
in renal collecting duct epithelial cells contributes to
inflammation and injury after unilateral ureteral obstruction. Am J
Pathol. 184:1287–1298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SJ and Lee SM: NLRP3 inflammasome
activation in D-galactosamine and lipopolysaccharide-induced acute
liver failure: role of heme oxygenase-1. Free Radic Biol Med.
65:997–1004. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo YP, Jiang L, Kang K, et al: Hemin
inhibits NLRP3 inflammasome activation in sepsis-induced acute lung
injury, involving heme oxygenase-1. Int Immunopharmacol. 20:24–32.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han WQ, Zhu Q, Hu J, Li PL, Zhang F and Li
N: Hypoxia-inducible factor prolyl-hydroxylase-2 mediates
transforming growth factor beta 1-induced epithelial-mesenchymal
transition in renal tubular cells. Biochim Biophys Acta.
1833:1454–1462. 2013. View Article : Google Scholar
|
|
21
|
Yu X, Deng L, Wang D, et al: Mechanism of
TNF-α autocrine effects in hypoxic cardiomyocytes: initiated by
hypoxia inducible factor 1α, presented by exosomes. J Mol Cell
Cardiol. 53:848–857. 2012.
|
|
22
|
Zhu Q, Wang Z, Xia M, Li P-L, Zhang F and
Li N: Overexpression of HIF-1α transgene in the renal medulla
attenuated salt sensitive hypertension in Dahl S rats. Biochim
Biophys Acta. 1822:936–941. 2012.
|
|
23
|
Zinchuk V, Zinchuk O and Okada T:
Quantitative colocalization analysis of multicolor confocal
immunofluorescence microscopy images: pushing pixels to explore
biological phenomena. Acta Histochem Cytochem. 40:101–111. 2007.
View Article : Google Scholar
|
|
24
|
Zhong Y, Liu T, Lai W, Tan Y, Tian D and
Guo Z: Heme oxygenase-1-mediated reactive oxygen species reduction
is involved in the inhibitory effect of curcumin on
lipopolysaccharide-induced monocyte chemoattractant protein-1
production in RAW264.7 macrophages. Mol Med Rep. 7:242–246.
2013.
|
|
25
|
Tsai CH, Yang SF, Lee SS and Chang YC:
Augmented heme oxygenase-1 expression in areca quid
chewing-associated oral submucous fibrosis. Oral Dis. 15:281–286.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milward MR, Chapple IL, Wright HJ, Millard
JL, Matthews JB and Cooper PR: Differential activation of NF-kappaB
and gene expression in oral epithelial cells by periodontal
pathogens. Clin Exp Immunol. 148:307–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haines DD, Lekli I, Teissier P, Bak I and
Tosaki A: Role of haeme oxygenase-1 in resolution of oxidative
stress-related pathologies: focus on cardiovascular, lung,
neurological and kidney disorders. Acta Physiol (Oxf). 204:487–501.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tseng HC, Lee IT, Lin CC, et al: IL-1β
promotes corneal epithelial cell migration by increasing MMP-9
expression through NF-κB- and AP-1-dependent pathways. PLoS One.
8:e579552013.
|
|
29
|
Viñuales C1, Gascón S, Barranquero C,
Osada J and Rodríguez-Yoldi MJ: Interleukin-1beta reduces galactose
transport in intestinal epithelial cells in a NF-κB and protein
kinase C-dependent manner. Vet Immunol Immunopathol. 155:171–181.
2013.PubMed/NCBI
|
|
30
|
He D, Su Y, Usatyuk PV, et al:
Lysophosphatidic acid enhances pulmonary epithelial barrier
integrity and protects endotoxin-induced epithelial barrier
disruption and lung injury. J Biol Chem. 284:24123–24132. 2009.
View Article : Google Scholar : PubMed/NCBI
|