1. Introduction
Macrophages are vital components of the innate
immune system and are ubiquitous throughout the body. First
identified and elucidated by Metchnikoff in the 19th century,
macrophages have been recognised for their pivotal roles in the
phagocytosis and elimination of microorganisms (1). Their multifaceted functions
encompass the maintenance of tissue equilibrium, orchestration and
resolution of immune responses during pathogenic assaults and the
facilitation of tissue repair and restructuring in both
developmental and injury-induced contexts (2,3).
Furthermore, macrophages exhibit diverse cellular responses and
adapt to distinct stimuli or sources within tissues or the
environment. For instance, exposure to microbial stimulation
triggers an M1 or inflammatory state in macrophages, which is
characterised by increased production of pro-inflammatory cytokines
and microbe eradication. Conversely, during helminthic or parasitic
infections, macrophages transition to an M2 or alternative state,
specialising in tissue regeneration and remodelling (4).
The eye is a remarkably specialised sensory organ
that encompasses several intricately interconnected tissue types,
each of which is pivotal for the formation of clear visual images
by the neural retina. Among the intraocular tissues, the pigmented
iris, ciliary body and choroid collectively form the uvea. Similar
to the brain, the retina represents neural tissue sheltered by the
blood-eye barrier, comprising a complex network of interconnected
neurons. Positioned adjacent to the neural retina, the choroid, a
highly vascularised connective tissue, serves a crucial role in
providing metabolic and nutritional support to the outer retina. In
the unique microenvironment of intraocular tissue, there are
different populations of resident tissue macrophages that are adept
at maintaining tissue homeostasis and coordinating inflammatory
responses when encountering abnormal stimuli.
The neural retina contains specialised resident
macrophages known as microglia (5). Originating early in embryogenesis
from precursor cells in the embryonic yolk sac, microglia migrate
to specific regions within the central nervous system (CNS) at
approximately embryonic day 8.5 (6,7).
Their developmental pathways overlap with those of tissue
macrophages. In cases of radiation-induced complete microglial
apoptosis, bone marrow-derived macrophages (BMDMs) can supplement
and express a phenotype similar to that of microglia. However,
these cells constitute a distinct population capable of
self-renewal and are not typically substituted for BMDMs (8). In the retina, microglia are
primarily found in three specific locations: The nerve fibre, inner
and outer plexiform layers (5).
Macrophages are crucial components of the innate
immune system and exhibit various functions. They serve as a
primary defence against microorganisms and orchestrate adaptive
immune responses. Apart from generating essential pro-inflammatory
cytokines and chemokines, macrophages also have pivotal roles in
phagocytosis, clearing apoptotic cells and tissue debris (9). In addition, macrophages engage in
immune regulation by expressing anti-inflammatory cytokines like
interleukin (IL)-10, transforming growth factor (TGF-β) and lipid
mediators, such as lipoxins. Macrophages are implicated in tissue
remodelling and the development of various organs, such as the
breast tissue, bones, kidneys and brain (10). Macrophage dysregulation may
trigger autoimmune conditions and persistent inflammatory diseases
(11).
In the CNS, microglia constitute 5-12% of total
brain cells and share functional similarities with peripheral
macrophages. Microglia actively contribute to synaptic plasticity
and debris clearance in the healthy brain (12,13). Studies revealed that even in a
relatively quiescent state, microglia have pivotal roles in tissue
repair and infection control (14). Following injury or infection,
microglia in the CNS promptly respond to stimuli by releasing
cytokines that induce phagocytosis and direct cytotoxicity
(15). Peripheral macrophages
can replenish the microglia. Although microglia and macrophages
share several functions, such as antigen presentation and
production of cytokines, including oxidative free radicals,
chemokines and nitric oxide (NO), they possess distinct
characteristics. In the initial stages of CNS inflammatory
responses, microglia demonstrate lower cytokine production levels
(CD45, C-C chemokine receptor type (CCR)1 and CCR5) alongside
higher TGF-β expression. Conversely, infiltrating macrophages
exhibit elevated expression of CD45, CCR1, CCR2 and CCR5,
accompanied by reduced TGF-β expression (6,16). These differences in biomarker
profiles aid in distinguishing the CNS-resident microglia from the
infiltrating macrophages (17).
Nonetheless, both resident microglia and infiltrating macrophages
have analogous roles in the CNS during inflammatory responses.
Macrophages demonstrate responsiveness to endogenous
signals after infection or injury, assuming both pathogenic and
protective roles (2,18,19). Upon appropriate stimulation, M1
macrophages serve as the frontline defence of the innate immune
system during the early stages of a disease. Microglia share
phenotypic traits with peripheral macrophages and detect
detrimental stimuli through various immune receptors, including
Toll-like receptors (TLRs), nucleotide-binding oligomerisation
domains (NODs) and NOD-like receptors (20,21). Microglia exhibit different
activation states within injured tissues (22,23). Upon injury, microglia or
macrophages infiltrating from the circulation polarise toward a
pro-inflammatory (M1) phenotype upon exposure to pro-inflammatory
cytokines such as interferon-γ (IFN-γ) and tumour necrosis factor
(TNF)-α.
Typically, M1 classically activated macrophages
express TNF-α, IL-1α, IL-1β, IL-6, IL-12, IL-23, C-X-C motif
chemokine ligand (CXCL)9, CXCL10 and other cytokines and
chemokines. They are distinguished by their high secretion ratio of
IL-12 and IL-23 but produce relatively less IL-10. Furthermore, M1
macrophages engage in the type I T-helper cell (Th1) immune
response as both inducer and effector cells, apart from their roles
in defence against parasites and tumours (24-26). Similar to infiltrating
macrophages, microglia respond by producing M1-associated factors,
including pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, IL-12,
IL-23 and TNF-α), chemokines, redox molecules [e.g. NADPH oxidase
and inducible NO synthase (iNOS)], macrophage receptors with
collagen structure, costimulatory proteins (CD40) and major
histocompatibility complex class II (18,21,27-30).
M2 macrophages have multifaceted roles in allergic
responses, parasite clearance, inflammation suppression, tissue
remodelling, angiogenesis, immune regulation and tumour promotion
(31). Within this subset, M2
macrophages consist of four distinct subpopulations: i) M2a,
predominantly induced by IL-4 and IL-13; ii) M2b, primarily
triggered by immune complexes, IL-1β and TLR ligands; iii) M2c
macrophages, produced in response to IL-10, glucocorticoids and
TGF-β (25,32-36); and iv) M2d, primarily induced by
TLR antagonists (33). The
polarisation of microglia toward the M2 phenotype mirrors that of
peripheral macrophages (37-40), leading to distinctive mRNA
profiles following stimulation with IL-4 and IL-10, including the
expression of arginase 1 (Arg-1), chitinase-like protein 3, Fizz1
and peroxisome proliferator-activated receptor (PPAR) (41). Although these connections have
been demonstrated in vitro, the induction of M2 occurs in
vivo in sterile wounds, even in the absence of IL-4 or IL-13
(42). In this model, M2
macrophages were observed to originate from M1 macrophages
transitioning into repair-oriented macrophages within the tissue
after recruitment from circulation (43). As a result, the intrinsic
phenotype of these cells may diverge based on their origin and
local microenvironment.
By contrast, although M2 macrophages are divided
into different subpopulations, they share a common phenotype
characterised by low production of IL-12 and IL-23 but a high
release of IL-10. M2a macrophages express IL-10, TGF-β, C-C motif
chemokine ligand (CCL1)7, CCL22 and other cytokines. In general, M2
macrophages are unique in that they release a low proportion of
pro-inflammatory cytokines, such as IL-1, TNF-α and IL-6. However,
M2b subpopulations are distinctive for high expression of IL-10 and
CD86 but low production of IL-12 and Arg-1. Like M1 macrophages,
they are proficient producers of IL-1, TNF-α and IL-6 (35,36). Furthermore, M2b macrophages
express high levels of reactive nitrogen intermediates and iNOS
(35,36).
M2c macrophages, also known as inactivated
macrophages, secrete IL-10, TGF-β, CCL16 and CCL18, having a key
role in the phagocytosis of apoptotic cells (34). In addition, M2d macrophages
induce IL-10 and vascular endothelial growth factor (VEGF)
production, thereby promoting angiogenesis and pathological tumour
processes (33). The phenotypes,
inducer factors, surface markers and functions of macrophage
polarisation are shown in Table
I, and a schematic diagram of the M1 and M2 macrophage subsets
is shown in Fig. 1.
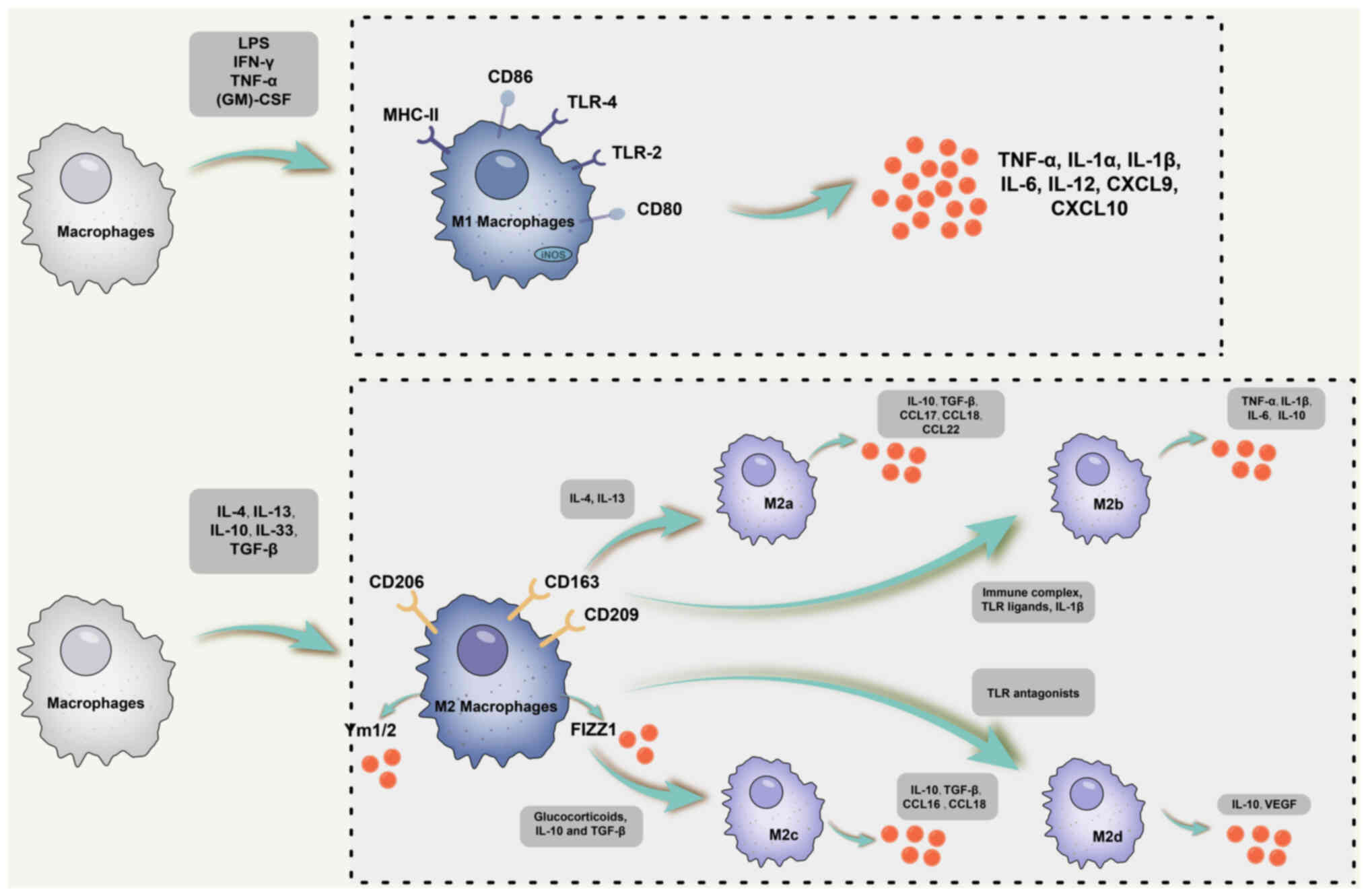 | Figure 1Schematic diagram of M1 and M2
macrophage subsets. Typically, M1-classically activated macrophages
are polarized in response to IFN-γ, TNF-α and other cytokines, and
express TNF-α, IL-1α, IL-1β, IL-6, IL-12, IL-23, CXCL9, CXCL10 and
other cytokines and chemokines. M2 macrophages consist of four
distinct subpopulations: i) M2a, induced primarily by IL-4 and
IL-13; ii) M2b is mainly triggered by immune complexes, IL-1β and
TLR ligands; iii) M2c macrophages, produced in response to IL-10,
glucocorticoids and TGF-β; iv) M2d, induced primarily by TLR
antagonists. Although M2 macrophages are divided into distinct
subpopulations, they share a common phenotype characterized by low
production of IL-12 and IL-23 but high release of IL-10. M2a
macrophages express IL-10, TGF-β, CCL17 as well as CCL22 and other
cytokines. However, the M2b subpopulation is unique in its high
expression of IL-10 and CD86 but lower production of IL-12 and
Arg-1. Like M1 macrophages, they are capable of producing IL-1,
TNF-α and IL-6. In addition, M2b macrophages express high levels of
RNI and iNOS. As for M2c macrophages, also known as inactivated
macrophages, these cells secrete IL-10, TGF-β, CCL16 and CCL18 and
have a key role in the phagocytosis of apoptotic cells.
Furthermore, M2d macrophages induce IL-10 and VEGF production,
thereby promoting angiogenesis and tumor pathological processes.
CXCL9, C-X-C motif chemokine ligand 9; TLR, Toll-like receptor;
iNOS, inducible nitric oxide synthase; RNI, reactive nitrogen
intermediates; (GM)-CSF, granulocyte-macrophage colony-stimulating
factor; MHC, major histocompatibility complex; LPS,
lipopolysaccharide; Arg-1, Arginase-1. |
 | Table IPhenotypes, inducible factors,
surface markers and functions of macrophage polarization. |
Table I
Phenotypes, inducible factors,
surface markers and functions of macrophage polarization.
| Cell type | Inducible
factor | Surface marker | Phenotype | Function | (Refs.) |
|---|
| M1 | IFN-γ, TNF-α,
(GM)-CSF, LPS | TLR-2, TLR-4, CD80,
CD86, iNOS, MHC-II | TNF-α, IL-1α,
IL-1β, IL-6, IL-12, IL-23, CXCL1-3, CXCL8-10, CCL2-5, CCL11 | Th1 immune
reaction, proinflammation, antitumor | (24-26) |
| M2 | | | | | |
| M2a | IL-4, IL-13 | CD206, MHC-II,
IL-1R, Arg-1, Ym1/2, FIZZ1 | TGF-β, Arg-1,
IL-10, CCL17, CCL22, CCL18 | Cell growth,
anti-inflammation, tissue repair, Th2 immune response, anaphylaxis,
fibrosis | (25,35,36) |
| M2b | Immune complex,
TLR, IL-1β | CD206, MHC-II,
CD86, IL-10R, IL-12R, IL-6R | TNF-α, IL-1β,
IL-10, IL-6, IL-12 | Regulation of
immune responses, inflammatory reactions | (32,35,36) |
| M2c | IL-10,
glucocorticoids, TGF-β | CD206, CD163,
TLR-1, TLR-8, Arg-1 | IL-10, TGF-β,
Arg-1, CXCL13, CCL16, CCL18 | Phagocytosis,
immunosuppression, tissue remodeling | (34-36) |
| M2d | TLR
antagonists | CD206, IL-10R,
IL-12R | VEGF, TNF-α, IL-10,
IL-12 | Promotion of
angiogenesis and tumor progression | (33,35,36) |
2. Intraocular inflammation-related
diseases
Macrophages have pivotal roles in maintaining tissue
homeostasis and regulating inflammation (44,45). M1 macrophages undergo
polarisation triggered by lipopolysaccharide (LPS) alone or in
conjunction with Th1 cytokines such as IFN-γ and
granulocyte-macrophage colony-stimulating factor (GM-CSF).
