Introduction
Pemetrexed is a potent chemotherapeutic agent with
known high efficacy and relatively low toxicity in the treatment of
non-squamous non-small-cell lung cancer (NSCLC) and malignant
pleural mesothelioma (MPM). As first-line therapy for advanced lung
adenocarcinoma, pemetrexed-based chemotherapy (PBC) has yielded an
average overall survival (OS) of 12.6 months (1). As second-line therapy for advanced
NSCLC, pemetrexed has yielded an overall response rate of 9.1%, a
median progression-free survival (PFS) of 2.9 months and a median
survival time of 8.3 months (2).
As first-line therapy for MPM, the median survival time with
pemetrexed/cisplatin treatment was 12.1 months and the response
rate was significantly higher in the pemetrexed/cisplatin group
compared to that in the control group (41.3 vs. 16.7%,
respectively; P<0.0001) (3).
Maintenance therapy with pemetrexed is an effective
strategy and is recommended as standard treatment for patients who
achieve disease control following first-line chemotherapy with 4–6
cycles of platinum-based doublet chemotherapy regimens. However, a
considerable number of patients are not administered pemetrexed
maintenance therapy due to concerns regarding toxicity or high
treatment cost. Therefore, it is imperative to investigate PBC as
potential second-line therapy upon disease progression following
first-line chemotherapy.
It was recently demonstrated that PBC may be used as
rechallenge treatment in MPM patients who achieve a PFS ≥12 months
with first-line PBC (4–6). We have also noticed in clinical
practice that certain patients with non-squamous NSCLC benefited
from rechallenge with PBC as second- or further-line treatment.
Therefore, the aim of this study was to investigate the efficacy
and safety of rechallenge with PBC in patients with advanced
non-squamous NSCLC.
Materials and methods
Patient selection
We evaluated patients with histologically confirmed
advanced non-squamous NSCLC who underwent PBC rechallenge as
second- or further-line therapy. The presence of unidimensionally
measurable disease was mandatory for inclusion in the study. The
eligibility criteria also included age ≥18 years, Eastern
Cooperative Oncology Group (ECOG) performance status (PS) ≤2 and an
estimated life expectancy of ≥3 months. An adequate bone marrow
reserve was required, with absolute neutrophil count
≥2.0×109/l, platelet count ≥100×109/l and
hemoglobin ≥9 g/dl. Creatinine clearance ≥60 ml/min, bilirubin
≤1.5-fold the upper limit of normal (ULN) and alanine
aminotransferase or asparate aminotransferase (AST) ≤3-fold ULN
were also required, whereas AST was allowed to be ≤5-fold ULN in
patients with known hepatic metastases. Patients with severe
comorbidities or other malignancies were excluded from this study.
Written informed consent was obtained from each patient prior to
the initiation of the PBC rechallenge. This study conformed to the
ethical rules of the Declaration of Helsinki and the protocol was
approved by the Institutional Review Board of Beijing Cancer
Hospital and Institute.
Study design
We conducted a retrospective study on PBC
rechallenge in a consecutive series of patients who had received
initial PBC in the Department of Thoracic Medical Oncology at the
Beijing Cancer Hospital. This was an observational study, thus, no
statistical design was used. We conducted descriptive analyses of
PFS, OS, tumor response rate and toxicity.
Treatment
The PBC regimens included single-agent pemetrexed
(500 mg/m2 every 3 weeks) and pemetrexed plus a platinum
compound, such as carboplatin (area under the plasma concentration
time curve of 5 mg/ml/min administered on day 1 of every 21-day
cycle) or cisplatin (75 mg/m2 administered on days 1–2
of every 21-day cycle). All the patients received supplemental
folic acid and vitamin B12. Standard prophylaxis for nausea and
vomiting was also administered as dexamethasone and intravenous
5-hydroxytryptamine type 3 receptor antagonists. The chemotherapy
dose was reduced by 20% for patients who experienced grade 4
toxicity. Platinum-containing regimens were repeated for a maximum
of 6 cycles; eligible patients were then allowed to switch to
single-agent pemetrexed maintenance therapy, which was continued
until disease progression or unacceptable toxicity.
Patient assessment
The baseline patient assessment included a complete
medical history, physical examination, complete blood count (CBC)
and blood chemistry tests. CBC and blood chemistry tests were
obtained prior to each course of chemotherapy. A chest computed
tomography (CT) scan, cerebral magnetic resonance imaging and
cervical and abdominal ultrasound (US) examinations were performed
at baseline and after every 2 cycles of treatment. An abdominal CT
scan was required if the patient had developed abdominal
metastases. After completion of the study treatment, the patients
were evaluated every 3 months with chest CT and cervical and
abdominal US scans until disease progression. The patients were
observed for survival until death or last contact. The optimal
tumor response was evaluated according to the Response Evaluation
Criteria in Solid Tumors, version 1.1 (7). Treatment toxicity was evaluated
according to the National Cancer Institute Common Toxicity
Criteria, version 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
Sample collection and processing
DNA was extracted from formalin-fixed
paraffin-embedded (FFPE) tumor tissues using E.Z.N.A. FFPE DNA kits
(Omega Bio-Tek, Inc., Norcross, GA, USA). The quality and
concentration of the extracted DNA were determined using NanoDrop
2000 (Thermo Fisher Scientific, Wilmington, DE, USA). The extracted
DNA was then used for epithelial growth factor receptor (EGFR)
mutation analysis by denaturing high-performance liquid
chromatography (8). The
thymidylate synthase (TS) protein expression status was assessed by
immunohistochemistry using Thymidylate Synthase (D5B3)
XP® Rabbit mAb (Cell Signaling Technology, Danvers, MA,
USA). Immunohistochemical staining was performed according to the
manufacturer’s instructions. Two pathologists independently
quantified the staining intensity, which was graded on a scale from
0+ to 3+; the percentage of tumor cells was
noted within each intensity category. The percentage score was then
multiplied by its intensity category to obtain a final H-score,
which ranged from 0 to 300. The highest H-score of triplicate
scores for each patient was used for further analyses.
Echinoderm microtubule-associated
protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion
detection
Fluorescence in situ hybridization (FISH) was
performed on FFPE tumor tissues using the Vysis LSI ALK Dual Color
Break Apart Rearrangement Probe (Abbott Molecular, Abbott Park, IL,
USA). The assays were performed according to the manufacturer’s
instructions. The tumor sections were analyzed under a fluorescence
microscope equipped with a triple-pass filter (DAPI/Green/Orange).
A FISH-positive sample was defined as 15% of tumor cells with split
signals.
Statistical analysis
PFS was calculated as the time from the first day of
study treatment until disease progression, as indicated by
radiological or clinical examination, or death from any cause.
Patients without any evidence of progressive disease (PD) were
censored at the date of the last follow-up. OS was defined as the
time from the first day of study treatment until death from any
cause; patients who remained alive on the date of the last
follow-up were censored on that date. If survival status was
unknown at the final follow-up, the OS time was censored at the
last contact date. OS and PFS analyses were computed by the
Kaplan-Meier method. In all the cases, statistical significance was
established as P≤0.05. Statistical analyses were performed with the
SPSS 13.0 software package (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics and treatment