Consequently, M1 macrophages secrete pro-inflammatory cytokines,
including IL-1β, IL-6, IL-12, IL-23 and TNF-α, through the
activation of various transcription factors, such as signal
transducer and activator of transcription (STAT)1, nuclear factor
κB (NF-κB) and IFN regulatory factor 5. Thus, M1 macrophages are
characterised by a pro-inflammatory phenotype (46). Conversely, M2 macrophages receive
polarisation signals primarily from Th2 cytokines, such as IL-4 and
IL-13, and exhibit anti-inflammatory and immunomodulatory
phenotypes (47). M2 macrophages
produce anti-inflammatory cytokines, including IL-10 and TGF-β, by
activating multiple transcription factors such as STAT3, STAT6, IFN
regulatory factor 4 and PPAR-γ (48). The association between macrophage
polarisation and inflammatory cytokine levels is illustrated in
Fig. 2
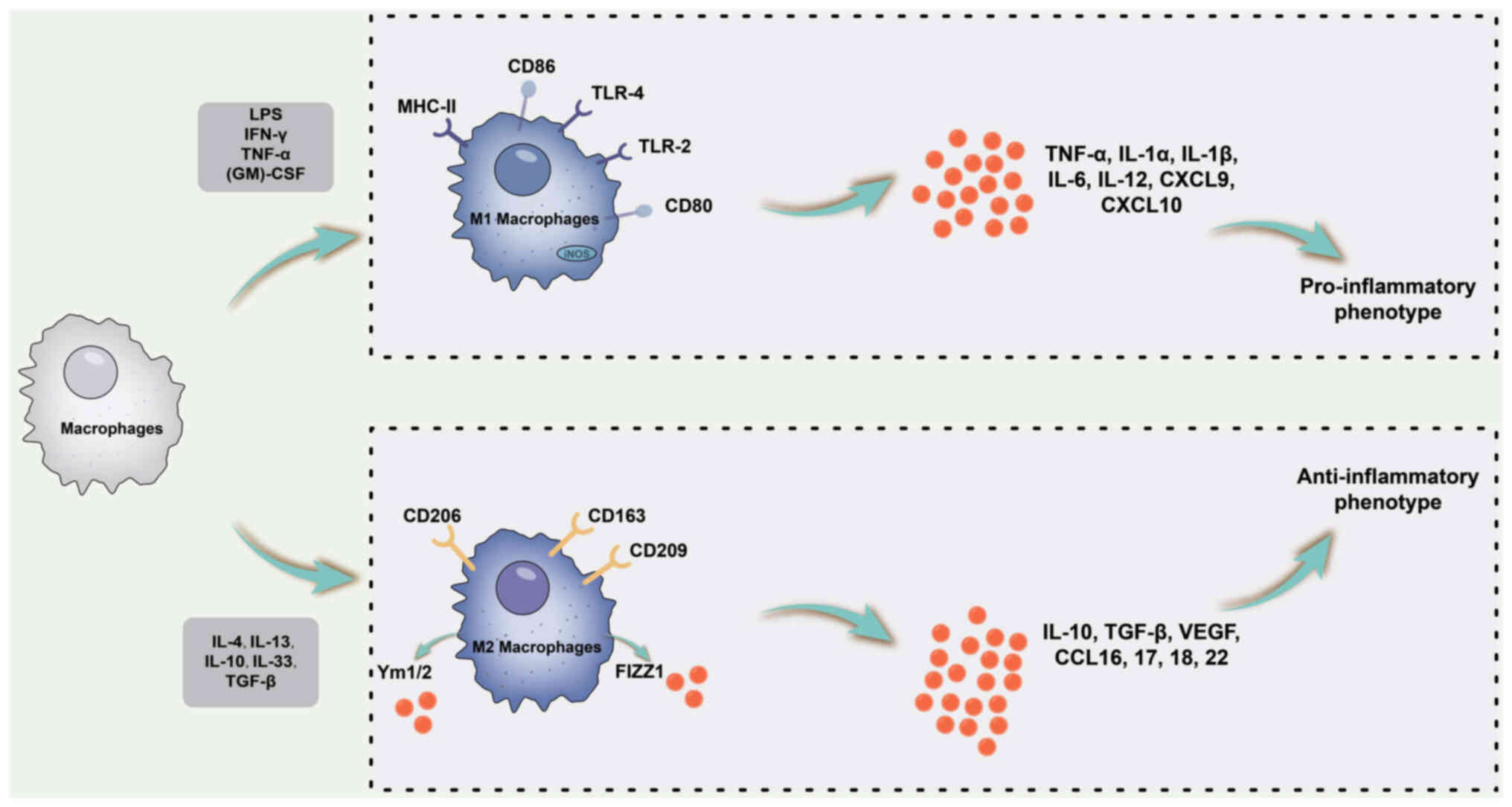 | Figure 2Schematic diagram of the association
between macrophage polarization and inflammatory cytokines. M1
macrophage polarization is primarily triggered by LPS alone or in
combination with Th1 cytokines, such as IFN-γ and GM-CSF. M1
macrophages are characterized by a pro-inflammatory phenotype and
secrete pro-inflammatory cytokines, including IL-1β, IL-6, IL-12,
IL-23 and TNF-α. By contrast, M2 macrophages primarily receive
polarizing signals from Th2 cytokines, such as IL-4 and IL-13,
exhibiting an anti-inflammatory and immunomodulatory phenotype. M2
macrophages produce anti-inflammatory cytokines including IL-10 and
TGF-β. MHC, major histocompatibility complex; LPS,
lipopolysaccharide; Th1, type 1 T-helper cell; CXCL9, C-X-C motif
chemokine ligand 9; TLR, Toll-like receptor; (GM)-CSF,
granulocyte-macrophage colony-stimulating factor; iNOS, inducible
nitric oxide synthase. |
Autoimmune uveitis
The experimental autoimmune uveitis (EAU) model is a
noninfectious uveitis animal model that closely resembles human
uveitis in both clinical and histological features (49-51). EAU in mice is induced through the
subcutaneous injection of an emulsified antigen, which disrupts
immune tolerance within the body. Following immunisation, naïve T
cells receive antigens delivered by presenting cells. Subsequently,
these cells are converted into Th0 cells, and T-cell subpopulations
such as Th1 and Th17 differentiated from these cells have important
functions in numerous autoimmune diseases, including EAU (52,53). These T cells multiply in the
peripheral system and translocate to the retina, where they release
inflammatory factors and promote macrophage migration, causing
tissue damage (50).
Diverse immune-cell infiltration is a hallmark of
the EAU retina, involving macrophages, neutrophils, dendritic cells
and other immune cells (54).
Furthermore, the proportions of various immune cells were observed
to vary across different phases of EAU. During the acute EAU phase,
macrophages accounted for 40% of all retinal immune cells. However,
in the late chronic stage, the percentage decreased to 19%. By
contrast, the percentage of immune cells, such as CD8 T cells and
myeloid-derived suppressor cells (MDSCs), increased during the
transition from the acute to the chronic phase (54).
In addition, the phenotypes of immune constituents
infiltrating different EAU stages undergo dynamic changes. For
instance, in the acute phase, most macrophages exhibit the M1
phenotype, whereas in the chronic (angiogenic) phase, M2
macrophages are predominant (55). These results suggest that the
retinal microenvironment under inflammatory conditions determines
the subsets of infiltrating cells, in addition to controlling the
phenotypes of different types of immune cell.
Studies have underscored the involvement of innate
immune cells, particularly macrophages and microglia, in antigen
presentation in EAU (56).
Macrophages are recognised as crucial effector cells in EAU and
contribute to the inflammatory process by releasing inflammatory
cytokines (57). Retinal
microglia exhibit phagocytic and pathogenic characteristics similar
to those of macrophages. Upon activation, both macrophages and
retinal microglia release pathogenic factors such as TNF-α and
iNOS, resulting in the nitration of cytochrome c, which is known to
cause EAU-cell apoptosis (58-60).
The aryl hydrocarbon receptor (AhR), a
high-molecular-weight transcription factor, has been demonstrated
to exert a negative regulatory effect on LPS-mediated inflammatory
responses in macrophages (61).
This suggests that AhR may be involved in the negative regulation
of M1 polarisation. After EAU induction, AhR-/mice had more severe
clinical and histopathological manifestations of uveitis than AhR
mice. Compared with AhR EAU mice, AhR+/+−/− EAU mice showed
evidence of a significant increase in macrophages/microglia and a
greater polarisation of phenotypes from M2 to M1 (62).
Furthermore, research has shown that the use of
2,3,7,8-tetrachlorodibenzo-p-dioxin (an AhR activator) can activate
AhR through the NF-κB, STAT1 and STAT3 signalling pathways. This
induces macrophage M2 polarisation, reducing the production of
apoptotic cells and the release of pro-inflammatory factors.
Consequently, the clinical manifestations of EAU are alleviated
(62).
IL-33 is a member of the IL-1 cytokine family and
signals through a heterodimeric receptor composed of suppression of
tumorigenicity 2 (ST2) and IL-1R accessory protein (63). Studies have highlighted the
crucial role of the IL-33/ST2 pathway in enhancing the polarisation
of alternatively activated macrophages (M2) (64).
Following 21 days of EAU induction, ST2-deficient
mice showed worse clinical symptoms than non-knockout mice, whereas
treatment of wild-type (WT) mice with IL-33 significantly improved
uveitis lesions. This improvement was accompanied by a significant
increase in the proportion of CD206 and CD273 cells, suggesting
that the upregulation of the IL-33/ST2 signalling pathway drives
macrophage (M2) polarisation, thus attenuating the clinical
manifestations of EAU (65).
Furthermore, glucocorticoids have been reported to
mediate the P38-MAPK/myocyte enhancer factor-2c axis, thereby
promoting the polarisation transition of macrophages from M2 to M1
and the release of anti-inflammatory factors. Consequently, this
process inhibits EAU and fosters the healing of damaged eye tissue
(66).
Suppressor of cytokine signalling (SOCS) proteins,
particularly SOCS1 and SOCS3, regulate macrophage polarisation and
cytokine expression. For instance, in BMDMs, SOCS3 acts as a
negative regulator of GM-CSF-induced expression of CCL2, Arg-1 and
matrix metallopeptidase 12 (67,68).
LysMCre/+SOCS3fl/fl mice
(LysMCre/+SOCS3fl/fl mice were obtained by
crossing SOCS3fl/fl mice with LysM-Cre mice, a type of
mouse with SOCS3 deficiency in myeloid cells) showed an increased
proportion of GM-CSF in the intraretinal milieu, which may have
triggered the release of CCL2 and Arg-1 from macrophages.
Research has demonstrated that mice deficient in
SOCS3 (LysMCre/+SOCS3f l/f l) experience
enhanced retinal degeneration and accelerated retinal angiogenesis
owing to inflammation (69). In
the acute phase of EAU, LysMCre/+SOCS3fl/fl
mice exhibited increased numbers of infiltrating neutrophils and
decreased numbers of macrophages compared to WT mice. Real-time
reverse transcription PCR analysis revealed a significant
upregulation in the release of TNF-α, IL-1β, IFN-γ, GM-CSF and
Arg-1 in the retina of LysMCre/+SOCS3fl/fl
mice compared to that in WT mice. Furthermore, the percentage of
Arg-1+ infiltrating cells was notably higher in
LysMCre/+SOCS3fl/fl EAU retinas than in WT
EAU retinas. In the absence of SOCS3, both macrophages and
neutrophils expressed higher levels of Arg-1, CCL2, IL-6 and VEGF,
promoting angiogenesis, suggesting that deletion of SOCS3 partially
induced M2 polarisation. Both isoforms of arginase have been
implicated in vascular cell dysfunction and vessel wall remodelling
in various diseases (70). Arg-1
is a characteristic marker of M2-type macrophages. To treat EAU,
researchers utilised an Arg inhibitor, amino-2-borono-6-hexanoic
acid, which effectively inhibits retinal angiogenesis without
improving inflammation (69).
This suggests that the development of retinal fibrovascular
membranes in EAU is associated with the polarisation of macrophages
to the M2 phenotype.
Optic neuritis
Optic neuritis, an acute inflammatory demyelinating
disease of the optic nerve, is an initial symptom of multiple
sclerosis (MS). It is characterised by optic nerve degeneration and
loss of retinal ganglion cells (RGCs), resulting in permanent
visual impairment; however, reliable treatments for this condition
are currently lacking. The well-established experimental autoimmune
encephalomyelitis (EAE) mouse model used for studying MS has also
proven useful for investigating optic neuritis. The mouse model is
characterised by the upregulation of molecules involved in
inflammation, gliosis and macrophage infiltration (71). Accumulation of inflammatory
factors leads to macrophage infiltration, which subsequently
produces a large number of potentially harmful cytokines, further
fuelling the inflammatory process (72).
EAE, triggered primarily by autoimmune Th1 and Th17
cells, is an inflammatory disease of the CNS (73). These cells produce various
cytokines, including IFN-γ, TNF and GM-CSF, which participate in
the M1 polarisation process of macrophages. Approximately 70% of
the immune cells in the inner environment of the CNS in an
inflammatory state are macrophages that are responsible for most
neuronal tissue damage by releasing TNF, NO and other inflammatory
factors (74-77). During EAE pathology, macrophages
exhibit a dual-activated phenotype that expresses both M1 and M2
markers, such as CD86 and chitinase-like protein 3 (78). During EAE, both M1 (IFN-γ) and M2
(IL-4) cytokines are present in the inflamed CNS. Therefore,
promoting the conversion of M1 subpopulation macrophages to the M2
subpopulation can promote the repair of MS-related damage and
ameliorate functional impairment (6). Studies have demonstrated that fatty
acids (FAs) have a positive impact on neuronal rescue by modulating
macrophage phenotypes, reducing pro-inflammatory capacity and
enhancing tissue recovery capacity (79).
Among M1-related factors, IL-12 and IL-23 are
largely involved in the progression of EAE (80) by inducing macrophage recruitment
through the upregulation of CXCL-10 and CXCL-11 release (81). Conversely, M2-related markers,
such as CCL-2, promote the repair of neuronal axons in the EAE
model (82), and CCL-22
upregulates the migration of anti-inflammatory immune cells during
EAE progression (83).
Furthermore, studies have demonstrated that treatment with ω-3 FAs
reduces RGC damage by regulating the conversion of the M1 to the M2
subpopulation (84).
In the context of optic neuritis in an EAE model,
suppressing M1 subpopulations and activating M2 subpopulations can
effectively prevent retinal inflammatory processes (85). This intervention holds promise
for hindering optic nerve damage and protecting RGCs from
death.
Sympathetic ophthalmia (SO)
SO is a type of uveitis characterised by
granulomatous lesions that occur after ocular surgery or
penetrating trauma (86). It
appears to occur as a delayed-type hypersensitivity reaction to
antigens in tissues exposed to traumatic events (87,88). The histopathology of SO is often
characterised by choroidal capillary involvement, the presence of
eosinophils, inflammation within the scleral canal, and substantial
infiltration by B lymphocytes and macrophages (89,90).
To further investigate the role of macrophages in
the inflammatory process of SO, researchers have performed
immunohistochemical staining of choroid tissue obtained from
patients clinically diagnosed with SO. Their analysis revealed a
significant presence of infiltrating CD68 cells, along with the
infiltration of TNF-α, INF-γ and other cytokines (91). These findings strongly suggest
that macrophages are involved in the pathological processes
underlying SO.
The presence of Dalen-Fuchs nodules suggests
granulomatous inflammation in the middle of the retinal pigment
epithelial (RPE) and Bruch's zones (88,89,92). Granulomas primarily consist of
activated macrophages (93,94) and may arise within the retina.