regimens
Between January, 2009 and June, 2013, a total of 31
patients underwent a PBC rechallenge in our department. Of the 31
patients, 16 were male and 15 were female. The median age was 58.5
years (range, 37–83 years). Of the 31 patients, 30 were diagnosed
with adenocarcinoma and 1 patient was diagnosed with
undifferentiated non-squamous NSCLC. A total of 19 patients
received initial PBC as first-line therapy and 12 received PBC as
second- or further-line therapy. A total of 11 patients received
the PBC rechallenge as second-line therapy and 20 patients received
the rechallenge as third- or further-line therapy. The clinical
characteristics of the patients are summarized in Table I.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | No. of patients
(n=31) | % |
|---|
| Age, years |
| Median (range) | 58.5 (37–83) | |
| Gender |
| Male | 16 | 51.6 |
| Female | 15 | 48.4 |
| Histotype |
| Adenocarcinoma | 30 | 96.8 |
|
Undifferentiated | 1 | 3.2 |
| ECOG performance
status |
| 0 | 0 | 0.0 |
| 1 | 30 | 96.8 |
| 2 | 1 | 3.2 |
| Response to initial
PBC |
| PR | 19 | 61.3 |
| SD | 12 | 38.7 |
| Response to PBC
rechallenge |
| PR | 5 | 16.1 |
| SD | 17 | 54.8 |
| PD | 9 | 29.1 |
| PFS after initial
PBC, months |
| <10 | 14 | 45.2 |
| ≥10 | 17 | 54.8 |
| TFS after initial
PBC, months |
| <3 | 8 | 25.8 |
| ≥3 | 23 | 74.2 |
| Line of therapy with
rechallenge |
| 2nd | 11 | 35.5 |
| 3rd | 7 | 22.6 |
| ≥4th | 13 | 41.9 |
The initial PBC regimens included pemetrexed plus
carboplatin (8 cases), pemetrexed plus cisplatin (17 cases) and
pemetrexed as single-agent therapy (6 patients). A total of 19
patients achieved a partial response (PR) and 12 patients achieved
stable disease (SD) following initial PBC. The median PFS following
initial PBC (PFS1) was 10.2 months (range, 1–23 months).
The PBC rechallenge regimens included pemetrexed as
single-agent therapy (11 cases), pemetrexed plus carboplatin (8
cases) and pemetrexed plus cisplatin (12 cases). A median of 4
cycles was administered (range, 2–8 cycles). Intermediate regimens,
which were administered between initial PBC and PBC rechallenge,
included single-agent docetaxel, gemcitabine and EGFR-tyrosine
kinase inhibitors (TKIs). A total of 7 patients received
further-line therapies following disease progression after PBC
rechallenge, including 2 patients who received an additional line
of PBC.
Outcomes of PBC rechallenge
After the PBC rechallenge, 5 patients (16.1%)
achieved a PR, 17 patients (54.8%) achieved SD and 9 patients
(29.1%) experienced PD. Overall, the disease control rate was
70.9%. Rechallenge with PBC was generally well tolerated. Grade 3/4
hematological toxicity was observed in 6 patients (19.4%), grade
1/2 fatigue was observed in 8 patients (25.8%), grade 3
gastrointestinal upset was reported in 1 patient and grade 1
proteinuria was observed in 1 patient. However, unlike single-agent
pemetrexed treatment, the doublet regimens were associated with
more severe fatigue, bone marrow depression and gastrointestinal
adverse effects, which led to treatment discontinuation in 4
patients.
The patients were followed up for a median of 34
months. During follow-up, 18 patients succumbed to the disease. Of
the 13 patients who remained alive on the date of the last
follow-up, 4 had no evidence of disease progression. The median PFS
for all the patients who received a PBC rechallenge was 3.8 months
(range, 1.3–15.1 months). There were no significant differences in
the overall response rate or PFS between single-agent and combined
chemotherapy regimens for the rechallenge (P=0.129 and P=0.201,
respectively).