Studies have shown that M1 macrophage-specific cytokines, such as
IL-23 and CCL19, account for a large proportion of granulation
tissue in SO (93), suggesting
that most inflammatory cells in SO Dalen-Fuchs nodules and
granulation tissue are M1 macrophages.
Retinitis pigmentosa (RP)
RP is a retinal degenerative disease accompanied by
the apoptosis of photoreceptor cells, often leading to severe
visual impairment. Degeneration of photoreceptors is initiated by
microglial activation, infiltration of macrophages, and
accumulation of immunoglobulins and complement factors, resulting
in persistent inflammation, proliferation of macroglial cells and
progressive apoptosis of retinal neurons (95,96). Consequently, it is crucial to
explore avenues for RP intervention therapy that involve the
regulation of microglial activation and suppression of the
inflammatory response.
As RP advances, the blood-retinal barrier becomes
disrupted, leading to the recruitment of macrophages into the
retina. This recruitment has an important role in activating immune
cells and triggering the release of pro-inflammatory factors, which
further exacerbates disease progression and ultimately leads to the
loss of the retinal photoreceptor layer (97). Blood-borne immune cells have a
significant role in the microenvironment associated with RP and are
considered key mediators of the development of neurodegenerative
diseases (98,99).
Resident microglia and invading macrophages
coordinate responses to CNS injury by restoring tissue loss and
causing neuroinflammation (14,100). These immune cells have distinct
phenotypes, such as M1 macrophages that foster inflammation and M2
macrophages that facilitate tissue repair and regeneration
(101). Given the contrasting
roles of these macrophage subsets, recent therapeutic approaches to
nervous system inflammation are being tuned from immune cell
suppression to achieving a balance through molecules that regulate
the M1/M2 phenotypic polarisation switch (102).
Studies have demonstrated that olfactory ensheathing
cell transplantation holds promise for regulating the polarisation
of retinal macrophages from M1 to M2 in Royal College of Surgeons
rats via the JAK2/STAT3 pathway. This approach reduces the
infiltration of activated M1 macrophages and fosters a less
inflammatory microenvironment (103). Significant improvements in both
the functional and structural aspects of vision can be achieved
through this intervention. Similarly, essential FA supplementation
improves retinal dysfunction and degeneration by reducing
inflammation and microglial activation, weakening M1 markers, and
inducing the transformation of rd10 mice (a model of autosomal
recessive RP) retinas and LPS-stimulated BV10 cells to the M2
phenotype (104).
Glaucoma
Glaucoma is a neurodegenerative disease
characterised by optic nerve atrophy and irreversible loss of RGCs
(105). Secondary degeneration
of RGCs has a critical role in the progression of glaucomatous
damage (106), as RGC apoptosis
may continue even after intraocular pressure is reduced. Hence,
delaying secondary RGC degeneration holds promise as a potential
therapeutic approach for glaucoma treatment.
Damage to RGCs can be categorised as primary
(resulting from direct injury to the axon or cell body, such as
axonal extrusion or transection) or secondary (resulting from the
release of toxic effectors from adjacent dying cells) (107-110). To investigate the secondary
degeneration of RGCs, researchers have developed the partial optic
nerve transection (PONT) model. In comparison with the complete
optic nerve transection and optic nerve crush models, which damage
all axons simultaneously, the PONT model offers an advantage, as it
only damages a portion of the inner axons of the optic nerve,
leaving others intact. This enables the separation of the primary
from the secondary injury (111).
In glaucoma, the mechanisms leading to the death of
RGCs are multifaceted and encompass the activation of
microglia/macrophages, autophagy, disturbances in calcium
regulation, apoptosis, oxidative stress, expression of
pro-apoptotic proteins and neurotrophic deprivation (112). Microglia and macrophages have
essential roles in inflammation, tissue restoration and homeostasis
regulation following inflammation or CNS injury (113). Various subsets of macrophages
contribute to the pro-inflammatory, anti-inflammatory, cell growth
and tissue repair processes. Therefore, manipulating the activation
state of microglia/macrophage subsets to foster favourable
cytoprotection in response to injury may be a promising approach
for glaucoma treatment.
The study revealed a significant increase in the
release of CD68, iNOS and Arg-1 one week after PONT modelling,
indicating an increase in M1 microglia/macrophages, which may
contribute to RGC death. However, researchers found that
polysaccharides extracted from Lycium barbarum could delay
RGC degeneration by four weeks after PONT. This delay was
accompanied by an increase in the number of activated
microglia/macrophages and a higher count of M2-type
microglia/macrophages. This suggests that L. barbarum
regulates microglia/macrophage phagocytic activity and induces M2
polarisation, ultimately leading to delayed RGC damage (111).
Furthermore, in a glaucoma ganglion cell injury
model (N-methyl-D-aspartic acid-induced retinal injury model),
studies demonstrated that pituitary adenylate cyclase-activating
polypeptide (PACAP) increased the proportion of M2 subpopulations.
In addition, PACAP promoted the release of factors such as TGF-β1
and IL-10 mRNA (112), both of
which are markers of the M2 subtype of microglia/macrophages. The
M2 subtype is associated with an acquired inactive state and is
linked to reduced tissue damage, enhanced phagocytosis, increased
synthesis of trophic factors, and decreased secretion of
pro-inflammatory cytokines (25). These findings suggest that PACAP
regulates the activation of microglia/macrophages toward the M2
subtype, thereby offering retinal protection against damage.
Ischaemic optic neuropathy
Clinically, non-arteritic anterior ischaemic optic
neuropathy (NAION) often presents as optic disc oedema accompanied
by acute painless visual loss (114). NAION is thought to arise from
ischaemic damage to the optic nerve, which triggers an inflammatory
response and oedema (115,116).
The optic nerve head is highly sensitive to changes
in blood flow and is easily affected by factors such as
autoregulation, vasospasm and systemic vascular diseases (117). In the context of NAION,
inflammation is thought to be partially responsible for optic nerve
damage (115,118). In a rat model of anterior
ischaemic optic neuropathy (rAION), extracellular macrophages in
the hypoxic region were recruited early and activated the resident
microglia (119). Macrophages
improve neuronal survival by secreting relevant factors and
effectively phagocytosing myelin components to promote axonal
regeneration (120).
Furthermore, activated M2 microglia/macrophages have been linked to
neuroprotection (121).
However, it is important to acknowledge that in CNS diseases,
activated microglia/macrophages may also release harmful factors,
including pro-inflammatory cytokines and free radicals, which can
cause damage to the nervous system (72). The proportion and subpopulation
type of M2 microglia/macrophages in different pathological states
may have distinct effects on the survival of RGCs and/or axon
repair.
Research findings indicate that early treatment of
the rAION model with G-CSF stabilises optic nerve vascular
permeability, reduces macrophage recruitment near the optic nerve
and induces M2 microglia/macrophage polarisation within the optic
nerve (122). This treatment
approach subsequently leads to a decreased expression of
pro-inflammatory factors, prevents apoptosis induced by such
factors and exerts neuroprotective effects in the rAION model.
Another study demonstrated that the binding complex
of icariin and CCAAT enhancer-binding protein β significantly
induces endogenous G-CSF expression by promoting alternative
phosphorylation of IκB kinase-β, inhibitor of NF-κB (123). The elevated G-CSF expression
then triggers noncanonical NF-κB activation, which further
activates the PI3K/serine/threonine protein kinase B-a (AKT1)
signalling pathway and promotes M2 microglia/macrophage
polarisation, thereby preventing neuroinflammation and RGC
apoptosis after optic nerve infarction in a rAION. In addition, ω-3
polyunsaturated FAs have also been found to possess neuroprotective
effects in rAION by promoting the transformation of M1 macrophages
into M2 macrophages. This transformation subsequently reduces the
release of pro-inflammatory factors, such as TNF-a, iNOS and IL-1β,
exerting anti-inflammatory effects that mitigate cytokine-induced
optic nerve damage and help maintain RGC survival after infarction
(84).
Furthermore, puerarin treatment has been found to
stimulate the PI3K-AKT signalling pathway and sustain AKT1
activation, resulting in microglia/macrophages releasing CCAAT
enhancer-binding protein β and hallmark M2 markers, such as Arg-1
and IL-10 (124,125). Polarisation of M1
microglia/macrophages into M2 microglia/macrophages reduces the
proportion of TNF-α and IL-1β after optic nerve infarction,
effectively preventing subsequent cytokine-induced optic nerve
injury.
3. Intraocular fibrosis-related
diseases
Intraocular fibrosis-related diseases exhibit
molecular mechanisms similar to fibrosis in organs, including the
lungs, liver, kidneys, heart and skin (126). Following tissue injury,
epithelial cells have a pivotal role in the recruitment and
activation of inflammatory cells, endothelial cells and
fibroblasts. Furthermore, epithelial cells undergo
epithelial-mesenchymal transition (EMT), facilitating their
transdifferentiation into myofibroblasts (127,128). These myofibroblasts are
responsible for extracellular matrix (ECM) production,
proliferation and migration across the basal layer, facilitating
the coverage and regeneration of damaged tissue.
During this stage, M2 macrophages emerge either
through the differentiation of recruited infiltrating monocytes or
through the polarisation of infiltrating M1 macrophages. STAT6
activation occurs during this period, fostering IL-4/IL-13-mediated
M2 macrophage differentiation by upregulating the expression of
Arg-1 and various other profibrotic phenotype genes. M2 macrophages
exhibit an anti-inflammatory phenotype and stimulate fibroblasts to
enhance ECM production (129).
The processes related to macrophage polarisation and fibrosis are
illustrated in Fig. 3.
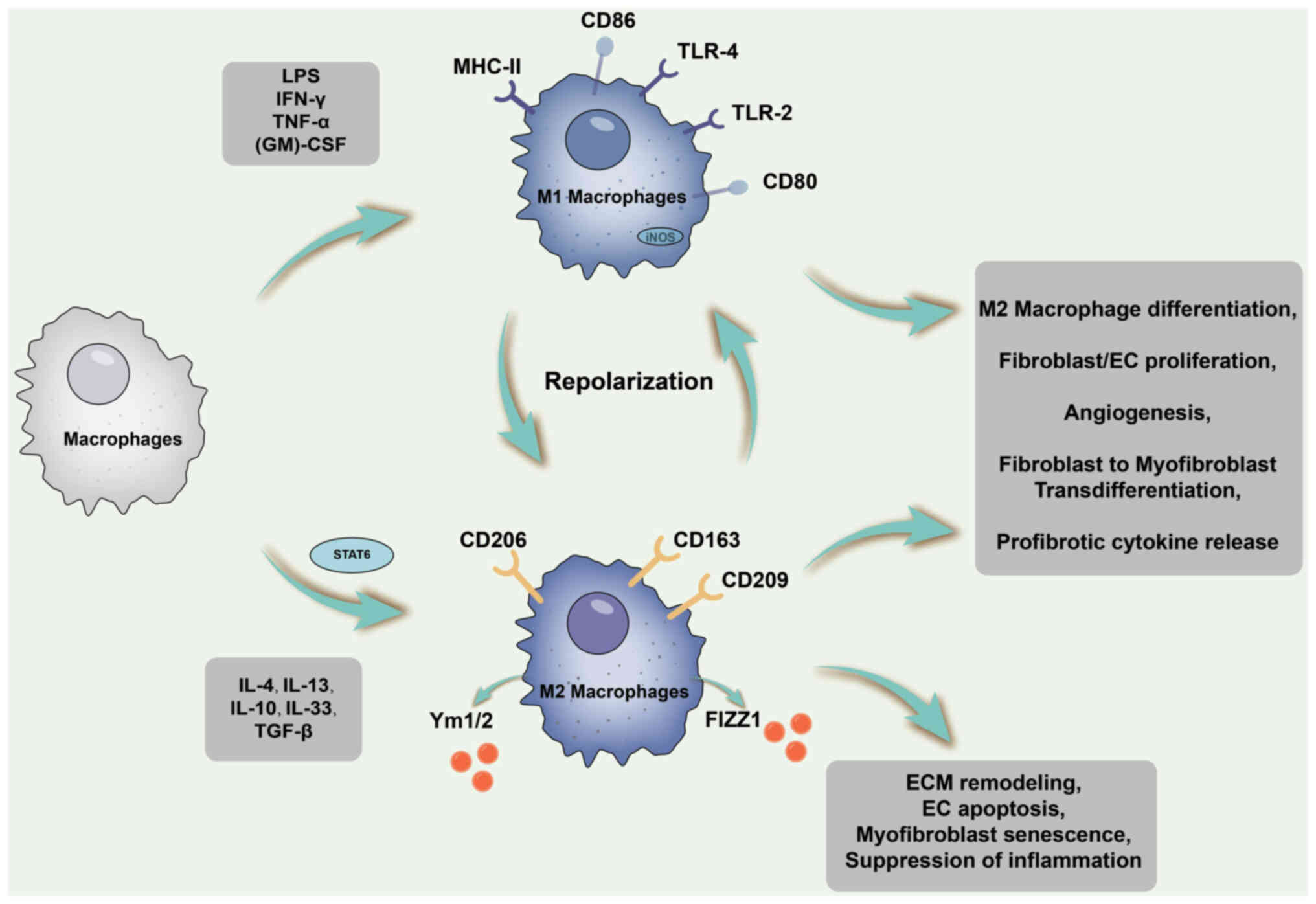 | Figure 3Schematic diagram of the processes
related to macrophage polarization and fibrosis. During tissue
remodeling and profibrotic phases, M2 macrophages emerge through
differentiation of recruited infiltrating monocytes or polarization
of infiltrating M1 macrophages. STAT6 is activated during this
period to promote IL-4/IL-13-mediated M2 macrophage differentiation
by upregulating the expression of Arg-1 and various other
pro-fibrotic phenotype genes. M2 macrophages exhibit an
anti-inflammatory phenotype and stimulate fibroblasts to enhance
ECM production, among other effects. ECM, extracellular matrix;
MHC, major histocompatibility complex; LPS, lipopolysaccharide;
TLR, Toll-like receptor; (GM)-CSF, granulocyte-macrophage
colony-stimulating factor; iNOS, inducible nitric oxide synthase;
STAT6, signal transducer and activator of transcription 6; Arg-1,
Arginase-1; EC, endothelial cell. |
Proliferative vitreoretinopathy
(PVR)
PVR is characterised by extensive proliferation and
shrinkage of cell tissues at the posterior interface of the
vitreous and inner surface of the retina (130). Shrinkage of these cell
membranes can lead to traction retinal detachment or the reopening
of previously treated retinal tears, resulting in severe visual
impairment. Although the exact pathogenesis of PVR remains to be
fully elucidated, the prevailing view is that it is a long-term
injury repair process involving the activation of inflammatory
cells, cytokine production, ocular cell proliferation and scarring
(131).
A pivotal characteristic of PVR is the formation of
myofibroblast membranes from transdifferentiated RPE cells and
other cell types, including macrophages (132). Macrophages are considered one
of the most crucial inflammatory cell types (133). By comparing vitreous samples
from patients with PVR and uncomplicated retinal detachment,
researchers have noted a significant increase in the number of
monocytes/macrophages in vitreous samples from patients with PVR.
Monocytes/macrophages were found to peak in the first 30 days after
symptom onset in PVR and gradually decline over the subsequent
three months (134). The
abundance of macrophages in the intraocular microenvironment in the
early stages of PVR and their sustained presence during progression
underscore their vital role in the pathological process of PVR.
Macrophages have an irreplaceable role in the
development of PVR through their ability to phagocytose damaged
cells and tissues and release various growth factors and cytokines
that mediate fibroblast chemotaxis and proliferation (135). Among the macrophage subsets, M2
macrophages, identified by the expression of Arg-1 and CD206, are
particularly important in tissue repair and fibrogenesis (136).
In studies conducted in a rabbit model of PVR
(137), researchers observed a
swift onset of intense inflammation within the initial two weeks
following PVR induction, with inflammation continuing to escalate
until the 4-week mark post-induction. After the inflammatory phase,
RPE cells undergo EMT, transitioning into fibroblast-like cells,
which then give rise to contractile membranes. Throughout this
process, the levels of growth factors, such as IFN-γ, VEGF,
platelet-derived growth factor BB, placental growth factor and
angiopoietin-2, surge, potentially fostering the survival,
proliferation and EMT of RPE cells. The formation of contractile
membranes and the secretion of growth factors are closely linked to
M2 macrophages (133,138). Studies based on vitreous
samples from human patients have indicated that M2
macrophage-derived microparticles can stimulate the proliferation
and migration of RPE cells by activating the PI3K/AKT/mTOR
signalling pathway (139),
thereby contributing to the pathogenesis of vitreoretinal
diseases.
Furthermore, studies based on vitreous samples from
human patients suggested that M2 macrophages may contribute to the
development of fibrovascular membranes in diabetic proliferative
retinopathy (140). In a mouse
model of PVR, CD206-positive M2 macrophages were found near the
surface of the fibrous proliferation membrane, and the γ-secretase
inhibitor DAPT was shown to inhibit RPE cell-induced PVR formation
(decreased α-smooth muscle actin expression) and inhibit the
infiltration of M2 macrophages by specifically targeting the Notch
signalling pathway, thereby ameliorating PVR (141). These findings underscore the
critical involvement of M2 macrophages in the pathogenesis of PVR
and present a potential therapeutic target for intervention.
Subretinal fibrosis
Choroidal neovascularisation (CNV) is the primary
cause of vision loss in neovascular age-related macular
degeneration (nAMD), with CNV possibly progressing to end-stage
fibrous plaques and disc scarring (142). In nAMD, the accumulation of
drusen may lead to reduced oxygen diffusion in the choriocapillary
plexus, eventually culminating in CNV. The subsequent growth of new
abnormal blood vessels in the subretinal space often results in
haemorrhage, triggering a wound-healing response that eventually
leads to subretinal fibrosis (126).
Fibrosis is a healing process that occurs in
response to tissue injury (128). During the healing phase,
angiogenesis is triggered to facilitate tissue repair, enhance
oxygen supply and facilitate the migration of inflammatory cells to
the lesion area (143). In
nAMD, CNV develops in the subretinal and/or subpigmented epithelial
spaces, causing haemorrhages and leaks that ultimately lead to
subretinal fibrosis. This process involves the recruitment and/or
migration of various cell types. These cells interact with
inflammatory factors, causing significant remodelling of the ECM
(144).
Histopathological examination of human eyes revealed
significant recruitment of macrophages during CNV, where they have
a role in the development of pathological neovascularisation,
drusen formation and fibroblast scaffolds (145). Direct anatomical and functional
evidence suggests that circulating macrophages rather than resident
macrophages are responsible for laser-induced CNV. To construct
subretinal fibrosis models, researchers commonly use a
laser-induced acute wound-healing model of CNV (146,147). In this experimental setup,
macrophages were found to promote the formation of fibroblast
scaffolds during the early wound-healing response of laser-affected
CNV lesions, with most macrophages at the laser injury sites being
activated M2 macrophages (147). CNV membranes infiltrated by M2
macrophages were more susceptible to fibrosis than those with M1
macrophage infiltration (148).
Studies based on the laser-induced subretinal
fibrosis model have demonstrated that inhibiting macrophage
transition to the M2 subpopulation via the PI3K/Akt axis
contributes to the improvement of fibrotic lesions in subretinal
fibrosis (146). In addition,
the application of triptolide has shown promise in reducing
subretinal fibrosis by inhibiting the polarisation of M2
subpopulations and suppressing the activation of the TGF-β1/Smad
axis, thereby downregulating TGF-β1-induced EMT/endothelial-MT
(149).
4. Intraocular malignancy
Macrophage phagocytic activity has a crucial role in
clearing dead and dying cells. However, tumours can regulate
macrophage function, thwart macrophage-triggered inflammation and
kill tumour cells. This metabolic reprogramming drives the
transformation of macrophages into either the M1 or M2
subpopulations, which are influenced by various cytokine stimuli.
Although tumour-associated macrophages do not strictly conform to
the M1 and M2 subpopulations, they often have similarities to M2
and actively promote tumour growth by upregulating
immunosuppression (150).
Research indicates that, in the tumour
microenvironment, M1-polarised macrophages primarily depend on
glucose flux and the conversion of glucose to lactate, along with
the production of reactive oxygen species and NO to combat tumours.
Conversely, M2-polarised macrophages predominantly rely on FA
β-oxidation and the tricarboxylic acid cycle while stimulating the
production of polyamines and L-proline to facilitate tumour growth
(150). Macrophage polarisation
and tumour-related processes are shown in Fig. 4.
Uveal melanoma
Uveal melanoma is the most prevalent primary
intraocular malignancy in adults, with an incidence of ~6-7 new
cases per million individuals (151). The current treatment modalities
include enucleation and radiation therapy. Although these
treatments can curtail primary tumour growth, they remain
ineffective in preventing tumour metastasis, leading to death
within ~1-3 years (152).
Studies have provided compelling evidence for the
pivotal role of macrophages in melanoma growth and survival.
Melanoma-derived exosomes have also been identified as mediators of
immunosuppression (153). These
exosomes exert their effects by directly interacting with and
suppressing various lymphocytes or by inducing MDSCs. In turn,
MDSCs promote M2 subset transformation and recruit tumour-promoting
regulatory T cells (154).
Researchers have postulated that lysing IFN-γ
released by both tumour cells and immune cells in the
microenvironment may be a contributing factor in transforming
macrophages from a tumour-promoting M2 phenotype to an anti-tumour
M1 phenotype, primarily through the IFN-γ/JAK-STAT1 pathway
(155,156). In a mouse xenograft model,
treatment with the oncolytic herpes simplex virus 1-enhanced green
fluorescence protein through vitrectomy injection led to increased
IFN-γ levels, an elevation in M1 macrophages and a reduction in M2
macrophages in peripheral blood, intraocular sites and distant
tumours. In vitro experiments have further demonstrated a
significant increase in IFN-γ at both the RNA and protein levels
following oncolytic virus infection (157). Consequently, this treatment
approach effectively reduced intraocular and subcutaneous tumours
throughout the body.
However, it has been observed that melanoma exosomes
can induce both M1 and M2 representative factors, namely TNF-α and
IL-10, respectively (154).
Furthermore, macrophage function assays have revealed an increasing
trend from iNOS (M1) to Arg-1 (M2) activity, indicating that
melanoma exosomes can induce a 'mixed' M1 and M2 tumour-promoting
macrophage activation phenotype. Thus, in the pathological
progression of uveal melanoma, M1 and M2 macrophage subpopulations
seem to have flexible adaptability to tumour survival.
5. Intraocular neovascularisation-related
diseases
Macrophages have a role in angiogenesis, albeit to a
limited extent, by promoting the production of pro-angiogenic and
growth factors, such as VEGF-A and fibroblast growth factor 2
(FGF2). Research indicates that M1 macrophages may facilitate
vascular sprouting through the secretion of VEGF, IL-1β and TNF-α
(158). Conversely,
investigations have demonstrated that M2 macrophages, rather than
M1 macrophages, enhance angiogenesis in vivo with increased
expression of VEGF, FGF2, insulin-like growth factor 1, CCL2 and
placental growth factor (33,159). Furthermore, a study determined
that M2-polarised macrophages exhibit greater angiogenic potential
than other subpopulations (159). However, the precise mechanisms
underlying macrophage-mediated angiogenesis and the cellular
interactions between endothelial cells and macrophage subsets
remain to be fully elucidated. Although the categorisation of
macrophages into distinct subpopulations offers a simplified
overview of their intricate functional activities in the body, the
specific mechanisms involved have not been determined. Macrophage
polarisation and angiogenesis-related cytokines are shown in
Fig. 5.
 | Figure 5Schematic diagram of the macrophage
polarization process and angiogenesis-related cytokines. M1
macrophages may promote vascular sprouting by secreting VEGF, IL-1β
and TNF-α. By contrast, M2 macrophages enhance angiogenesis in
vivo and enhance the expression of VEGF, FGF-2, IGF-1, CCL-2
and PGF. LPS, lipopolysaccharide; TLR, Toll-like receptor;
(GM)-CSF, granulocyte-macrophage colony-stimulating factor; iNOS,
inducible nitric oxide synthase; IGF, insulin-like growth factor;
CCL2, C-C motif chemokine ligand 2; FGF, fibroblast growth factor;
PGF, placental growth factor. |
Diabetic retinopathy (DR)
Diabetes is a metabolic disorder primarily
characterised by hyperglycaemia stemming from abnormal insulin
secretion and insulin resistance. Among patients with DR,
microvascular complications are the most common manifestation. DR
is categorised into non-proliferative DR (non-PDR) and PDR (PDR),
with a distinction based on the presence of retinal
neovascularisation (160).
Non-PDR is typically characterised by asymptomatic microvascular
changes, whereas PDR is involved in angiogenesis (161). DR is characterised by the
abnormal growth and leakage of small blood vessels, leading to
local oedema and associated tissue dysfunction. Dysregulation of
vascular regeneration and inflammation are thought to be involved
in the pathogenesis of DR (162,163).
Researchers have observed that upon high-glucose
stimulation, microglia initially polarise toward the M2a phenotype,
a response that initially mitigates tissue damage (164). However, as time progresses,
there is an escalation in the production of M1 pro-inflammatory
factors, accompanied by a decrease in the production of M2
anti-inflammatory factors, gradually shifting the macrophages
toward the M2b phenotype. In advanced stages, microglia tend to
exhibit an M1 phenotype with pronounced pro-inflammatory effects.
In a rat model of streptozotocin-induced DR, there was an increase
in M1 polarisation and a decrease in M2 polarisation, and microglia
tended toward M1 polarisation with increasing glucose
concentrations (165). The
levels of iNOS (an M1 marker) and Arg-1 (an M2 marker) were higher
in the retinas of db/db mice at five weeks of age. However, at
eight weeks of age, iNOS levels continue to increase, whereas Arg-1
levels return to baseline (166).
It has been indicated that melatonin inhibits the
excessive activation of microglia in the retina of diabetic rats by
inhibiting the PI3K/AKT/Stat3/NF-κB signalling pathway, i.e.,
reducing the number of microglia cells and promoting their
anti-inflammatory properties (167). It was speculated that this is
related to melatonin promoting the transformation of microglia from
pro-inflammatory (M1) to anti-inflammatory (M2) cells. Similarly,
it was demonstrated that inhibiting M1 polarisation and promoting
M2 polarisation of retinal microglia in DR rats through the
TLR4/MyD88/NF-κB p65 pathway can effectively improve early DR
(168).
Retinopathy of prematurity (ROP)
ROP is characterised by impaired retinal blood
vessel growth and development in preterm infants, which frequently
leads to visual impairment and blindness (169). The pathological process of ROP
is divided into two stages: An initial phase marked by delayed
vascular growth after birth accompanied by vascular regression,
followed by a second phase of hypoxia-induced pathological
angiogenesis (170). An
abnormal vascular state disrupts the inner retinal environment,
thereby exacerbating the ischaemic state and leading to retinal
leakage, scarring and eventually blindness. Although treatments
such as laser and cryotherapy have improved ROP-related blindness,
visual outcomes remain suboptimal for treated patients. Laser
treatment has been successful in resolving most threshold ROP cases
(171) and 100% of
pre-threshold ROP cases (172).
However, eyes treated with cryofixation or laser photocoagulation
often manifest structural sequelae (171), underscoring the pressing need
for preventive and less invasive therapeutic approaches.
Vascular abnormalities and inflammatory cell
recruitment are primary contributors to the progression of abnormal
retinal vascular diseases, including PDR and ROP (173). Studies have shown that
macrophages have a role in promoting abnormal angiogenesis during
pathological retinal vessel growth and that M1 and M2
subpopulations of macrophages are present in the intraretinal
environment of ROP (174).
Studies have demonstrated that microglia/macrophages
are activated after P12 once oxygen-induced retinopathy (OIR)
models are established. M1 microglia/macrophages were observed in
neovascular tufts located in the retina, starting at P12 and
reaching their peak at P17 upon returning to normoxic conditions.
At this time-point, the NF-κB/STAT3 axis is triggered, which
results in an increased proportion of M1 microglia/macrophages and
an enhanced proportion of TNF-α and IL-6. Consequently, the
neovascular clusters exhibit a progressive increase in volume from
P12 to P17. However, a shift to M2-type microglia/macrophage
activity occurs from P17 onwards during the advanced stages of OIR.
The IL-4/STAT6/PPAR-γ axis is triggered from P17 and reaches its
maximum at P20, promoting M2 microglia/macrophage transformation.
This, in turn, results in a decrease in inflammatory factors and
regression of neovascular clusters (175).
Furthermore, investigations have revealed that
cytokines TNF-α and VEGF, released by the M1 subpopulation, promote
abnormal angiogenesis through interactions with endothelial cells.
By contrast, M2 macrophages promote vascular anastomosis. The
involvement of Notch1 signalling has been reported, although the
exact secretory factors remain to be elucidated (176). These findings underscore the
coordinated engagement of M1 and M2 macrophage subsets in guiding
retinal neovascularisation.
Promoting macrophage transition from the M1 to the
M2 phenotype during the pathological process of OIR is thought to
have anti-angiogenic benefits. To investigate this, Marchetti et
al (177) used human
umbilical cord blood to obtain enriched progeny CD14(+) cell
populations, which were then injected into the eyes of OIR mice.
The results demonstrated that only CD14(+) cells polarised into
M2-type macrophages could promote the normalisation of retinal
vasculature and control pathological neovascularisation.
Consequently, areas of vascular occlusion and associated tissue
hypoxia were reduced. A separate study found that in the OIR
retina, activated microglia/macrophages were predominantly of the
M1 type rather than the M2 type. Treatment with ferulic acid was
shown to decrease the proportion of iNOS+ microglia/macrophages
while increasing the release of Arg-1, suggesting its potential to
transform microglia/macrophages from the M1 type (expressing iNOS,
CD86, IL-6 and TNF-α) to the M2 type (expressing Arg-1, IL-10 and
CD206), thus exerting a strong anti-angiogenic effect (178).
It has been demonstrated that blocking the
activation of NF-κB signalling can effectively promote the
transformation of M1 macrophages into the M2 phenotype in OIR mice
and subsequently reduce the number of neovascular clusters
(179). In addition, IL-17A
neutralisation attenuates ocular neovascularisation by increasing
the proportion of M2 macrophages and downregulating the release of
VEGF from M1 macrophages (180).
To explore macrophage polarisation in an OIR mouse
model, researchers assessed the retina and found a significant
increase in both M1and M2-like macrophages compared to normal
controls. Both M1 and M2 macrophages exhibit a pro-angiogenic
effect, promoting human umbilical vein endothelial cell (HUVEC)
proliferation and contributing to retinal pathological
neovascularisation (181).
Similarly, Ma et al (174) investigated patients with
advanced ROP and revealed a pro-angiogenic and pro-inflammatory
microenvironment, with M1 macrophages predominant over M2.
In studies focusing on the role of pigment
epithelium-derived factor, it was shown to inhibit macrophage
polarisation in the retinas of an OIR mouse model through the
regulation of adipose triglyceride lipase in the MAPK and Notch1
pathways. Specifically, pigment epithelium-derived factor
suppressed the Notch1 and MAPK signalling pathways by inhibiting
adipose triglyceride lipase, leading to a significant reduction in
the release of iNOS and Arg-1, which are characteristic factors of
M1 macrophages and M2 subpopulations, respectively. This ultimately
resulted in a reduction in retinal neovascularisation (181).