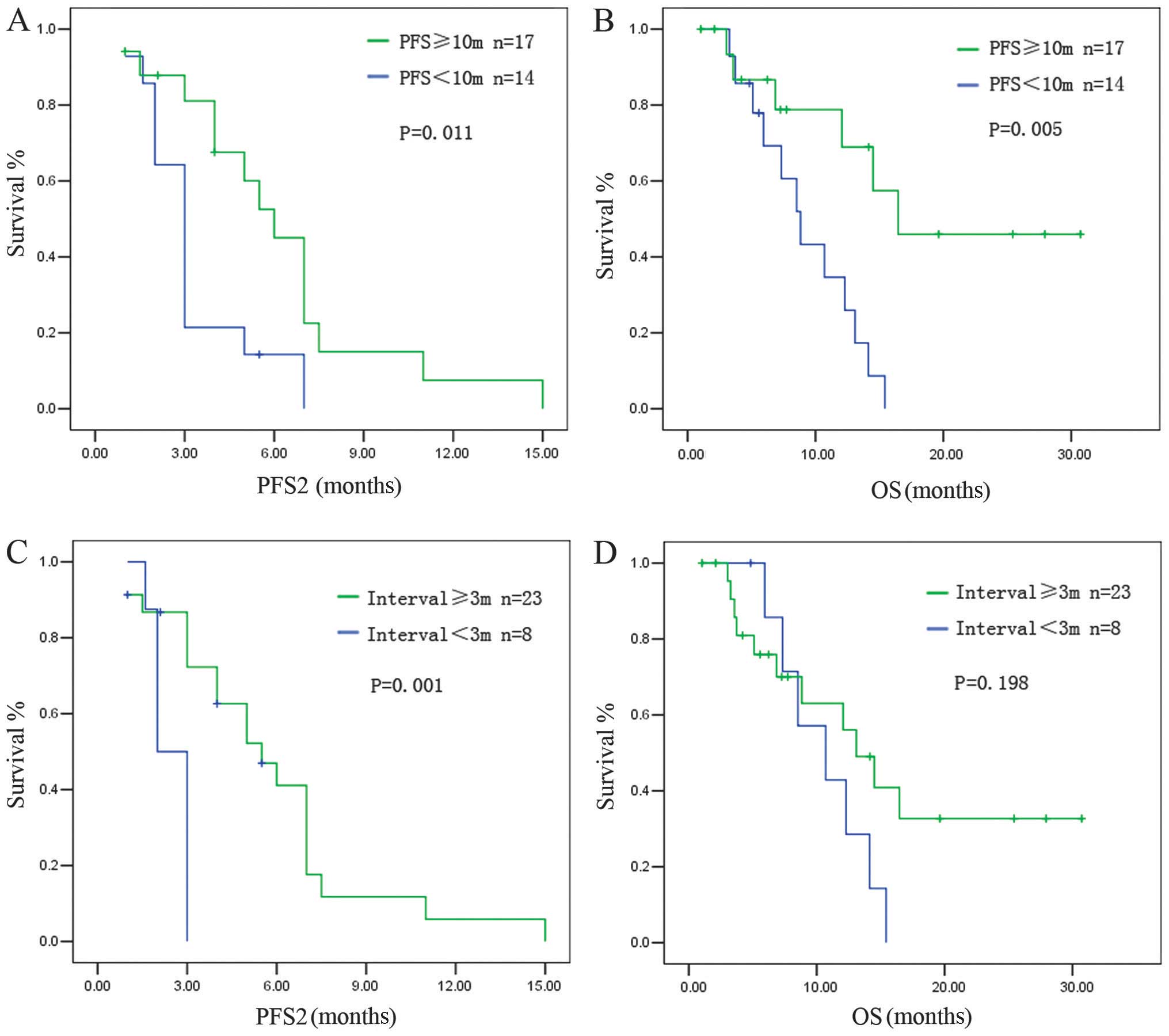
The PFS achieved after the PBC rechallenge (PFS2)
was correlated with PFS1. Patients with a PFS1 of ≥10 months had a
median PFS2 of 6.2±0.33 months, whereas patients with a PFS1 of
<10 months had a median PFS2 of 3.1±0.26 months (P=0.011)
(Fig. 1A). The OS for the
rechallenge was also correlated with PFS1. Patients with a PFS1 of
≥10 months had a longer OS compared to those with a PFS1 of <10
months (median OS: 16.47±3.2 vs. 8.83±1.24 months, respectively;
P=0.005) (Fig. 1B).
The treatment-free survival (TFS), calculated from
the last dose of the initial PBC to radiologic evidence of disease
progression, was also associated with survival after the
rechallenge. Patients with a TFS of ≥3 months had a longer PFS2
compared to those with a TFS of <3 months (median PFS2: 5.5±0.90
vs. 2.10±0.28 months, respectively; P=0.001) (Fig. 1C). Patients with a TFS of ≥3 months
tended to have a longer OS compared to patients with a TFS of <3
months, although statistical significance was not reached (median
OS: 13.10±1.93 vs. 10.70±2.79 months, respectively; P=0.198)
(Fig. 1D).