Consequently, the role of macrophages in
neovascularisation in OIR models remains a subject of debate, as
both M1 and M2 macrophages may be involved. On the one hand, in the
OIR model, retinal neovascularisation can manifest in two forms:
Pathological neovascularisation, characterised by the emergence of
abnormal blood vessels sprouting from the retinal surface into the
vitreous, and physiological revascularisation, which involves the
restoration of avascular regions with functional intraretinal
vessels (182). Therefore,
their proportions at different pathological stages may have
contradictory effects. Ritter et al (183) found that a large number of
migrating cells were localised in the retinal ischaemic area with a
large loss of microglia, which may replace the function of
microglia and promote vascular remodelling in the damaged area by
releasing an appropriate amount of VEGF. Therefore, their location
within the retina may also be one of the reasons for their
contradictory roles in OIR models.
CNV
Wet AMD is a disease that causes vision loss due to
the growth of CNV in the macula (184). Aberrant neovascularisation
initially proliferates under the RPE band and then breaches the RPE
band, causing intraocular haemorrhage and exudative serous retinal
detachment, and later, discoid scarring (126). This localised loss of the
retinal photoreceptor layer and RPE zone results in irreversible
macular function loss and vision impairment.
CNV is considered involved in the submacular
healing process (126,144). Angiogenesis has a crucial role
in this process, and current clinical strategies predominantly
focus on reducing the levels of VEGF, which is the primary factor
that promotes angiogenesis (185,186). However, despite these efforts,
only ~30% of patients with exudative AMD experience a three-line
improvement in visual acuity, and ~15% of patients experience
progressive deterioration, leading to legal blindness, even after
receiving VEGF-inhibiting drugs (187-189). These results were expected,
considering angiogenesis is an integral part of the complex healing
phase. Hence, the search for alternative therapeutic approaches for
CNV beyond anti-angiogenic treatments continues.
Multiple studies have explored AMD pathology and
identified inflammation as a key driver of neovascular AMD
progression (99,126,144,190,191). AMD is characterised by a
chronic inflammatory response. Within this inflammatory milieu,
macrophage recruitment and cytokine regulation are key mediators of
CNV development (191).
Studies have indicated that macrophages are
involved in abnormal angiogenesis in the pathology of CNV. M1
macrophages, characterised by specific markers such as iNOS, IL-6
and TNF-α, have been shown to inhibit angiogenesis (159). Conversely, M2 macrophages,
identified using specific markers such as Arg-1, CD206 and CD163,
promote pathological angiogenesis in CNV (192). Nakamura et al (193) revealed that increased IL-10
release in the eyes of aged mice activates associated signalling
pathways, resulting in an increased proportion of M2 macrophages
and the activation of vascular proliferative processes. Macrophage
polarisation has emerged as a potential therapeutic target for CNV
treatment.
In laser-impacted CNV, dynamic patterns of M1
macrophages and M2 subpopulations were observed, showing an early
and immediate shift to M1, followed by a sustained shift to M2. M1
macrophages appear to be involved in the initial stages of CNV,
whereas M2 macrophages have a critical role in the middle and late
stages of CNV development and remodelling (194). For instance, during
experimental CNV, upregulation of M1 signature factors (TNF-α and
iNOS) was observed at day 3, suggesting inflammation at the onset
of CNV lesions. By contrast, CD206 reached its maximum expression
on day 7 of CNV formation, whereas CD86 and CD163 reached their
maximum expression on day 14 of lesion formation. These marker
genes represent different M2 macrophage subpopulations, suggesting
that different macrophage subtypes have distinct roles at different
time-points during the pathological process of CNV. Specifically,
M2a macrophages may be associated with neovascularisation, whereas
M2b and M2c macrophages may be involved in fibrous scarring
(195).
CSF1, also known as macrophage CSF, has a crucial
role in macrophage recruitment (196) and the transition to the M2
subpopulation (197). When the
CSF1 receptor receives CSF1, the PI3K/AKT/forkhead box (FOX)O1 axis
is activated, promoting M2 polarisation. Furthermore, under hypoxic
conditions, HUVECs release more CSF1, thereby promoting macrophage
migration and transition to the M2 subpopulation by upregulating
the PI3K/AKT/FOXO1 axis. In a CSF1/CSF1 receptor (CSF1R)-associated
manner, the M2 subpopulation upregulates the proliferation,
recruitment and lumen formation of HUVECs. Inhibition of the
CSF/CSFR axis has been shown to suppress M2 polarisation of
macrophages and attenuate laser-induced CNV formation in mice
(198).
In addition, studies have revealed that long
non-coding RNA nuclear paraspeckle assembly transcript 1 (NEAT1)
promotes the expression of M2 macrophage markers by targeting
phosphatase and tensin homolog via microRNA (miR)-148a-3p.
Downregulation of NEAT1 can effectively inhibit CNV by suppressing
the transformation of M2 macrophage subsets (199).
Researchers have proposed that the Rho-associated
protein kinase (ROCK) signalling pathway is a key pathway in
regulating macrophage polarisation, and that the expression of ROCK
pathway-related factors and pathway signal transduction processes
affect the pathological process of CNV. They conducted experiments
by differentiating mouse BMDMs into M1 or M2 phenotypes and
injecting them into the eyeballs of laser-modelled WT mice. They
observed that CNV lesions were not altered by native-morphological
macrophages but that M2 subpopulation macrophages promoted lesion
progression, which was reversed in ROCK2 inhibitor-treated animals.
By contrast, the M1 subpopulation ameliorated the damage caused by
the CNV. Further intravitreal injection of the M1 subpopulation in
laser-modelled mice treated with a ROCK2 inhibitor did not
ameliorate CNV-induced damage, confirming that ROCK2 inhibits CNV
lesions in vivo by promoting the polarisation transition of
macrophages to M1 (200). Ras
homolog family member A (RhoA) expression and myosin phosphatase
target subunit 1 and myosin light chain phosphorylation are also
upregulated in CNV and decreased by melatonin administration
(201). The RhoA/ROCK axis
promotes the transition of macrophages to the M2 subpopulation and
prevents conversion of the M1 subpopulation, thereby triggering CNV
lesions. Melatonin converts M2 microglia/macrophages to the M1
subset by inhibiting the RhoA/ROCK axis, resulting in the
downregulation of CNV lesions, reduced associated vascular leakage
and inhibition of abnormal vascular status in laser-affected CNV
lesions.
Other studies have shown that miR-505 is abnormally
upregulated in laser-induced CNV lesions. Transmembrane protein 229
B (TMEM229B) was identified as a direct target of miR-505-5p, and
administration of an miR-505 inhibitor significantly upregulated
the expression of endogenous TMEM229B in CNV mice. This specific
inhibition of M2 polarisation in mice with CNV led to reduced VEGF
expression and suppressed CNV formation. In vitro
experiments further demonstrated that exogenous TMEM229B
significantly inhibited the expression of the M2-specific markers
Ym-1 and Arg-1 (202).
In addition, the IL-4 mutant protein IL-4/Q116E was
found to regulate the inflammatory response of laser-induced CNV
through the Notch/delta-like canonical Notch ligand 4/monocyte to
macrophage differentiation-associated signalling pathway,
increasing the expression of CD68 and CD80 and reducing the
expression of Arg-1 in RPE choroidal tissue. Induction of
macrophage polarisation from M2 to M1 attenuated CNV development
(203).
Furthermore, in the context of a laser-induced CNV
model, injured RPE upregulated
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(PFKFB3)-driven glycolysis in macrophages, resulting in the
induction of hypoxia-inducible factor (HIF)-1α/HIF-2α and NF-κB.
This subsequently induced the expression of macrophage
subset-associated signature factors and pro-angiogenic factors,
ultimately promoting the transformation of the M1 subpopulation of
macrophages into the M2 subpopulation and promoting CNV
development. However, the PFKFB3 inhibitor AZ67 effectively
downregulated the expression of HIF-1α/HIF-2α and NF-κB signalling
and largely prevented laser-affected CNV lesions (204).
Furthermore, researchers have used small
interfering RNAs (siRs) to suppress TIMP metallopeptidase inhibitor
3 (TIMP-3) expression in BMDMs and RPE/choroidal tissues in a
laser-induced mouse model of CNV. They found that the release of M2
biomarkers CD206, CD163, Arg-1 and Ym-1 was correspondingly
upregulated in vitro and in vivo in the siR-TIMP-3
group, indicating that a lack of TIMP-3 may promote M2 macrophage
differentiation. Furthermore, intraocular injection of siR-TIMP-3
was shown to upregulate the progression of CNV lesions, as detected
using optical coherence tomography angiography, suggesting that
TIMP-3 inhibition is associated with the M2 macrophage subset and
has a key role in CNV formation (205).
Finally, studies have shown a greater proportion of
M2 macrophages compared with the M1 subpopulation during three and
seven days of buffer treatment in a laser-induced mouse model
(206). Triptolide
significantly downregulated the accumulation of the M2
subpopulation at the lesion site over the 3 and 7-day periods.
Triptolide also reduced the proportion of M2 macrophages during the
same periods. In addition, triptolide decreased the release of
VEGF, intercellular adhesion molecule 1 and TNF-α in local CNV
injury, consistent with a reduction in the total number of
aggregated macrophages and a lower ratio of the M2 subpopulation.
Consequently, intraperitoneal injection of triptolide inhibited the
transformation of the M2 subpopulation in CNV focal lesion areas,
resulting in the downregulation of inflammatory and angiogenic
factors, thereby inhibiting CNV progression and macrophage
infiltration in CNV focal areas (207).
6. Conclusion
The present review provides an overview of key
findings on the role of microglia/macrophage polarisation in
intraocular diseases. It also provides ideas for further research
on macrophage polarisation and the role of different subpopulations
in intraocular diseases and a summary of the association between
macrophage polarisation and different diseases. In intraocular
tissues, there are not only BMDMs but also specialised resident
macrophages called microglia, which provide the initial defence
against microorganisms and participate in immune regulation. They
have key roles in phagocytosis by clearing apoptotic cells and
tissue debris. Dysfunction of macrophages/microglia may lead to
autoimmune and persistent inflammatory diseases. An increasing
number of studies have shown that macrophage or microglial
polarisation has a key role in the pathological process of
intraocular diseases and that regulating the polarisation process
can effectively delay the progression of related diseases.
The present study provides the first review of the
association between macrophage polarisation and intraocular
disease. Although studies have been published on the relationship
between macrophage polarisation and intraocular neovascular
diseases, the publication time was relatively early and the
literature included was not comprehensive. Simultaneously, there is
a lack of an overview of the relationship between macrophage
polarisation and intraocular fibrosis- and inflammation-related
diseases. In addition, the present review covers, as much as
possible, the typical literature on the association between
intraocular disease and macrophage polarisation to provide a more
comprehensive overview.
The present study also has certain limitations. On
the one hand, certain studies have divided macrophages into
different subgroups during the study to simplify their complex
functional activities in the body, but not enough to elucidate the
specific mechanisms involved. In addition, most studies simply
divided macrophages into M1/M2 subgroups and did not further
subdivide M2a, M2b or other subgroups in terms of research methods.
Therefore, only we can an overview of the relevant mechanisms can
be provided, rather than discussing them in depth. Although all
attempts were made to unify the differences in research models in
the process of literature inclusion, there may still be differences
in methods among certain studies. Different models can only
simulate the pathological process of diseases locally, which may
lead to differences in the relevant research conclusions. In the
present study, it was attempted to determine the association
between macrophage polarisation and intraocular diseases.
Therefore, the discussion section provided a simplified overview of
the mechanism and did not discuss its relevance to other cell types
(neutrophils, T cells, fibroblasts, etc.) in depth. Furthermore,
because there are few clinical studies on the association between
macrophage polarisation and intraocular diseases, it is difficult
to collect clinical literature on the pathogenesis of diseases.
As mentioned earlier, the current study lacks
further delineation of the macrophage subsets; therefore, future
studies need to identify the macrophage subsets involved in the
disease process to further elucidate the specific mechanisms
involved. Although numerous basic studies are related to
intraocular diseases, relevant clinical studies are still lacking.
Therefore, in the future, more clinical studies on the association
between macrophage polarisation and intraocular diseases are
required to further explore the disease mechanism.
Availability of data and materials
Not applicable.
Authors' contributions
HL analyzed and summarized the literature on the
association of macrophage polarization with intraocular disease and
was a major contributor in writing the manuscript. BL is
responsible for the search and collection of literature related to
macrophage polarization and intraocular diseases. YLZ optimized the
writing structure and developed the idea of the article. All
authors read and approved the final manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
RPE
|
retinal pigment epithelium
|
|
HIF-1α
|
hypoxia-inducible factor-α
|
|
TNF-α
|
tumor necrosis factor-α
|
|
LPS
|
lipopolysaccharide
|
|
TLR
|
Toll-like receptor
|
|
PPARγ
|
peroxisome proliferator-activated
receptor γ
|
|
NO
|
nitric oxide
|
|
iNOS
|
inducible NO synthase
|
|
VEGF
|
vascular endothelial growth
factor
|
|
EAU
|
experimental autoimmune uveitis
|
|
MDSCs
|
myeloid-derived suppressor cells
|
|
ROS
|
reactive oxygen species
|
|
AhR
|
aryl hydrocarbon receptor
|
|
BMDMs
|
bone marrow-derived macrophages
|
|
MS
|
multiple sclerosis
|
|
RGCs
|
retinal ganglion cells
|
|
EAE
|
experimental autoimmune
encephalomyelitis
|
|
CNS
|
central nervous system
|
|
FA
|
fatty acids
|
|
SO
|
sympathetic ophthalmia
|
|
RP
|
retinitis pigmentosa
|
|
PONT
|
partial optic nerve transection
|
|
LBP
|
Lycium barbarum
|
|
PACAP
|
pituitary adenylate
cyclase-activating polypeptide
|
|
PVR
|
proliferative vitreoretinopathy
|
|
CNV
|
choroidal neovascularization
|
|
nAMD
|
neovascular age-related macular
degeneration
|
|
ECM
|
extracellular matrix
|
|
Arg-1
|
Arginase-1
|
|
NAION
|
nonarteritic anterior ischemic optic
neuropathy
|
|
rAION
|
rat AION
|
|
ROP
|
retinopathy of prematurity
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
CSF1/M-CSF
|
macrophage colony-stimulating factor
1
|
|
CSF2/(GM)-CSF
|
granulocyte-macrophage
colony-stimulating factor 2
|
Acknowledgments
Not applicable.
Funding
The study was supported by the Sichuan Province International
Science and Technology Innovation Cooperation/Hong Kong, Macao and
Taiwan Science and Technology Innovation Cooperation Project (grant
no. 2019YFH0117).
References
|
1
|
Yan J and Horng T: Lipid metabolism in
regulation of macrophage functions. Trends Cell Biol. 30:979–989.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murray PJ and Wynn TA: Protective and
pathogenic functions of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murray PJ: Macrophage polarization. Annu
Rev Physiol. 79:541–566. 2017. View Article : Google Scholar
|
|
5
|
Chinnery HR, McMenamin PG and Dando SJ:
Macrophage physiology in the eye. Pflugers Arch. 469:501–515. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu F, Shi M, Zheng C, Shen D, Zhu J,
Zheng X and Cui L: The roles of macrophages and microglia in
multiple sclerosis and experimental autoimmune encephalomyelitis. J
Neuroimmunol. 318:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Norden DM, Muccigrosso MM and Godbout JP:
Microglial priming and enhanced reactivity to secondary insult in
aging, and traumatic CNS injury, and neurodegenerative disease.
Neuropharmacology. 96:29–41. 2015. View Article : Google Scholar :
|
|
8
|
Kabba JA, Xu Y, Christian H, Ruan W,
Chenai K, Xiang Y, Zhang L, Saavedra JM and Pang T: Microglia:
Housekeeper of the central nervous system. Cell Mol Neurobiol.
38:53–71. 2018. View Article : Google Scholar
|
|
9
|
Das A, Sinha M, Datta S, Abas M, Chaffee
S, Sen CK and Roy S: Monocyte and macrophage plasticity in tissue
repair and regeneration. Am J Pathol. 185:2596–2606. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biswas SK, Chittezhath M, Shalova IN and
Lim JY: Macrophage polarization and plasticity in health and
disease. Immunol Res. 53:11–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strauss O, Dunbar PR, Bartlett A and
Phillips A: The immunophenotype of antigen presenting cells of the
mononuclear phagocyte system in normal human liver-a systematic
review. J Hepatol. 62:458–468. 2015. View Article : Google Scholar
|
|
12
|
McMurran CE, Jones CA, Fitzgerald DC and
Franklin RJ: CNS remyelination and the innate immune system. Front
Cell Dev Biol. 4:382016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tay TL, Hagemeyer N and Prinz M: The force
awakens: Insights into the origin and formation of microglia. Curr
Opin Neurobiol. 39:30–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Subramaniam SR and Federoff HJ: Targeting
microglial activation states as a therapeutic avenue in Parkinson's
disease. Front Aging Neurosci. 9:1762017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du L, Zhang Y, Chen Y, Zhu J, Yang Y and
Zhang HL: Role of microglia in neurological disorders and their
potentials as a therapeutic target. Mol Neurobiol. 54:7567–7584.
2017. View Article : Google Scholar
|
|
16
|
Jiang HR, Milovanović M, Allan D, Niedbala
W, Besnard AG, Fukada SY, Alves-Filho JC, Togbe D, Goodyear CS,
Linington C, et al: IL-33 attenuates EAE by suppressing IL-17 and
IFN-gamma production and inducing alternatively activated
macrophages. Eur J Immunol. 42:1804–1814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Butovsky O, Landa G, Kunis G, Ziv Y,
Avidan H, Greenberg N, Schwartz A, Smirnov I, Pollack A, Jung S and
Schwartz M: Induction and blockage of oligodendrogenesis by
differently activated microglia in an animal model of multiple
sclerosis. J Clin Invest. 116:905–915. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boche D, Perry VH and Nicoll JA: Review:
Activation patterns of microglia and their identification in the
human brain. Neuropath Appl Neuro. 39:3–18. 2013. View Article : Google Scholar
|
|
19
|
Mills CD: M1 and M2 macrophages: Oracles
of health and disease. Crit Rev Immunol. 32:463–488. 2012.
View Article : Google Scholar
|
|
20
|
Ransohoff RM and Brown MA: Innate immunity
in the central nervous system. J Clin Invest. 122:1164–1171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ransohoff RM and Perry VH: Microglial
physiology: Unique stimuli, specialized responses. Annu Rev
Immunol. 27:119–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Graeber MB: Changing face of microglia.
Science. 330:783–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stout RD, Jiang C, Matta B, Tietzel I,
Watkins SK and Suttles J: Macrophages sequentially change their
functional phenotype in response to changes in microenvironmental
influences. J Immunol. 175:342–349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang N, Liang H and Zen K: Molecular
mechanisms that influence the macrophage m1-m2 polarization
balance. Front Immunol. 5:6142014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Varnum MM and Ikezu T: The classification
of microglial activation phenotypes on neurodegeneration and
regeneration in Alzheimer's disease brain. Arch Immunol Ther Exp
(Warsz). 60:251–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Colton C and Wilcock DM: Assessing
activation states in microglia. CNS Neurol Disord Drug Targets.
9:174–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henkel JS, Beers DR, Zhao W and Appel SH:
Microglia in ALS: The good, the bad, and the resting. J Neuroimmune
Pharm. 4:389–398. 2009. View Article : Google Scholar
|
|
30
|
Hanisch UK and Kettenmann H: Microglia:
Active sensor and versatile effector cells in the normal and
pathologic brain. Nat Neurosci. 10:1387–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang LX, Zhang SX, Wu HJ, Rong XL and Guo
J: M2b macrophage polarization and its roles in diseases. J Leukoc
Biol. 106:345–358. 2019. View Article : Google Scholar
|
|
33
|
Ferrante CJ, Pinhal-Enfield G, Elson G,
Cronstein BN, Hasko G, Outram S and Leibovich SJ: The
adenosine-dependent angiogenic switch of macrophages to an M2-like
phenotype is independent of interleukin-4 receptor alpha (IL-4Rα)
signaling. Inflammation. 36:921–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zizzo G, Hilliard BA, Monestier M and
Cohen PL: Efficient clearance of early apoptotic cells by human
macrophages requires M2c polarization and MerTK induction. J
Immunol. 189:3508–3520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Edwards JP, Zhang X, Frauwirth KA and
Mosser DM: Biochemical and functional characterization of three
activated macrophage populations. J Leukoc Biol. 80:1298–1307.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chhor V, Le Charpentier T, Lebon S, Oré
MV, Celador IL, Josserand J, Degos V, Jacotot E, Hagberg H, Sävman
K, et al: Characterization of phenotype markers and neuronotoxic
potential of polarised primary microglia in vitro. Brain Behav
Immun. 32:70–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Freilich RW, Woodbury ME and Ikezu T:
Integrated expression profiles of mRNA and miRNA in polarized
primary murine microglia. PLoS One. 8:e794162013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fenn AM, Henry CJ, Huang Y, Dugan A and
Godbout JP: Lipopolysaccharide-induced interleukin (IL)-4
receptor-α expression and corresponding sensitivity to the M2
promoting effects of IL-4 are impaired in microglia of aged mice.
Brain Behav Immun. 26:766–777. 2012. View Article : Google Scholar
|
|
40
|
Liu HC, Zheng MH, Du YL, Wang L, Kuang F,
Qin HY, Zhang BF and Han H: N9 microglial cells polarized by LPS
and IL4 show differential responses to secondary environmental
stimuli. Cell Immunol. 278:84–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Michelucci A, Heurtaux T, Grandbarbe L,
Morga E and Heuschling P: Characterization of the microglial
phenotype under specific pro-inflammatory and anti-inflammatory
conditions: Effects of oligomeric and fibrillar amyloid-beta. J
Neuroimmunol. 210:3–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Crane MJ, Daley JM, van Houtte O, Brancato
SK, Henry WJ Jr and Albina JE: The monocyte to macrophage
transition in the murine sterile wound. PLoS One. 9:e866602014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Italiani P, Mazza EM, Lucchesi D, Cifola
I, Gemelli C, Grande A, Battaglia C, Bicciato S and Boraschi D:
Transcriptomic profiling of the development of the inflammatory
response in human monocytes in vitro. PLoS One. 9:e876802014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Orecchioni M, Ghosheh Y, Pramod AB and Ley
K: Macrophage polarization: Different gene signatures in M1(LPS+)
vs. classically and M2(LPS-) vs. alternatively activated
macrophages. Front Immunol. 10:10842019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Watanabe S, Alexander M, Misharin AV and
Budinger GRS: The role of macrophages in the resolution of
inflammation. J Clin Invest. 129:2619–2628. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Atri C, Guerfali FZ and Laouini D: Role of
human macrophage polarization in inflammation during infectious
diseases. Int J Mol Sci. 19:18012018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
He C and Carter AB: The metabolic
prospective and redox regulation of macrophage polarization. J Clin
Cell Immunol. 6:3712015. View Article : Google Scholar
|
|
49
|
Bansal S, Barathi V, Iwata D and Agrawal
R: Experimental autoimmune uveitis and other animal models of
uveitis: An update. Indian J Ophthalmol. 63:211–218. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Meng X, Fang S, Zhang Z, Wang Y, You C,
Zhang J and Yan H: Preventive effect of chrysin on experimental
autoimmune uveitis triggered by injection of human IRBP peptide
1-20 in mice. Cell Mol Immunol. 14:702–711. 2017. View Article : Google Scholar
|
|
51
|
Bousquet E, Camelo S, Leroux Les Jardins
G, Goldenberg B, Naud MC, Besson-Lescure B, Lebreton L, Annat J,
Behar-Cohen F and de Kozak Y: Protective effect of intravitreal
administration of tresperimus, an immunosuppressive drug, on
experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci.
52:5414–5423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Haruta H, Ohguro N, Fujimoto M, Hohki S,
Terabe F, Serada S, Nomura S, Nishida K, Kishimoto T and Naka T:
Blockade of interleukin-6 signaling suppresses not only th17 but
also interphotoreceptor retinoid binding protein-specific Th1 by
promoting regulatory T cells in experimental autoimmune
uveoretinitis. Investigative Invest Ophthalmol Vis Sci.
52:3264–3271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Luger D, Silver PB, Tang J, Cua D, Chen Z,
Iwakura Y, Bowman EP, Sgambellone NM, Chan CC and Caspi RR: Either
a Th17 or a Th1 effector response can drive autoimmunity:
Conditions of disease induction affect dominant effector category.
J Exp Med. 205:799–810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao J, Chen M and Xu H: Experimental
autoimmune uveoretinitis (EAU)-related tissue damage and
angiogenesis is reduced in CCL2-/-CX3CR1gfp/gfp mice. Invest
Ophthalmol Vis Sci. 55:7572–7582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen M, Copland DA, Zhao J, Liu J,
Forrester JV, Dick AD and Xu H: Persistent inflammation subverts
thrombospondin-1-induced regulation of retinal angiogenesis and is
driven by CCR2 ligation. Am J Pathol. 180:235–245. 2012. View Article : Google Scholar
|
|
56
|
Lipski DA, Dewispelaere R, Foucart V,
Caspers LE, Defrance M, Bruyns C and Willermain F: MHC class II
expression and potential antigen-presenting cells in the retina
during experimental autoimmune uveitis. J Neuroinflamm. 14:1362017.
View Article : Google Scholar
|
|
57
|
Miura-Takeda S, Tashiro-Yamaji J, Oku H,
Takahashi T, Shimizu T, Sugiyama T, Ikeda T, Kubota T and Yoshida
R: Experimental autoimmune uveoretinitis initiated by
non-phagocytic destruction of inner segments of photoreceptor cells
by Mac-1(+) mononuclear cells. Microbiol Immunol. 52:601–610. 2008.
View Article : Google Scholar
|
|
58
|
Niven J, Hoare J, McGowan D, Devarajan G,
Itohara S, Gannagé M, Teismann P and Crane I: S100B up-regulates
macrophage production of IL1β and CCL22 and influences severity of
retinal inflammation. PLoS One. 10:e1326882015. View Article : Google Scholar
|
|
59
|
Nguyen AM and Rao NA: Oxidative
photoreceptor cell damage in autoimmune uveitis. J Ophthalmic
Inflamm Infect. 1:7–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu GS, Lee TD, Moore RE and Rao NA:
Photoreceptor mitochondrial tyrosine nitration in experimental
uveitis. Invest Ophthalmol Vis Sci. 46:2271–2281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kimura A, Naka T, Nakahama T, Chinen I,
Masuda K, Nohara K, Fujii-Kuriyama Y and Kishimoto T: Aryl
hydrocarbon receptor in combination with Stat1 regulates
LPS-induced inflammatory responses. J Exp Med. 206:2027–2035. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huang Y, He J, Liang H, Hu K, Jiang S,
Yang L, Mei S, Zhu X, Yu J, Kijlstra A, et al: Aryl hydrocarbon
receptor regulates apoptosis and inflammation in a murine model of
experimental autoimmune uveitis. Front Immunol. 9:17132018.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chackerian AA, Oldham ER, Murphy EE,
Schmitz J, Pflanz S and Kastelein RA: IL-1 receptor accessory
protein and ST2 comprise the IL-33 receptor complex. J Immunol.
179:2551–2555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kurowska-Stolarska M, Stolarski B, Kewin
P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B,
van Rooijen N, et al: IL-33 amplifies the polarization of
alternatively activated macrophages that contribute to airway
inflammation. J Immunol. 183:6469–6477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Barbour M, Allan D, Xu H, Pei C, Chen M,
Niedbala W, Fukada SY, Besnard AG, Alves-Filho JC, Tong X, et al:
IL-33 attenuates the development of experimental autoimmune
uveitis. Eur J Immunol. 44:3320–3329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qu R, Zhou M, Qiu Y, Peng Y, Yin X, Liu B,
Bi H, Gao Y and Guo D: Glucocorticoids improve the balance of M1/M2
macrophage polarization in experimental autoimmune uveitis through
the P38MAPK-MEF2C axis. Int Immunopharmacol. 120:1103922023.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tortorella C, Simone O, Piazzolla G,
Stella I and Antonaci S: Age-related impairment of GM-CSF-induced
signalling in neutrophils: Role of SHP-1 and SOCS proteins. Ageing
Res Rev. 6:81–93. 2007. View Article : Google Scholar
|
|
68
|
Jost MM, Ninci E, Meder B, Kempf C, Van
Royen N, Hua J, Berger B, Hoefer I, Modolell M and Buschmann I:
Divergent effects of GM-CSF and TGFbeta1 on bone marrow-derived
macrophage arginase-1 activity, MCP-1 expression, and matrix
metalloproteinase-12: A potential role during arteriogenesis. FASEB
J. 17:2281–2283. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen M, Zhao J, Ali IHA, Marry S,
Augustine J, Bhuckory M, Lynch A, Kissenpfennig A and Xu H:
Cytokine signaling protein 3 deficiency in myeloid cells promotes
retinal degeneration and angiogenesis through arginase-1
up-regulation in experimental autoimmune uveoretinitis. Am J
Pathol. 188:1007–1020. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Benitez JT and Bouchard KR: Brain stem
auditory evoked response correlates in patients with
spinocerebellar lesions. Am J Otol. 7:183–187. 1986.PubMed/NCBI
|
|
71
|
Horstmann L, Schmid H, Heinen AP, Kurschus
FC, Dick HB and Joachim SC: Inflammatory demyelination induces glia
alterations and ganglion cell loss in the retina of an experimental
autoimmune encephalomyelitis model. J Neuroinflamm. 10:1202013.
View Article : Google Scholar
|
|
72
|
Cui Q, Yin Y and Benowitz LI: The role of
macrophages in optic nerve regeneration. Neuroscience.
158:1039–1048. 2009. View Article : Google Scholar
|
|
73
|
Starossom SC, Veremeyko T, Yung AWY,
Dukhinova M, Au C, Lau AY, Weiner HL and Ponomarev ED: Platelets
play differential role during the initiation and progression of
autoimmune neuroinflammation. Circ Res. 117:779–792. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Martinez FO and Gordon S: The M1 and M2
paradigm of macrophage activation: Time for reassessment.
F1000Prime Rep. 6:132014. View
Article : Google Scholar : PubMed/NCBI
|
|
75
|
McGeachy MJ: GM-CSF: The secret weapon in
the T(H)17 arsenal. Nat Immunol. 12:521–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ponomarev ED, Shriver LP, Maresz K,
Pedras-Vasconcelos J, Verthelyi D and Dittel BN: GM-CSF production
by autoreactive T cells is required for the activation of
microglial cells and the onset of experimental autoimmune
encephalomyelitis. J Immunol. 178:39–48. 2007. View Article : Google Scholar
|
|
77
|
Ponomarev ED, Shriver LP, Maresz K and
Dittel BN: Microglial cell activation and proliferation precedes
the onset of CNS autoimmunity. J Neurosci Res. 81:374–389. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ponomarev ED, Maresz K, Tan Y and Dittel
BN: CNS-derived interleukin-4 is essential for the regulation of
autoimmune inflammation and induces a state of alternative
activation in microglial cells. J Neurosci. 27:10714–10721. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang HL, Hassan MY, Zheng XY, Azimullah
S, Quezada HC, Amir N, Elwasila M, Mix E, Adem A and Zhu J:
Attenuated EAN in TNF-α deficient mice is associated with an
altered balance of M1/M2 macrophages. PLoS One. 7:e381572012.