The response to initial PBC (PR vs. SD) did not
affect PFS2 or OS after the PBC rechallenge (median PFS2: 5.00±1.03
vs. 3.20±0.39 months, respectively; P=0.598; and median OS:
12.30±0.96 vs. 10.70±4.01 months, respectively; P=0.589) (Fig. 2).
Molecular analysis and PBC
rechallenge
Molecular markers were analyzed in the 31 patients
in this study who were classified as sensitive to PBC. The EGFR
mutation rate (exons 19, 21) was 25.8% (n=8). Of the 10 patients
who provided available FFPE sections for further analysis, 2 cases
were positive for EML4-ALK gene fusion and 4 patients exhibited
positive staining for TS, which were considered potential
predictors of pemetrexed efficacy (9–11).
We analyzed the same markers in another group of
patients (n=55) who were primarily resistant to PBC and experienced
PD within 4 cycles of initial treatment with PBC. The EGFR mutation
rate in this group was 29.1% (n=16). Of the 21 cases submitted for
further analysis, 7 patients exhibited positive TS expression and 2
patients were positive for EML4-ALK gene fusion (Table II). No significant differences
were observed between the PBC-sensitive and PBC-resistant
groups.
 | Table IIMolecular analyses. |
Table II
Molecular analyses.
| Markers | Group Aa | Group Bb |
|---|
| EGFR | 25.8% (8/31) | 29.1% (16/55) |
| TS | 40% (4/10) | 30% (7/21) |
| EML4-ALK | 20% (2/10) | 9.5% (2/21) |
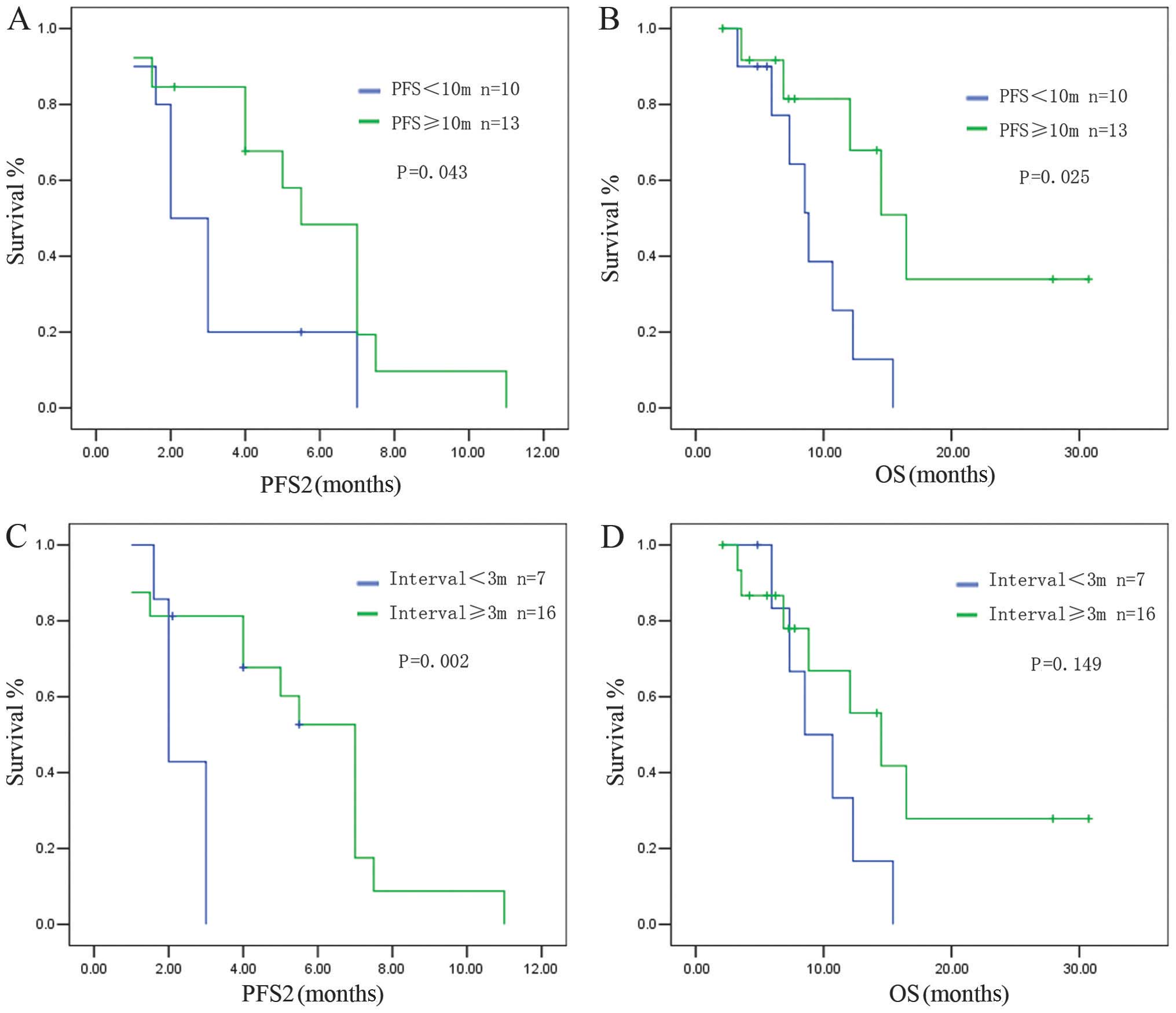
The EGFR mutation status may affect the clinical
outcome of PBC; therefore, we subsequently analyzed the subgroup of
23 EGFR wild-type patients in the original study group of 31
patients (we excluded the 8 patients with EGFR-sensitive
mutations). The results were similar to those observed in the
entire group.
Patients with a PFS1 of ≥10 months had a median PFS2
of 5.52±0.77 months, whereas patients with a PFS1 of <10 months
had a median PFS2 of 2.13±0.36 months (P=0.043) (Fig. 3A). A longer OS was observed in