View Article : Google Scholar
|
|
80
|
Kroenke MA, Carlson TJ, Andjelkovic AV and
Segal BM: IL-12- and IL-23-modulated T cells induce distinct types
of EAE based on histology, CNS chemokine profile, and response to
cytokine inhibition. J Exp Med. 205:1535–1541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ha Y, Liu H, Zhu S, Yi P, Liu W, Nathanson
J, Kayed R, Loucas B, Sun J, Frishman LJ, et al: Critical Role of
the CXCL10/C-X-C chemokine receptor 3 axis in promoting leukocyte
recruitment and neuronal injury during traumatic optic neuropathy
induced by optic nerve crush. Am J Pathol. 187:352–365. 2017.
View Article : Google Scholar :
|
|
82
|
Kwon MJ, Shin HY, Cui Y, Kim H, Thi AH,
Choi JY, Kim EY, Hwang DH and Kim BG: CCL2 mediates
neuron-macrophage interactions to drive proregenerative macrophage
activation following preconditioning injury. J Neurosci.
35:15934–15947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Butti E, Bergami A, Recchia A, Brambilla
E, Del Carro U, Amadio S, Cattalini A, Esposito M, Stornaiuolo A,
Comi G, et al: IL4 gene delivery to the CNS recruits regulatory T
cells and induces clinical recovery in mouse models of multiple
sclerosis. Gene Ther. 15:504–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Georgiou T, Wen YT, Chang CH, Kolovos P,
Kalogerou M, Prokopiou E, Neokleous A, Huang CT and Tsai RK:
Neuroprotective effects of Omega-3 polyunsaturated fatty acids in a
rat model of anterior ischemic optic neuropathy. Invest Ophthalmol
Vis Sci. 58:1603–1611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Locri F, Cammalleri M, Pini A, Dal Monte
M, Rusciano D and Bagnoli P: Further evidence on efficacy of diet
supplementation with fatty acids in ocular pathologies: Insights
from the EAE model of optic neuritis. Nutrients. 10:14472018.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sen HN and Nussenblatt RB: Sympathetic
ophthalmia: What have we learned? Am J Ophthalmol. 148:632–633.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Castiblanco CP and Adelman RA: Sympathetic
ophthalmia. Graefes Arch Clin Exp Ophthalmol. 247:289–302. 2009.
View Article : Google Scholar
|
|
88
|
Jakobiec FA, Marboe CC, Knowles DN II,
Iwamoto T, Harrison W, Chang S and Coleman DJ: Human sympathetic
ophthalmia. An analysis of the inflammatory infiltrate by
hybridoma-monoclonal antibodies, immunochemistry, and correlative
electron microscopy. Ophthalmology. 90:76–95. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shah DN, Piacentini MA, Burnier MN, McLean
IW, Nussenblatt RB and Chan CC: Inflammatory cellular kinetics in
sympathetic ophthalmia a study of 29 traumatized (exciting) eyes.
Ocul Immunol Inflamm. 1:255–262. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Abu El-Asrar AM, Struyf S, Van den Broeck
C, Van Damme J, Opdenakker G, Geboes K and Kestelyn P: Expression
of chemokines and gelatinase B in sympathetic ophthalmia. Eye
(Lond). 21:649–657. 2007. View Article : Google Scholar
|
|
91
|
Aziz HA, Flynn HW Jr, Young RC, Davis JL
and Dubovy SR: Sympathetic ophthalmia: Clinicopathologic
correlation in a consecutive case series. Retina. 35:1696–1703.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chan CC, Nussenblatt RB, Fujikawa LS,
Palestine AG, Stevens G Jr, Parver LM, Luckenbach MW and Kuwabara
T: Sympathetic ophthalmia. Immunopathological findings.
Ophthalmology. 93:690–695. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Furusato E, Shen D, Cao X, Furusato B,
Nussenblatt RB, Rushing EJ and Chan CC: Inflammatory cytokine and
chemokine expression in sympathetic ophthalmia: A pilot study.
Histol Histopathol. 26:1145–1151. 2011.PubMed/NCBI
|
|
94
|
Marak GE Jr: Recent advances in
sympathetic ophthalmia. Surv Ophthalmol. 24:141–156. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ben M'Barek K and Monville C: Cell therapy
for retinal dystrophies: From cell suspension formulation to
complex retinal tissue bioengineering. Stem Cells Int.
2019:45689792019.PubMed/NCBI
|
|
96
|
Athanasiou D, Aguila M, Bellingham J, Li
W, McCulley C, Reeves PJ and Cheetham ME: The molecular and
cellular basis of rhodopsin retinitis pigmentosa reveals potential
strategies for therapy. Prog Retin Eye Res. 62:1–23. 2018.
View Article : Google Scholar :
|
|
97
|
Kyger M, Worley A and Adamus G: Autoimmune
responses against photoreceptor antigens during retinal
degeneration and their role in macrophage recruitment into retinas
of RCS rats. J Neuroimmunol. 254:91–100. 2013. View Article : Google Scholar :
|
|
98
|
Sevenich L: Brain-resident microglia and
blood-borne macrophages orchestrate central nervous system
inflammation in neurodegenerative disorders and brain cancer. Front
Immunol. 9:6972018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xu H, Chen M and Forrester JV:
Para-inflammation in the aging retina. Prog Retin Eye Res.
28:348–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wohleb ES: Neuron-microglia interactions
in mental health disorders: 'For better, and for worse'. Front
Immunol. 7:5442016. View Article : Google Scholar
|
|
101
|
Edholm ES, Rhoo KH and Robert J:
Evolutionary aspects of macrophages polarization. Results Probl
Cell Differ. 62:3–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Neves J, Zhu J, Sousa-Victor P, Konjikusic
M, Riley R, Chew S, Qi Y, Jasper H and Lamba DA: Immune modulation
by MANF promotes tissue repair and regenerative success in the
retina. Science. 353:aaf36462016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Xie J, Li Y, Dai J, He Y, Sun D, Dai C, Xu
H and Yin ZQ: Olfactory ensheathing cells grafted into the retina
of RCS rats suppress inflammation by down-regulating the JAK/STAT
pathway. Front Cell Neurosci. 13:3412019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Olivares-González L, Velasco S, Gallego I,
Esteban-Medina M, Puras G, Loucera C, Martínez-Romero A,
Peña-Chilet M, Pedraz JL and Rodrigo R: An SPM-enriched marine oil
supplement shifted microglia polarization toward M2, ameliorating
retinal degeneration in rd10 mice. Antioxidants (Basel). 12:982022.
View Article : Google Scholar
|
|
105
|
Quigley HA, Nickells RW, Kerrigan LA,
Pease ME, Thibault DJ and Zack DJ: Retinal ganglion cell death in
experimental glaucoma and after axotomy occurs by apoptosis. Invest
Ophthalmol Vis Sci. 36:774–786. 1995.PubMed/NCBI
|
|
106
|
Levkovitch-Verbin H, Dardik R, Vander S,
Nisgav Y, Kalev-Landoy M and Melamed S: Experimental glaucoma and
optic nerve transection induce simultaneous upregulation of
proapoptotic and prosurvival genes. Invest Ophthalmol Vis Sci.
47:2491–2497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Prilloff S, Henrich-Noack P and Sabel BA:
Recovery of axonal transport after partial optic nerve damage is
associated with secondary retinal ganglion cell death in vivo.
Invest Ophthalmol Vis Sci. 53:1460–1466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Levkovitch-Verbin H, Quigley HA, Martin
KRG, Zack DJ, Pease ME and Valenta DF: A model to study differences
between primary and secondary degeneration of retinal ganglion
cells in rats by partial optic nerve transection. Invest Ophthalmol
Vis Sci. 44:3388–3393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Levkovitch-Verbin H, Quigley HA,
Kerrigan-Baumrind LA, D'Anna SA, Kerrigan D and Pease ME: Optic
nerve transection in monkeys may result in secondary degeneration
of retinal ganglion cells. Invest Ophthalmol Vis Sci. 42:975–982.
2001.PubMed/NCBI
|
|
110
|
Yoles E and Schwartz M: Degeneration of
spared axons following partial white matter lesion: Implications
for optic nerve neuropathies. Exp Neurol. 153:1–7. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li HY, Huang M, Luo QY, Hong X,
Ramakrishna S and So KF: Lycium barbarum (wolfberry) increases
retinal ganglion cell survival and affects both
microglia/macrophage polarization and autophagy after rat partial
optic nerve transection. Cell Transplant. 28:607–618. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wada Y, Nakamachi T, Endo K, Seki T,
Ohtaki H, Tsuchikawa D, Hori M, Tsuchida M, Yoshikawa A, Matkovits
A, et al: PACAP attenuates NMDA-induced retinal damage in
association with modulation of the microglia/macrophage status into
an acquired deactivation subtype. J Mol Neurosci. 51:493–502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Schwartz M: Macrophages and microglia in
central nervous system injury: Are they helpful or harmful? J Cereb
Blood Flow Metab. 23:385–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hayreh SS: Ischemic optic neuropathy. Prog
Retin Eye Res. 28:34–62. 2009. View Article : Google Scholar
|
|
115
|
Salgado C, Vilson F, Miller NR and
Bernstein SL: Cellular inflammation in nonarteritic anterior
ischemic optic neuropathy and its primate model. Arch Ophthalmol.
129:1583–1591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang C, Guo Y, Miller NR and Bernstein
SL: Optic nerve infarction and post-ischemic inflammation in the
rodent model of anterior ischemic optic neuropathy (rAION). Brain
Res. 1264:67–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Nicholson JD, Leiba H and Goldenberg-Cohen
N: Translational preclinical research may lead to improved medical
management of non-arteritic anterior ischemic optic neuropathy.
Front Neurol. 5:1222014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hayreh SS and Zimmerman MB: Nonarteritic
anterior ischemic optic neuropathy: Natural history of visual
outcome. Ophthalmology. 115:298–305.e2. 2008. View Article : Google Scholar
|
|
119
|
Bernstein SL, Johnson MA and Miller NR:
Nonarteritic anterior ischemic optic neuropathy (NAION) and its
experimental models. Prog Retin Eye Res. 30:167–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yin Y, Cui Q, Li Y, Irwin N, Fischer D,
Harvey AR and Benowitz LI: Macrophage-derived factors stimulate
optic nerve regeneration. J Neurosci. 23:2284–2293. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Orihuela R, McPherson CA and Harry GJ:
Microglial M1/M2 polarization and metabolic states. Brit J
Pharmacol. 173:649–665. 2016. View Article : Google Scholar
|
|
122
|
Wen YT, Huang TL, Huang SP, Chang CH and
Tsai RK: Early applications of granulocyte colony-stimulating
factor (G-CSF) can stabilize the blood-optic-nerve barrier and
ameliorate inflammation in a rat model of anterior ischemic optic
neuropathy (rAION). Dis Model Mech. 9:1193–1202. 2016.PubMed/NCBI
|
|
123
|
Desai TD, Wen YT, Daddam JR, Cheng F, Chen
CC, Pan CL, Lin KL and Tsai RK: Long term therapeutic effects of
icariin-loaded PLGA microspheres in an experimental model of optic
nerve ischemia via modulation of CEBP-β/G-CSF/noncanonical NF-κB
axis. Bioeng Transl Med. 7:e102892022. View Article : Google Scholar
|
|
124
|
Nguyen Ngo Le MA, Wen YT, Ho YC, Kapupara
K and Tsai RK: Therapeutic effects of puerarin against anterior
ischemic optic neuropathy through antiapoptotic and
anti-inflammatory actions. Invest Ophthalmol Vis Sci. 60:3481–3491.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Arranz A, Doxaki C, Vergadi E, Martinez de
la Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A,
Venihaki M, Margioris AN, et al: Akt1 and Akt2 protein kinases
differentially contribute to macrophage polarization. Proc Natl
Acad Sci USA. 109:9517–9522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Friedlander M: Fibrosis and diseases of
the eye. J Clin Invest. 117:576–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wynn TA: Common and unique mechanisms
regulate fibrosis in various fibroproliferative diseases. J Clin
Invest. 117:524–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wermuth PJ and Jimenez SA: The
significance of macrophage polarization subtypes for animal models
of tissue fibrosis and human fibrotic diseases. Clin Transl Med.
4:22015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Pastor JC, de la Rúa ER and Martin F:
Proliferative vitreoretinopathy: Risk factors and pathobiology.
Prog Retin Eye Res. 21:127–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Cordeiro MF, Schultz GS, Ali RR,
Bhattacharya SS and Khaw PT: Molecular therapy in ocular wound
healing. Br J Ophthalmol. 83:1219–1224. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Agrawal RN, He S, Spee C, Cui JZ, Ryan SJ
and Hinton DR: In vivo models of proliferative vitreoretinopathy.
Nat Protoc. 2:67–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Esser P, Heimann K and Wiedemann P:
Macrophages in proliferative vitreoretinopathy and proliferative
diabetic retinopathy: Differentiation of subpopulations. Br J
Ophthalmol. 77:731–733. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Martin F, Pastor JC, De La Rúa ER,
Mayo-Iscar A, García-Arumí J, Martínez V, Fernández N and Saornil
MA: Proliferative vitreoretinopathy: Cytologic findings in vitreous
samples. Ophthalmic Res. 35:232–238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Garweg JG, Tappeiner C and Halberstadt M:
Pathophysiology of proliferative vitreoretinopathy in retinal
detachment. Surv Ophthalmol. 58:321–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: Cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wong CW, Cheung N, Ho C, Barathi V, Storm
G and Wong TT: Characterisation of the inflammatory cytokine and
growth factor profile in a rabbit model of proliferative
vitreoretinopathy. Sci Rep. 9:154192019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ishikawa K, Kannan R and Hinton DR:
Molecular mechanisms of subretinal fibrosis in age-related macular
degeneration. Exp Eye Res. 142:19–25. 2016. View Article : Google Scholar
|
|
139
|
Song Y, Liao M, Zhao X, Han H, Dong X,
Wang X, Du M and Yan H: Vitreous M2 macrophage-derived
microparticles promote RPE cell proliferation and migration in
traumatic proliferative vitreoretinopathy. Invest Ophthalmol Vis
Sci. 62:262021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kobayashi Y, Yoshida S, Nakama T, Zhou Y,
Ishikawa K, Arita R, Nakao S, Miyazaki M, Sassa Y, Oshima Y, et al:
Overexpression of CD163 in vitreous and fibrovascular membranes of
patients with proliferative diabetic retinopathy: possible
involvement of periostin. Br J Ophthalmol. 99:451–456. 2015.
View Article : Google Scholar
|
|
141
|
Zhang J, Zhou Q, Yuan G, Dong M and Shi W:
Notch signaling regulates M2 type macrophage polarization during
the development of proliferative vitreoretinopathy. Cell Immunol.
298:77–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Lim LS, Mitchell P, Seddon JM, Holz FG and
Wong TY: Age-related macular degeneration. Lancet. 379:1728–1738.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Greaves NS, Ashcroft KJ, Baguneid M and
Bayat A: Current understanding of molecular and cellular mechanisms
in fibroplasia and angiogenesis during acute wound healing. J
Dermatol Sci. 72:206–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kent D and Sheridan C: Choroidal
neovascularization: A wound healing perspective. Mol Vis.
9:747–755. 2003.
|
|
145
|
Cherepanoff S, McMenamin P, Gillies MC,
Kettle E and Sarks SH: Bruch's membrane and choroidal macrophages
in early and advanced age-related macular degeneration. Br J
Ophthalmol. 94:918–925. 2010. View Article : Google Scholar
|
|
146
|
Bo Q, Shen M, Xiao M, Liang J, Zhai Y, Zhu
H, Jiang M, Wang F, Luo X and Sun X: 3-Methyladenine alleviates
experimental subretinal fibrosis by inhibiting macrophages and M2
polarization through the PI3K/Akt pathway. J Ocul Pharmacol Ther.