patients with a PFS1 ≥10 months compared to patients with a PFS1 of
<10 months (median OS: 16.47±2.42 vs. 8.83±1.01 months,
respectively; P=0.025) (Fig.
3B).
Patients with a TFS of ≥3 months exhibited a longer
PFS2 after the PBC rechallenge compared to those with a TFS of
<3 months (PFS2: 7.2±0.52 vs. 2.0±0.18 months, respectively;
P=0.002) (Fig. 3C). The OS also
tended to be longer in the group with a longer TFS interval,
although statistical significance was not reached (OS: 14.5±2.94
vs. 8.53±2.06 months, respectively; P=0.149) (Fig. 3D).
Discussion
Malignancies such as ovarian cancer and SCLC, may be
retreated with the first-line chemotherapeutic regimen if a patient
develops a sensitive relapse, which is defined as disease that
responds to first-line chemotherapy, but relapses >6 months
after the last dose of the first-line treatment (12,13).
However, in NSCLC, there are currently no available studies on
rechallenging with certain first-line regimens.
In this study, we analyzed a total of 31 NSCLC
patients who underwent rechallenge with PBC. All the patients had
achieved disease control after the initial PBC. Rechallenging with
PBC was associated with acceptable tolerability, while doublet
regimens are generally associated with more severe adverse effects,
including bone marrow depression, liver damage and fatigue. Our
results suggested that combined PBC regimens should be selectively
reserved for patients with good PS scores and who have experienced
few toxicities with prior chemotherapy regimens.
We observed that the patients who achieved a PFS1 of
≥10 months exhibited a longer PFS2 and OS compared to those with a
PFS1 of <10 months. Patients with a TFS of ≥3 months also
exhibited a longer PFS2 compared to those with a TFS of <3
months. Such results are similar to those reported by previous
studies on MPM patients (4,6).
However, the cut-off value of PFS1 in this study was shorter
compared to that in the study of Ceresoli et al (4) (10 vs. 12 months, respectively). This
difference may be attributed to the differences in the physical
condition of the patients. In the Ceresoli study, all the patients
received PBC as first-line therapy; the PS score was 0 in 12
patients (38.7%) and 1 in 18 patients (58.1%). However, in the
present study, 12 patients (38.7%) received PBC as second- or
further-line therapy and the PS score was 1 in 30 patients and 2 in
1 patient. This gap in the physical condition of the patients is
likely to have caused the difference in the cut-off value of PFS1
between the two studies.
EGFR-TKIs play an important role in the treatment of
advanced non-squamous NSCLC and TKI therapy may significantly
affect OS. We observed that, in the subgroup with a PFS1 of <10
months, 4 patients were EGFR mutation-positive, 13 patients
received EGFR-TKI therapy and the median PFS was 5.38 months; in
the subgroup with PFS1 ≥10 months, 4 patients were EGFR
mutation-positive, 9 patients received EGFR-TKI therapy and the
median PFS was 4.33 months. No significant differences were
observed between these two subgroups. Furthermore, the PBC
rechallenge also increased survival among EGFR wild-type patients,
indicating that the survival results were likely not confounded by
EGFR-TKI therapy.
Continuous maintenance treatment with pemetrexed is
an effective and well-tolerated option for patients with advanced
non-squamous NSCLC with good PS who achieve disease control
following induction therapy with PBC. In the PARAMOUNT study
(14), a significant reduction in
the risk of disease progression was observed in the pemetrexed
group compared to the placebo group (HR=0.62; 95% CI: 0.49–0.79;
P<0.0001). The median PFS was 4.1 months (95% CI:3.2–4.6 months)
for pemetrexed and 2.8 months (95% CI: 2.6–3.1 months) for placebo.
The median OS was 13.9 months for pemetrexed and 11.0 months for
placebo. Pemetrexed maintenance therapy resulted in a 22% reduction
in the risk of death (HR=0.78, 95% CI: 0.64–0.96; P=0.0195). In the
AVAPERL study (15), after a
median follow-up of 8.1 months, the PFS from random assignment was
significantly improved in the bevacizumab plus pemetrexed group
compared to that in the bevacizumab alone group (median PFS: 3.7
vs. 7.4 months, respectively; HR=0.48; 95% CI: 0.35–0.66;
P<0.001).
However, not all patients require maintenance
therapy, since some patients experience a long PFS after initial
PBC without any sign of disease progression. For those who do not
receive maintenance therapy after induction therapy, a PBC
rechallenge may be the optimal second-line option, provided the
PFS1 or TFS is sufficiently long, as demonstrated by the present
study. These two modalities, maintenance and rechallenge therapy,
may apply to different patients. We hypothesized that, for patients
who experience disease progression within a short time after PBC
induction, maintenance therapy is required, whereas patients who
achieve long-term SD are candidates for rechallenge therapy.
However, this hypothesis requires confirmation by further
studies.
There were certain limitations to our study. First,
all the enrolled patients responded well to initial PBC, resulting
in a longer PFS1 (median, 10.2 months) compared to that previously
reported by studies on Asian patients (4.3–6.8 months) (16,17).
In addition, due to the relatively small sample size, we did not
observe any significant differences between PBC-sensitive and
PBC-resistant groups in the analyses of molecular markers.
To the best of our knowledge, this study was the
first on PBC rechallenge in lung cancer. We demonstrated that
rechallenging with PBC is tolerable and may be a viable option for
patients with advanced non-squamous NSCLC with a good PS and a PFS1
of ≥10 months or a TFS of ≥3 months after initial treatment with
PBC. Our findings may provide another effective strategy for
patients who had benefited from PBC induction chemotherapy but did
not receive pemetrexed maintenance therapy. Further large-scale
clinical trials are required to determine the best criteria for PBC
rechallenge and identify potential biomarkers that predict
sensitivity to pemetrexed.