36:618–628. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
He L and Marneros AG: Macrophages are
essential for the early wound healing response and the formation of
a fibrovascular scar. Am J Pathol. 182:2407–2417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Cao X, Shen D, Patel MM, Tuo J, Johnson
TM, Olsen TW and Chan CC: Macrophage polarization in the maculae of
age-related macular degeneration: A pilot study. Pathol Int.
61:528–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Lai K, Li Y, Li L, Gong Y, Huang C, Zhang
Y, Cheng L, Xu F, Zhao H, Li C, et al: Intravitreal injection of
triptolide attenuates subretinal fibrosis in laser-induced murine
model. Phytomedicine. 93:1537472021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Mehla K and Singh PK: Metabolic regulation
of macrophage polarization in cancer. Trends Cancer. 5:822–834.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Egan KM, Seddon JM, Glynn RJ, Gragoudas ES
and Albert DM: Epidemiologic aspects of uveal melanoma. Surv
Ophthalmol. 32:239–251. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Johnson DB and Daniels AB: Continued poor
survival in metastatic uveal melanoma: Implications for molecular
prognostication, surveillance imaging, adjuvant therapy, and
clinical trials. JAMA Ophthalmol. 136:986–988. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Hood JL: The association of exosomes with
lymph nodes. Semin Cell Dev Biol. 67:29–38. 2017. View Article : Google Scholar
|
|
154
|
Bardi GT, Smith MA and Hood JL: Melanoma
exosomes promote mixed M1 and M2 macrophage polarization. Cytokine.
105:63–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Delwar ZM, Kuo Y, Wen YH, Rennie PS and
Jia W: Oncolytic virotherapy blockade by microglia and macrophages
requires STAT1/3. Cancer Res. 78:718–730. 2018. View Article : Google Scholar
|
|
156
|
Zhou D, Huang C, Lin Z, Zhan S, Kong L,
Fang C and Li J: Macrophage polarization and function with emphasis
on the evolving roles of coordinated regulation of cellular
signaling pathways. Cell Signal. 26:192–197. 2014. View Article : Google Scholar
|
|
157
|
Liu S, Zhang J, Fang S, Zhang Q, Zhu G,
Tian Y, Zhao M and Liu F: Macrophage polarization contributes to
the efficacy of an oncolytic HSV-1 targeting human uveal melanoma
in a murine xenograft model. Exp Eye Res. 202:1082852021.
View Article : Google Scholar
|
|
158
|
Spiller KL, Anfang RR, Spiller KJ, Ng J,
Nakazawa KR, Daulton JW and Vunjak-Novakovic G: The role of
macrophage phenotype in vascularization of tissue engineering
scaffolds. Biomaterials. 35:4477–4488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Jetten N, Verbruggen S, Gijbels MJ, Post
MJ, De Winther MPJ and Donners MMPC: Anti-inflammatory M2, but not
pro-inflammatory M1 macrophages promote angiogenesis in vivo.
Angiogenesis. 17:109–118. 2014. View Article : Google Scholar
|
|
160
|
Cheung N, Mitchell P and Wong TY: Diabetic
retinopathy. Lancet. 376:124–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Jenkins AJ, Joglekar MV, Hardikar AA,
Keech AC, O'Neal DN and Januszewski AS: Biomarkers in diabetic
retinopathy. Rev Diabet Stud. 12:159–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Wu JH, Li YN, Chen AQ, Hong CD, Zhang CL,
Wang HL, Zhou YF, Li PC, Wang Y, Mao L, et al: Inhibition of
Sema4D/PlexinB1 signaling alleviates vascular dysfunction in
diabetic retinopathy. EMBO Mol Med. 12:e101542020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Madonna R, Balistreri CR, Geng YJ and De
Caterina R: Diabetic microangiopathy: Pathogenetic insights and
novel therapeutic approaches. Vasc Pharmacol. 90:1–7. 2017.
View Article : Google Scholar
|
|
164
|
Chen C, Wu S, Hong Z, Chen X, Shan X,
Fischbach S and Xiao X: Chronic hyperglycemia regulates microglia
polarization through ERK5. Aging (Albany NY). 11:697–706. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Chen T, Zhu W, Wang C, Dong X, Yu F, Su Y,
Huang J, Huo L and Wan P: ALKBH5-mediated m6A
modification of A20 regulates microglia polarization in diabetic
retinopathy. Front Immunol. 13:8139792022. View Article : Google Scholar
|
|
166
|
Yao Y, Li J, Zhou Y, Wang S, Zhang Z,
Jiang Q and Li K: Macrophage/microglia polarization for the
treatment of diabetic retinopathy. Front Endocrinol (Lausanne).
14:12762252023. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Tang L, Zhang C, Lu L, Tian H, Liu K, Luo
D, Qiu Q, Xu GT and Zhang J: Melatonin maintains inner
blood-retinal barrier by regulating microglia via inhibition of
PI3K/Akt/Stat3/NF-κB signaling pathways in experimental diabetic
retinopathy. Front Immunol. 13:8316602022. View Article : Google Scholar
|
|
168
|
Fang M, Wan W, Li Q, Wan W, Long Y, Liu H
and Yang X: Asiatic acid attenuates diabetic retinopathy through
TLR4/MyD88/NF-κB p65 mediated modulation of microglia polarization.
Life Sci. 277:1195672021. View Article : Google Scholar
|
|
169
|
Good WV, Hardy RJ, Dobson V, Palmer EA,
Phelps DL, Quintos M and Tung B; Early Treatment for Retinopathy of
Prematurity Cooperative Group: The incidence and course of
retinopathy of prematurity: Findings from the early treatment for
retinopathy of prematurity study. Pediatrics. 116:15–23. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Dogra MR, Katoch D and Dogra M: An update
on retinopathy of prematurity (ROP). Indian J Pediatr. 84:930–936.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Dhawan A, Dogra M, Vinekar A, Gupta A and
Dutta S: Structural sequelae and refractive outcome after
successful laser treatment for threshold retinopathy of
prematurity. J Pediatr Ophthalmol Strabismus. 45:356–361. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Katoch D, Sanghi G, Dogra MR, Beke N and
Gupta A: Structural sequelae and refractive outcome 1 year after
laser treatment for type 1 prethreshold retinopathy of prematurity
in Asian Indian eyes. Indian J Ophthalmol. 59:423–426. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Lee J, Kim KE, Choi DK, Jang JY, Jung JJ,
Kiyonari H, Shioi G, Chang W, Suda T, Mochizuki N, et al:
Angiopoietin-1 guides directional angiogenesis through integrin
αvβ5 signaling for recovery of ischemic retinopathy. Sci Transl
Med. 5:203ra1272013. View Article : Google Scholar
|
|
174
|
Ma J, Mehta M, Lam G, Cyr D, Ng TF, Hirose
T, Tawansy KA, Taylor AW and Lashkari K: Influence of subretinal
fluid in advanced stage retinopathy of prematurity on proangiogenic
response and cell proliferation. Mol Vis. 20:881–893.
2014.PubMed/NCBI
|
|
175
|
Li J, Yu S, Lu X, Cui K, Tang X, Xu Y and
Liang X: The phase changes of M1/M2 phenotype of
microglia/macrophage following oxygen-induced retinopathy in mice.
Inflamm Res. 70:183–192. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Outtz HH, Tattersall IW, Kofler NM,
Steinbach N and Kitajewski J: Notch1 controls macrophage
recruitment and Notch signaling is activated at sites of
endothelial cell anastomosis during retinal angiogenesis in mice.
Blood. 118:3436–3439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Marchetti V, Yanes O, Aguilar E, Wang M,
Friedlander D, Moreno S, Storm K, Zhan M, Naccache S, Nemerow G, et
al: Differential macrophage polarization promotes tissue remodeling
and repair in a model of ischemic retinopathy. Sci Rep. 1:762011.
View Article : Google Scholar :
|
|
178
|
Sun X, Ma L, Li X, Wang J, Li Y and Huang
Z: Ferulic acid alleviates retinal neovascularization by modulating
microglia/macrophage polarization through the ROS/NF-κB axis. Front
Immunol. 13:9767292022. View Article : Google Scholar
|
|
179
|
Sui A, Chen X, Demetriades AM, Shen J, Cai
Y, Yao Y, Yao Y, Zhu Y, Shen X and Xie B: Inhibiting NF-κB
signaling activation reduces retinal neovascularization by
promoting a polarization shift in macrophages. Invest Ophthalmol
Vis Sci. 61:42020. View Article : Google Scholar
|
|
180
|
Zhu Y, Tan W, Demetriades AM, Cai Y, Gao
Y, Sui A, Lu Q, Shen X, Jiang C, Xie B and Sun X: Interleukin-17A
neutralization alleviated ocular neovascularization by promoting M2
and mitigating M1 macrophage polarization. Immunology. 147:414–428.
2016. View Article : Google Scholar :
|
|
181
|
Gao S, Li C, Zhu Y, Wang Y, Sui A, Zhong
Y, Xie B and Shen X: PEDF mediates pathological neovascularization
by regulating macrophage recruitment and polarization in the mouse
model of oxygen-induced retinopathy. Sci Rep. 7:428462017.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Zhou Y, Yoshida S, Nakao S, Yoshimura T,
Kobayashi Y, Nakama T, Kubo Y, Miyawaki K, Yamaguchi M, Ishikawa K,
et al: M2 macrophages enhance pathological neovascularization in
the mouse model of oxygen-induced retinopathy. Invest Ophthalmol
Vis Sci. 56:4767–4777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Ritter MR, Banin E, Moreno SK, Aguilar E,
Dorrell MI and Friedlander M: Myeloid progenitors differentiate
into microglia and promote vascular repair in a model of ischemic
retinopathy. J Clin Invest. 116:3266–3276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Fine SL, Berger JW, Maguire MG and Ho AC:
Age-related macular degeneration. New Engl J Med. 342:483–492.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Rosenfeld PJ, Shapiro H, Tuomi L, Webster
M, Elledge J and Blodi B; MARINA and ANCHOR Study Groups:
Characteristics of patients losing vision after 2 years of monthly
dosing in the phase III ranibizumab clinical trials. Ophthalmology.
118:523–530. 2011. View Article : Google Scholar
|
|
186
|
CATT Research Group; Martin DF, Maguire
MG, Ying GS, Grunwald JE, Fine SL and Jaffe GJ: Ranibizumab and
bevacizumab for neovascular age-related macular degeneration. New
Engl J Med. 364:1897–1908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Takeda A, Baffi JZ, Kleinman ME, Cho WG,
Nozaki M, Yamada K, Kaneko H, Albuquerque RJ, Dridi S, Saito K, et
al: CCR3 is a target for age-related macular degeneration diagnosis
and therapy. Nature. 460:225–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Rosenfeld PJ, Brown DM, Heier JS, Boyer
DS, Kaiser PK, Chung CY and Kim RY; MARINA Study Group: Ranibizumab
for neovascular age-related macular degeneration. N Engl J Med.
355:1419–1431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Brown DM, Kaiser PK, Michels M, Soubrane
G, Heier JS, Kim RY, Sy JP and Schneider S; ANCHOR Study Group:
Ranibizumab versus verteporfin for neovascular age-related macular
degeneration. N Engl J Med. 355:1432–1444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Grisanti S and Tatar O: The role of
vascular endothelial growth factor and other endogenous
interplayers in age-related macular degeneration. Prog Retin Eye
Res. 27:372–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zarbin MA: Current concepts in the
pathogenesis of age-related macular degeneration. Arch Ophthalmol.
122:598–614. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
He L and Marneros AG: Doxycycline inhibits
polarization of macrophages to the proangiogenic M2-type and
subsequent neovascularization. J Biol Chem. 289:8019–8028. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Nakamura R, Sene A, Santeford A, Gdoura A,
Kubota S, Zapata N and Apte RS: IL10-driven STAT3 signalling in
senescent macrophages promotes pathological eye angiogenesis. Nat
Commun. 6:78472015. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Yang Y, Liu F, Tang M, Yuan M, Hu A, Zhan
Z, Li Z, Li J, Ding X and Lu L: Macrophage polarization in
experimental and clinical choroidal neovascularization. Sci Rep.
6:309332016. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Wang C, Zhang R, Zhang Q, Jin H, Wei C, Wu
C, Mei L, Liu Y and Zhang P: Cytokine profiles and the effect of
intravitreal aflibercept treatment on experimental choroidal
neovascularization. Ophthalmic Res. 65:68–76. 2022. View Article : Google Scholar
|
|
196
|
Manthey CL, Moore BA, Chen Y, Loza MJ, Yao
X, Liu H, Belkowski SM, Raymond-Parks H, Dunford PJ, Leon F, et al:
The CSF-1-receptor inhibitor, JNJ-40346527 (PRV-6527), reduced
inflammatory macrophage recruitment to the intestinal mucosa and
suppressed murine T cell mediated colitis. PLoS One.
14:e2239182019. View Article : Google Scholar
|
|
197
|
Braza MS, Conde P, Garcia M, Cortegano I,
Brahmachary M, Pothula V, Fay F, Boros P, Werner SA, Ginhoux F, et
al: Neutrophil derived CSF1 induces macrophage polarization and
promotes transplantation tolerance. Am J Transplant. 18:1247–1255.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Zhou Y, Zeng J, Tu Y, Li L, Du S, Zhu L,
Cang X, Lu J, Zhu M and Liu X: CSF1/CSF1R-mediated crosstalk
between choroidal vascular endothelial cells and macrophages
promotes choroidal neovascularization. Invest Ophthalmol Vis Sci.
62:372021. View Article : Google Scholar
|
|
199
|
Zhang P, Lu B, Zhang Q, Xu F, Zhang R,
Wang C, Liu Y, Wei C and Mei L: LncRNA NEAT1 sponges MiRNA-148a-3p
to suppress choroidal neovascularization and M2 macrophage
polarization. Mol Immunol. 127:212–222. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Zandi S, Nakao S, Chun KH, Fiorina P, Sun
D, Arita R, Zhao M, Kim E, Schueller O, Campbell S, et al:
ROCK-isoform-specific polarization of macrophages associated with
age-related macular degeneration. Cell Rep. 10:1173–1186. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Xu Y, Cui K, Li J, Tang X, Lin J, Lu X,
Huang R, Yang B, Shi Y, Ye D, et al: Melatonin attenuates choroidal
neovascularization by regulating macrophage/microglia polarization
via inhibition of RhoA/ROCK signaling pathway. J Pineal Res.
69:e126602020. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Zhao S, Lu L, Liu Q, Chen J, Yuan Q, Qiu S
and Wang X: MiR-505 promotes M2 polarization in choroidal
neovascularization model mice by targeting transmembrane protein
229B. Scand J Immunol. 90:e128322019. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Gao L, Jiang W, Liu H, Chen Z and Lin Y:
Receptor-selective interleukin-4 mutein attenuates laser-induced
choroidal neovascularization through the regulation of macrophage
polarization in mice. Exp Ther Med. 22:13672021. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Liu Z, Mao X, Yang Q, Zhang X, Xu J, Ma Q,
Zhou Y, Da Q, Cai Y, Sopeyin A, et al: Suppression of myeloid
PFKFB3-driven glycolysis protects mice from choroidal
neovascularization. Br J Pharmacol. 179:5109–5131. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Cheng Y, Cheng T and Qu Y: TIMP-3
suppression induces choroidal neovascularization by moderating the
polarization of macrophages in age-related macular degeneration.
Mol Immunol. 106:119–126. 2019. View Article : Google Scholar
|
|
206
|
Lai K, Gong Y, Zhao W, Li L, Huang C, Xu
F, Zhong X and Jin C: Triptolide attenuates laser-induced choroidal
neovascularization via M2 macrophage in a mouse model. Biomed
Pharmacother. 129:1103122020. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Chanmee T, Ontong P, Konno K and Itano N:
Tumor-associated macrophages as major players in the tumor
microenvironment. Cancers (Basel). 6:1670–1690. 2014. View Article : Google Scholar : PubMed/NCBI
|



