Acknowledgements
This study was supported by the National Natural
Science Foundation Fund for Distinguished Young Scholars
(81025012); the National Natural Science Foundation Key Program
(81330062); and the Beijing Health Systems Academic Leader
(2011-2-22). We would like to thank our colleagues at the
Department of Thoracic Medical Oncology, Peking University School
of Oncology, Beijing Cancer Hospital and Institute, for their
support.
References
|
1
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanna N, Shepherd FA, Fossella FV, et al:
Randomized phase III trial of pemetrexed versus docetaxel in
patients with non-small-cell lung cancer previously treated with
chemotherapy. J Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
et al: Phase III study of pemetrexed in combination with cisplatin
versus cisplatin alone in patients with malignant pleural
mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ceresoli GL, Zucali PA, De Vincenzo F, et
al: Retreatment with pemetrexed-based chemotherapy in patients with
malignant pleural mesothelioma. Lung Cancer. 72:73–77. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zucali PA, Simonelli M, Michetti G, et al:
Second-line chemotherapy in malignant pleural mesothelioma: results
of a retrospective multicenter survey. Lung Cancer. 75:360–367.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bearz A, Talamini R, Rossoni G, et al:
Re-challenge with pemetrexed in advanced mesothelioma: a
multi-institutional experience. BMC Res Notes. 5:4822012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
8
|
Bai H, Wang Z, Chen K, et al: Influence of
chemotherapy on EGFR mutation status among patients with
non-small-cell lung cancer. J Clin Oncol. 30:3077–3083. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CY, Chang YL, Shih JY, et al:
Thymidylate synthase and dihydrofolate reductase expression in
non-small cell lung carcinoma: the association with treatment
efficacy of pemetrexed. Lung Cancer. 74:132–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Christoph DC, Asuncion BR, Mascaux C, et
al: Folylpoly-glutamate synthetase expression is associated with
tumor response and outcome from pemetrexed-based chemotherapy in
malignant pleural mesothelioma. J Thorac Oncol. 7:1440–1448. 2012.
View Article : Google Scholar
|
|
11
|
Monica V, Scagliotti GV, Ceppi P, et al:
Differential thymidylate synthase expression in different variants
of large-cell carcinoma of the lung. Clin Cancer Res. 15:7547–7552.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pfisterer J and Ledermann JA: Management
of platinum-sensitive recurrent ovarian cancer. Semin Oncol.
33(Suppl 6): S12–S16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garassino MC, Torri V, Michetti G, et al:
Outcomes of small-cell lung cancer patients treated with
second-line chemotherapy: A multi-institutional retrospective
analysis. Lung Cancer. 72:378–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paz-Ares LG, de Marinis F, Dediu M, et al:
PARAMOUNT: Final overall survival results of the phase III study of
maintenance pemetrexed versus placebo immediately after induction
treatment with pemetrexed plus cisplatin for advanced nonsquamous
non-small-cell lung cancer. J Clin Oncol. 31:2895–2902. 2013.
View Article : Google Scholar
|
|
15
|
Barlesi F, Scherpereel A, Rittmeyer A, et
al: Randomized phase III trial of maintenance bevacizumab with or
without pemetrexed after first-line induction with bevacizumab,
cisplatin, and pemetrexed in advanced nonsquamous non-small-cell
lung cancer: AVAPERL (MO22089). J Clin Oncol. 31:3004–3011. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawano Y, Ohyanagi F, Yanagitani N, et al:
Pemetrexed and cisplatin for advanced non-squamous non-small cell
lung cancer in Japanese patients: phase II study. Anticancer Res.
33:3327–3333. 2013.PubMed/NCBI
|
|
17
|
Xu B, Liu P, Yin Y, Liu P and Shu Y:
Pemetrexed as the first-line therapy for Chinese patients with
advanced non-squamous non-small-cell lung cancer. Biomed
Pharmacother. 67:763–769. 2013. View Article : Google Scholar : PubMed/NCBI
|